Health.go.ke
Republic of Kenya
Kenya
Essential
Medicines
List 2016
Ministry of Health
Kenya
Essential
Medicines
List 2016
Kenya Essential Medicines List 2016
Published by the Ministry of Health
Ministry of Health
Afya House, Cathedral Rd
Box 300 16-00100
+254 20 271 7077
Any part of this document may be freely reviewed, quoted, reproduced, or
translated in full or in part, provided that the source is acknowledged.
It may not be sold, or used for any commercial purpose.
Users of this publication are encouraged to send
comments, queries and proposals for amendment1 to the following
address from which additional information and copies may be obtained:
The Chief Pharmacist
Ministry of Health
1 Proposals for amendments to the list should be submitted using the KEML
Proposed Amendment Form
Table of Contents
KEML 2016
Foreword
This update of the Kenya Essential Medicines List (KEML) is most welcome. It is a key tool which should effectively be used to promote access to essential medicines, and through their correct selection, management and use to achieve maximum therapeutic benefit and optimise patient outcomes.
The KEML is an investment guide - a guide for the investment of healthcare funds in financing the most appropriate medicines to achieve therapeutic aims in response to prioritised public health need.
It is also meant to guide policy, focus of attention and resources (time, financial, technical and human) in areas and activities which support the above aims, such as training, quality assurance, financing & insurance, regulation & monitoring, appropriate use (including control of antimicrobial resistance), operational research and local production.
As such the KEML must be fully responsive to the aims and objectives of national health policies and strategies. In this respect, the KEML has incorporated the most current guidance to adequately address the heavy but gradually decreasing burden of communicable diseases (such as malaria, TB and HIV). In addition, particular attention has been paid to medicines to manage the ever-increasing numbers of those with non-communicable diseases (especially heart disease, diabetes, cancers and chronic respiratory diseases) which already account for over half of hospital admissions and deaths. Furthermore, medicines for other key (but often neglected or less well managed) areas of public health such albinism and jiggers, have been included in this KEML.
The evidence for listing medicines on the KEML 2016 was derived from a globally coordinated process of the World Health Organization (WHO), which develops the Model List of Essential Medicines, and makes the relevant information and knowledge available to countries for their own adaptation. The National Medicines and Therapeutics Committee
KEML 2016
(NMTC), through a Technical Working Group (TWG) coordinated the adaptation of the evidence, and extensive stakeholder consultations for the updated KEML.
The KEML should therefore be used with confidence and commitment as a highly relevant, evidence-based and up to date reference document. The systematic and well-managed consensus process through which it has been produced has ensured the incorporation of current evidence-based best therapeutic practice backed by extensive scientific data and robust application of selection criteria. Therefore the selection of the items listed is well justified and suitably adapted to the prevailing health sector context.
The KEML is meant to guide medicines investments for all relevant actors in Kenya. Because of the strong evidence base, the KEML represents best practice in the selection of medicines for optimum therapeutic outcomes. Therefore, it is applicable to, and recommended for use by policymakers and public sector providers at national and county levels, by private, faith-based and NGO actors, and by development partners.
The listing of medicines in a national list such as the KEML is only the initial step of a series of measures which must be implemented to ensure that the expected benefits and substantial health impact are realised.
Given its critical importance, the Ministry of Health is committed to support the KEML and to institutionalize the underlying principles and concepts, in respect of evidence-based priority-setting for medicines and other health technologies.
This arduous and technically complex task was completed well only through the sustained commitment and dedicated work of many individuals who contributed their time and expertise at the various stages of its development.
KEML 2016
On behalf of the Ministry of Health, I would wish to acknowledge and sincerely thank all the contributors, reviewers and editors who have made this KEML a reality2.
I also wish to thank WHO for the solid and objective evidence base and ready guidance, and for ongoing policy guidance to optimize the KEML as a priority-setting tool for Universal Health Coverage (UHC).
Finally I would like to thank the USAID-MSH/Health Commodities and Services Management Programme (HCSM) for their financial and technical support and the DANIDA Health Sector Programme Support (HSPS) for continuous technical advice throughout this complex process.
The KEML provides a key tool in support of efforts to attain equity and high standards in healthcare. It is intended to guide medicines development, production, procurement and supply, prescribing, dispensing and use, as well the development, monitoring and evaluation of strategies, thereby enhancing Appropriate Medicines Use (AMU).
It is for use by all disciplines of healthcare workers, general practitioners, specialists and healthcare management personnel as well as students and interns.
This KEML comes at a time when Kenya is defining strategies to attain the Sustainable Development Goals (SDGs)3, to which the country is committed. In this regard, access to medicines and vaccines is one of the cornerstones of universal health coverage (UHC), and is critical to the achievement of the health-related SDGs.
2 See Annex 1 for a list of all the individuals involved 3 Goal 3 is ‘Ensure healthy lives and promote well-being for all at all ages' with a key target ‘Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all'

KEML 2016
The KEML is a key component of the Kenya Essential Package for Health (KEPH), which defines the comprehensive priority services and interventions for UHC.
The regular and consistent use of the KEML can be expected to improve healthcare, and to contribute to the attainment of the Constitutional right to health.
I therefore strongly encourage all relevant health professionals to make the best use of this KEML in their daily work, to provide feedback on its use, and any suggestions towards its improvement and future revisions.
Rationale for Development of the KEML
Healthcare management and therapeutics are highly dynamic fields,
with new approaches, treatment protocols and therapeutic products
entering the market on a continuous basis. Providing comprehensive
healthcare services to the population requires heavy investments,
which constitute a major and ever-increasing cost to governments,
households and individuals. Therefore, effective mechanisms are
needed to prioritize the various health interventions and products, in
order to maximize therapeutic benefits and optimize patient outcomes.
Such mechanisms must be anchored on the best available scientific
evidence of cost-effectiveness, in order to objectively guide investment
decisions.
In this regard, clinical management guidelines, national formularies and essential medicines lists should be developed to guide and standardize healthcare delivery and these should be regularly updated to keep pace with best practice, and to optimize investments in healthcare. The Kenya Essential Medicines List was last revised and produced in 2010, but there was no effective mechanism for promoting and monitoring its use, and for subsequent regular review and revision. Consequently, this important guide to best practice in medicines selection became progressively outdated and consequently its relevance and usefulness in the health sector gradually diminished.
The Kenya Essential Package for Health (KEPH)
KEPH4 is a life-cohort based approach to the delivery of healthcare
services, which defined in a comprehensive manner, the services which
the sector is to prioritize so as to maintain health at all the different
stages of life. It defines the priority services that are necessary to be
4 For details of KEPH see Health Sector Strategic and Investment Plan (KHSSP) July 2013-
June 2017, The Second Medium Term Plan for Health: Transforming Health: Accelerating Attainment of Health Goals (Available a
KEML 2016
provided at 6 distinct levels of care – from the community level up to tertiary hospitals - for each of 6 defined life cohorts: pregnancy and the newborn (up to 2 weeks), early childhood (to 5 years), late childhood (6-12 years), adolescence and youth (13-24 years), adulthood (25-59 years) and the elderly (60+ years). KEPH also provides the framework for referral of clients across the sector providers, and it aims to improve utilization of health services at lower levels of care, beginning at the community level, as well as networking among providers and facilities across the different levels and between the public and non-public providers. KEPH also guides the types of medicines and other health inputs to be made available at each level and for each cohort, in line with the services to be provided and the corresponding expertise for the level, as defined in the sector norms and standards. The revised KEML strives to align with these strategic orientations in the health sector.
