Forum-link.org.uk


www . i-base . info
a guide for treatment
i-Base treatment training manual for advocates
HIV drug resistance
2. Key concepts: genetics and HIV life cycle
3. Mutations with and without drugs: 'selective pressure' and
'survival of the fittest‘
4. Resistance tests and interpreting the results
5. When to use resistance tests?
6. Research into drug resistance
AppendicesAppendix 1: Questions on resistance
Appendix 2: Supplementary information about genetics
Appendix 3: List of amino acids and their abbreviations
Appendix 4: DNA codes for amino acid
Appendix 5: HIV genome map with example mutations
Appendix 6: Stanford Drug Resistance Database online tables
Appendix 7: IAS-USA guidelines and cross resistance
Appendix 8: Summary of UK Guidelines (BHIVA)
Appendix 9: Example of a resistance test report
Recording resistance test results
This booklet is one section of the i-Base training manual for advocates, available online (www.i-
Base.info). Other sections include: The immune system and CD4 count; Virology, HIV and viral
load; Introduction to ARVs; Side effects of ARVs; OIs and co-infections; HIV and pregnancy;
Drug users and ARVs, Understanding clinical trials and other learning resources.
This resource is part of a copyright-free project that is available on the i-Base website to
download in various formats, or to work online. As with other treatment information produced by
i-Base we encourage translations into other languages.
Thanks to an advisory group of community advocates, and to David Dunn and researchers at
UK HIV Drug Resistance Database group for comments.
Written and compiled by: Simon Collins for HIV i-Base, Third edition, May 2014.
Thanks to The Monument Trust for their support in funding this publication
Phoneline 0808 800 6013
a guide for treatment
This is an introduction to HIV drug resistance.
It is written for people who want to understand this aspect of their treatment.
It was original y developed as a training course for treatment advocates.
Although the subject sounds technical, this guide is written in mainly non-technical language.
HIV drug resistance
Phoneline 0808 800 6013
1.1 Introduction
Resistance can be a daunting subject. It sounds complicated, scientific,
technical and difficult.
• Drug resistance is important. It determines whether your treatment will
work and whether it will fail. It determines which drugs you can use.
• Most people use HIV treatment for years without developing resistance.
• However, if resistance develops, it stays with you for life. Avoiding
resistance makes sure you keep the widest choice of drugs.
• The principles behind resistance are simple. If an organism (i.e. a virus)
continues to reproduce in the presence of a drug, resistance will develop.
1.2 Resistance on a personal level
If you are reading this guide for your own health, then there may be more
information, including technical information, than you expect or need.
One of the aims of the resource is to collect and explain information that
is not usually easy to find in one place. So has more detail than most
community guides to drug resistance. It was designed as a course based on
reading one section each week. Take time to understand each section.
However, if you like to know more about this geeky stuff, more technical
information is included in the appendices. We use non-technical language
throughout but when technical terms are important, we explain them. We
also include a glossary.
Luckily, most people are able to use HIV combination for many years without
developing resistance.
This is an important point.
In the UK, less than 5% of people each year develop resistance, once they
have had an undetectable viral load for more than a year. This depends on
continuing to take treatment.
If resistance does develop, in many cases this is linked to difficulties with
adherence.
The most active thing you can do to avoid resistance is therefore to get into
a good routine for taking your meds on time.
HIV drug resistance
1.3 Questions about resistance
This guide started with a list of over 30 questions about resistance from a
group of HIV-positive people. • Which drugs can someone use if they have resistance?• How are treatment choices made?• Can resistance be passed from mother to child?• Can someone develop resistance even with perfect adherence?• What is "wild-type" virus and what does it do?• When should you have a resistance test?• How expensive (or cheap) are resistance tests?• Are the tests always 100% accurate?• Is resistance inevitable?• What should I expect to hear back from my resistance test? • How does resistance affect me? • How can I avoid resistance? • Is resistance permanent? • What happens if I get resistance?These are questions that lots of people have. The guide was designed to
work through the science behind the answers.
These and other questions are listed in Appendix 1. The answers are in the
online version of this guide but they are also answered in the text through
the guide.
If you have question after reading this resource, please email the i-Base
Q&A service and we will do our best to help.
[email protected]
Phoneline 0808 800 6013
1.4 Course outline
Each section in this booklet was written as part of a course. Each section looks
at a different aspect of resistance.
Most people do not have a medical background, so we start with basics. Each
section describes a different aspect of drug resistance.
• The resource requires active participation.
• Each section involves reading, taking your own notes, and responding to
questions. Each section should take about 30-60 minutes. The additional
material in appendices does not need to be learned by heart. These are
references that will be referred to through the course.
• We asked participants from the original course to ask questions and
complete online evaluations.This involved sending at least one email back
for each section. We included these questions as part of the training.
This course was developed over several months.
As well as learning, it is meant to be fun.
1.5 Learning objectives
By the end of the training you should have an understanding of:
• Key concepts: genetics, HIV structure and lifecycle.
• Basic mechanisms of how and why resistance to HIV drugs occurs.
• How resistance is measured, when to test and how test results are
• The impact of resistance on HIV treatment and treatment options; Treatment
strategies for people with drug resistance; How new drugs can overcome
• Transmission of drug resistance.
• Examples from research into drug resistance.
The training should help advocates advise on resistance research from a
community perspective. For example, by working with researchers on local or
national research studies.
It should help HIV positive people who want to understand this aspect of
treatment in more detail.
HIV drug resistance
1.6 Introductory reading
The following three short sections from the i-Base Introduction to
Combination Therapy are included as background reading. This information
should help prompt questions that we cover later in the course.
http://i-base.info/guides/starting/resistancehttp://i-base.info/guides/starting/avoiding-resistancehttp://i-base.info/guides/starting/missed-doseIf you are not reading this guide online or do not have internet access, don't
worry about this reading. Everything will be covered anyway ;)
1.7 Feedback
The online version of this guide includes a short online survey for each
section.
If you are reading this as a booklet in one go, there is a single feedback
survey for the whole guide at this link:http://www.surveymonkey.com/s/L8ZJM7PA paper version in included on page 67 that you can send back by FREEPOST if you
prefer this format.
Your feedback is important.
Thank you for your help us in this way.

Phoneline 0808 800 6013
2: Key concepts: genetics and HIV lifecycle
2.1 Recap from previous section
The introductory reading in section 1 was general information about some of
the practical issues about HIV resistance.
2.2 Introduction to section 2
For the first proper section we need to start with basics and learn about a
few important concepts.
This includes an introduction to genetics, how HIV makes copies of itself
when it reproduces or replicates and how it makes tiny mistakes each time.
2.3 Genetics
The structure of things that reproduce, grow and die is usually dependent
on genetic material. This is the case for bacteria, viruses, insects, animals, a
tomato, a beanstalk or a human.
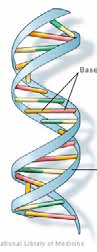
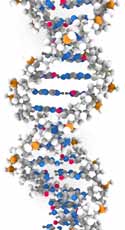
This is usually a double strand of RNA called DNA (see Figure 1).
Figure 1: Illustrations of DNA: (a) simplified to show bases, and (b) showing molecular
structure of the bases and the sugar and phosphate groups that form the backbone
ribbon strips
Source: US National Library of Medicine. [http://ghr.nlm.nih.gov/handbook/basics/dna]
HIV drug resistance
DNA is like a recipe book for how to make a new organism (tomato/human/
virus etc). For humans, DNA is in cells that have a nucleus - skin cells, bone
cells, brain cells, liver cells, blood cells and many others.
The genetic structure of HIV is slightly different because it is single-stranded
RNA. Before it can replicate inside the nucleus of a human cell, it needs to
be transformed into double stranded DNA. To do this, HIV mainly uses CD4
cells. These are a type of blood cells that are part of the immune system.
DNA is made up of a chain of chemicals called nucleotides (or bases). There
are only four bases and the order of the bases determine what they do. The
chain of bases are held together by a backbone of two strands of sugar
and phosphate molecules. This makes the familiar double helix structure in
Figure 1.
Human DNA is a chain of 3,000,000,000 bases. The four bases are
abbreviated to letters: A (adenine), T (thymine), C (cytosine) and G
(guanine).
The code for a human will look very similar (but is much longer):
CATTGAGGT etc (continuing for 3,000,000,000 letters).
Because DNA is a double strand, this is actually a double chain of
3,000,000,000 base pairs. The pairs always twin A with T, and C with G. So the chain looks like:
HIV is a similar chain, but much shorter with about 9,700 bases.
This is the recipe for HIV to replicate. If these letters change for any reason,
it is like changing the the recipe. The next generation of HIV will then be
slightly different.
Changes in each generation is called evolution. Evolution occurs for every
living thing - for humans, tomatoes and viruses.
See Appendix 2 for more information about DNA.
Phoneline 0808 800 6013
2.4: Life cycles and replication
Every living thing, by definition, has a life cycle. This is repeated from
generation to generation. At it's most basic, this includes: 1. early development and birth, 2. replication, perhaps many times, and then 3. death.
The life cycle for HIV is very fast. HIV in an active CD4 cell only survives for
1–2 days. Over this time, a cell is infected, the virus replicates and then the
cell dies. Infected cells also signal to uninfected cells to die more quickly.
In an HIV negative person, CD4 cells live for 3-4 days, so HIV causes all
activated CD4 cells to live for a shorter time. However, most of the immune
system is resting or asleep. HIV in a resting cell is also resting.
