1475-2875-7-95.fm
Research
Substandard anti-malarial drugs in Burkina Faso
Maike Tipke*1, Salou Diallo2, Boubacar Coulibaly2, Dominic Störzinger3,
Torsten Hoppe-Tichy3, Ali Sie2 and Olaf Müller1
Address: 1Department of Tropical Hygiene and Public Health, Ruprecht-Karls-University Heidelberg, Im Neuenheimer Feld 324, 69120 Heidelberg, Germany, 2Centre de Recherche en Santé de Nouna (CRSN), Nouna, POB 2, Burkina Faso and 3University Pharmacy, Ruprecht-Karls-University Heidelberg, Im Neuenheimer Feld 670, 69120 Heidelberg, Germany
Email: Maike Tipke* -
[email protected]; Salou Diallo -
[email protected]; Boubacar Coulibaly -
[email protected]; Dominic Störzinger -
[email protected]; Torsten Hoppe-Tichy -
[email protected]; Ali Sie -
[email protected]; Olaf Müller -
[email protected]
* Corresponding author
Published: 27 May 2008
Received: 7 February 2008Accepted: 27 May 2008
Malaria Journal 2008,
7:95
2008 Tipke et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: There is concern about an increasing infiltration of markets by substandard and fake
medications against life-threatening diseases in developing countries. This is particularly worrying
with regard to the increasing resistance development of
Plasmodium falciparum against affordable
anti-malarial medications, which has led to a change to more expensive drugs in most endemic
countries.
Methods: A representative sample of modern anti-malarial medications from licensed (public and
private pharmacies, community health workers) and illicit (market and street vendors, shops)
sources has been collected in the Nouna Health District in north-western Burkina Faso in 2006.
All drugs were tested for their quality with the standard procedures of the German Pharma Health
Fund-Minilab. Detected low standard drugs were re-tested with European Pharmacopoeia 2.9.1
standards for disintegration and ultraviolet-visible spectroscopy at the laboratory of the Heidelberg
University for confirmation.
Results: Overall, 86 anti-malarial drug samples were collected, of which 77 samples have been
included in the final analysis. The sample consisted of 39/77 (50%) chloroquine, 10/77 (13%)
pyrimethamine-sulphadoxine, 9/77 (12%) quinine, 6/77 (8%) amodiaquine, 9/77 (12%) artesunate,
and 4/77 (5%) artemether-lumefantrine. 32/77 (42%) drug samples were found to be of poor
quality, of which 28 samples failed the visual inspection, nine samples had substandard
concentrations of the active ingredient, four samples showed poor disintegration, and one sample
contained non of the stated active ingredient. The licensed and the illicit market contributed 5/47
(10.6%) and 27/30 (90.0%) samples of substandard drugs respectively.
Conclusion: These findings provide further evidence for the wide-spread existence of
substandard anti-malarial medications in Africa and call for strengthening of the regulatory and
quality control capacity of affected countries, particularly in view of the now wider available and
substantially more costly artemisinin-based combination therapies.
(page number not for citation purposes)
Malaria Journal 2008,
7:95
The World Health Organization (WHO), thus, recom-
Malaria remains the globally most important parasitic dis-
mended taking action against substandard drugs at the
ease. Out of the approximately one million deaths caused
individual country leis recommendation is
by malaria each year, the great majority occurs in children
supported by the German Pharma Health Fund (GPHF)
under the age of five years living in sub-Saharan Africa
that designed an affordable laboratory kid for screening
drugs even under tropical conditions. The GPHF-Minilabis working with widely available reagents and can also be
Malaria, if treated early with effective drugs, is fully cura-
run without electricThe GPHF-Minilab oper-
ble. However, most of the affected children live in remote
ates in a four step procedure, including two physical
and poor communities with low access to functioning
methods (visual inspection and disintegration test) and
modern health services ildren are
two chemical methods (qualitative colour reaction test
either not treated at all, receive traditional treatment of
and semi-quantitative thin-layer chromatograp].
uncertain efficacy, or are treated with drugs primarilybought at the informal drug sector [
The aim of this study was to evaluate the presence of sub-standard anti-malarial drugs in the public, private, and
In recent years, increasing numbers of substandard and
informal markets of Burkina Faso.
fake medications were detected in the international mar-kets, but precise figures of the global situation are lacking.