The KEML in the Context of Devolved Health Care
As described in the following sections, the KEML 2016 is derived from a
robust and globally recognized process of scientific assessment of
efficacy, safety and quality; as well as cost-effectiveness evaluation. The
investments required for such evaluations are massive, and the
processes require standardization of the evidence, in order to promote
uniformity in clinical care, disease control and public health protection.
Therefore, the KEML is a critical tool in ensuring the right to health by
ensuring optimum therapeutic interventions. Because of this, for the
national and county governments, the KEML 2016 should be the basis
for selecting the medicines for procurement using public funds.
Furthermore, the national and county governments have a duty to
ensure that essential medicines are available within the context of a
functioning health system, at all times in adequate amounts, in the
appropriate dosage forms, with assured quality and adequate
information, and at a price the individual and the community can afford.
To attain this, it is imperative that national and county governments develop sustainable solutions for financing essential medicines through
KEML 2016
increased budget allocations for health, and robust priority-setting mechanisms to optimize efficiency of the health budget. This should be accompanied by the regular updating of the KEML through a robust and evidence-based national process.
The WHO Model List of Essential Medicines
WHO is the secretariat for the Expert Committee on Selection and Use
of Essential Medicines, the group of experts responsible for revising and
updating the Model List of Essential Medicines (EML) and the Model
List of Essential Medicines for Children (EMLc). Every medicine listed is
vetted for efficacy, safety and quality, and is subjected to a comparative
cost-effectiveness evaluation with other alternatives in the same class
of medicines. WHO updates the lists every two years and the lists have
become an important guide for governments and institutions around
the world, in the development of their own essential medicines lists.
The 2015 edition (ie. the 19th EML and 5th EMLc) includes inter alia ground-breaking new treatments for hepatitis C, various cancers (including breast cancer and leukaemia) and multi-drug resistant tuberculosis (TB). Placing a new medicine on the WHO EML is a first step towards improving access to innovative medicines that show clear clinical benefits and could have enormous public health impact globally.
The purpose of the Model List is to provide guidance for the prioritization of medicines from a clinical and public health perspective. The hard work begins with efforts to ensure that those medicines are actually available to patients. This requires collaborative effort between governments, the private sector, civil society, WHO and other international partners.
The National Medicines and Therapeutics Committee (NMTC)
The role of NMTCs is critically important in identifying appropriate
medicines for use throughout the system and for guiding the use of
those medicines. When operating well, a NMTC is the leading clinical
coordinating body, as well as the reference point for all activities with
medicines-related components. They are considered a vital important
structure for ensuring evidence-based therapeutics, as part of a
comprehensive quality of care program.
KEML 2016
The first NMTC was established after the formulation of the Kenya National Drug Policy in 1994 but since then, the functioning of the NMTC has been erratic and ineffective, because of inadequate understanding of its critical role and multiple functions, a perception that it was primarily a pharmaceutical body, and a focus only on the intermittent development of therapeutic documents such as essential medicines lists and clinical guidelines rather than the provision of continuous advice and guidance on medicines and health technologies management and utilisation. It has suffered from a lack of enabling legislation, which would entrench the evidence-based guidance into decision-making for healthcare financing and service provision. Going forward, the Ministry of Health is committed to actively supporting all NMTC-coordinated initiatives to ensure that these challenges are minimised, in order to obtain the maximum value from its work including review and revision the KEML. In addition, healthcare institutions and facilities are encouraged to form similar medicines therapeutics committees (MTCs), to promote evidence-based processes that ensure the selection and use of those medicines that address the needs and priorities of the community in that area.
The KEML Development Process5
a) Background
Kenya developed its first Essential Medicines List in 1981. Over the years,
the Essential Medicines List (EML) concept has become increasingly
entrenched into the health system, with successive revisions of the
KEML in 1993, 2003 and 2010. Although the KEML review and
development process has encountered various challenges (see below),
the document is nevertheless considered a key policy and reference for
the sector, and efforts are constantly made to ensure that it is updated,
effectively disseminated and its regular and routine use promoted.
5 The first Kenya Essential Medical Supplies List (KEMSL) was simultaneously developed
alongside the KEML with the two retreats involving both TWGs and continuous close coordination between the two processes
KEML 2016
b) Past experience with KEML 2010
The previous KEML (2010) was successfully developed by an ad-hoc
technical working group under the supervision of the then NMTC,
following a well-managed process. It was the product of extensive,
diligent, highly creditable and relevant work, but it suffered from a
number of challenges which compromised the expected benefits.
These included: insufficient distribution & dissemination; inadequate
advocacy & promotion of its multiple uses and potential benefits;
absence of monitoring & evaluation to guide future revision and lack of
active solicitation of feedback from users to verify its continuing
relevance.
In the following years, the NMTC became dormant and suffered from uncertainties, disruption and un-coordination during a period of enormous changes and restructuring within the health sector. These changes included the promulgation of the Constitution 2010, various re-organizations of the Ministry of Health, and the onset of devolution. As a result, the intended 2-yearly review did not take place.
Further, the potential impact of the KEML as a guideline is limited by the lack of enabling legislation to mandate evidence-based cost-effectiveness evaluation in the determination of the public financing of medicines. This lack of legal status of the KEML (and the associated clinical guidelines) has also contributed to a failure to establish sustainable structures and processes within the health system, for the necessary periodic, regular and timely update. Consequently, these health system gaps have led to increasing obsolescence, ever-decreasing relevance of, and low levels of adherence to, these very useful tools.
c) Preliminary review
As a result of the above, the intended 2-yearly review did not take place.
Preparatory work for updating the KEML started in late 2012, using an
advance copy of the WHO Model List (ML) 2013, and compiling all the
comparisons and deviations with the KEML 2010, as a key review tool
and focus for selection discussions.
KEML 2016
d) Re-establishment of the NMTC
In February 2014, after months of preparation, the NMTC6 was
reconstituted and members appointed by the Principal Secretary. The
composition of the membership was closely guided by best practice in
this area to ensure the correct representation of all key MoH
departments. The NMTC responsibilities were described as: policy
development in the evaluation, selection & use of medicines & health
products; standards & guidelines development & dissemination; rational
prescribing and cost-effective use; IEC for health providers in matters
related to medicines & their use.
In the following months, the NMTC had numerous meetings and two
retreats in the course of which TORs were developed, 7 Technical
Working Groups (TWGs) established (with their own TORs & members
identified) and a prioritised action plan developed to guide the
sequence of work in multiple areas of interest. Amongst these TWGs
was one for the review and update of the KEML and another for the
preparation of a first ever Kenya Essential Medical Supplies List
(KEMSL) covering the selection of non-medicines items.
e) Preparation of key KEML review tools
In December 2014, the new WHO Model List 2015 (19th edition for adults, 5th edition for children) was made available online, necessitating a complete re-review of the KEML 2010 in comparison with this, and also with the Kenyatta National Hospital Formulary which was produced in 2013, and which gave an additional useful comparison representing a more recent picture of medicines utilisation in Kenya than the KEML 2010.
Work on preparing the tools which would be required for the eventual review and update of the KEML proceeded throughout 2015. The key tools developed as spreadsheets were
a Yes List (comparing the WHO ML with the KEML 2010 and
identifying all items on the ML but not the KEML for consideration for possible inclusion)
6 See Annex 5 for details
KEML 2016
a No List comparing the KEML 2010 with the ML and identifying
all items on the KEML but not the ML for consideration for deletion
f) Establishment of the review Technical Working Groups
In September 2015, again after several months of preparation, the TWGs
for the KEML and KEMSL were established with the required
representative membership (in line with WHO guidelines) appointed by
the Director of Medical Services.