HIV is also very prolific–it replicates a lot! Each infected CD4 cell produces
several hundred new infectious particles of HIV (called virions). A virus is
called a virion when it is not inside a cell. These virions infect new CD4 cells
and the cycle repeats. When not on treatment, millions of CD4 cells become
infected every day and at least 100 million new HIV virions are produced
each day.
HIV has one of the highest and fastest replication rates of all viruses. It
replicates a lot in a very short time. HIV has to reproduce its genetic code which is in the form of a strand of
9,200 bases. Small mistakes in copying the genetic RNA is like print errors
in a recipe. Because HIV does not have a way to proofread, mistakes are
common. In every reproduction cycle it makes at least one mistake.
By comparison, human DNA replication usually has very accurate proof-
reading. If it detects an error it goes back to correct it. In humans an error
occurs only once in every 10-100 million bases. In humans, many changes
are not important and the role of much of human DNA is not understood. Although 90% of DNA was thought to be junk more recent research thinks it
may be more important and that we just have not yet understood it.
If a recipe spelled ‘sugar' as ‘suger' you would probably guess right and still
make a good cake. But changing ‘2 eggs' to ‘20 eggs' would make a mess.
With HIV, some changes are important and some make no noticeable
difference. Sometimes, one change affects the way a drug works.


HIV drug resistance
The lack of proof reading, together with the vast
amount of new viruses produced each day, makes it
likely that at least one HIV mutation will be produced
in every cycle (when not on treatment).
Sometimes dual mutations may occur on the same
strand of HIV. Luckily, even with so much virus being
produced triple mutations relating to drug resistance
rarely occur by chance.
To understand how different mutations affect drug
resistance it is useful to use a different diagram for
the structure of HIV. (See Appendix 5).
This shows the genetic structure of the single strand
of RNA for HIV as nine main genes. In order to
picture this structure, the genetic structure of HIV RNA that shows each
gene, is shown as a block, some of which overlap.
Each of these main genes plays an important role in making new HIV. You
don't need to learn about the function of each gene but it is useful to know
that they exist.
By comparison, the chains of nucleotides in human DNA is organised into
over 20,000 genes (in 23 pairs of chromosomes).
2.5 HIV replication
The third point in this section involves combining points one and two:
i) HIV is a chain of 9,200 bases that replicates every 1–2 days.
ii) Even with a viral load of only 10,000 copies/mL, over 100 million new
viruses are produced each day.
iii) Every reproduction cycle includes at least one mistake: somewhere
an A could change to a C; or a G to an A etc; just by accident. HIV does
not proofread.
Before starting treatment (ie before viral load is dramatically reduced) every
single base change is likely to be present. Some of these mutations cause
drug resistance.
Phoneline 0808 800 6013
2.6 Section 2: Learning points
• HIV is a virus made up of two single strands of genetic material, called
• The genetic material in HIV is much shorter than human DNA. It is like
comparing a pea to the Titanic.
• The order of the four bases determines everything about the structure of
an organism (whether this is a virus, a tomato or a human).
• The lifecycle for HIV is short (only 1-2 days).
• The natural process of replication sometimes involves slight changes to
the genetic structure. These are called mutations.
• HIV doesn't have a way to proofread for these mutations. This means that
everyday slightly different new versions of HIV are produced.
• Often these new mutations make no difference, but some can stop an
HIV drug from working.
HIV drug resistance
3. Drug resistant mutations (with and without drugs):
'selective pressure' and 'survival of the fittest‘
3.1 Recap of previous section
The previous section set the stage:
• We have a genetic organism – in this case a virus (HIV).
• After infecting a cell, the virus can replicates many times. HIV produces
more than 100 million new virions every 1–2 days.
• But HIV has no proofreading mechanism. It makes at least one mistake
(mutation) just by chance in each replication cycle.
• Someone who is not on treatment is likely to have every possible single
mutation the HIV genome. We don't have one virus but a pool or soup of
thousands of slightly different types of HIV.
3.2 Introduction to section 3
Section 3 looks at how HIV mutations behave when HIV drugs are around.
3.3 Wild-type virus and drug pressure
In someone not on treatment, mutations that develop that can affect how a
drug works are made at random.
Mutations generally make HIV less fit at replicating. Wild type HIV is
therefore stronger and fitter that drug resistant HIV.
When not on treatment, drug resistant HIV has no advantage over the wild
type. It is less fit and so wild type continues to be dominant. The muation
may still be in the pool of viruses but it will stay a minority.
• The main strain will be the fittest virus (ie wild type when not on
• A mutation that stops a drug working is called ‘a mutation associated with
drug resistance' or, more commonly, ‘a drug resistant mutation'.
• As long as you are not taking the drug associated with this resistance,
this mutation will have no relative advantage over wild type virus.
Phoneline 0808 800 6013
Now think about how the pool of viruses will change if you start taking
this drug.
• The drug will be able to kill most of the viruses in the pool. However, it will
not be active against the virus that is resistant to the drug.
• The drug resistant virus will continue to replicate. This resistant virus now
has an advantage over the other viruses. It is relatively more fit.
• Slowly, the drug resistant HIV will become dominant in the pool.
• In the presence of the drug, further mutations can develop and make the
resistance even stronger.
Before taking treatment the resistant HIV had no advantage over wild-type.
Now, it is stronger than the other viruses. See Figure 2.
This major concept is called ‘survival of the fittest'.
Figure 2: How resistant mutations respond to treatment
HIV drug resistance
3.4 Survival of the fittest
‘Survival of the fittest' is central to the concept of evolution.
Whether talking about how life developed from tiny organisms into plants,
fish, birds, animals and humans, or how a virus changes, life evolves. It has
taken millions of years for humans to evolve, but HIV does this in days.
Each generation evolves and adapts in relation to the surrounding
environment.
This is the same for HIV. HIV evolves and when the environment changes
this affects how HIV evolves. By taking treatment, the environment for the
virus changes because the virus is now in the presence of drugs.
This idea is sometimes explained another way. Treatment is referred to as
exerting a selective pressure on the virus to change.
• This pressure is encouraging the resistant virus to reproduce.
• It is a selective pressure, because it is encouraging the mutations linked
to resistance to that drug to be selected over those without resistance.
3.5 Selective pressure
Sections 3.1 to 3.4 involved important ideas. Take a break. Let these ideas
sink in.
These ideas will be the foundations for the next sections, which should be
easier. Take time to recap the following key points.
Key point 1: HIV drugs do not initially cause resistance. Mutations
occur because the virus makes mistakes. However, a drug exerts
selective pressure for resistance to develop and expand.
Although the first mutations occur by chance, often before treatment, if the
virus continues to replicate in the presence of a drug, this can now generate
new and more complicated patterns of resistant mutations.
The second and third mutations would not be likely to occur naturally if the
treatment wasn't present. This means that if you stay on a failing treatment
then more complicated mutations can occur.
Key point 2: is that selective pressure of drugs can work in two ways.
If you stop or switch treatment then the drug pressure is taken away.
Phoneline 0808 800 6013
This will reduce the amount of resistant virus in the pool. Without the drug,
the resistant HIV no longer has a relative advantage over wild type.
• Wild type virus is better at replicating than resistant HIV, when there are
• When the drug pressure is removed, wild type HIV then becomes the
majority virus.
• The time this takes depends on the specific mutation. Mutations that
develop easily are sometimes the fastest to reduce when a drug is
Figure 3: Resistance reduces if treatment is stopped but it remains at low levels
Question: Does the resistant virus disappear when you stop taking a drug?Answer: No. Drug resistant HIV becomes a minority in the pool (see Figure
3) and is archived in CD4 cells that are sleeping. Sometimes this can happen
within weeks and sometimes it might take years. The resistant HIV then
becomes too difficult to detect with a standard resistance test. (See Section
5: When to use resistance tests).
HIV drug resistance
Key point 3: A resistance test only tells you about resistance to the
drugs that were being taken when the blood sample was taken. When
looking for transmitted drug resistance, the sample closest to the
infection is most likely to show resistance.
This point is important when advocating for resistance testing.
If you are infected with drug resistant HIV, this will initially be your dominant
strain. Over time, some mutations become a minority at a level that is too
low to be detected. Even though the majority population may become wild type HIV, drug
resistance will still be present. This is called ‘archived resistance'.
• UK guidelines recommend resistance testing for everyone who is newly
diagnosed with HIV.
• If a resistance test is not provided, a blood sample needs to be taken and
stored. This is so it can be tested before starting treatment.
• UK guidelines also recommend resistance testing when viral load has
rebounded on treatment and when treatment has never reduced viral
load to undetectable.
Key point 4: Drug resistant HIV can be transmitted in the same ways
as wild type HIV. This includes sexually, through shared injecting
equipment, from needlestick injuries and from mother to baby. HIV
positive people can be reinfected with a different drug resistant strain.
Many cases of re-infection are only detected because the new infection was
with drug-resistant HIV.
About 10% of new infections in the UK are with HIV that has one or more
major mutations. A smaller percentage are infected with HIV that is resistant
to two or more drugs. Although rare, some people are infected with HIV that
is resistant to three or more drugs or drug classes. • The longer the time from infection to diagnosis, the more difficult it is to
detect some types of transmitted resistance.
• Some mutations drop below the levels that can be detected by resistance
tests within a few months but some mutations can still be detected after
several years.
Phoneline 0808 800 6013
Question: What happens if someone was infected with HIV that was drug
resistant and starts treatment that includes one of those drugs?Answer: If they are using a combination with three drugs, then only two of
those drugs will be fully active. This increases the risk that the combination
may not be strong enough. It will be more difficult to get an undetectable
viral load and resistance to the other drugs can develop.