It is estimated that more than 10% of the globally traded
medicines are counterfeits ]. In developing countries,
The study was carried out in the Nouna Health District
where regulatory and control mechanisms are weak, peo-
(NHD), a rural, multi-ethnic area of north-western
ple are at highest risk to purchase substandard medica-
Burkina Faso, West Africa. Most of the estimated 280,000
tions d in 2005: "Pharmaceutical
inhabitants of NHD are living under self-subsistence con-
products are attractive candidates for illegal trade, espe-
ditions. Malaria is the main cause of morbidity and mor-
cially in developing countries. They are easily transporta-
tality in the NHD as in the whole of Bu].
ble, have high value per unit, and most importantly, their
Formal health services in NHD comprise of 25 primary
quality cannot be assessed readily by lay persons or even
health care facilities, which are covering the needs of a var-
experts without the aid of a quality testing laboratory" ].
iable number of villages, and the district hospital in
Drugs to treat infectious diseases, like malaria, pneumo-
Nouna town D is holoendemic for malaria with
nia or diarrhoea, are frequently a target of criminal action
elevated transmission rates during and shortly after the
rainy season, which typically lasts from June to October].
After the marketing of the artemisinin-based combinationtherapy (ACT) against malaria in Asia, these costly drugs
Study design and procedures
were found to be counterfeit in 38% and 53% in two stud-
This cross-sectional study was carried out during the rainy
ies conducted in different countries of south-east Asia
season of 2006. Drugs were sampled in August and Sep-
]. In Cambodia, for example, it was shown that fake
tember which are known to be the months of most
artesunate was sold by 71% of local drug vendor
intense drug sale in this area of West].
Globally it is estimated that more than US$ 30 billion peryear are earned by the overall trade of substandard and
Anti-malarial drugs were collected from a sample of 16
counterfeit drugs and this will probably increase to US$
villages (based on specific information from the results of
75 billion by the year.
a representative anti-malarial drug provider study con-ducted in early 2006 in the whole of NHD), and in Nouna
The intake of fake medicaments can lead to life-threaten-
town. After identification of all points of drug sale in these
ing consequences [Especially for diseases carrying
villages, anti-malarial drug samples were purchased from
a high mortality if left untreated, like malaria, substand-
markets, street vendors, shops, private pharmacies, com-
ard drugs will raise death rates. Furthermore, such drugs
munity health workers, and the governmental pharmacies
can lead to numerous adverse drug effects due to under-
attached to the peripheral health centres. Each market
dosing, over-dosing, and unexpected or toxic substances
place was defined as one point of sale and the drug collec-
]. Moreover, it will influence the economic welfare of
tion was carried out on respective market days. For the
patients, health systems, and drug companies that pro-
analysis, private pharmacies, community health workers,
duce genuine pr]. Finally, it will most likely
and the health centre and hospital pharmacies were
increase the risk of selection and spreading of drug resist-
defined as licensed market, while markets, street vendors,
an. Fake drugs, furthermore, can lead to biased data
and shops were summarized as illicit market. The sample
on drug resistance or drug efficacy studies].
consisted of tablets and capsules of chloroquine, amodi-
(page number not for citation purposes)
Malaria Journal 2008,
7:95
aquine, sulphadoxine/pyrimethamine, quinine, artesu-
the Heidelberg University. The confirmatory disintegra-
nate and, artemether-lumefantrine.
tion tests were performed according to European Pharma-copoeia 2.9.1 [ntification of the active
Drugs were purchased by one field worker who behaved
ingredient of respective samples was done with the ultra-
like a normal client. Each sample of a drug was labelled
violet-visible spectroman investiga-
with an identification number and put into a plastic bag
tors were blinded to the origin of respective samples.
without any further manipulation. A drug collectionsheet, which provided information on the date, place, and
conditions of purchase, the name of the drug indicated by
All data were transferred into a Microsoft Excel database.
the vendor and the name stated on the product, the active
The descriptive analysis was done by SPSS 12.0 for Win-
ingredient and the price, was filled in immediately. After
purchase, all drug samples have been stored in a dark, dryand air conditioned place inside the laboratory building
of the
Centre de Recherche en Santé de Nouna (CRSN).
