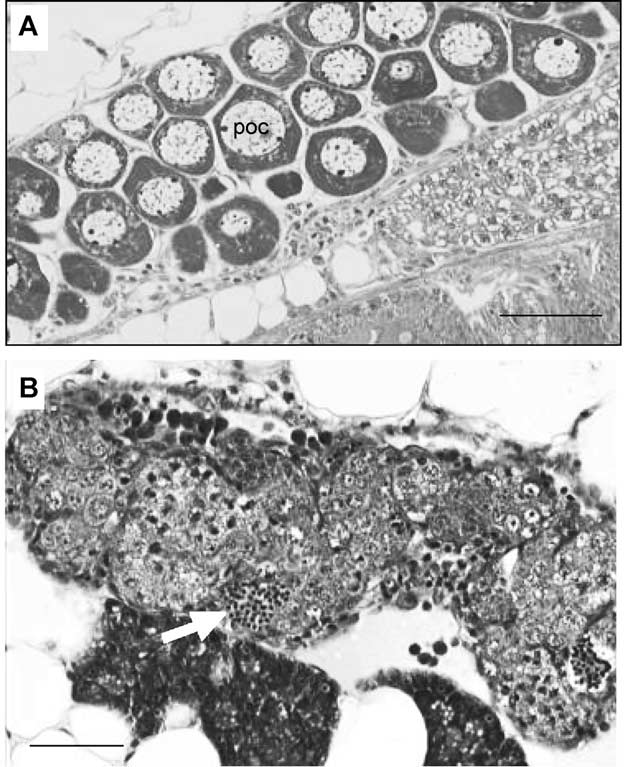
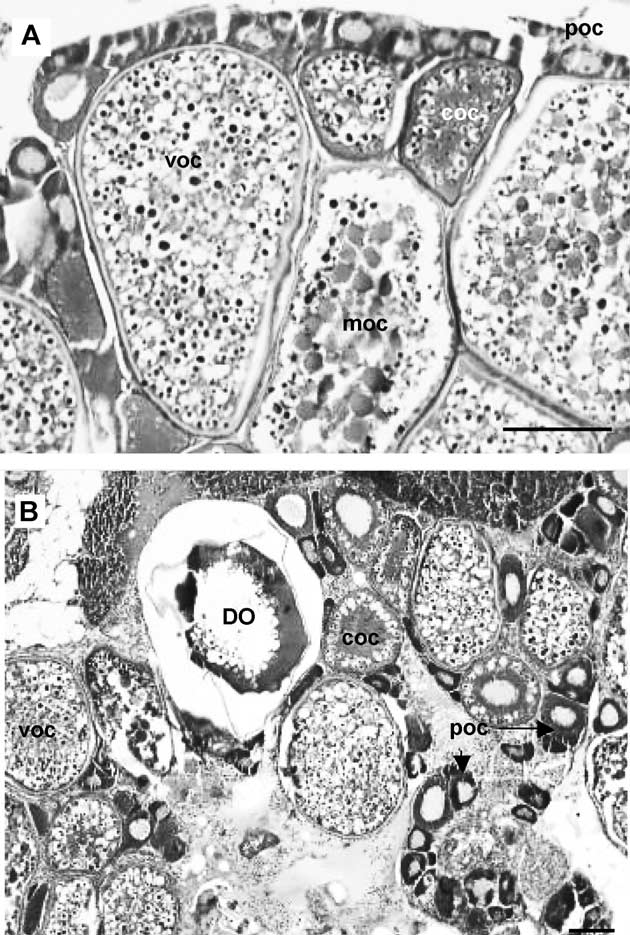
roche-plus.co.nz
The GAZYVA® dosing and administration guideA guide for health care professionals administering GAZYVA to patients with chronic lymphocytic leukaemia (CLL). DAYS 1 AND 2 CYCLE 1, DAYS 8 AND 15, CYCLES 2-6, DAY 1 All patients Patients Patients Patients with a