3.6 Using three drugs in HIV treatment
The example in Figure 2 shows that using a single drug provides pressure
for pre-existing resistance to become dominant.
This is one reason why combination therapy uses three drugs. See Figure 4.
Single mutations are all present when someone starts treatment. Some dual
resistant mutations may be present on a few viruses. But triple resistance
will not be present on the same viruses. Even with HIV being such a rapid
and prolific virus, this is very unlikely.
Figure 4: Combinations with three drugs work against low-level pre-existing resistance
HIV drug resistance
In Figure 4, virus R-1 is resistant to Drug-1, R-2 is resistant to D-2 and R-3 is
resistant to D-3. Although R-1, R-2 and R-3 mutations are all present when
starting treatment, these three different mutations are very unlikely to have
all developed on the same virus.
HIV that is already resistant to Drug-1, will be killed by D-2 and D3. The virus
resistant to Drug-2 will be killed by D-1 and D3.
Even if R-1 and R-2 have developed on the same virus (dual resistance)
then D-3 will still be active against this virus.
D-1, D-2 and D-3 will also all be active against wild-type HIV, which is
hopefully the majority population.
3.7 Section 3: Learning points
• HIV drugs do not initially cause resistance. Mutations occur because HIV
makes mistakes and there is no proofreading mechanism.
• A drug exerts selective pressure for resistance to develop.
• Under continued drug pressure, more complicated patterns of resistance
mutations develop that only occur because of this continued drug
• Results from resistance tests only accurately tell you about resistance to
the drugs that were being taken when the blood sample was drawn.
• Without drugs, wild type virus is more fit than resistant virus.
• Drug resistant HIV can be transmitted in all the same ways the wild type
virus can be transmitted. See section 6.5.
• Someone can be infected by HIV that is already resistant to one or more
• An HIV-positive person can be reinfected with a different strain of HIV.
Cases of reinfection are only usually detected when the new infection (or
superinfection) is with drug-resistant HIV. See section 6.8 and Appendix
• Reinfection with drug resistant HIV can have serious health implications
because there are fewer drugs to chose from.
• Combination therapy uses three active drugs to try to ensure it is active
against pre-existing resistance.
Phoneline 0808 800 6013
4. Resistance tests and interpreting test results
4.1 Recap of previous section
The previous section looked at how the presence or absence of drugs
interacts with the evolution of HIV and that HIV is a virus with a very rapid
and prolific life cycle.
Instead of thinking about one virus, we have talked about how this is really
a pool of thousands of slightly different viruses. Which viruses dominate
depends on the drug pressure. When drugs are started, continued or
stopped, this changes the environment.
When drug levels are too low to keep the viral load suppressed, HIV can
develop resistance to those drugs. These resistant viruses are then fitter
than wild type HIV and most able to survive. The pool then becomes mainly
resistant virus.
More complicated patterns of mutations can then occur under the ‘selective
pressure' of the drugs. These combinations of mutations evolve in ways that
would be unlikely if drugs were not present.
When on treatment, new resistance only seems to develop when viral load
is detectable (greater than 50 copies/mL). If viral load is undetectable and
adherence is good, then resistance is rare.
Learning how resistance tests work will help understand this.
4.2 Introduction to section 4
Section 4 explains two main types of resistance tests: genotype tests and
phenotype tests. A third type of resistance test that is marketed as ‘a virtual
phenotype' combines both approaches.
Both genotype tests and phenotype tests work from blood samples but
they work in different ways (see Figure 5). The results are also interpreted
differently.
These two words are often used in other aspects of science. Genotype
refers to a genetic sequence and specific changes. Phenotype refers to
observations related to the changes (i.e. in a test tube).
Section 4 also includes technical information, so work through at your own
pace. Please write down questions when anything is not clear.
HIV drug resistance
Figure 5: How genotype and phenotype tests work
Genotype tests
look to see how
tests see
the structure
of a sample of
HIV drugs
your HIV may
still work to
control your
type of HIV.
4.3 Genotype tests and genotypic resistance: numbers and
letters
Genotype tests (also called genotypic tests) look for changes in the structure
of the virus.
The test compares genetic sequences to those seen in wild type HIV.
Mutations are described with numbers and letters.
This is easier to imagine if you think of HIV as a long chain of amino acids.
Each group of three bases is an amino acid. The example in Appendix 2
shows that the three bases for the amino acid called methionine (M) only
needs one base change to make the amino acid valine (V). This is a simple
change.
If the three bases change from ATA to GTA, the amino acid at that junction
changes from methionine to valine. Appendix 5 illustrates mutations in
relation to the HIV genome.
The section of the genome targetted by nukes (and NNRTIs) is called
reverse transcriptase (RT). If the change for ATA to GTA occurs at junction
number 184 in the RT gene, this will affect how some drugs work. The
mutation described above is written as M184V (in RT).
If the virus changes at junction 103 on the RT genome, from AAA or AAG
to AAC or AAT, the amino acid at that junction changes from lysine (K) to
asparagine (N). This mutation is written as K103N in RT.
You don't need to know all these names but a key to the abbreviation letters
for different amino acids is in Appendix 3.
Phoneline 0808 800 6013
These two mutations are good examples to start with because:• They only need one base change to change the amino acid.
• They are both very common mutations• Their impact is like an on-off switch. Without the mutation the drug works.
With the mutation the drug doesn't.
M184V results in high level resistance to 3TC and FTC. It stops both these
drugs from working.
K103N results in high level resistance to NNRTIs like nevirapine and
efavirenz. It stops both these drugs from working.
Table 1 lists key mutations and the impact they have on treatment. The
integrase mutation is included as a more complicated example.
Table 1: Important drug resistance mutations
M41L usually occurs with T215Y. Together these mutations
confer intermediate-to-high resistance to AZT and d4T, and
a lower resistance to ddI, abacavir, and tenofovir.
K65R causes intermediate resistance to ddI, abacavir, 3TC,
FTC, and tenofovir, and low-level resistance to d4T. K65R
causes AZT to be more active (called hypersensitive or
High level resistance to 3TC and FTC and low level
resistance to abacavir and ddI
K103N causes high-level resistance to nevirapine,
delavirdine, and efavirenz. By itself it does not affect
etravirine efficacy. However, it increases the effect on
etravirine from 3-fold to 15-fold reduced sensitivity when
L100I is also present.
I50L causes intermediate-to-high level resistance to
atazanavir/r and increases susceptibility to other PIs.
L90M causes resistance to nelfinavir, saquinavir/r,
atazanavir/r, and indinavir/r. When present with other
mutations it also reduces the activity of fosamprenavir/r and
HIV drug resistance
Q148H/ Integrase Q148H/K or R are mutations selected by raltegravir and
elvitegravir. By itself Q148H reduces susceptibility to both
these drugs by about 5-10 fold and Q148RK reduces
susceptibility by >30-100 fold. With G140S, Q148HRK
reduces susceptibility by more than 100-fold. Q148HKR
alone have minimal effects on dolutegravir, but causes
more than 10-fold reduced susceptibility in combination with
E138K, with or without G140S.
Key point 5: Some mutations stop a drug from working completely
(high level resistance). However, some only have a moderate impact
(intermediate) and some only have little impact (low level resistance).
Key point 6: Mutations that are associated with resistance to one drug
can also have resistance to similar drugs in the same family. This is
called ‘cross-resistance'. For example, if you develop resistance to one
NNRTI like efavirenz it is likely you will be cross-resistant to nevirapine,
even though you have never taken nevirapine.
There are too many mutations to remember but it is good to know the
common examples in Table 1.
Luckily, several research groups publish comprehensive tables and
explanations online. Use these if you need to find about about a specific
mutation or drug. See: Appendix 6: Stanford Drug Resistance Database
online tables.
As you learn more about drug resistance, it gets easier to remember key
mutations, especially knowing what the letters and numbers mean.
The IAS-USA guidelines illustrate resistance to each drug in a different way.
Each drug has a bar representing the section of the HIV genome where
resistance develops. The numbers inside the bar are the junctions where
mutations are linked to resistance. The letters on the top are the amino acids
at that junction for wild type HIV. The letters underneath are the amino acid
changes linked to drug resistance.
Phoneline 0808 800 6013
This is a good way to visually compare the resistance profile of drugs in the
same class. It is a quick way to get an idea of cross-resistance. Appendix 7
includes these charts and links to the original resources.
Mutations that show resistance is reverting back to wild type are called
revertant mutations.
For example, the T215Y mutation is associated with AZT resistance. If this
mutation is transmitted to someone who is not taking AZT, the virus changes
back closer to wild type. The mutations T215D, T215N or T215S are seen as
‘tracks' in this change. They are interpreted as previously having T215Y and
so imply resistance to AZT.
Question: How do researchers find out about the mutations associated with
each drug?Answer: Every new drug is tested in test tube studies to see which mutations
occur (called in vitro passaging). These are usually similar to the mutations
seen in human studies when treatment fails (ie in people whose viral load
stays detectable). For example, in studies that include 3TC, the M184V
mutation is one of the first changes seen if viral load is not reduced to
undetectable (less than 50 copies/mL). Using 3TC without any other HIV
drugs (i.e. 3TC monotherapy), results in M184V within a couple of weeks.
This 3TC resistant HIV will be cross-resistant to FTC. New drugs are often
developed to specifically work against resistant virus.
Question: Are resistance tests perfect? Are all mutations known?Answer: Resistance tests are not perfect, but major mutations usually
accurately predict when a drug will not work. Given the number of possible
combinations of mutations, this is an area of research that is always
HIV drug resistance
4.4 Phenotype tests and phenotypic resistance: x-fold resistance
Phenotype resistance tests look at resistance in a very different way.
