Appliedecology.cals.ncsu.edu
SRAC Publication No. 473
Southern regional
Medicated Feed for Food Fish
Anita M. Kelly1
Medicated feed is frequently recommended to control
drugs for use in aquaculture, preventing bacterial disease
bacterial disease outbreaks in cultured fish. Medicated
outbreaks with proper disease management strategies is
feeds are commercially prepared, and contain an anti-
the best method to avoid bacterial diseases in fish. For
biotic to control specific bacterial infections by either
more information on preventing diseases on fish farms
killing the bacteria or preventing the bacteria from
see SRAC Publication No. 4703,
Disease Prevention on
reproducing. Antibiotics do not control parasites, fungus,
Fish Farms; SRAC Publication No. 4707,
Biosecurity in
Aquaculture, Part 1: An Overview; and SRAC Publication
No. 4708,
Biosecurity in Aquaculture, Part 2: Recirculating
Bacterial disease
Bacterial diseases of fish are usually a result of a
Bacterial infections occur in fish just like any other
stressful event such as periods of low dissolved oxygen or
animal. Several different pathogenic bacteria are associ-
spawning stress. In nature, fish are generally less prone
ated with disease in cultured freshwater food fish such as
to bacterial disease outbreaks as they can seek the least
channel catfish, hybrid striped bass, and salmon. For spe-
stressful conditions. In aquaculture, fish often are unable
cific information on bacterial diseases see SRAC Publica-
to reduce their own stressful conditions, and thus, are
tion No. 477,
ESC - Enteric Septicemia of Catfish; SRAC
weakened by increased fish density, inadequate nutrition,
Publication No. 478,
Aeromonas Bacterial Infections -
poor water quality (i.e., low dissolved oxygen or high
Motile Aeromonad Septicemia; and SRAC Publication No.
ammonia and nitrite), parasite infestation, and handling.
479b,
Columnaris Disease: Flavobacterium columnare.
When stress occurs in fish, their immune system is
Fortunately, many bacterial diseases of cultured fish
suppressed, increasing susceptibility to bacterial infec-
can be successfully treated with medicated feeds that con-
tions. As a result, cultured fish are more susceptible to
tain U.S. Food and Drug Administration (FDA) approved
disease than free-ranging animals. Minimizing stressful
antibacterial drugs. These compounds have undergone
conditions often reduces the incidence of disease. Fail-
extensive animal, human-food, and environmental test-
ing to correct stressful conditions that lead to a disease
ing prior to approval for use in fish. Unfortunately, there
outbreak, even while treating sick fish with medicated
are very few drugs approved by the FDA for use in fish
feed, will usually prevent the medication from being fully
in the United States. Compounding this issue can be the
effective or will result in a reinfection of disease after
improper use of antibiotics which has resulted in devel-
treatment is completed. Therefore, medications should
opment of many antibiotic resistant strains of bacteria.
only be thought of as part of the strategy in controlling
Therefore, medicated feeds should only be used when
and preventing disease. Prior to and during medication,
absolutely necessary and according to label or veteri-
fish culturists should review all husbandry and environ-
narian instructions. Because there are so few approved
mental factors that may have contributed to the disease
outbreak and correct them to prevent the disease from
continuing or reoccurring.
1 University of Arkansas at Pine Bluff
Bacteria are either opportunistic or obligate patho-
ensure that the antibiotic is out of the fish and that it is
gens. Opportunistic bacteria are present in the water and
safe for human consumption. It is a good idea to designate
inside the fish, and generally cause no problem. When
this date on the pen, tank, or raceway with a clearly visible
culture conditions deteriorate, these bacteria will take the
and prominent sign in order to eliminate any potential
opportunity to cause disease in infected fish. Common
error of a premature harvest.
examples of opportunistic bacteria which can cause dis-
The U.S. Food and Drug Administration (FDA) has
ease and death of food fish include:
Aeromonas hydroph-
approved only four antibiotics for use in food fish. The
ila, Flexibacter columnaris, and
Pseudomonas fluorescens.