Following this a secretariat was established to support the TWGs and
developed Standard Operating Procedures (SOPs) for the review &
update process of the KEML and the preparation of the KEMSL. These
were adapted from WHO SOPs for Guidelines Development which
involve the establishment of a robust, scientific methodology in order
to ensure the production of a credible and reliable output anchored
firmly in best scientific (evidence-based) practice. Given the time
available and deadlines applicable for the current review, it was not
possible to fully implement the SOP this time around, but key review
and selection principles and methodologies were identified for
implementation by the TWGs. To make the methodology explicit and
set the rules for the process, ten key criteria were identified for
application during the selection process as detailed on pIn November 2015, as preparation for the forthcoming review retreat,
the members of both TWGs were assembled for an intensive induction
meeting which oriented them to essential medicines concepts and
guided them through the selection principles, criteria, process and
tools (with adaptations to include medical supplies) and completed
planning for the retreat.
g) Undertaking the KEML review
Following this, in relation to the KEML, discussions were held with national disease control programmes7, two 3-day retreats were convened with the TWGs and numerous consultations made with
7 HIV/AIDs, TB, Malaria, Cancer, Palliative Care, Reproductive Health, and Vaccines
KEML 2016
specialists in all key therapeutics areas8. Using the WHO Model List and the tools developed for the review, and through careful application of Essential Medicines principles and selection criteria, discrepancies and issues requiring clarification were identified and discussed and consensus reached on required amendments to the KEML. In regard to the retreats, members of the TWGs were reminded of the steps to be followed and the criteria to be applied. They also each signed a Declaration of Interest form to ensure transparency, impartiality and objectivity in their work. At the retreats members of the KEML TWG carried out a systematic and thorough review of each Essential Medicines item by item, and section by section, having received relevant inputs from the consultations. During the course of the review process important practice issues (especially relating to current irrational use of medicines or medical supplies by health professionals) were identified for urgent attention. Following incorporation of all the agreed changes, the updated drafts of the KEML was circulated to the TWG members for final review and confirmation of its completeness and correctness and a few corrections made based on comments received.
h) Feedback to stakeholders
A month after the second retreat, a half-day meeting was convened at
which key stakeholders9 were taken through the draft KEML, its process
of development and intended multiple uses.
Participants expressed appreciation for the high quality and thoroughness of the work done, but were most concerned that the KEML should (this time) be fully implemented in order to achieve the intended benefits and maximum therapeutic impact. They were
8 These included antimalarials, psychotherapeutics, anaesthetics, dermatologicals,
ophthalmologicals, anti-cancer medicines, ARVs, cardiology, immunologicals, nutritional products, radiologicals, ENT medicines, gastroenterology, hepatitis, renal medicines/dialysis fluids, and endocrinology
9 Including senior MoH management, heads of national disease control programmes,
CEOs of major hospitals, regulatory and supply organisation officials, some County Executives for Health or their representatives, and NMTC members
KEML 2016
informed that a comprehensive implementation plan would be put
together to ensure that this would indeed be the case.
i) Finalisation of the document for printing
Simultaneous post-retreat work was completed to produce a print-ready version for signing-off by the Cabinet Secretary, prior to printing, official launch and dissemination.
Challenges faced during the review and revision process
Despite the systematic, scientific approach and best efforts of the TWG,
a number of challenges presented themselves during the process of
review and revision of the KEML including:
Lack of required information. For certain proposed items information
was missing or incomplete in terms of such aspects as: (relative) cost, availability, cost-effectiveness, numbers of patients expected to require/benefit from the item (to assist in making a judgement on public health priority), limited published scientific information on an item and its use, and lack of written submissions for proposed list amendments. These constraints highlight a lack of the health technology assessment (HTA) required to ensure fully evidence-based (scientific) selection (ie. investment) decisions and thus robust justification for each of these decisions
Inadequate orientation of some contributors to essential medicines
concepts. Although members of the TWG were well-oriented having
undergone an intensive and comprehensive induction programme,
most of the specialists engaged to provide inputs into the process
had not had the benefit of such orientation. Efforts were made to
provide a brief explanation of the purpose of the KEML and the key
criteria to be applied, but not surprisingly many proposals were
received which, although mostly representing good clinical practice,
did not fit the criteria for listing as an essential medicine on the KEML
Inconsistencies between the national treatment guidelines, hospital
formulary and essential medicines lists. Although the Clinical
KEML 2016
Management and Referral Guidelines10 (2009) and the Kenyatta National Hospital Formulary (2013) were produced in good faith and through extensive and inclusive technical consultative processes, the management of the processes was not fully in line with best international good practice as for example defined by the WHO Handbook for Guideline Development11 and the resulting output inevitably compromised by this. For example, the documents contain many more medicines than are listed on the WHO Model List, some non-recommended and obsolete medicines and multiple medicines class members, eg. numerous beta-blockers, where best practice would dictate identifying a first choice medicine and strictly limited, well-justified second-line options.
The Clinical Management and Referral Guidelines were not fully responsive to essential medicines criteria in the selection of medicines for use in managing the conditions covered and have, with the passage of time, inevitably become progressively obsolete. Thus they are not well-aligned with the new KEML and are in need of urgent review and update following a well-defined, systematic and evidence-based process.
The NMTC is expected to oversee the next review of the clinical guidelines following a Standard Operating Procedure being prepared in line with WHO recommendations12 and international best practice. The NMTC will also be expected to supervise the preparation of a National Formulary derived from the KEML. This is also urgently required to provide all the necessary prescribing and other information on the medicines in the KEML to ensure (together with the clinical guidelines) that they are used appropriately, so as to derive the maximum therapeutic benefit.
Time constraints. Lengthy delays in undertaking the required regular
review of the obsolete KEML 2010 and the increasing urgency to
10 Clinical Management and Referral Guidelines for Level 1 Community, for Levels 2-3
Primary Care, and for Levels 4-6 Hospitals (3 books) (Ministry of Health, 2009)
11 Available a12 See Handbook for Guideline Development, WHO 2012 (available at
KEML 2016
produce an updated list as soon as possible - for example, to guide medicines procurement decisions and improve current medicines utilisation practices – meant that the full SOP for the review and revision process could not be applied for the current version. Thus, compromises had to be made to enable the process to be completed expeditiously. For example, it was not possible to insist on full written justifications to be submitted in support of list amendment proposals. Nor was it feasible to place proposals in the public domain for review and comment.
Prohibitive costs. This was probably the greatest challenge facing
selection decisions and it presents a major dilemma. A medicine may satisfy all other criteria but be unaffordable given the likely continuing limited resources available for medicines procurement for the public health system. It was the considered opinion of the TWG that no matter how effective a medicine might be, if its cost was so prohibitive as to be out of consideration for public sector procurement, there was no point listing it on the KEML. However, it is intended that medicines costs will be closely monitored and future selection decisions reconsidered accordingly as part of the planned continuous review process.
For medicines already on the WHO Model List, the above challenges did not apply, since the ML incorporates best current practice backed up by extensive scientific evidence. So the major criteria remaining to be considered were: is the proposed medicine required/suitable for use in Kenya, is it (cost-)effective and is it (likely to be) available. The bigger challenge involved medicines not on the Model List. For these, a selection decision was based on a reasonable judgement given the best available information - with a proviso that the NMTC monitors the effect of the decision, utilisation and impact of the medicine, and strives to obtain more supportive evidence for its continued inclusion in the list.