Instead of looking at mutations, phenotype tests measure how active a drug
is in a sample of HIV compared to how active it is in a sample of wild-type
HIV.
So, an HIV drug is added to a sample of HIV in a test tube, and the test
measures how much HIV continues to be produced. The quantity of the drug
is then slowly increased to see how much extra is needed to have the same
impact on reducing viral replication compared to a normal dose on wild-type
HIV.
Phenotype tests are more difficult to run. They are more expensive and take
longer to get a result. For these reasons phenotype tests are mainly used
when the result from a genotype test is unclear.
Phenotype results are given as a fold change (or cut-off) with an
interpretation of what this means. For example, if a sample needed four
times the quantity of drug to have the same impact on stopping the virus
replicating, the result would be 4-fold resistance (or 4-fold loss in sensitivity).
In practice, you would need to increase the daily dose by four times to get
the same effect on viral load.
Sometimes resistance can be overcome by increasing the dose of
medication in a person. In practice, this only tends to be when someone
has complicated resistance and fewer drug choices. For most drugs and in
most circumstances this would cause too many side effects to be an option.
Some HIV drugs, including darunavir and dolutegravir, have higher doses for
people that have drug resistance.
The clinical impact of phenotypic resistance varies depending on each drug.
For example, 4-fold resistance to one drug may still be sensitive while 4-fold
resistance to another may be resistant. Each drug has a different cut-off
for when a drug is sensitive, partially resistant or completely resistant.
Nukes generally become resistant at low fold-changes while PIs have higher
thresholds.
Each make of phenotype test has its own reference chart for cut-off values
for each drug. These numerical values can differ between tests.
Phoneline 0808 800 6013
4.5 How are genotype and phenotype resistance related?
The clinical interpretation of both genotype and phenotype resistance tests are
based on results collected from large numbers of responses from real patients,
collected in various databases.
These databases relate the average impact that a mutation (or pattern of
mutations) has on the phenotypic resistance.
For example, M184V is associated with high level resistance to 3TC and FTC,
and results in a more than 300-fold reduction in drug sensitivity. With this
mutation, it would take a dose 300 times higher than the standard dose to have
the same anti-HIV effect. This would be physically impossible and too toxic.
M184V may still have a benefit in continuing 3TC because M184V replicates
less well than wild type and because it increases susceptibility to AZT, d4T and
tenofovir. However, the benefit of continued 3TC will be less than the benefit of
3TC in patients with wild-type HIV.
This is an unusual feature of M184V that is not shared with most other
mutations. Although other mutations also reduce fitness, this effect is usually
overcome by new mutations that compensate for this. Continuing to take 3TC or FTC to keep the M184V mutation may keep viral
load a little lower. This is because the virus is let fit. It reproduces less well so
there is less virus.
4.6 Virtual phenotype tests
Virtual phenotype tests are a third type of resistance test. They are really
genotype tests, and the mutations that are detected are included in the results. However, the pattern of mutations is also compared to a huge database of
matched genotype and phenotype results and a phenotypic result is predicted
from the database.
As with the other tests, an interpretation comes with the results that explains
whether each drug is likely to be sensitive, intermediate or resistant.
These tests produced very sophisticated individual results. However, they
are now rarely used because drug options to treat resistant HIV and now
much better. One of the main virtual phenotype tests, produced by Virco, was
withdrawn at the end of 2013 becasue of low use.
HIV drug resistance
4.7 Primary and secondary vs major and minor mutations
The terms primary and secondary resistance mutations, are now rarely
used.
Instead, major and minor mutations are used.
Major mutations have a big impact on drug resistance.
Minor mutations only have a small impact on drug resistance.
The terms primary and secondary are confusing because they sound like
primary mutations occur first. Sometimes however, the first mutations make
little difference.
For example, with protease inhibitors the first mutations have little impact
on how well a drug works. Then, as more mutations accumulate, the impact
becomes more important.
Finally, after 5 or 6 or more mutations, the clinical impact becomes more
significant. With protease inhibitors the first mutations to occur are minor
(secondary) mutations and major (primary) mutations occur later.
4.8 Resistance testing: practical issues
Resistance testing is widely used in most western countries. The information
they provide helps choose drugs that have the best chance of working.
However, resistance tests are more accurate in showing which drugs will not
work, than guaranteeing which drugs will work.
• Resistance tests can only detect resistance to drugs that you are
currently taking or have recently been taking. Remember that when a
drug is stopped, wild-type HIV becomes relatively more fit (see section 3)
and many mutations reduce to levels that are too low to detect. Usually
a mutation has to be present in more than 20% of viral population to be
detected with routine tests.
• Treatment choice needs to be based on someone's lifetime history
of treatment and resistance, not just the single result of one current
resistance test.
Phoneline 0808 800 6013
• In addition to the report given by the test lab, results need to be
interpreted by an expert who has your treatment and resistance history.
Experts do not always agree and different databases sometimes report
different results. Even though the tests may not all agree, it is better to
have this information to inform your treatment choices.
• There is less information about resistance to newer drugs. This is
because fewer people have developed resistance to those treatments.
There is less information in resistance databases to predict how new or
old mutations will affect how these drugs will work.
4.9 Section 4: Learning points
This has been a complicated technical section.
• There are three main types of resistance tests but genotype tests are
used most frequently.
• Different mutations have different clinical implications. Some are
associated with high level resistance, some with intermediate and some
with low resistance.
• Resistance for some drugs develops on a sliding scale, but for some
drugs it only takes one key mutation results in complete resistance.
• Genotype tests report mutations and phenotype tests report fold-
changes. All resistance tests should include a detailed interpretation for
each drug. You can ask for a copy of this report.
• Resistance to one drug can result in resistance to similar drugs in the
same class. This is called cross-resistance.
• Viral load needs to be detectable to get a result. How ‘detectable'
depends on the specific lab and test. This used to be above 500 copies/
mL but some labs can get a result when viral load is between 50 and 500
• The interpretation of complicated results requires expert advice.
HIV drug resistance
Resistance 5: When to use resistance tests
5.1 Recap of previous section
The last session looked at how resistance is measured and how test results
are interpreted.
• There are two main types of resistance tests but genotype tests are used
most often. Genotype tests report mutations (ie M184V) and phenotype
test report fold-changes (ie 4-fold resistance). Both tests should include
a detailed interpretation – ie whether each drug is likely to be active
(sensitive), partially active (reduced sensitivity) or inactive (resistant).
• Each mutation has a different clinical implication. Some are associated
with high level resistance and some with lower resistance.
• Resistance to one drug in a class often means you have resistance to
similar drugs in the same class. This is called cross-resistance.
• Resistance can only be tested when viral load is detectable but different
labs have different lower viral load cut-offs for the test to work.
• It is important to consider the history of resistance and not just the results
of the current resistance test. This include previous treatment history and
previous resistance tests.
• The interpretation of complicated results requires expert advice.
5.2 Introduction to section 5
This section look at when resistance tests should be used.
• UK recommendations are based the monitoring guidelines, adult
guidelines and pregnancy guidelines produced by BHIVA and the
paediatric guidelines produced by PENTA.
http://www.bhiva.org/PublishedandApproved.aspxhttp://www.pentatrials.org/guidelines.htmOther guidelines include:• European HIV Drug Resistance Guidelines (2009)http://regaweb.med.kuleuven.be/publications/european_guidelines• US treatment guidelines (DHHS)http://www.aidsinfo.nih.gov/guidelines/Most treatment guidelines for Western countries have similar
Phoneline 0808 800 6013
recommendations for resistance testing. The resistance section of the BHIVA
adult guidelines is reprinted in Appendix 8.
Section 5 also includes access to resistance testing.
• Why are these tests not always given?
• When to advocate for someone who has not been given a test.
5.3 When to use resistance tests
Genotype resistance tests are recommended when first diagnosed and
before most treatment changes (see Table 3), including:1. When first diagnosed (to check for transmitted drug resistance).
2. Before starting treatment (to help with the choice of treatment).
3. Before changing treatment, as long as viral load is detectable.
Table 3: When to use resistance tests
Yes, all patients. Both for recent In the UK, 5-15% of newly
and older infections.
diagnosed people have at
least one mutation.
Yes, BEFORE STARTING.
i) Test the earliest sample. If
i) People who have never had
this is not available, a current
a resistance test should have
sample should be used.
a sample tested before starting ii) If someone has had other
exposures since diagnosis,
ii) People who may have been
a resistance test will limit the
reinfected with a new strain of
chance that the first treatment
HIV since their first resistance
test may be retested before
starting treatment.
Yes. If viral load rebounds on
A resistance test BEFORE
treatment (viral treatment, test for resistance
CHANGING treatment will
provide an indication of how
treatment. Resistance testing
much resistance developed
can help determine if treatment while the treatment was
failure is due to HIV reinfection.
failing. Some low level
resistance may not be
HIV drug resistance
No. A resistance test is not
Resistance only develops
treatment (side needed if your viral load is
on failing treatment. Never
undetectable. If this is soon
test when viral load is
after starting treatment and viral undetectable.
load is still reducing, resistance
testing is not needed.
Women who start treatment
As for starting treatment.
during pregnancy should be
Testing if viral load remains
tested for drug resistance.
detectable is important for
Resistance testing should
be done if viral load is still
Although treatment should be
detectable at delivery. If
stopped carefully to reduce
the women decides to stop
the risk of resistance, this
treatment after the birth,
should be confirmed with a
resistance should be tested six
resistance test.
weeks after stopping treatment.