The study is part of the A8 study of the SFB 544, which hasbeen approved by the local Ethical Committee of the
At the time-point of the study no artemisinin drugs or
CRSN, Burkina Faso, and by the Ethical Committee of the
ACT were available in the NHD, except in the two private
Heidelberg University. The specific study procedures were
pharmacies in Nouna town. To get a broader impression
discussed and approved by the responsible authorities of
on the quality of artemisinin drugs or ACT in Burkina
the NHD. At each point of sale, not more than 50% of the
Faso, a convenience sample of such products was taken
total amount of available malaria drugs was purchased.
from market places and private pharmacies in randomlyselected quarters of Ougadougou, the capital of Burkina
A total of 86 anti-malarial drug samples have been col-lected, 79 came from the NHD and seven from Ouaga-
dougou (Figure these, 48/86 (56%) were
The initial testing of all drug samples was performed at the
chloroquine samples, 6/86 (7%) were amodiaquine sam-
laboratory of the CRSN with the German Pharma Health
ples, 10/86 (12%) were pyrimethamine-sulphadoxine
Fund (GPHF)-Minilab. Two physical methods (visual
samples, 9/86 (10%) were quinine samples, and 13/86
inspection and disintegration test) and two chemical
(15%) were artemisinin or ACT samples (of those, six
methods (qualitative colour reaction test and semi-quan-
were from NHD and seven from Ouagadougou).
titative thin-layer chromatography) were performedaccording to the existing GPHF-Minilab standards. All
Overall, 18/86 (21%) samples, which had failed the
data was noted down in the specific "reporting forms" of
GPHF-Minilab disintegration and/or semi-quantitative
the GPHF-Minilab.
thin-layer chromatography tests, were re-tested for confir-mation. Another 9/86 (10%) drug samples showed tailing
GPHF-Minilab procedures were performed by the two
phenomena in the GPHF-Minilab semi-quantitative thin-
investigators (MT and SD). In case a sample failed the vis-
layer chromatography. Tailing phenomena indicate possi-
ual inspection by one of the investigators it was re-exam-
ble auxiliary substances in the respective drug samples (all
ined by the second investigator for confirmation.
nine samples were chloroquine). These samples were also
Furthermore, in cases of doubt external aid was consulted
referred for further investigation to the pharmacy of the
(e.g. internet research on manufactures information and
University of Heidelberg. Here it was concluded that these
figures provided on homepages). Drug samples which
phenomena were most probably caused by povidone,
failed the colour reaction by the first investigator, i.e. none
which are widely used as excipient in tablets. As it cannot
of the stated and expected active ingredient was present,
be excluded that other unknown substances have caused
were retested in a second run by the respectively second
the tailing phenomena, these nine samples were excluded.
investigator for confirmation. The aim of this procedure
Thus, 77 anti-malarial drug samples were finally included
was to confirm the coherence of the stated and actually
in the analysisf these, 47/77 (61%)
present active ingredient. Further investigations to deter-
were from the licensed market and 30/77 (39%) were
mine potential other present active ingredients, which
from the illicit market (Tab
were not indicated, were therefore, not performed.
In total, 32/77 (41.6%) anti-malarial drug samples were
Drug samples that failed the disintegration test or semi-
found to be of substandard quality, of which 28/32 (88%)
quantitative thin-layer chromatography in the GPHF-
samples failed the visual inspection. These samples have
Minilab testings, were sent to Germany, where confirma-
been chloroquine, 23/28 (82%); sulphadoxine/pyrimeth-
tory tests took place at the laboratory of the pharmacy of
amine, 4/28 (14%); and artesunate, 1/28 (4%). 4/32
(page number not for citation purposes)
Malaria Journal 2008,
7:95
Anti-malarial drug samples
Samples contained
auxiliary substances
Samples referred for
identification testing
Samples excluded
18/ 32 samples reference tested at
from final analysis
the University of Heidelberg
Samples included in the final
GPHF = German Pharma Health Fund, TLC = Thin Layer Chromatography
Flow chart of study sample.