three antibiotics that are commercially available are
Ter-
Obligate pathogenic bacteria can cause disease even
ramycin® (Terramycin® 200 for Fish),
Romet® (Romet®30
in the absence of stressors. Examples include
Aeromonas
and Romet® TC) and
Florfenicol (Aquaflor®). Sulfamera-
salmonicida, Edwardsiel a ictaluri, Renibacterium salmo-
zine® is also approved but is no longer available. FDA
ninarum, and
Yersinia ruckeri. However, they can become
approves specific products that contain the antibiotics
more problematic under stressful environmental condi-
and only those specific products can be purchased and
used for species listed on the label. For example, if the fish
species is not listed in Table 1 for use with Terramycin®,
Use of medicated feeds
it cannot be legally used to treat the bacterial infection in
Once a bacterial infection has been diagnosed in
Each of the currently approved antibiotics and the
fish, an approved antibiotic feed can be determined. The
approved product is discussed separately below. This list
treatment should always be the maximum recommended
may change as new antibiotics obtain approval for use
dose for that species and should be fed for the total
in food fish. To determine if an antibiotic not on the list
number of days recommended (even if the fish appear to
below has obtained approval, please visit the FDA Center
have recovered before the end of the treatment period).
for Veterinary Medicines website at: http:/ www.fda.gov/
Feeding lower concentrations of antibiotics or decreas-
ing the number of days the drug is fed can allow bacterial
pathogens to develop a resistance to the antibiotic. If this
occurs, the antibiotic would likely not be able to control
certain infections that may occur later at a fish farm or
Terramycin® has been used for treatment of food fish
for many years. The approved product for fish is Terra-
Fish often stop eating as a bacterial disease pro-
mycin®200 for Fish, which contains the active ingredient
gresses, so early diagnosis and treatment are essential to
oxytetracycline dihydrate. This drug is usually effective
ensure that infected fish consume the medicated feed.
against a number of bacteria which cause disease in food
Once a bacterial disease is diagnosed, and the appropri-
ate medicated feed is determined, the feed should be used
Terramycin® 200 for Fish is incorporated into the feed
immediately. Furthermore, doses have been calculated
by commercial feed mills licensed by the FDA. The label
for an antibiotic to maintain a certain level in the blood-
(Table 2) includes a feeding rate to achieve the desired
stream for a certain period of time in order to be effective.
dosage levels required to attain adequate therapeutic
Depending upon the compound, it takes a day or so to
treatment. Terramycin® must be fed for 10 days to control
reach this level. Treatment should be done for the pre-
the infection. Once the treatment is completed, the fish
scribed time and never stopped prematurely because the
must be held for an additional 21 days before they can be
fish "look better".
marketed for food or released into the wild in order to
Prophylactic use of antibiotics is prohibited. Such use
allow elimination of the drug from the fish. Marketing
can lead to increased disease resistance and higher resi-
fish for human consumption before the end of the 21-day
dues of the antibiotic in the tissues of fish. Prophylactic
withdrawal period is a violation of federal law. As a result,
use of antibiotics has not been shown to increase growth
marketing plans must be considered before treating fish
rates in fish. For these reasons, only use antibiotics when
with Terramycin®. Once treated, fish cannot be sold for a
absolutely necessary to treat a bacterial infection.
minimum of 31 days (10-day treatment period plus 21-day
It is important to emphasize to all involved, the
withdrawal period). An additional consideration when
proper withdrawal time for the antibiotic used (see Table
feeding Terramycin® medicated feed manufactured at a
1), and that all understand that no food fish harvest will
feed mill is that it is only available as a sinking feed. The
occur prior to that period being completed. This is to
drug is broken down by the higher temperatures needed
Table 1: Antibiotics approved for use in medicated feed for foodfish.
Trade name
Indications
(For the control of:)
2.5 – 3.75 g per 100
21-day withdrawal time
lbs fish per day for
furunculosis (Aeromonas
salmonicida), bacterial
hemorrhagic septicemia
(A. liquefaciens), and
pseudomonas disease
Freshwater-raised Mortality due to
3.75 g per 100 lbs
21-day withdrawal time
coldwater disease caused
fish per day for 10
by
Flavobacterium
Mortality due to columnaris
3.75 g per 100 lbs
21-day withdrawal time
fish per day for 10
Oncorhynchus
Bacterial hemorrhagic
2.5 - 3.75 g per 100
Water temperature not
septicemia
(A. liquefaciens)
lbs fish per day for
and pseudomonas disease
21-day withdrawal time
Furunculosis due to
50 mg per kg fish per 42-day withdrawal time
Enteric septicemia due to
50 mg per kg fish per 3-day withdrawal time
Edwardsiel a ictaluri
Mortality due to enteric
10 mg per kg fish per 15-day withdrawal time
septicemia associated with
Edwardsiel a ictaluri
Freshwater-raised 1) Mortality due to
10 mg per kg fish per 15-day withdrawal time
furunculosis associated
with
Aeromonas
2) Mortality due to coldwater
disease associated with
F.