KEML 2016
Recommendations for future KEML review and revision
Legal establishment of the NMTC. In the context of the SDGs, the
KEML and the clinical guidelines are critical tools for the attainment of UHC. Therefore, in order to ensure that health financing decisions are based on sound and robust evidence, it is imperative that the NMTC and its associated processes of economic evaluation become legally entrenched into the health system, through an appropriate statutory committee or agency
Full application of the SOP for the KEML review and revision process
including: the requirement for written amendment proposals backed up by scientific justification, greater involvement of stakeholders in general and key stakeholders in particular, publishing of announcements of the process, invitations for submissions and amendment proposals on the Ministry of Health website, and early commissioning of expert (specialist) reviews of priority sections of the list
Development of the required health technology assessment13 (HTA)
capacity to facilitate comprehensive and complete assessment of
medicines proposed for addition to the list, particularly where these
are not on the WHO Model List (which have been subjected to
adequate HTA to support their inclusion)
Regular review and proper alignment. The KEML should be kept
under constant review and a new edition published every 2 years in line with the updates of the WHO Model List (ML). It is important that efforts be made to ensure that future editions of the national clinical guidelines and KEML are properly aligned, in order to realize the full benefits of evidence-based health care
Continued and intensified advocacy for the KEML, and improved
awareness and application of essential medicines principles,
13 HTA is ‘Health technology assessment (HTA) is a multidisciplinary activity that
systematically examines the technical performance, safety, clinical efficacy, and effectiveness, cost, cost-effectiveness, organizational implications, social consequences, legal, and ethical considerations of the application of a health technology' [Report from the EUR-ASSESS Project, Int J Technol Assess Health Care 1997, 13(2)]
KEML 2016
especially in making medicines selection decisions in preparation of clinical guidelines, essential medicines lists and formularies
Active monitoring and assessment of the utilisation of the KEML for
the uses described on and of the utilisation and impact of listed medicines, especially those which are newly introduced
Utilise the NMTC as a key resource in contributing to the development
of health financing strategies. Kenya is reviewing its health financing
strategy to move towards insurance-based financing, as part of the
reforms to facilitate UHC. A key requirement is to ensure that the
health interventions and technologies listed in the KEPH are derived
from systematic and objective cost-effectiveness evaluation. In this
regard, it is important that clear criteria be established to guide
evidence-based decision-making on which medicines and other
health technologies can be procured and/or reimbursed with public
funds. The current NMTC processes and procedures provide a good
starting point, and can be further refined by adapting from similar
successful processes in other countries
KEML Revision & Amendment Procedure
It is anticipated that the KEML will be reviewed constantly, and the full
list updated at least every 2 years, depending on the nature and extent
of cumulative amendments required. Urgent amendments will be
disseminated as required through the already established coordination
forums or other mechanisms for communication within the healthcare
system.
The NMTC (or an equivalent statutory entity) will undertake the review
and revision of future editions of the Clinical Guidelines and KEML. In
this strictly evidence-based process, the NMTC will be well guided by:
feedback obtained from operational research on KEML use in
each of the key medicines management areas identified in the
Main Uses of the KEML section on p
reports on KEML use obtained through feedback by users and
during the course of supportive supervision
MoH-approved changes in disease management protocols
(with concurrent changes to the relevant Clinical Guidelines)
KEML 2016
changes made to the biannual WHO Model Lists results of other relevant health research into disease
management and medicines utilisation
new information provided by medicines manufacturers on their
new information arising through quality assurance systems, eg.
pharmacovigilance and post-market surveillance
KEML Amendment Proposal Forms (see received from
In order to understand fully the relevance and wide range of application
of the KEML, readers are urged to become familiar with the Main Uses
of an EML as summarised on pand to study the Selection Criteria
used as listed on p This will definitely enrich the review & revision
needed to keep the KEML relevant and useful as a tool for improving
the quality, reliability and cost-effectiveness of health care services.
Presentation of Information
Medicines on the KEML are listed by broad therapeutic categories (Sections). Within each Section, medicines appear in alphabetical order and with the appropriate dosage forms indicated. The listing does not imply preference for one medicine over another.
Core List
The Core List represents the priority needs for the health‐care system.
Medicines on the Core List are:
Considered to be the most efficacious, safe and cost‐effective
for the relevant conditions
Those which do not require specialist inputs (see Specialist List
Expected to be routinely available in health facilities (at the
appropriately designated levels of care)
Expected to be affordable to the majority of the population. All
efforts should be made to ensure equitable access to medicines
on the Core List (and the most critical Specialist List items).
Priority conditions corresponding to the Core List were identified on the basis of current and anticipated future public health relevance and their potential for safe and effective treatment.
Specialist List
These items listed in italics are essential medicines for priority
conditions for which specialized diagnostic or monitoring facilities,
and/or specialist medical care, and/or specialist training are needed.
No level of use is indicated for such items as the level at which particular required specialist inputs are available will vary over time depending on the level of development of, and investment in, the health system in response to the aim of greatly improving access to specialist services.
Special efforts should be made to acquire Specialist List items, with particular priority on those recommended as 1st line medicines.
KEML 2016
Level of Use
This indicates the lowest level of the healthcare delivery system at
which each particular medicine may reasonably be expected to be
appropriately used (ie. after correct diagnosis and a correct decision on
management of the condition according to current best therapeutic
practice).
It is thus the lowest level at which the medicine is expected to be available for use (ie. distributed, stored, prescribed and dispensed).
The current levels are as follows:
1 = Community Health Services
2 = Dispensary/Clinic 3 = Health Centre
4 = Primary Hospital14 5 = Secondary Hospital15
6 = Tertiary Hospital16
14 Formerly District Hospitals, now (Sub-)County Hospitals 15 Formerly Provincial Hospitals, now County Referral Hospitals
16 National (Referral) Hospitals
KEML 2016
Abbreviations & Acronyms
Used in the text:
ADRs
Adverse Drug Reactions
Essential Medicine
Kenya Essential Package for Health
Medicines & Therapeutics Committee (Institutional)
NMTC National MTC TWG
Technical Working Group
Used in the KEML table:
ads adsorbed
amp ampoule
conc concentrate(d)
BP
British Pharmacopoeia (current edition)
Dispersible tablet
enteric-coated (tablet)
film coated (tablet)
HCl hydrochloride salt hyd hydrogen IM
international units
m/r modified (prolonged, delayed, slow) release n/a
MU mega (million) units PFI
powder for injection (to be reconstituted with diluent)
PFOL powder for oral liquid (to be reconstituted with diluent) SC
Tuberculin units
KEML 2016
Essential Medicines List (EML)
Background Information
Enhancing Access to Essential Medicines (EM)
Access to Essential Medicines is a core component of the right to health,
and a requisite to the attainment of national health goals. This national
Essential Medicines List (EML) defines the priority focus for investment
in medicines by the public health sector, towards ensuring the provision
of equitable healthcare to the population in line with defined sector
policies, strategies, norms and standards.