(13%) samples were found to be of poor disintegration
quantitative thin-layer chromatography tests and ultravio-
(three chloroquine, one sulphadoxine/pyrimethamine)
let-visible spectroscopy. Here, 9/31 (29%) anti-malarial
drug samples with substandard concentrations of therespective active ingredient were detected. Of these, 5/9
One of 32 (3%) samples (sulphadoxine/pyrimethamine)
(55.6%) were chloroquine, 3/9 (33.3%) were quinine,
failed the colour reaction, indicating neither sulphadox-
and 1/9 (11.1%) were sulphadoxine/pyrimethamine
ine nor pyrimethamine was present in this sample. This
sample, thus, was excluded from the following semi-
Table 1: Origin of anti-malarial drugs included into the final analysis
NHD except Nouna town
Nouna town
NHD = Nouna Health District
(page number not for citation purposes)
Malaria Journal 2008,
7:95
Table 2: Points of sale and numbers of anti-malarial drugs included in the sample
NHD except Nouna town
Nouna town
Public health facility
Community health worker
Market place
NHD = Nouna Health District
Substandard drugs in the licensed and illicit anti-malarial
health servicesAlso private pharmacies in this country
reported difficulties in the constant supply with ACT, due
Overall, 47/77 (61.0%) anti-malarial drug samples were
to shortages at the wholesalers. Increasing demand,
purchased at the licensed market and 30/77 (39.0%) at
accompanied by unavailability through legal sources, will
the illicit market. 5/47 (10.6%) anti-malarial drug sam-
be likely to contribute to the growing problem of fake
ples from licensed and 27/30 (90.0%) samples from illicit
medica]. Beside this, criminal greed, the lack of
sources respectively were found to be of substandard qual-
legislation, lack of law enforcement, corruption, and com-
ity. Due to signs of decline of tablets, one artesunate sam-
plex trade arrangements, are facilitating the influx of sub-
ple in our study was found to be substandard. The
standard and counterfeit products in the developing
artesunate sample was labelled with the letters "not for
sale" and purchased at a market place in Ougadougou.
The main result from this study is the large proportion of
The visual inspection was failed by 27/30 (90.0%) anti-
anti-malarial drugs found to be substandard in Burkina
malarial drug samples from the illicit market, while one
Faso. Nearly half of the sample showed varying degrees of
sample purchased in the licensed market did not pass.
impaired quality and, probably not surprising, the great
Taprovides detailed information on the reasons for
majority of those were from the illicit market. However,
not passing the visual inspection differentiated by the two
all failures detected in nevertheless which step of the qual-
different market types. All products that failed the disinte-
ity testing procedure were treated alike in our analysis.
gration (4/77, 5.2%) or colour reaction (1/77, 1.3%) were
This is despite the fact that the different types of reduced
purchased in the illicit market. Substandard concentra-
quality may well have different impacts (e.g. a not cor-
tions of the active ingredient were detected in 5/29
rectly labelled and/or sticky, broken and dirty tablet might
(17.2%) and 4/47 (8.5%) of anti-malarial samples from
have contained the proper amount of active ingredient,
the illicit and licensed markets respectively.
and thus would not have necessarily reduced treatmentsuccess). Nevertheless, international drug quality stand-
ards should be applied worldwide.
In Burkina Faso, ACT is recommended for the treatmentof uncomplicated malaria since 2005, but until recently,
Our results support the findings from other studies which
these drugs were not available through governmental
have addressed the topic of substandard drugs in SSA
Table 3: Number (%) of failed samples listed by the active ingredient and test
Thin-layer chromatography
* Some samples failed more than one test
(page number not for citation purposes)
Malaria Journal 2008,
7:95
Table 4: Real value analysis of samples with substandard concentrations of the active ingredient
Place of purchase
Real value analysis (mg)
Chloroquine Phosphate (100 mg)
Chloroquine Phosphate (100 mg)
Chloroquine Phosphate (250 mg)
Chloroquine Phosphate (250 mg)
Chloroquine Phosphate (250 mg)
Sulfadoxine/Pyrimethamine (500/25 mg)
Quinine Sulfate (300 mg)
public health facility
Quinine Sulfate (300 mg)
public health facility
Quinine Sulfate (300 mg)
TLC = Thin Layer Chromatography, min. = minimal detected content, max. = maximal detected content, mid. = average detected content
-m Cameroon for example, 32% of
This is partly explained by tablets being carried around in
chloroquine, 10% of quinine, and 13% of sulphadoxine/
huge bags from one market to the other. The substandard
phyrimethamine samples were found to be substandard
physical composition of products from illegal drug
a study on private pharmacies in Nigeria, 48% of
sources was also confirmed by the results of the disinte-
anti-infective drugs, including anti-malarial drugs, were
gration test, which is most likely related to poor storage
found to be of impair
conditions. High temperatures and humidity can influ-ence the dissolution rate, thus leading to suboptimal
In the illicit market, drugs are sold as blister packs, as sin-
activity of the drug due to reduced bio-availability
gle tablets cut off from a blister, or just taken from big con-
,,]. Another reason that can prevent proper
tainers or plastic bags without any labelling, packaging or
disintegration is related to the formulation of the drug
instruction leaflets A large proportion of anti-
itself []. Deliberately counterfeit drugs might contain
malarial drugs obtained in the illicit market failed the vis-
substances such as flour, baking powder or other sub-
ual inspection due to signs of decline and destruction.