Mortality due to columnaris
15-day withdrawal time
disease associated with
per kg fish per day
for 10 daysOthers – 10 mg per
kg fish per day for 10
Freshwater-raised 1) Mortality due to
15 mg per kg fish per
warmwater finfish
streptococcal septicemia
15-day withdrawal time
Table 2: Label rates of Terramycin® 200 for fish to use at various feeding rates.
Feeding Rate
To achieve a dose rate of 2.5 – 3.75 g/100 pounds of fish
Pounds feed/
Terramycin in
Terramycin for Fish
Total biomass that one ton
100 Pounds fish (%)
Finished Feed
per ton of feed
of medicated feed will treat
to make a floating pellet. Feeding a sinking food to sick
pond fish makes it difficult to determine if they are eating
the medicated feed. Terramycin® 200 can be top dressed
The Veterinary Feed Directive (VFD) is a new cat-
on floating feeds with vegetable oil, but the stability of the
egory of medicated feeds created by the Animal Drug
antibiotic on the feed may be inferior to the stability in a
Availability Act of 1996. It provides an alternative to pre-
manufactured feed.
scription status for certain animal drugs for use in feed,
while requiring participation of a veterinarian to issue a
directive to enable producers to acquire VFD medicated
feeds. Antibiotics listed under the VFD listing cannot be
Romet® (Romet-30®, Romet-TC®) is approved for
used as extra label, meaning it cannot be used on fish spe-
use in salmonids and catfish. This product contains two
cies other than those listed on the label.
drugs, sulfadimethoxine and ormetoprim. These drugs
Florfenicol, sold under the trade name of Aquaflor®,
in combination are more effective than either drug used
is the first antibiotic used in aquaculture that falls within
alone, with both acting on different parts of the folic acid
the VFD. This means that florfenicol can only be used
metabolism pathway. Bacteria need to manufacture folic
under the supervision of a licensed veterinarian in the
acid for cell reproduction, where fish and humans can
context of a valid veterinarian-client relationship. Addi-
receive theirs from the diet. This makes the compound
tionally, florfenicol cannot be used under extra-label drug
very safe for animals
use options unless the producer is using it under an FDA
Romet® is specifically approved for treatment of
Investigational New Animal Drug (INAD).
bacterial diseases listed in Table 1. Romet® medicated
Florfenicol is specifically approved for treatment of
feed is only fed for 5 days as opposed to the 10 days for
bacterial diseases listed in Table 1. Florfenicol medicated
Terramycin®. The withdrawal period for Romet® is 3 days
feed is fed for 10 days. The withdrawal period for flor-
for channel catfish. With a 5-day treatment period and
fenicol is 15 days for channel catfish, salmonids raised in
a 3-day withdrawal period, catfish treated with Romet®
freshwater, and warmwater finfish raised in freshwater.
can be slaughtered in as little as eight days after the drug
As a result, fish cannot be sold for human consumption or
treatment is initiated. Salmonids have a required 42-day
stocked into natural waters for 25 days (10 day treatment
withdrawal period from Romet® before being slaughtered.
period and 15 day withdrawal time).
Another advantage of Romet® is its availability from com-
mercial mills in a floating pellet. This allows direct pond
observation of the fish eating the medicated feed.