This EML is based on the Concept of Essential Medicines, defined by
WHO as:
those that meet priority health care needs of the population
carefully and systematically selected using an evidence-based process
with due consideration of:
o public health relevance
o clear evidence on efficacy and safety
o comparative cost-effectiveness
meant to be always available in a functioning health-care system:
o in adequate amounts
o in appropriate dosage forms
o with assured quality and adequate information
o at an affordable price for the individual & community
This EML is derived from the WHO Model List #19 (Adults) and #5 (Children) of 2015 and various national guidelines for specific conditions (eg. malaria, hepatitis, TB/leprosy, HIV/AIDS, STI, and IMCI) which represent the best current therapeutic practice in each of the priority conditions covered
Benefits of an EML to a Country
Priority Setting
The EML represents priority-setting on two levels:
KEML 2016
Careful identification of the priority health interventions, and the
careful selection of a limited range of EM results in a higher quality of care, better medicines management (including improved quality) and more cost-effective use of health resources
General benefits
Many studies show the positive impact of clinical guidelines and EMLs
on the availability and proper use of medicines within health care systems. This is very important in resource-poor settings where public sector medicines availability is often erratic
Measures to ensure regular EM supply will result in real health gains
and in increased public confidence in health services - and in the government of the day
Specific benefits
Supply system: use of an EML leads to easier and more-efficient
procurement, storage, distribution, stock management & record keeping; lower stocks (smaller item range, predictable procurement with reduced level of safety stocks); better quality assurance (can focus on fewer items); easier dispensing (greater familiarity with fewer items); more effective local production (efficiency in producing fewer items for a more predictable market)
Prescribing: use of an EML enables prescriber training to be more
focused & easier to deliver, more experience to be gained with fewer medicines, production of more focused medicines information (eg. National Formulary), minimizing of irrational treatment alternatives and better recognition of adverse drug reactions (ADRs)
Cost: use of an EML should lead to lower treatment costs (through
selection of the most cost-effective items), more competition (through identification of key items for national investment and therefore a substantial market for potential suppliers) and lower supplies management costs (fewer items to manage)
Patient Use: use of an EML will result in focused education efforts on
fewer, well known medicines, improved patient knowledge on medicines use, increased treatment adherence and improved medicines availability
KEML 2016
Essential Medicines Selection Criteria
Inclusion of a medicine on the EML should be considered if the
medicine, as far as reasonably possible, meets the following criteria:
1. Relevance/Need: Public health relevance and contributes towards
meeting the priority health care needs of the population
2. Safety: Scientifically proven and acceptable safety (side-effects &
toxicity) in its expected way of use
3. Comparative Efficacy: Proven and reliable efficacy compared with
available alternatives (based on adequate and scientifically sound data from clinical studies)
4. Quality: Compliance with internationally acceptable quality standards,
as recognized by the national medicines regulatory authority - currently the Pharmacy and Poisons Board (including stability under expected conditions of storage & use)
5. Performance: Sufficient evidence of acceptable performance in a
variety of settings (eg. levels of health care)
6. Comparative cost-benefit: a favourable cost-benefit ratio (in terms
of total treatment costs) compared with alternatives
7. Single ingredient: Unless there is no suitable alternative available, a
medicine should have only a single active ingredient
8. Local Suitability/Appropriateness: Preference should be given to a
medicine which is well known to health professionals, suitable for local use (eg. dose-form, staff training, support facilities) and socio-culturally appropriate (eg. method of use/administration)
9. Pharmacokinetic Profile: Wherever possible the medicine should
distribution, metabolism and excretion; drug interactions)
10. Local Production: Wherever possible the medicine should have the
possibility of being manufactured locally (for improved availability, reduced procurement costs)
Main Uses of the KEML
The KEML is a cornerstone of the national healthcare system, and a key
component of both the national health and national pharmaceutical
KEML 2016
policies. It is a vitally important tool and reference source for guiding
the management of common health conditions in the country, as well as
the management and utilization of medicines at national, county and
institutional (health facility) levels.
The KEML aims to support the smooth functioning of the healthcare
system and radically improve the availability and appropriate use of
medicines, for improved health status of the population. The health
sector will realize the full benefits of the KEML when it is routinely,
appropriately and fully utilized in the following key areas:
1. Healthcare Financing & Medicines Supply Budgeting: The KEML
should be used as a basis for prioritization of investment of available healthcare finances and, together with careful & systematic quantification of needs, for the estimation of required annual medicines supply budgets at all levels of the healthcare system. It should also form the basis for medicines financing by development partners.
2. Health Insurance Schemes: Medicines are a major cost element in
healthcare financing for Government, insurance schemes and partners. As the sector elaborates a comprehensive healthcare financing system, the KEML should be used as the basis for expanding coverage or reimbursement of medicines costs (e.g. positive lists with 100% reimbursement only for items on the EML).
3. Procurement, Supply & Distribution (including Donations): The
KEML should be used as a basis for determining medicines procurement requirements for all health care levels, ie. from dispensary level, to county level and up to the national referral level. This applies equally to public procurement by the national and county governments as well as procurement by the faith-based, NGO, private sector and other actors. The strong evidence-base for expected clinical benefits will help to guide investment of scarce health resources towards providing the most appropriate medicines, to patients and the public.
KEML 2016
Use of the KEML will help focus management efforts on a needs-based and prioritized list of critical items, and can greatly improve the functioning and efficiency of medicines supply & distribution systems. The KEML should be used as a basis for pre-printed order forms for the pull system of medicines supply to health facilities. The level of use (LOU) designation should be used to guide the supply and use of medicines at the appropriate levels of care, as defined in KEPH.
4. Donations: potential medicines donors and recipients should use
the KEML to determine the most appropriate types and presentations of medicines for donation to meet public health priorities, including health emergencies. This should be done in line with up-to-date national guidelines on donation of medicines and health products.
5. Healthcare Workforce Development: up-to-date clinical guidelines
and the KEML should be key references in the training of health care personnel, to provide correct orientation on evidence-based management of health conditions, as well as the appropriate prescribing, dispensing and medicines utilisation. This includes formal and in-service training, as well as continuing professional education for medical, pharmaceutical and nursing professionals. Use of these tools can help to correctly orientate health service delivery towards optimal utilization of medicines.
6. Medicines Regulation & Monitoring (including Quality Assurance):
The KEML should be used as a basis for ensuring an effective system of regulation of all activities involving medicines (including import, export, local production, registration, levels of distribution/use, quality monitoring, post-market surveillance [pharmacovigilance], prescribing and dispensing). The KEML should guide medicines regulatory decision-making, aimed at enhancing access to Essential Medicines. This may include fast-track registration and incentives to stimulate local pharmaceutical production of items listed on the KEML. Information that is comprehensive and unbiased should be made available to health workers and the public and due emphasis
KEML 2016
placed on market surveillance for quality, safety and efficacy of items listed on the KEML.
7. Appropriate Use of Medicines: The KEML should be used as a basis
for designing strategies and initiatives to promote the correct use of
medicines by health professionals, patients and the public. Such
activities should focus on promoting and improving utilization of
Essential Medicines (on the KEML) as the most appropriate for
attaining maximum health benefits.
In particular the KEML should be used as the focus of related
surveys, studies, operational research by the National Medicines &
Therapeutics Committee (NMTC) and institutional MTCs, with the
aim of improving the availability, affordability, prescribing,
dispensing and use of medicines for greater public health impact. It
should also be used as a basis for appropriate and effective
additional monitoring and control measures applicable to items
designated on the list for restricted use only.
Antimicrobial Resistance and Antibiotic Use Policies: The KEML
should be used to restrict antibiotic availability in health facilities to
those selected as the most appropriate for use at each level in the
current circumstances and context of changing resistance patterns.
Systematic data through drug efficacy monitoring and
pharmacovigilance, should inform future review of the KEML.
8. Medicines Policy Monitoring & Operational Research: up-to-date
clinical guidelines and the KEML should be used to identify parameters for monitoring, evaluation and operational research in the health sector, with the aim of ensuring the continued relevance of medicines and pharmaceutical policies to current health care requirements; as well as establishing the required evidence base for effective, systematic and regular KEML review and revision.