Table 5: Reasons for not passing the visual inspection separated by different kind of markets
Manufacture not identified
Signs of decline*
Manufacture not identified + missing tablets
Manufacture not identified + signs of decline*
missing tablets in blister + signs of decline*
Manufacture not identified + expired + signs of decline*
Manufacture not identified + package damaged + signs of decline*
Signs of decline = sticky, broken, dirty, or high abrasion
(page number not for citation purposes)
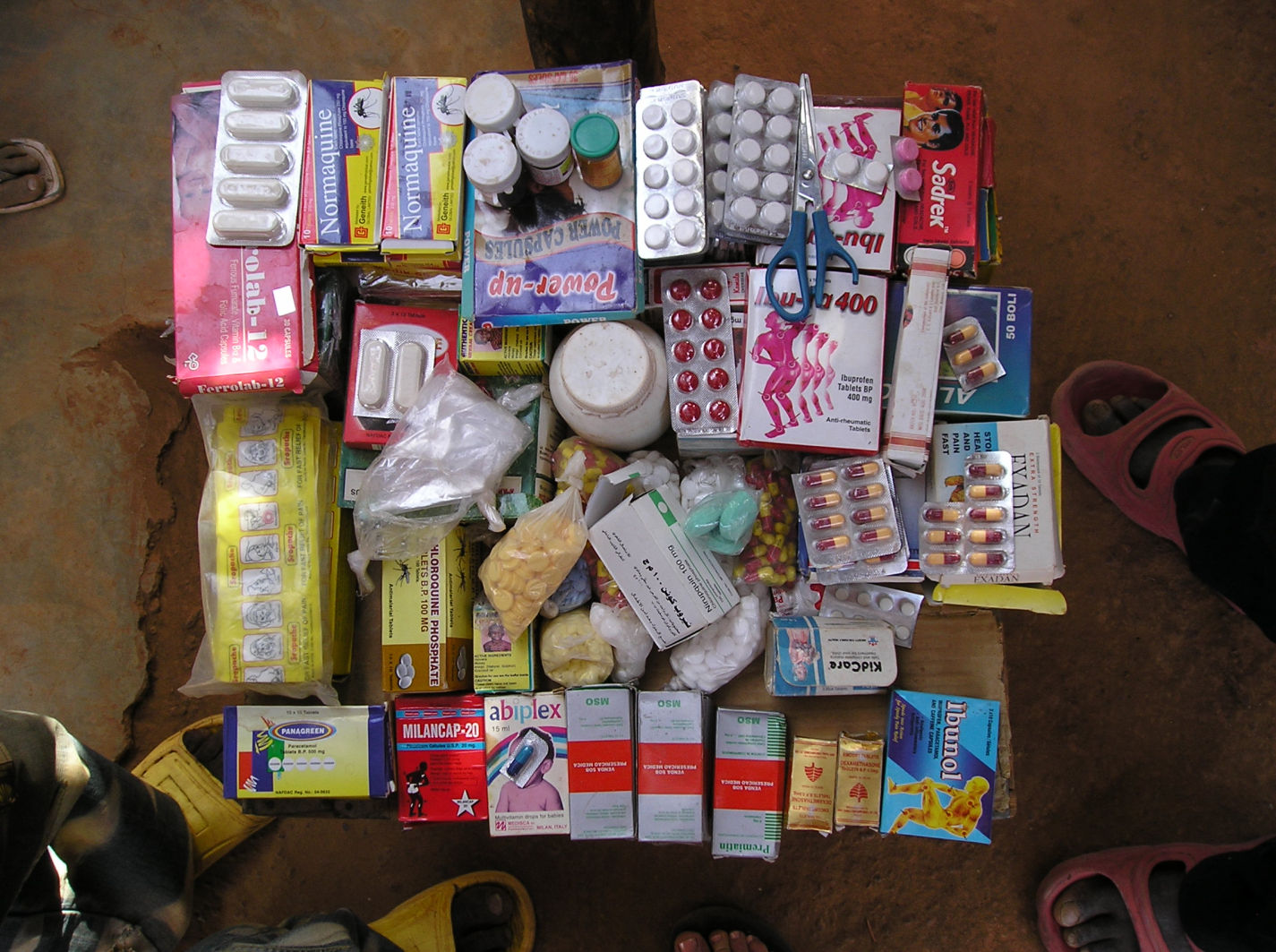
Malaria Journal 2008, 7:95
n illicit market of Burkina Faso
Drugs sold at an illicit market of Burkina Faso.