Guidelines for resistance testing Resistance develops in
in children are the same as for
children in the same way as
it does in adults. Any child on
In the rare cases (in Western
treatment with a detectable
countries) of infants born with
viral load is likely to have
HIV, resistance testing should
developed, or be developing
be included with the full panel of resistance.
other tests.
Before using a UK and European guidelines
CCR5 inhibitor only work
CCR5 inhibitor recommend using a type of
against CCR5-tropic virus.
genotype test to check viral
PEP should be started as
PEP combination usually
soon as possible. It should
include protease inhibitors as
not be delayed waiting for
transmitted PI resistance is
resistance test results. If the
HIV positive partner has drug
The urgency with PEP is to
resistance, this will affect the
first start any combination.
choice of drugs used for PEP.
If resistance is discovered later
the drugs can be modified.
Phoneline 0808 800 6013
5.4 Which tests to use: genotype or phenotype?
Recommendations for resistance testing always refer to using a GENOTYPE
test first. This is because genotype tests are cheaper (approximately £200 vs £700)
and quicker (1–2 weeks compared to more than two weeks), compared to
phenotype tests. Genotype results are also more widely understood.
A phenotype test is generally only recommended if the results from a
genotype test are difficult to interpret.
Phenotype tests (including virtual phenotype tests) are only used in people
who have very limited treatment choices. This is usually in a case where
there is extensive resistance to several different classes of HIV drugs.
Question: Are resistance tests used to see if the type of HIV in different
people is in some way linked – in prosecution cases of transmission, for
example?Answer: No. The tests comparing two different viruses are called
phylogenetic tests. They are more complicated and expensive tests. It is
important to remember that phylogenetic tests can show when people have
a similar virus, but not the direction of infection (ie whether one partner
5.5 How to access tests if the guidelines are not followed
Although guidelines are clear, resistance tests are not always provided
routinely. This is why it is important to know about the current UK guidelines.
If this is for cost reasons, then it is important to go back to the clinic to
ensure that the test is included as part of current standard of care.
i-Base sometimes get calls from people who were newly diagnosed but
didn't get resistance a resistance test until they asked for it.
• Usually it is sufficient to go back to the doctor and refer to the guidelines.
• Some clinics store a sample to test later, before starting treatment. In
theory this may be okay, but sometimes samples get lost, or old samples
may be difficult to test. In these cases, testing the current sample may not
pick up resistance which is present at low levels. There is no real cost-
saving from delaying this test.
HIV drug resistance
• If your doctor or clinic will not agree to the test when it is clearly
recommended, you can write to the head of your clinic and the head of
your health trust. If this is still not provided you may want to register at
another clinic to get this test. You can always change back to your local
clinic in the future for routine monitoring or treatment.
Please call the i-Base phoneline if you would like further information or
support in accessing resistance tests.
5.6 Section 5: Learning points
This section has been more practical and should help connect the previous
technical information to how this affects things in the clinic.
• Treatment guidelines are an important resource, because they state when
tests should be used. Most guidelines agree on the use of resistance
testing. Guidelines are free to access online.
• Genotype tests are used routinely. Phenotype tests are used when there
is more complicated resistance and fewer treatment options. They are
also used when results from a genotype test are unclear.
• Guidelines are not always followed, especially for newly diagnosed
• Resistance tests and the subsequent results often require active patient
or advocacy involvement.
Phoneline 0808 800 6013
Resistance 6: Research into drug resistance
6.1 Recap from previous section
This last section looked an when to use resistance tests.
We referred to the UK treatment guidelines, though other guidelines for
countries with access to resistance tests are similar. Even in the UK these
tests are not always provided, so this is an advocacy issue.
Key times include:• On first diagnosis (to test for transmitted resistance).
• Before starting treatment (if there is a risk of HIV reinfection).
• If viral load doesn't drop by more than 90% in the first month on
• If viral load rebounds to over 200-1000 copies/mL on treatment.
• Before any treatment change when viral load is over 1000 copies/mL.
• One month after discontinuing treatment (for women who only use
treatment during pregnancy).
The reason for testing at each time is included in Table 3 in section 5.3.
6.2 Introduction to section 6
This section looks at some research studies. These provide examples of the
ideas discussed in earlier sections.
They show some of the ways that research changed how we understand
the way HIV acts. They also show how new information led to changes in
guidelines and in the way that HIV positive people are treated.
6.3 Resistance with 3TC monotherapy
A good study to show how resistance to a drug develops in a person (rather
than in theory or in a test tube) comes from early studies of the first HIV
drugs. This is the perfect way to get resistance! You have a high viral load and take
a treatment that is not strong enough to reduce viral load to undetectable.
This was early research. Using only one or two drugs is not recommended
now, other than in some circumstances with a boosted protease inhibitor.
HIV drug resistance
In this example, 360 people with good CD4 counts (200-500) used one of
four treatment. The groups were:1)
AZT monotherapy (no other active drugs)
3TC monotherapy (no other active drugs)
AZT + standard dose 3TC (150 mg twice-daily)
AZT + high dose 3TC (300 mg twce-daily)
This early AZT dose was 200 mg every 8 hours (ie three times a day).
Placebo pills were used in the monotherapy groups so everyone in the study
was taking the same number of pills.
CD4 and viral load results were recorded for one year, see Figure 6.
Figure 6: Changes in viral load with mono and dual therapy (adapted from Eron et al.)
LAMIVUDINE, ZIDOVUDINE, OR BOTH IN PATIENTS WITH HIV INFECTION
AZT + low dose 3TC
AZT + high dose 3TC
Mean Change in HIV-1 RNA (log copies/ml) �1.5
viral load reduction (logs) –2.0 —�2.00 4 8 12 16 20 24 28 32 36 40 44 48 52
PATIENTS WHO COULD BE EVALUATED
Zidovudine only ( )
Lamivudine only ( )
Low-dose combined ( )
Figure 6 shows the impact of resistance when only one or two nucleosides
High-dose combined ( )
are active. Within two weeks viral load dropped in all groups. But then with
Figure 2. Mean (�SE) Changes from Base Line in the Log Concentration of HIV RNA, According to the Week of the Study.
The number of patients shown for each week in each treatment group is the number who could be evaluated at that time. After week
24, the numbers of patients indicate the numbers available for study at each point in the analysis; the numbers do not indicate rates
3TC monotherapy viral load rebounded by week four and rebounded in the
of withdrawal from the study. Some patients had not completed the extended phase of the study by the time of this analysis.
other groups by week eight).
When the change in HIV-1 RNA levels was analyzed in
cell count over the first 24 weeks of the study in patients
the 224 patients who had base-line levels 2 log or more
who received the combination therapy at either dose, as
Reference: Eron JJ et al.
above the threshold of detection of the r T
ev reatment with 3TC,
compared with zidovudine monotherapy. Unlike many
criptase PCR assay, the median peak decrease in levels
studies evaluating AZT
other or both in HIV
therapies,3,7-9,33-36 our s -positive patients with 200 to
500 CD4+ cells/mm3. NEJM V
among patients receiving combination therapy at ei ol 333 no 25 p1662-1669. (21 December 2005).
showed sustained increases from the base-line CD4�
dose was 2.1 log, and approximately two thirds of the
cell count with both combination treatments and no re-
patients in the co.nejm.org/doi/full/10.1056/NEJM199512213332502
mbination-therapy groups had at least
turn toward base-line values in patients followed for 52
one value that was 2 log or more below the base-line
weeks. The difference between the combination groups
value during the study. This double-blind, randomized
and the zidovudine-only group in the mean increase
clinical trial used changes in plasma levels of HIV-1
from base line at 52 weeks was more than 100 cells per
RNA as a primary end point. The plasma level of HIV-
cubic millimeter. Although conflicting results have been
1 RNA is a strong predictor of the progression of HIV-
reported,2 several earlier studies of antiretroviral thera-
1 infection, independently of the CD4� cell count.28,29
py have shown clinical benefit when the therapy being
In addition, retrospective analyses of several prospec-
evaluated produced changes in CD4� cell counts33-36
tive clinical trials have shown that a reduction in the
that were either less substantial or less prolonged than
plasma level of HIV-1 RNA in response to therapy is
the changes we observed. Given the greater magnitude
an independent predictor of clinical benefit.30-32
and duration of the effect of treatment combining lami-
The decreases in HIV-1 RNA levels were accompa-
vudine and zidovudine on the CD4� cell count and the
nied by substantial increases from the base-line CD4�
viral burden, it is possible that this combination will
The New England Journal of Medicine
Downloaded from nejm.org by SIMON COLLINS on February 10, 2011. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Phoneline 0808 800 6013
6.4 An undetectable viral load stops HIV evolving
In section 3 we looked at HIV not proofreading to check that new virus is
always the same. This results in someone having a vast mixture of slightly
different viruses before they start treatment.
This example is included to show the benefit of treatment driving viral load to
undetectable. [1]Lisa Frenkel is an American paediatrician who led a team that looked at viral
load in 37 children who generally had undetectable viral loads on treatment.
About 60% of children (21 out of 37) also had low-level blips (from 50 to 400
copies/mL) on treatment.
The group looked at the virus present during the blips and compared this
to the sample before treatment (using a phylogenetic test). In 8 out of
11 children the virus was exactly the same. The only evidence of virus
development was in two children whose viral load had blipped many times.
This was one of the first studies to show that ongoing replication effectively
stops when on treatment. Some of these children had been on treatment
for several years and their HIV was exactly the same as when they started
treatment. This research supported the theory that treatment is usually
as potent as it can be. Adding more drugs does not reduce viral load any
further. This has been demonstrated in other intensification studies. [2]This is important.