Selecting the proper medicated feed
salmoninarum, which is the cause of bacterial kidney
disease in salmonids, or Mycobacterium species, which
To optimize the response to medicated feed, the
can occur in many food fish species including salmon and
causative agent needs to be identified and a sensitivity test
hybrid striped bass. This is why it is important to work
should be performed to ensure that the correct antibiotic
with a fish health specialist/fish veterinarian for proper
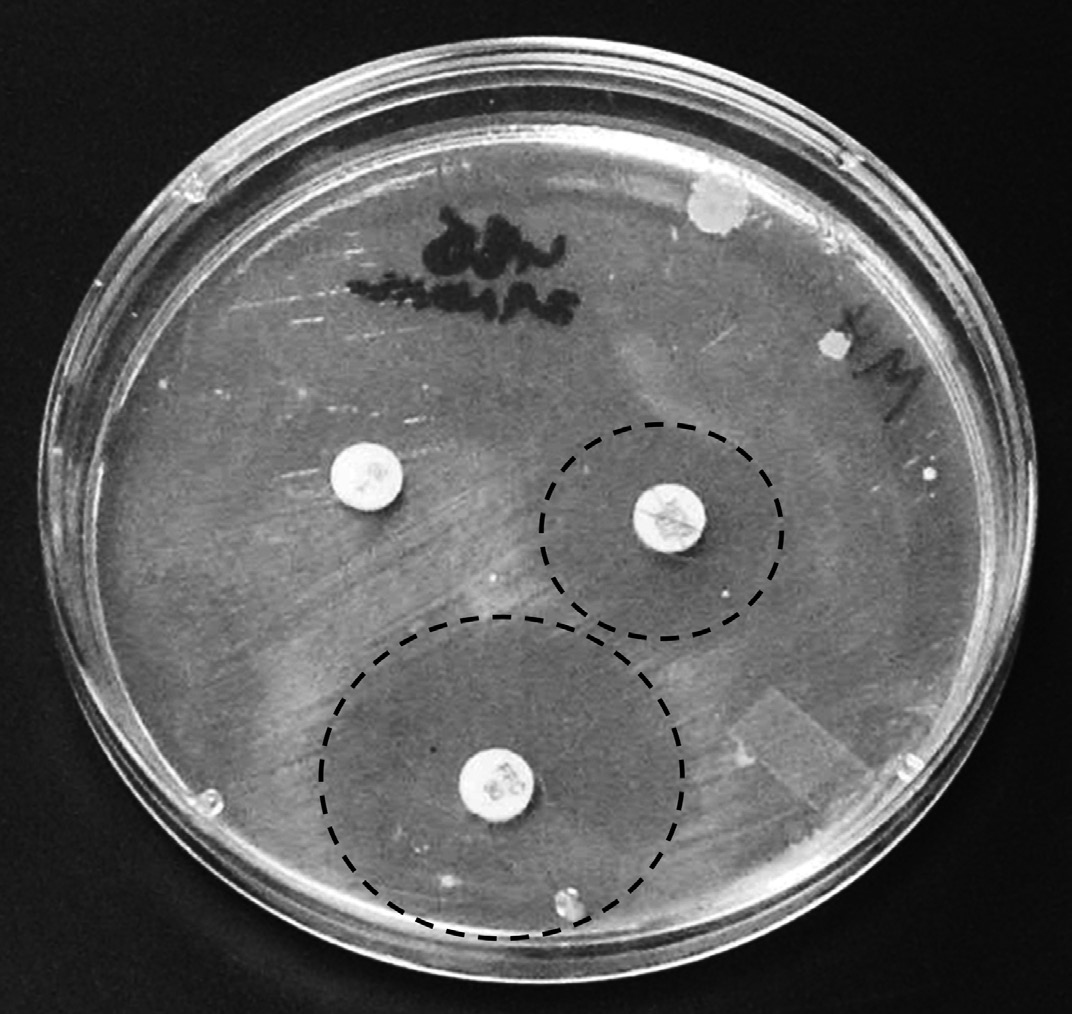
is used. A sensitivity test (Fig. 1) shows the relative suscep-
tibility of the disease-causing bacteria to various antibiot-
ics. Small discs, each containing a different antibiotic are
Treatment strategies
placed on an agar plate that has been recently inoculated
with the isolated disease causing bacteria. If bacteria are
Economics and other factors also help to determine
unable to grow in the presence of a particular antibiotic, a
the appropriateness of using medicated feed. If the cost
clear area is present surrounding the disc. If the drug has
of the treatment is more than the cost of the fish, it does
no effect, the bacteria will grow up to or over the top of the
not make economic sense to treat the fish. When possible,
disc. The clear area is measured and compared to a stan-
expensive treatments should be avoided unless they are
dard to determine if the antibiotic would be effective in
likely to save money for the producer. A good example is
treating the bacterial infection. A fish health professional
the treatment strategy for Enteric Septicemia of Catfish
or disease diagnostic laboratory can perform the sensitiv-
(ESC) caused by Edwardsiel a ictaluri. This disease occurs
ity test for you and recommend an antibiotic to be used.
when temperatures are between 68 and 82 °F (20 and 28
There are situations when antibiotic treatment may be
°C) when the bacteria are in their optimum growth range.
ineffective. Some disease outbreaks are caused by bacte-
Fish dying from ESC will usually stop dying as tempera-
ria that are resistant to particular antibiotics and some
tures rise above 82 °F (28 °C) or fall below 68 °F (20 °C).
bacterial diseases cannot be controlled with currently-
Medicating fish just before temperatures are forecast to
approved medicated feed. For example, there are no FDA
be in the 90s, for example, is often not advised, because
approved antibiotics currently available that are effective
the disease stops on its own due to the high temperatures.
against an active outbreak associated with Renibacterium
Using this type of strategy can save a significant amount
of money on medicated feed purchases.