stances. Poor disintegration is considered an important,
poor manufacturing processes. Nevertheless, to distin-
but neglected problem in the field of dr.
guish if this was performed deliberately or unintention-ally will be diffbeen reported that
For one product tested in this study, the presence of the
sometimes poor and good quality tablets are mixed in
stated active ingredient could not be confirmed. These
same batch, and even tablets in blister packs have been
tablets have been obtained in a shop in Nouna town; they
found to be of mixed standards [.
came from a plastic bag without label and were sold assulphadoxine/phyrimethamine. As the letters METRO
The sampling of this study was based on a large and rep-
were engraved into the tablets, these tablets were most
resentative household survey in the frame of a malaria
likely metronidazol case exemplifies the
control intervention study which was conducted in the
problem of unqualified drug selling practices in the illicit
whole NHD in early 2006. The results can thus be consid-
market, as it has been reported also from neighbouring
ered as representative for this area of rural West Africa. All
countries, too [.
drug quality testing in the field was conducted throughone experienced laboratory scientist (SD) supervised by
Although most substandard anti-malarials detected in this
the main investigator (MT), and the confirmation tests
study were found in the illicit market, some were also
were done in a reference laboratory of Germany. Thus, the
found in the licensed market. The majority of these were
validity of this study can be considered as high. As artem-
quinine tablets. Currently we cannot fully exclude that
isinin drugs and ACT were not available at the time of the
these results might be linked to a methodological failure,
study in the NHD apart from private pharmacies in
as other quinine samples of the same brand were found to
Nouna town, a convenience sample of these important
be of good quality. However, as results were confirmed in
drugs was collected in Ouagadougou, the major town of
the reference laboratory, it was concluded, that some of
Burkina Faso. However, as the overall number of drug
the quinine tablets were of substandard concentrations of
samples by drug class tested was rather small, results have
the active ingredient. As a consequence, a larger sample
to be interpreted with caution.
should be tested in the near future for validation. The factthat different samples of the same product passed or failed
It is reassuring, that of the artemisinin and ACT drugs col-
can probably be explained by the use of uncontrolled,
lected and tested only one has been found to be substand-
substandard active ingredients during manufacture or
ard. This product was most likely diverted from the
(page number not for citation purposes)

Malaria Journal 2008, 7:95
DS Sample preparation and data collection, production offinal manuscript
BC Conception and design of the study, supervision oflaboratory work, production of final manuscript.
THT Sample preparation and data collection, productionof final manuscript.
AS Conception and design of the study, supervision of thestudy project, production of final manuscript.
OM Conception and design of the study, supervision ofthe study project, analysis and interpretation of data,drafting of manuscript, production of final manuscript.
Acknowledgements
The study received funding from the Deutsche Forschungsgemeinschaft in
the frame of the SFB 544. We like to thank Dr. Wiedemann from the Uni-
versity Dresden for providing technical advice for analysis, Dr. Jähnke for
his advice and support during drug testing, and Noelle Konaté for her great
sed as sulphadoxine/pyrimethamine in a shop
engagement during anti-malarial drug collection.
Tablets purchased as sulphadoxine/pyrimethamine in a shop.