The HIV produced in most people with an undetectable viral load seems to
come from resting or latent CD4 cells that went to sleep before treatment
was started, and are periodically waking. These long-lived cells (some sleep
for more than 60 years) are referred to as the viral reservoir. This has led to
other researchers trying to find a way to target the resting CD4 cells. If this
can be done there is the chance to cure HIV.
References1. Frenkel, L. M. et al. Evidence that Low-Level Viremias during Effective Highly Active
Antiretroviral Therapy Result from Two Processes: Expression of Archival Virus and
Replication of Virus. Journal of Virology, August 2005, p. 9625-9634, Vol. 79, No. 15
2. See this HTB report on intensification studies. Lack of virological impact of treatment
intensification in suppressed patients supports latent viral reservoir as source of residual
viraemia. HTB August 2008.
HIV drug resistance
6.5 Trends in transmitted drug resistance over time
In section 3.5 we included the first reference to the fact that resistant HIV
can be transmitted. Lots of research groups keep databases of every resistance test in a country.
They can then look at trends in transmitted drug resistance each year.
This study, from the UK HIV drug resistance database (UK-HDRD), is
available online.
The group tracks and reports the results of resitance testing each year and
shows how this changes over time.
The study reported trends in resistance over time and differences between
newly diagnosed people and people with drug resistance.
This is only one of many studies produced by the group,
Figure 7. Percentage of tests showing drug resistance by drug class in the UK
Phoneline 0808 800 6013
6.6 Single dose nevirapine during pregnancy
This example shows the importance of timing when looking for resistance.
Section 4.3 showed how some mutations develop easily and that for some
drugs and drug classes resistance develops easily - in this example NNRTIs.
Nevirapine is an NNRTI and key mutations - Y181C and K103N - only need
one base change to develop. Once there they are like an on/off switch: the
NNRTI stops having any impact on the viral load.
Nevirapine is good at getting nearly everywhere in your body very quickly.
A single dose of nevirapine just before giving birth dramatically reduced the
chance of passing HIV to the baby. From 25% down to 12% with a single
dose. Previously, AZT needed to be taken twice-daily for several months to
have the same impact on reducing transmission to the baby.
For women in countries with no access to treatment this was potentially
very exciting. However, other researchers were cautious about the risk of
resistance for the mother. Using only a single dose of a drug meant that the
nevirapine was not supported by any other drugs. Viral load was still likely to
be high, and nevirapine also takes several weeks to leave the body. This left
a long time for HIV to develop NNRTI mutations by chance. They develop
and reproduce because they are more fit when nevirapine is still there.
In an important single-dose nevirapine study (called HIVNET 012), the
researchers first tested for resistance six months after the women used
nevirapine, and reported that resistance was only seen in 20% of women
(which was still very high). Resistance experts were more concerned. In
section 3.5 we learnt that resistance that develops when a drug is present
usually becomes difficult to find when the drug is stopped. Some researchers
talk about resistance ‘fading' but this is a confusing term because we know
that when someone restarts treatment that resistance quickly comes back.
It is nearly always archived. When the researchers went back and tested
earlier samples - this time only two weeks after the women had used
nevirapine - they found over 75% of mothers had nevirapine mutations.
Reference:Eshleman EH et al. HIV-1 variants with diverse nevirapine resistance mutations emerge rapidly
after single dose nevirapine: HIVNET 012. XII International HIV Drug Resistance Workshop, Los
Cabos, Mexico, 10-14 June 2003. Abstract 79.
Reported in HTB, September 2003. Single-dose nevirapine resistance in over 75% of mothers. http://i-base.info/htb/11689
HIV drug resistance
6.7 Importance of resistance testing before starting treatment
This study is interesting because it shows the importance of resistance
testing before starting treatment.
It looked at about 300 people who were treated during primary HIV infection
(within 6 months of infection). Resistance testing was not used before
starting treatment to choose drugs. However, 35 people were found to have
resistance when their pre-treatment (baseline) samples were subsequently
tested. Of these 35 people, 21 had resistance to one drug, 10 to two drugs
and 4 people had resistance to all three drugs in their combination.
The resistance group had a significantly poorer response to treatment. Only
16% had undetectable viral load after three months compared to 40% of
people with no resistance. After six months the difference was still significant:
57% vs 79%, even after allowing for age, gender and differences in CD4
count and viral load at baseline.
This study and similar others were used as evidence to support the
recommendation to provide resistance testing before starting treatment in
Reference: Chaix1 ML, Desquilbet L, Cottalorda J et al. Sub-optimal response to HAART
in patients treated at time of primary HIV-1 infection and infected with HIV resistant strains.
Abstract 114. Antiviral Therapy 2005; 10:S126.
http://i-base.info/htb/7232
6.8 Reinfection/superinfection: catching HIV twice
This example is a report on reinfection from an important conference in 2010.
These were certainly not the first studies to report reinfection (sometimes
called superinfection) but it is a report of four different studies that each
involved interesting cases.
The table in Section 5.3 includes testing for resistance in anyone whose
viral load rebounds on treatment. It specifically mentions the circumstance
for someone on stable treatment but who may have been reinfected with
a different strain of HIV. One of the studies included a couple where both
partners were HIV-positive and did not use condoms. However, one of the
partners has no resistance and an undetectable viral load. The other had
triple-class resistance and a generally detectable viral load.
Phoneline 0808 800 6013
In this case the partner with no resistance became infected with the resistant
HIV. The initial viral load rebound prompted the doctor to test for resistance
and to compare the results to earlier samples. This case is important because it occured in someone who had been HIV
positive for many years (ie not in early infection). It also had a dramatic
clinical impact - the person's previously active treatment stopped working.
Reference:Castro E et al. HIV-1 superinfection with a drug-resistant strain in a patient successfully
controlled with ART. Poster abstract 480.
http://www.retroconference.org/2010/Abstracts/37374.htmSee report in HTB, June 2010. HIV reinfection cases reported at CROI 2010. http://i-base.info/htb/10502
6.9 Section 6: Learning points
• HIV drug resistance is an important and essential field of HIV research.
• Studies can change the way that resistance tests are used.
• Research can change treatment guidelines.
• This research often has a direct impact on the way that HIV drugs are
prescribed and how HIV treatment is managed.
HIV drug resistance
Appendix 1: Questions on resistance
The questions below were posted by participants on the i-Base course on
HIV and drug resistance. All question are answered on online:http://i-base.info/home/appendix-1-questions-on-resistance/
1. Which drugs can someone use
10. You need virus present for
if they have already developed
resistance testing – so you
can't test if the viral load is
2. How are treatment choices made
undetectable. How high does the
for someone with resistance?
viral load need to be to be able
to use resistance testing?
3. Can drug resistance be passed
from mother to child?
11. Do you need to be currently
taking a drug to see if you
4. Can a person's genetic makeup
are resistant to that drug?
contribute to them becoming
Is resistance still detected if
resistant to a drug?
you have stopped or changed
5. Is it possible to develop
resistance to a drug even with
12. If you have developed resistance
perfect adherence?
to a drug, does that mean that
6. Are some drugs easier to
you are resistant to all the drugs
become resistant to than others?
7. I want to understand the terms
13. Is poor adherence the only factor
used about resistance.
that leads to developing drug-
8. If viral load in the blood is less
resistance? Are there any other
than 50 copies/mL, but is higher
in sanctuary or compartment
14. If your viral load is undetectable
sites (ie the brain or genital
but your CD4 still low, could that
compartments), can resistance
be a sign of drug-resistance?
develop in those sites?
Would the doctors consider
9. What is "wild type" virus and
doing a drug-resistance test?
15. What are the main signs of drug-
16. At what level is viral load
considered undetectable?
Phoneline 0808 800 6013
17. What is a viral load ‘blip' and
25. With all clinics trying to cut back
what should you do about it?
because of funding cuts, how
18. When should you have a
do we make sure that important
tests like the resistance tests are
offered to patients when they
19. Seeing as HIV "makes
mistakes" and does not have
"proof reading" abilities when
26. How expensive (or cheap) are
replicating, is it possible to
resistance tests?
introduce "defective or modified"
27. Are resistance tests available in
genetic material that would
developing countries?
render the virus ineffective?
28. What happens to a pregnant
20. What is the difference between
woman with resistance to the
the types of tests for resistance –
drugs used for PMTCT?
phenotype vs genotype? Which
29. Are the tests always 100%
21. Are resistance tests the same
30. Are all possible mutations
tests that are used to see if
the type of HIV in different
people is in some way linked
31. Why can boosted protease
– in prosecution cases of
inhibitors be used as
transmission, for example?
monotherapy without developing
22. If a woman takes ART during
pregnancy for prevention of
32. Is resistance inevitable?
mother to child transmission
33. How does wild type virus prevent
(PMTCT) and wants more
resistant virus from reproducing?
children, is she likely to become
34. As a patient, what should I
resistant to those drugs?
expect to hear back from my
23. What options does a woman
resistance test? What should the
have for future pregnancies?
24. Some people have never had
resistance tests and would not
know how to bring this up in a
discussion with a doctor. How
can this best be addressed?
HIV drug resistance
Appendix 2: Supplementary information about genetics
DNA as a recipe book – for making new HIV
Sometimes it helps to think of DNA as a recipe book.
• There are only four letters used in this book, (A, T, C and G: the four
nucleotides or BASES)
• There are only 20 different words (the 20 common AMINO ACIDS). Each
word only has three letters. The place that each of these words is printed
is also called a CODON.
• Each sentence (a PROTEIN) is made up of chains of many 3-lettered
words (AMINO ACIDS).