9. Pharmaceutical Manufacturing: the KEML should be used as a basis
for local manufacturing decisions focusing on priority public health items and formulations. Incentives for local production should primarily target products listed on the KEML.
Summary of Main Changes in
KEML 2016
The process of developing this KEML has resulted in significant changes to the items listed in the KEML 2010. The changes comprise additions of medicines that were previously not on the list, deletions of medicines that are either considered obsolete, or where other alternatives are considered more cost-effective based on available evidence; as well as changes to presentations to facilitate better administration and use.
The summary below highlights the main changes made in preparation of the KEML 2010.
Amendments Summary17
Deletions from KEML 2010
Additions to KEML 2016
KEML 2016 Totals
Total presentations19
Total list entries
17 These are expressed in terms of entries, ie. in a few cases there may be more than one
list entry for a given item
18 Drug combinations are counted separately 19 ie. all dose-forms, strengths, sizes of items; there are 67 multiple entries giving the
total of 687 entries on the KEML
KEML 2016
Kenya
Essential
Medicines
List 2016
Kenya Essential Medicines List 2016
Additions
Item Added
Inhalational anaesthesia; improved adverse
effect profile; replaced halothane in some countries Useful substitute for nitrous oxide in
Medical air inhalation (medicinal gas)
patients sensitive to oxygen toxicity IV anaesthesia (alt: thiopentone); improved
Propofol injection 10mg/mL
adverse effect profile, more rapid recovery; useful for diagnostic sedation
a) Lidocaine HCl injection 2%
Replaces lignocaine HCl injection 1%
(30mL vial) (preservative-free)
(can be diluted to make 1% as required)
b) Lidocaine HCl topical solution 2%
Replaces lignocaine topical solution 4%
(spray bottle) Chlorphenamine maleate injection20
Allergic reactions to morphine
Useful intraoperative medication
Fentanyl injection 50 micrograms/mL
(rapid onset, short-acting)
a) Midazolam injection 1mg/1mL amp
Replace diazepam presentations
b) Midazolam oral liquid 2mg/mL [c]
(faster onset and recovery)
c) Midazolam tablet 7.5mg a) Paracetamol injection 10mg/mL
Post-operative analgesia only
(100mL vial) b) Morphine oral liquid 1mg/mL
Severe chronic pain
c) Morphine oral liquid 10mg/mL
d) Morphine tablet 30mg
(prolonged-release)
(for greater dosing flexibility)
c) Diazepam gel or rectal solution
Alternative presentation
5mg/mL (0.5mL tube)
(where oral route not possible)
Gabapentin tablet 300mg a) Haloperidol injection 5mg/1mL amp
b) Haloperidol tablet 5mg
Improved symptom management in
Hyoscine butylbromide injection
Lactulose oral liquid 3.1-3.7mg/5mL [c]
Loperamide capsule 2mg
20 Added to this section (was already listed elsewhere in KEML 2010)
KEML 2016
Item Added
a) Metoclopramide injection 5mg/mL b) Metoclopramide oral liquid
5mg/5mL c) Metoclopramide tablet 10mg
Improved symptom management in
c) Midazolam oral liquid 2mg/mL [c]
d) Midazolam tablet 7.5mg a) Ondansetron injection 2mg/mL [c]
b) Ondansetron tablet 4mg [c] a) Cetirizine oral liquid 5mg/5mL
Replaces deleted chlorphenamine
b) Cetirizine tablet 10mg
(better adverse effect profile) Replaces activated charcoal tablet 250mg
Activated charcoal PFOL 50g
as preferred presentation
a) Deferasirox tablet 100mg
b) Deferasirox tablet 400mg
Flumazenil injection 100mcg/mL
Benzodiazepine poisoning
Protamine sulphate injection21
Heparin overdose
10mg/mL a) Ethanol injection 100%
Methanol poisoning
b) Ethanol oral liquid 95-96%
Fomepizole sulphate injection 5mg/mL
Methanol poisoning
Pralidoxime chloride PFI 1g
Organophosphate poisoning
Sodium nitrite injection 30mg/mL Sodium thiosulphate injection
Cyanide poisoning
250mg/mL Diazepam gel or rectal solution
Replaces diazepam injection in children
(preferable presentation)
Gabapentin tablet 300mg
Focal seizures Replaces diazepam injection in adults (recommended 1st line parenteral
Lorazepam injection 4mg/mL
anticonvulsant, better adverse effects profile)
a) Phenobarbital sodium injection
Paediatric emergencies
30mg/1ML amp [c]
a) Lamotrigine tablet 25mg
Refractory epilepsy
b) Lamotrigine tablet 100mg Albendazole tablet 400mg
Required for current management
21 Added to this section (was already listed elsewhere in KEML 2010)
22 Added to this section (was already listed elsewhere in KEML 2010)
KEML 2016
Item Added
Diethylcarbamazine tablet 100mg a) Amoxicillin tablet 250mg
Replaces PFOL 125mg/5mL
(dispersible, scored) a) Amoxicillin + clavulanic acid tablet
Replaces PFOL 250mg + 62.5mg/5mL
200mg + 28.5mg (DT, scored)
b) Amoxicillin + clavulanic acid tablet
Replaces tablet 625mg
875mg + 125mg Benzathine benzylpenicillin PFI
Preferred presentation; replaces 1.44g
900mg (1.2MU) vial
(2.4MU) vial For restricted use in surgical premedication
Cefazolin PFI 1g vial
prophylaxis For restricted use in sickle-cell prophylaxis & rheumatic heart disease prophylaxis
Phenoxymethylpenicillin tablet 250mg (as alternative to benzathine penicillin
where this is unsuitable)
a) Imipenem + cilastin PFI
For specialist 2nd line use only in life-
250mg + 250mg vial
threatening hospital-acquired infections due
b) Imipenem + cilastin PFI
to suspected or proven multi-drug resistant
500mg + 500mg vial
organisms
a) Ciprofloxacin oral liquid
For use in children
Alternative to metronidazole; longer action
Tinidazole tablet 500mg23
permits single daily dosing for improved adherence
Ciprofloxacin solution for IV infusion
Required additional dose form for use in
2mg/mL (50mL bottle) (as lactate) [c]
a) Clofazamine capsule 50mg
For use in recommended national
b) Clofazamine capsule 100mg
treatment regime
b) Rifampicin + isoniazid +
pyrazinamide + (RHZ) [c]
Required paediatric dose-form
75mg + 50mg + 150mg
Bedaquiline tablet 100mg
Capreomycin PFI 1g vial
Cycloserine tablet 250mg
Delamanid tablet 50mg
For specialist use in MDR TB
Levofloxacin tablet 500mg (scored)
Linezolid tablet 600mg
Moxifloxacin tablet 400mg
p-aminosalicylic acid granules 4g
23 Added to this section (was already listed elsewhere in KEML 2010)
KEML 2016
Item Added
Prothionamide tablet 250mg
Rifabutin capsule 150mg
For specialist use in MDR TB
Clotrimazole vaginal tablet 500mg
Replaces 200mg (improved adherence)
Aciclovir PFI 250mg vial
Required additional presentation
a) Tenofovir disoproxil fumarate (TDF) tablet 150mg