Mixing medicated feeds
All of the approved antibiotics for use in food fish are
Type A medicated feeds. Producers may purchase Type
A premixes only if they hold a valid feed mill license. The
VFD form issued by the veterinarian will contain mix-
ing or dilution instructions. Medicated feed mill license
applications (Forms FDA 3448) may be obtained from the
Public Health Service, Consolidated Forms and Publica-
tions Distribution Center, Washington Commerce Center,
3222 Hubbard Rd., Landover, MD 20785, or electroni-
cally from the Center for Veterinary Medicine home
page at http:/ www.fda.gov/cvm. Additionally, a set of
guidelines for manufacturing feed, referred to as Good
Manufacturing Practices (GMPs), are designed to prevent
feed contamination and provide reasonable assurance
that medicated feed additives are used properly. These
guidelines serve as Food and Drug Administration (FDA)
Figure 1. Antibiotic discs on a bacterial plate (white circles). Clear area
regulations. Everyone involved in producing medicated
around the disc (highlighted by the dash lines) indicates that the antibiotic
or non-medicated feed, whether at a commercial off-farm
is useful in fighting the infection. Clear areas are measured in mil imeters
plant or with an on-farm mill or grinder/mixer, must
and compared with standards in order to determine if antibiotic is effective
comply with the GMPs.
against the bacteria disease.
Storage of medicated feed
Use of medicated feed
As with all fish food, medicated feed should be stored
in alternative species
in a cool, dry place. If available, a freezer is ideal for stor-
ing fish feed for extended periods provided it does not
At the time of this writing, FDA will allow veterinar-
get moist or wet. Antibiotics and essential nutrients will
ians to prescribe the use of Terramycin® 200 for Fish and
deteriorate rapidly in a warm, moist environment. Exces-
Romet®30 or Romet® TC medicated feed for fish species or
sive decomposition of antibiotics as a result of improper
diseases other than those listed on the label. For example,
storage can result in unsuccessful treatment. Any unused
Terramycin® 200 for Fish, medicated feed that has been
medicated feed, stored at room temperature, should be
approved for use in catfish, may be prescribed extra-label
discarded after 3 to 4 months. However, a VFD medicated
for hybrid striped bass by a licensed veterinarian. Flor-
feed is not valid 5 days after the prescribed treatment
fenicol (Aquaflor®) cannot be legally used on species other
terminates. Thus florfenicol (Aquaflor®) is a one-time use
than those on the label, unless it is used under an INAD.
purchase and any extra feed must be discarded and not
Check with a qualified fish health professional or veteri-
kept for future use. Be sure to follow all state guidelines
narian on the current status of medicated feed use regula-
for disposal of unused or old medicated feed.
tions before treating your fish.
Additional information on use of medicated feeds
approved for use in food fish can be found at:
http:/ www.fda.gov/AnimalVeterinary/Development
http:/ www.fws.gov/fisheries/aadap/home.htm
Quick Reference Guide to Approved Drugs for Use in
Aquaculture. 2011. http:/ www.fws.gov/fisheries/aadap/
SRAC fact sheets are reviewed annual y by the Publications, Videos and Computer Software Steering Committee. Fact sheets are revised
as new knowledge becomes available. Fact sheets that have not been revised are considered to reflect the current state of knowledge.
The work reported in this publication was supported in part by the Southern Regional
Aquaculture Center through Grant No. 2010-38500-21142 from the United States
Department of Agriculture, National Institute of Food and Agriculture.
Source: https://appliedecology.cals.ncsu.edu/wp-content/uploads/473.pdf
ORIGINAL STUDIES, REVIEWS, Volume 19, Number 11, 2009ª Mary Ann Liebert, Inc. AND SCHOLARLY DIALOG THYROID CANCER AND NODULES Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer The American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer
100 The Open Nutrition Journal, 2008, 2, 100-105 Open Access Alkaline Mineral Supplementation Decreases Pain in Rheumatoid Arthri-tis Patients: A Pilot Study Regina Maria Cseuz1, Istvan Barna2, Tamas Bender3 and Jürgen Vormann*,4 1Revita Klinik, Budapest, Hungary; 2Institute of Experimental Medicine of the Hungarian Academy of Sciences, Budapest, Hungary; 3Hospital Brothers of St. John of God, Budapest, Hungary, 4Institute for Prevention and Nutrition, Ismaning, Germany