• Each recipe – a chapter – (a GENE) is made up of several thousand
• Each book (GENOME) is made up of many chapters (GENES)The HIV genome has nine short chapters, using about 3,000 words (AMINO
ACIDS/CODONS) and about 9,200 letters (BASES).
The human genome contains 23 large chapters (CHROMOSOMES), many
thousands of sentences, around one billion words and three billion letters.
If you read one word every second, 24 hours a day, it would take over 30
years to read the human genome.
In humans, only 10% of the 3 billion bases are thought to be important and
active. So some changes in DNA may not make any difference. This means
that a large percentage of human DNA is like advertisments or blank pages.
We know what some parts of the DNA chain relate to – ie one part will
determine the colour of your eyes. Other sections of DNA have been
linked to more critical functions including risk or protection from a range of
hereditary health complications. So, many things about you are determined
by the order of the four bases and 20 amino acids in your DNA.
Phoneline 0808 800 6013
Nucleotides (bases)
There are only four base chemicals that make up DNA:
A = adenine T = thymine C = cytosine G = guanine
The order of these bases determine the structure and function of all life. To
make it easier, in DNA these molecules only have two pairs of bonds:A always binds to T and C always binds to G.
Amino acids
Each group of three bases will be one of 20 different amino acids.
Amino acids are the chemical building blocks to make proteins and almost
everything in the body is either made of proteins or needs proteins to make
it.
See Appendix 3: List of amino acids and their abbreviations.
You do not need to learn these or know about the differences for each amino
acid. It is important to understand that each letter stands for a different
amino acid.
For example:The three bases ATG is the code for the amino acid called methionine (M)The three bases GTA or GTG are two of the codes for the amino acid called
valine (V)Most amino acids can be made up from different combinations of letters.
See Appendix 4: DNA codes for amino acids.
For example:Valine can be made from four different base combinations: GTT, GTA, GTG
and GTC• Strings of amino acids make up different proteins.
• Strings of proteins make up different genes.
• Strings of genes (in humans) are called chromosomes.
In this example, notice that only one letter needs to change to get from
methionine (M) to valine (V). This gives an indication that the mutation is a
simple change.
HIV drug resistance
Appendix 3: List of amino acids and their abbreviations
This table is included for reference only.
There is no need to learn these names and abbreviations. It is important that
you understand that the letter in drug resistance refers to different amino
acids. This table is included for future reference.
Table 4: Amino acids and their abbreviations
Single-letter 3-letter
abbreviation abbreviation

Phoneline 0808 800 6013
Appendix 4: DNA codes for amino acid
Table 5 is only for reference. You do not need to
learn any of these codes by heart.
It is included for two reasons.
1. Most amino acids can be made from more than one combination of bases.
2. That some mutations are easy and some are difficult. Easy mutations only
need one letter to change. The most complex mutations need to change
all three bases.
Table 5: DNA codes for amino acids
phenylalanine TTC
phenylalanine TTA leucine
GAT aspartic acid
GAC aspartic acid GGC glycine
GAA aspartic acid GGA glycine
GAG aspartic acid GTG glycine
Valine is coded by four different combinations: GTT, GTC, GTA and GTG.
This shows that some changes will not change the amino acid.
HIV drug resistance
If one mutation changes the third base from a T to C – as in GTT to GTC – the
amino acid at that junction will still be valine.
Some amino acid changes require one base change, some require two base
changes and some require all three bases to change.
The number of base changes needed to
change one amino acid to another is related
to how quickly a mutation develops.
This chart shows that the M184V is an easy
mutation - like getting one bell on a fruit
machine.
To change from methionine (M) to valine (V) only requires a change at one
base: ATG to GTG.
The mutation M184V that stops 3TC from working, is commonly the first
mutation to be detected in any 3TC-containing combination if that treatment
fails. As an easy mutation, it can occur
quickly. With 3TC monotherapy (ie when
3TC is the only drug) resistance would be
likely to occur within two weeks.
By comparison, the T215Y mutation is more
complex - like getting three cherries.
Threonine (T) can be made from four different base combinations: ACT, ACC,
ACA and ACG.
Tyrosine (Y) can be made from only two: TAT and TAC.
Importantly, to change from threonine to tyrosine needs at least the first two
bases to change (from ACT to TAT or ACC to TAC). In some cases, it might
need all three bases to change (from ACA or ACG to TAT or TAC).
The T215Y mutation is one of the main mutations associated with resistance to
AZT. However, as a more complex mutation, it is less likely to occur by chance.
In practice, this mutation might take six months to develop in someone taking
AZT monotherapy.
These mutations are like fruit machines: getting one bell is easier than getting a
row of three cherries to show. You need to play more often and for longer to get
the difficult combinations.
Phoneline 0808 800 6013
Appendix 5: HIV genome map with example mutations
This graphic is a map that simplifies HIV into nine main genes: gag, pol, vif,
vpr, vpu, env, tat, rev and nef. The map shows roughly how these genes are
Figure 8: HIV genome map with example mutations
HIV drug resistance
Each gene has a different role in the HIV life cycle. You don't need to learn
all these functions, just recognise that we know roughly what many of the
functions are. For example env is easiest to remember because it is the
recipe for spikey proteins that stick out from the ‘envelope' coating on the
outside of the virus.
Pol is important when talking about drug resistance. This is because this
section of the HIV genome that has the code for the enzymes that are
targetted by many HIV drugs. These include protease inhibitors (PIs),
reverse transcriptase inhibitors (nukes and non-nukes) and integrase
inhibitors.
The numbers underneath the main picture relate to the 9700 bases (roughly
3,200 amino acids) that make up the genetic structure of HIV. Pol starts at number 2550 and stretches to 5096.
The protease section stretches from 2253 to 2550. This is a chain of 297
nucleotides, which equals 99 amino acids (amino acids are groups of three
nucleotides).
Therefore, the protease gene is numbered from 1 to 99. The example here is
L90M which is a common PI mutation.
In a similar way the amino acids in reverse transcriptase are numbered from
1 to 296. A change at position 184 on the RT gene from ATA to GTA is the
M184V mutation.
Genotype resistance tests do not need to look at the whole of the HIV
genome. They only need to look at this short section to get information about
resistance to nukes, non-nukes and PIs.
Resistance tests for entry inhibitors like T-20 need to look at a section of
the env gene. Resistance to integrase inhibitors needs a test that looks at a
small section on the integrase section of the pol gene. A more detailed map of the HIV-1, HIV-2, and SIV genomes, available athttp://hiv-web.lanl.gov/content/immunology/pdf/2000/intro/GenomeMaps.pdfA more detailed explanation of what these different genes do is at this link:http://i-base.info/qa/faq/hiv-genome-explainedIf you feel really geeky, then check it out, but while learning about resistance
just to let the rest of the information in this section sink in first…
Phoneline 0808 800 6013
Appendix 6: Stanford Drug Resistance Database online tables
The Stanford Drug Resistance Database is one of several online research
resources that contain a vast amount of support information about drug
resistance.
One page in the resource includes links to each drug family (Drug resistance
mutation tables) and the related mutations and individual pages for each HIV
drug (Antiretroviral drug summaries).
http://hivdb.stanford.edu/pages/drugSummaries.html
For example the link to NRTI-associated mutations.
http://hivdb.stanford.edu/cgi-bin/NRTIResiNote.cgi
This link looks complicated but just needs explaining (see Table 4).
Table 4: Nucleoside RT inhibitor (NRTI) resistance mutations (Stanford database)
184 Thymidine analogue mutations Non-thymidine analogue Multi-NRTI resistance
75 115 69 151 62 75 77 116
F/Y Q/E RN
Cons stands for consensus sequence which means wild-type non-resistant
virus. The letters in the Cons row refer to the amino acids that should be at
those junctions.
The line of numbers above the ‘Cons' row are the positions or junctions
(codons) where important changes occur:41 67 70 210 215 219 65 70 74 75 115 69 151 62 75 77 116Each of these junctions in RT is linked to resistance to different members of
the family of HIV drugs called ‘nukes' (reverse transcriptase inhibitors). They
are arranged in three sections.
The first section is for mutations linked to AZT (ZDV, zidovudine) or d4T
(these drugs are both thymidine analogues). The second section relates to
other nukes (non thymidine analogues, ie 3TC, ddI, tenofovir, abacavir etc).
The third section includes mutations that have broad cross resistance to all
nukes (multi-drug resistance).
HIV drug resistance
Each row next to a drug name then shows the mutation at each junction that
is associated with drug resistance.
So reading across from 3TC the letters V and I mean that M184V or M184I
both indicate resistance to 3TC. Red letters indicate the resistance is high
level (it will have a big impact on stopping the drug form working). Regular
letters indicate lower level resistance, showing that the drug may still work a
little, but less than if it was wild-type.
The letters in black (representing K65R, K65N, K70E, K70G, T69Ins, Q151M
and A62V) list other mutation that all reduce how well HIV works.
Although this looks like a lot of mutations, amino acid changes at all the
other junctions are not associated with resistance. for example changes at
42, 43, 44, 45, 46 etc in RT have not been seen to be clinically relevant.
NOTE: ‘Ins' refers to insertion, which is a type of mutation where instead
of one base switching to another (technically called a ‘point mutation'), an
additional base in inserted. This shows the complexity of interpreting HIV drug resistance….Time for a
break to let this information settle :)The link to 3TC (lamivudine) drug summary explains the implications for
each of the mutations that have been associated with 3TC resistance AND
includes references for the studies which show this:http://hivdb.stanford.edu/pages/GRIP/3TC.htmlFor example:
M184V M184V causes high-level resistance to 3TC (>300-fold reduced
susceptibility). In patients with viruses containing M184V,
there is some benefit in continuing 3TC because viruses with
M184V replicate less well than wild-type viruses and because
this mutation increases susceptibility to ZDV, d4T, and TDF
(Campbell et al. 2005; Larder et al. 1995; Nijhuis et al. 1997).