Greenwood BM, Bojang K, Whitty CJM, Targett GA: Lancet
licensed market to the illicit market, a procedure fre-
quently observed in malaria endemic coun]. How-
Kouyaté B, Sie A, Yé M, De Allegri M, Müller O:
ever, it is likely that the increasing demand for artemisinin
PLoS Med 2007, 4:e127. doi:10.1371/jour-
and ACT drugs will be accompanied by increasing produc-
tion and distribution of substandard and fake products in
Müller O, Traoré C, Becher H, Kouyaté B:
the near futu]. There is already evidence for such a
Trop Med Int Health 2003,
development from a study conducted in East and Central
WHO: Counterfeit medicines. Fact sheet 2006:2
Africa on the quality of artemisinin and ACT drugs availa-
ble at private pharmacies [. Reports from Tanzania and
Cameroon also indicated the presence of counterfeit dihy-
Newton PN, White NJ, RozeBMJ 2002, 324:800-801.
droartemisinin and counterfeit artesunate in the African
maa consequence, appropriate anti-malarial
BMJ 2002, 324:698.
Cockburn R, Newton PN, Agyarko KE, Akunyili D, Withe NJ:
drug surveillance needs to become established in all coun-
tries of SSA. In addition, governmental drug control
PLoS Med 2005, 2:e100.
authorities need to be strengthened, access to affordable,
The World Bank: Pharmaceuticals: Counterfeits, Substandard
Drugs and Drug Diversion. HNP brief no 2 Report number 32192
quality controlled drugs needs to be improved, and the
population should become better informed on the risks
associated with buying drugs at the illicit market.
Am J Trop Med Hyg 2004, 70:245-250.
Ahmad K: WHO fights fake pharmaceuticals. Lancet Infect Dis
2006, 6:195.
Newton PN, Green MD, Fernández FM, Day NPJ, White NJ:
The authors declare that they have no competing interests.
Lancet Infect Dis 2006, 6:602-613.
Hall KA, Newton PN, Green MD, De Veij M, Vandenabeele P, Pizza-
nelli D, Mayxay M, Dondorp A, Fernandez F:
All authors read and approved the final manuscript.
Am J Trop Med Hyg 2006, 75:804-811.
Rozendaal J: Lancet 2001,
357:890.
MT Conception and design of the study, sample prepara-
Tran P: Counterfeit Drug Sales in Africa Strong, Threaten
tion, data collection, analysis and interpretation of data,
Public Health. VOA News
drafting of manuscript, production of final manuscript.
. Accessed: 01.11.2007.
G J Postgrad Med 2006, 52:288-290.
SD Sample preparation, data collection and interpretation
WHO, Malaria Control Department & Essential Drugs and Medicines
of data, production of final manuscript.
Policy Department: Access to antimalarial medicines improv-
(page number not for citation purposes)
Malaria Journal 2008, 7:95
ing the affordability and financing of artemisinin-based com-
bination therapies. WHO/CDS/MAL/2003.1095 2003:12-14.
Das GPHF-Minilab – Schutz vor gefälschten und qualitativ
minderwertigen Arzneimitteln ccessed: 26.04.2007
Jähnke R: Couterfeit Medicines and the GPHF-Minilab for
Rapid Drug Quality Verification. Pharm Ind 2004, 66:1187-1193.
IFPMA: Counterfeit Medicines. International Federation of Pharma-
ceutical Manufactures Association 20].
Accessed: 17.04.2007.
Jähnke R, Küsters G: Low-cost quality assurance of medicines
using the GPHF-Minilab. Drug Inf J 2001, 35:941-945.
Mukhopadhyay R: The hunt for counterfeit medicines. Anal
Chem 2007:2623-2627.
Müller O, Becher H, Baltussen A van Zweeden, Yé Y, Diallo DA,
Konaté AT, Gbangou A, Kouyaté B, Garenn BMJ 2001, 322:1567-1572.
Hamani AI: Les médicaments de la rue à Niamey: Modalités de
vente et contrôle de qualité de quelques médicaments anti-
infectieux. Université de Bamako, Faculté de Medecine de Pharmacie et
d'Odonto-stomatologie Masters thesis 2005.
Europäisches Arzneibuch: 2.9.1 Zerfallszeit von Tabletten und
Kapseln. 5.0th edition. 2005, 1:283-4.