b) Tenofovir disoproxil fumarate (TDF) tablet 200mg a) Efavirenz tablet 200mg
b) Efavirenz tablet 400mg c) Efavirenz tablet 600mg a) Etravirine tablet 25mg
b) Etravirine tablet 100mg Atazanavir + ritonavir tablet 300mg +
100mg a) Darunavir (TCM) oral liquid (susp) 100mg/mL
b) Darunavir tablet 75mg (f/c) c) Darunavir tablet 100mg (f/c) d) Darunavir tablet 600mg (f/c) a) Ritonavir oral liquid 400mg/5mL
b) Ritonavir tablet 100mg
Required for updated HIV management
Raltegravir tablet 25mg
Raltegravir tablet 100mg Raltegravir tablet 400mg (f/c) a) Abacavir + lamivudine tablet 60mg + 30mg b) Abacavir + lamivudine tablet
120mg + 60mg c) Abacavir + lamivudine tablet 600mg + 60mg a) Efavirenz + lamivudine + tenofovir tablet 400mg + 300mg + 300mg
b) Efavirenz + lamivudine + tenofovir tablet 600mg + 300mg + 300mg Emtricitabine + tenofovir tablet
200mg + 300mg Lamivudine + tenofovir tablet
a) Lamivudine + zidovudine tablet
KEML 2016
Item Added
Ganciclovir PFI 500mg vial
Cytomegalovirus retinitis (CMV)
a) Ribavirin tablet 200mg (film coated)
Viral haemorrhagic fevers and hepatitis C
b) Ribavirin tablet 400mg (film coated) Pegylated interferon alfa-2a injection
6.4.4.1.1
180 micrograms (vial or prefilled syringe)
Hepatitis B
Tenofovir disoproxil fumarate
6.4.4.1.2
tablet 300mg Pegylated interferon alfa-2a injection
6.4.4.2.1
180 micrograms (vial or prefilled syringe)
Hepatitis C
a) Ribavirin tablet 200mg (f/c)
6.4.4.2.2
b) Ribavirin tablet 400mg (f/c) b) Artemether + lumefantrine tablet
For patients <24kg
20mg + 120mg (dispersible)
c) Artemether + lumefantrine tablet
For patients >35kg; reduced pill burden
80mg + 480mg a) Artesunate injection 30mg vial
Reduced waste in paediatric use
b) Artesunate injection 60mg vial
For 1st line treatment of severe malaria
Dihydroartemisinin + piperaquine
For 2nd line treatment of uncomplicated
(DHA+PPQ) tablet (scored)
malaria (only after confirmed treatment
failure with artemether + lumefantrine)
Primaquine tablet 7.5mg
For radical cure of P. vivax infection
Proguanil HCl tablet 100mg
For malaria prophylaxis in sicklers and TSS
Sulfadoxine + pyrimethamine tablet
Use instead of deleted sulphadiazine and
Paracetamol suppository 125mg [c]
Required paediatric presentation
Ciclosporin capsule 100mg
For reduced pill burden with higher doses
a) Methylprednisolone PFI 125mg (as sodium succinate)
Kidney transplantation
b) Methylprednisolone PFI 500mg (as sodium succinate) a) Mycophenolic acid tablet 180mg (as mycophenolate sodium) e/c
Kidney transplantation; better side-effect
b) Mycophenolic acid tablet 360mg
profile than mofetil salt
(as mycophenolate sodium) e/c
Prednisolone tablet 5mg
Kidney transplantation Kidney transplantation (induction phase;
a) Tacrolimus concentrate for IV
when vomiting, and oral route not possible in
infusion 5mg/1mL amp
postoperatively)
KEML 2016
Item Added
b) Tacrolimus capsule 500 micrograms
Kidney transplantation; various strengths
c) Tacrolimus capsule 1mg
required for titrated dose reduction
d) Tacrolimus capsule 5mg
Alendronic acid tablet 70mg
See list entry for indications
a) Capecitabine tablet 150mg
See list entry for indications
b) Capecitabine tablet 500mg b) Carboplatin injection 10mg/mL
See list entry for indications
(45mL vial)
a) Cyclophosphamide PFI 200mg vial
See list entry for indications See list entry for indication;
Dacarbazine PFI 200mg vial
replaces 100mg vial
a) Docetaxel injection 10mg/mL (2mL vial)
See list entry for indications
b) Docetaxel injection 10mg/mL (8mL vial)
a) Doxorubicin PFI 10mg vial
See list entry for indications
a) Filgrastim injection 120
micrograms/0.2ml prefilled syringe
See list entry for indications
b) Filgrastim injection 300
micrograms/0.5ml prefilled syringe
a) Gemicitabine PFI 200mg vial
See list entry for indications
b) Gemicitabine PFI 1g vial a) Hydroxycarbamide (hydroxyurea)
See list entry for indication
capsule 250mg a) Ifosfamide PFI 1g vial
See list entry for indications
b) Ifosfamide PFI 2g vial
Imatinib tablet 400mg
See list entry for indications
a) Irinotecan injection 20mg/mL
(2mL vial)
See list entry for indication
b) Irinotecan injection 20mg/mL
(5mL vial)
Melphalan tablet 2mg
See list entry for indications
Mesna injection 100mg/mL (2mL amp)
See list entry for indications
a) Oxaliplatin inj. 2mg/mL (25mL vial)
See list entry for indications
b) Oxaliplatin inj. 2mg/mL (50mL vial) a) Paclitaxel injection 6mg/mL (5mL
See list entry for indications
b) Paclitaxel injection 6mg/mL (16.7mL
a) Rituximab injection 10mg/mL
See list entry for indications
KEML 2016
Item Added
(10mL vial)
b) Rituximab injection 10mg/mL
See list entry for indications
(50mL vial)
Thalidomide capsule 100mg
See list entry for indications
a) Trastuzumab PFI 150mg vial
See list entry for indications
b) Trastuzumab PFI 440mg vial Vinblastine sulphate injection 1mg/mL
See list entry for indications
(10mL vial) a) Vinorelbine injection 10mg/mL
(1mL vial)
See list entry for indications
a) Vinorelbine injection 10mg/mL
(5mL vial)
Zoledronic acid injection 800
See list entry for indications
micrograms/mL (5mL vial)
Anastrozole tablet 1mg
Post-menopausal breast cancer
Bicalutamide tablet 50mg
See list entry for indication
b) Dexamethasone tablet 500
See list entry for indication
micrograms
Diethylstilboestrol (DES) tablet 5mg
See list entry for indication
a) Goserelin implant 3.6mg
See list entry for indications
b) Goserelin implant 10.8mg
Methylprednisolone PFI 500mg
See list entry for indications
a) Prednisolone oral liquid
See list entry for indications
15mg/5mL [c]
Tamoxifen tablet 20mg (as citrate)
See list entry for indications
Finasteride tablet 5mg
Benign prostatic hyperplasia
Tamsulosin tablet 400 micrograms a) Levodopa + carbidopa tablet 100mg + 10mg
Parkinsonism (1st line)
b) Levodopa + carbidopa tablet
a) Pramipexole tablet 180 micrograms
Refractory parkinsonism
b) Pramipexole tablet 700 micrograms Ferrous salt oral liquid (drops)
Required paediatric presentation
25mg/mL elemental iron
Periconceptual prevention of first
a) Folic acid tablet 400 micrograms
occurrence of neural tube defects
a) Phytomenadione (Vit K1) injection
Prevention of neonatal vitamin K deficiency
1mg/1mL amp [c]
bleeding (replaces 10mg/0.2mL amp)
b) Phytomenadione (Vit K1) injection
Warfarin-associated bleeding
10mg/mL (5mL amp)
KEML 2016
Item Added
Tranexamic acid injection 100mg/mL
Prevention/treatment of bleeding
associated with excessive fibrinolysis
a) Enoxaparin injection (prefilled
Safe, effective and more convenient