However, the benefit of continued 3TC will be less than the
benefit of 3TC in patients with wild-type virus.
This explanation includes new terms which relate to phenotype resistance
including ‘fold changes' and ‘reduced susceptibility'.
Phoneline 0808 800 6013
Appendix 7: IAS-USA mutations and cross resistance
The following tables from the International Antiviral Society USA (IAS-USA)
show mutations for each drug and cross resistance between drugs in each
class.
The IAS-USA website includes free download of the latest guidelines to HIV
drug resistance published in 2013 in Topics in HIV Medicine that includes
Table 5: Key to IAS-USA drug resistance mutation tables (2013)
HIV drug resistance
Table 5: IAS-USA resistance tables for NRTI and NNRTI drugs
Table 6: IAS-USA resistance tables for NNRTI drugs
Phoneline 0808 800 6013
Table 7: IAS-USA resistance tables for PI drugs
Table 8: IAS-USA resistance tables for entry inhibitors and integrase inhibitors
HIV drug resistance
Appendix 8: Summary of UK Guidelines (BHIVA)
Section 14 in the UK monitoring guidelines (BHIVA 2011) includes the
current recommendations for resistance testing. They are summarised in Table 9 below. Please see the online version for full
details and references.
Ref: Asboe D et al. British HIV Association guidelines for the routine investigation and
monitoring of adult HIV-1-infected individuals 2011. HIV Medicine (2012), 13, 1–44. http://www.bhiva.org
Table 9: Summary recommendations on when to perform resistance testing
diagnosis Starting ART
Recommended, if not already carried out.
Repeat testing not routinely recommended but
can be considered if superinfection likely.
After starting Consider resistance testing if suboptimal
response to first-line therapy (<1 log10 copies/
mL reduction after 4 weeks of therapy).
Consider resistance testing if viral load >50
copies/mL at 12–16 weeks after starting
Recommend resistance testing if viral load >50
copies/mL at 24 weeks after starting
Recommended to guide treatment changes.
Perform while on treatment (or not more than 2
weeks after stopping)
*Consider phenotype or virtual phenotype if multiple regimen failure and/or multiple mutations
on genotype where interpretation is uncertain.
Source: BHIVA monitoring guidelines, 2011, Table 14.1.
Phoneline 0808 800 6013
Appendix 9: Example of a resistance test report
Most resistance test in the UK use
The sample below shows the section
the Stanford database to generate a of a sample report for nukes and
resistance report.
NNRTIs. The online version includes
The list of mutations is put into an
online resource that generates the
A virtual phenotype test result is also
included online.
HIV drug resistance
The i-Base website glossary includes
DNA – an abbreviation for the
more detail for some of these terms.
scientific word for genes and genetic
active – an active drug is a drug that
material. It is the abbreviation for
still works to reduce viral load. The
virus is still sensitive to that drug.
drug resistant mutation – a mutation
amino acids – amino acids are the
or change that occurs in the HIV
building blocks of proteins. DNA codes genome that reduces a drugs ability to
for amino acids. Three nucleotides
(segments of the genetic code) make
escape mutation – a change in
HIV that evades the immune system
baseline test – a blood test taken
(rather than stoppin a drug from
before treatment is started to see if
there is any resistance.
genome – the complete genetic
CD4 count – a blood test that
information (RNA or DNA) of an
indicates the strength of the immune
system. The CD4 test is one of the
genotype – the genetic makeup of a
most important indicators for deciding
cell, an organism, or an individual.
when to start HIV treatment.
genotype test – a test that looks at
clinical cut-off (CCO) – a test result
how the genetic structure of a sample
that is associated with an impact on
of HIV and whether the virus has
clinical care. With resistance tests a
changed with drug resistant mutations.
lower CCO is the level below which
high level resistance – when an HIV
a drug is still sensitive or active. An
drug no longer works against the virus.
upper CCO is the level above which
the drug is not considered active.
intermediate level resistance – when
a drug still has some impact on HIV,
codon – the word for the junction
but when this is reduced (compared to
on genetic material (DNA or RNA)
wild-type HIV) because there is some
occupied by three nucleotides (or
drug resistance.
bases) to form an amino acid.
low level resistance – when there is
combination therapy – three or more some resistance but it does not have
HIV drugs to treat HIV.
any significant impact on how well a
compensatory mutation – an
additional mutation, that return the
major mutation – a drug resistance
virus to a greater fitness.
mutation that has a big impact on
cross-resistance – when resistance
whether a drug continues to work. This
to one drug causes resistance to other used to be called a primary mutation.
similar drugs.
Phoneline 0808 800 6013
minor mutation – a drug resistance
reverse transcriptase – an enzyme
mutation that has a small impact on
unique to HIV. It is used to convert
whether a drug continues to work.
single-stranded RNA into double-
This used to be called a secondary
stranded DNA. This is needed
before HIV's genetic material can be
monotherapy – using only one drug.
integrated in the human DNA.
mutation – a change in the genetic
revertant mutation – this term is used
structure of an organism (including a
virus like HIV).
i) a genetic change that shows
nucleotide – the building blocks of the the virus is returning to a wild-type
genetic code (DNA/RNA). Also called
ii) a compensatory mutation as it
partially active – a treatment that has compensates for the reduced fitness
some resistance and some sensitivity.
caused by the first mutations.
PEP (Post-Exposure Prophylaxis) –
RNA – an abbreviation for the
a course of HIV treatment (usually
scientific word for genetic material
one month) taken after a potential
found in some types of viruses
exposure to reduce the chance of
(ribonucleic acid). It is very similar to
DNA but is single-stranded rather than
phenotype — relating to how an
organism behaves, based on how its
selective pressure – this is when
genotype relates to the environment.
a factor in the environment causes
one type of organism to develop and
phenotype test – a resistance test
grow in preference to another. With
that looks at how well a drug works
HIV drug resistance, the presence of
against HIV in a test tube.
a drug exerts selective pressure for
reinfection – catching HIV a second
resistance to develop. It is based on
time. Sometimes called superinfection.
evolution and the concept of ‘survival
resistance – when the genetic
of the fittest'.
structure of a virus or organism
sensitive – when referring to the
changes so that treatment no longer
activity of a drug, sensitive means that
a drug still works.
viral load – the amount of virus (for
example in blood, genital fluids of
tissue sample).
wild-type – HIV without any drug
HIV drug resistance
Record your resistance test results
UK guidelines recommend that HIV-positive people should be encouraged to know
their resistance test results. They also recommend that HIV clinics and laboratories
should make sure that resistance test results are permanently recorded.
Keeping a personal record of the important summaries will help if you change clinics
or if there is ever a problem with your notes. The treatment history is as important as resistance test results for interpretting the
Resistance test results
Date Results (include main mutations and which drugs are resistant)
Phoneline 0808 800 6013
Feedback
Your feedback on this guide helps us develop new resources and improve this
resource. All comments are really appreciated. Comments can be posted free to: FREEPOST RSJY-BALK-HGYT, i-Base, 57 Great Suffolk Street, London SE1 0BB.
Or made directly online at: http://www.surveymonkey.com/s/L8ZJM7P
1. How easy was the information in this guide to understand? Too easy
2. How much of the information did you already know? None
3. Did the information help you feel more confident speaking to your doctor? Yes, a lot Yes, a little
4. Which information did you find most useful?
5. Do you still have questions after reading this guide? Please give
examples.
Please include a contact email address if you would like us to reply.
6. Any other comments?
Contact details (if you would like a reply): Name
HIV drug resistance
All i-Base publications are available free
Treatment guides are written in everyday language
HTB is written in more technical medical language
Please photocopy or cut out this form and post to
HIV i-Base
4th Floor, 57 Great Suffolk Street, London, SE1 0BB
or fax to 020 7407 8489
or order online www.i-Base.info
Please send me
Introduction to Combination Therapy .
Guide to hepatitis C for people living with HIV .
Changing treatment: guide to second-line therapy .
Pregnancy and womens health .
HIV & your quality of life: side effects and other complications .
HIV testing and risks of sexual transmission .
HIV Treatment Bulletin (HTB) .
Address .
Postcode . Tel .
Phoneline 0808 800 6013
HIV drug resistance
Source: http://forum-link.org.uk/files/pdf/i-base-training-resistance-may14.pdf
Shoulder & Elbow2015, Vol. 7(4) 299–307 ! The Author(s) 2015Reprints and permissions:sagepub.co.uk/journalsPermissions.navDOI: 10.1177/1758573215601779sel.sagepub.com BESS/BOA Patient Care Pathways Amar Rangan, Lorna Goodchild, Jo Gibson, Peter Brownson,Michael Thomas, Jonathan Rees and Ro Kulkarni of stiffness. End range pain may persist until full Frozen shoulder is an extremely painful and debilitat-
Design of a prospective, randomized evaluation of an integrated nutrition program Design of a prospective, randomized evaluation of an integrated nutrition program in rural Viet Nam* David R. Marsh, Helena Pachón, Dirk G. Schroeder, Tran Thu Ha, Kirk Dearden, Tran Thi Lang, Nguyen Dhanh Hien, Doan Anh Tuan, Tran Duc Thach, and David Claussenius