Europäisches Arzneibuch: 2.2.25 UV-Vis-Spektroskopie. 5.0th
edition. 2005, 1:46-8.
Atemnkeng MA, Chimanuka B, Plaizier-Vercammen J: J Clin Pharm Ther 2007, 32:123-132.
Amin AA, Kokwaro GO: J
Clin Pharm Ther 2007, 32:429-440.
Ogwal-Okeng JW, Owino E, Obua C: Afr
Health Sci 2003, 3:2-6.
Legris C: La détection des médicaments contrefaits par inves-
tigation de leur authenticité. Étude pilote sur le marché
pharmaceutique illicite de Cote d'Ivoire. In Université Henri
Poincaré, Nancy I France; Phd Thesis; 2005.
Gaudiano MC, Di Maggio A, Cocchieri E, Antoniella E, Bertocchi P,
Alimonti S, Valvo L:Malar J 2007, 6:22.
Minzi OM, Moshi MJ, Hipolite D, Massele AY, Tomson G, Ericsson O,
Gustafsson LL: J Clin Pharm Ther 2003,
28:117-122.
Shakoor O, Taylor RB, Behrens RH: Trop Med Int
Health 1997, 2:839-884.
Taylor RB, Shakoor O, Behrens RH, Everard M, Low AS, Wangboon-
skul J, Reid RG, Ko Lancet 2001, 357:1933-1936.
WHO: The quality of antimalarials. A study in selected Afri-
can countries. Geneva: World Health Organization 2003:1-67.
Amin AA, Snow RW, Kokwaro GO J Clin Pharm Ther 2005, 30:559-65.
Kelesidis T, Kelesides I, Rafailidis PI, Falagas ME:
scientist can read your work free of charge
J Antimicrob Chemother 2007, 60(2):214-236.
Risha PG, Shewiyo D, Msami A, Masuki G, Vergote G, Vervaet C,
"BioMed Central will be the most significant development for
disseminating the results of biomedical researc h in our lifetime."
Trop Med Int Health 2002, 7:701-707.
Sir Paul Nurse, Cancer Research UK
Newton PN, Mc Gready R, Fernandez F, Green MD, Sunjio M, Bru-neton C, Phanouvong S, Millet P, Whitty CJM, Talisuna AO, Proux S,
Your research papers will be:
Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H,
available free of charge to the entire biomedical community
Palmer K, Day NPJ, Greenwood BM, Nosten F, White NJ
peer reviewed and published immediately upon acceptance
PLos Med 2006, 3:e197.
cited in PubMed and archived on PubMed Central
Atemnkeng MA, De Cock K, Plaizier-Vercammen
yours — you keep the copyright
Trop Med Int Health 2007, 12:68-74.
Submit your manuscript here:
(page number not for citation purposes)
Source: http://www.crsn-nouna.bf/IMG/pdf/antimalarial_drug_typke_-2.pdf
GDP and Integrity of The Supply Chain Presented by: Karen S Ginsbury B.Pharm, MSc, MRPharmS IFF, October 2010 What are the Risks What are the Risks in GDP? • For a formal assessment need to use one of the tools in ICH Q9 / Annex 20: – HACCP– FMEA– Ishikawa (Fishbone) diagram + one of the • Will take you to a lot of the points already Every Picture Tells a Story
TREATING LIKE ALIKE: THE PRINCIPLE OF NON- DISCRIMINATION AS A TOOL TO MANDATE THE EQUAL TREATMENT OF REFUGEES AND BENEFICIARIES OF COMPLEMENTARY PROTECTION [Recent years have seen academics in the field of international law demonstrate a growing interest in the subject of complementary protection, particularly in the rights that follow from complementary protection as opposed to from refugee status. While scholars have largely focused on the ‘protection gap' between the rights granted to refugees and those granted to beneficiaries of complementary protection in the context of international law, they often overlook the fact that this ‘gap' is most significant at a domestic level. They also overlook the potential usefulness of the principle of non-discrimination codified under art 26 of the International Covenant on Civil and Political Rights as a way to close the ‘protection gap' between the two groups. This paper seeks to promote art 26 as a valuable tool for the elimination of differentiation between refugees and beneficiaries of complemen-tary protection, thereby enhancing its role as a source of rights for highly vulnerable individuals and enriching the way we think about entitlement to protection at international law.]