syringe) 40mg/0.4mL
alternative to heparin; no requirement for
b) Enoxaparin injection (prefilled
routine monitoring
syringe) 80mg/0.8mL
Required low dose presentation, especially
a) Warfarin sodium tablet 0.5mg
for paediatric use
a) Deferasirox tablet 100mg
b) Deferasirox tablet 400mg
Iron overload
Deferoxamine mesilate PFI 500mg vial a) Hydroxycarbamide (hydroxyurea)
capsule 250mg
Sickle cell disease
b) Hydroxycarbamide capsule 500mg
Plasma, fresh frozen
New section added in line with
Whole blood a) Normal immunoglobulin injection IV
5% protein solution (100mL vial)
Primary immune deficiency &
b) Normal immunoglobulin injection IV
Kawasaki disease
10% protein solution (100mL vial)
Plasma volume expansion;
Polygeline IV infusion 3.5% (500mL)
replaces dextran 70%
a) Carvedilol tablet 6.25mg
Replace atenolol tablet 50mg;
b) Carvedilol tablet 12.5mg
improved benefit/risk profile
Isosorbide dinitrate tablet 20mg
Useful longer acting vasodilator
a) Carvedilol tablet 6.25mg
Replace atenolol tablet 50mg;
b) Carvedilol tablet 12.5mg
improved benefit/risk profile
b) Verapamil tablet 80mg (scored)
Replaces 40mg for better dose flexibility
Epinephrine injection 100 micrograms/
Cardiac arrest
mL (10mL amp) a) Carvedilol tablet 6.25mg
Replaces atenolol tablet 50mg; improved
b) Carvedilol tablet 12.5mg
benefit/ risk profile 1st line antihypertensive with improved
Losartan tablet 50mg
adverse effect profile and prolonged action
a) Carvedilol tablet 6.25mg
Useful additions as per WHO Model List
b) Carvedilol tablet 12.5mg b) Furosemide oral liquid
Useful paediatric presentation
KEML 2016
Item Added
Spironolactone tablets 25mg (scored)
Useful addition as per WHO ML
Clopidogrel tablet 75mg
Useful addition as per WHO ML
Atorvastatin tablet 20mg (scored)
Useful addition as per WHO ML
Terbinafine HCl cream 1%
Refractory fungal infections
Aciclovir cream 5% (5g)
Herpes infections
Mupirocin ointment 2% (15g)
Prophylaxis during dialysis procedures
Clobetasone butyrate ointment 0.05%
Very potent topical steroid
Crotamiton cream 10%
Pruritis (especially after scabies)
Desonide gel 0.05%
Mild topical steroid
Mometasone furoate ointment 0.1%
Potent topical steroid Moderate-severe atopic eczema, especially
Tacrolimus ointment 0.03%
Benzoyl peroxide gel 5%
Coal tar + salicylic acid ointment
Crotamiton cream 10%
Scabies and related pruritis
Ivermectin tablet 6mg (scored)
Refractory scabies
White soft paraffin 100%
Sunscreening cream or lotion
Albinism & photodermatoses
Fluorescein test strip 0.6mg
Replaces fluorescein eye drops 1%
Iopromide injection (solution for IV
Required radiocontrast media
infusion) 150-370mg iodine/mL Gadobutrol IV injection (solution)
1 mmol/mL Gadodiamide IV injection (solution)
New section: MRI contrast media
0.5 mmol/mL Gadopentate dimeglumine IV injection
(solution) 0.5 mmol/mL b) Chlorhexidine gel 4%
Umbilical cord care
(as digluconate 7.1%) [c]
Alcohol-based hand rub solution,
Hand hygiene; key element of infection
isopropyl alcohol 75%
prevention & control
(500mL dispenser) b) Furosemide oral liquid [c]
For paediatric use
Spironolactone tablet 25mg
Potassium-sparing diuretic
Lansoprazole dispersible tablet 15mg
For paediatric use Severe peptic ulcer; and peptic ulcer when
b) Omeprazole PFI 40mg vial
oral route is not possible
KEML 2016
Item Added
Alternative for young children who cannot
a) Domperidone oral liquid 5mg/5mL
tolerate metoclopramide or who require an
oral liquid antiemetic Alternative for adult patients who cannot
b) Domperidone tablet 10mg
tolerate metoclopramide
a) Ondansetron injection 2mg/mL
base as HCl (2mL amp)
Recommended 1st line treatment for post-
b) Ondansetron tablet
operative nausea & vomiting (PONV)
(orally disintegrating) 4mg (base equivalent)
Continuation oral steroid after initial
Dexamethasone tablet 500 micrograms
parenteral therapy Supersedes sulfasalazine (deleted); improved
Mesalazine tablet 400mg (e/c)
side-effect profile
Prednisolone tablet 5mg
Chronic inflammatory bowel disease Alternative presentation when oral route
b) Bisacodyl suppository 10mg
ORS (4 sachets) + zinc sulphate
Required for updated treatment protocols
tablets 20mg dispersible (10) co pack Rehydration solution for malnutrition
Required for updated treatment protocols
(ReSoMal) PFOL (sachet for 1L)
Required for treatment of disorders of sexual
Testosterone gel 1%
development Required for current family planning
Levonorgestrel tablet 30 micrograms
Estradiol cypionate +
Required for current family planning
medroxyprogesterone acetate
injection 5mg + 25mg b) Medroxyprogesterone acetate
Required for current family planning
(DMPA) depot injection (SC)
104mg/0.65mL (prefilled syringe)
Levonorgestrel- releasing Intrauterine
system (LNG-IUS), reservoir with
Required for current FP protocols
52mg Progesterone-releasing vaginal ring
Required for current FP protocols
Conjugated oestrogens tablets 0.3mg
Required for treatment of delayed puberty For patients >60 years & as 2nd line in others
Gliclazide tablet 40mg
if glibenclamide not suitable
Insulin, rapid acting injection 100 IU/mL
For restricted paediatric use, where a
(10mL vial) (lispro, aspart or glulisine)
particularly rapid effect is required
KEML 2016
Item Added
Medroxyprogesterone acetate tablet
For menstrual conditions and endometriosis
5mg Lugol's iodine oral liquid 130mg total
For pre-operative use in hyperthyroidism
iodine/mL [c]
For 2nd line use if carbimazole not
Propylthiouracil tablet 50mg
HPV vaccine injection (suspension)
Required for current vaccination protocols
1mL vial (2 doses) Polio vaccine (IPV) injection
Required for current vaccination protocols
5mL vial (10 doses) Rotavirus vaccine (oral suspension)
Required for current vaccination protocols
1.5mL (single dose) Cholera vaccine (oral suspension)
For use in epidemics
1.5mL vial (single dose) Varicella vaccine (PFI + diluent) 0.5mL
For use in high-risk groups
vial (single dose) Pneumococcal vaccine (23-valent
adsorbed conjugate) injection
For specialist use in high-risk groups
(suspension) 0.5mL vial (single dose) Atracurium besilate injection
Neuromuscular blocker
(short-intermediate duration)
Cisatracurium injection 2mg/mL (10mL
Neuromuscular blocker (intermediate
duration; replaces vecuronium)
Glycopyrronium bromide +
Reversal of non-depolarising neuromuscular
neostigmine metilsulfate injection 500 blockade (especially young and elderly micrograms + 2.5mg/1mL amp
Pancuronium bromide injection
Neuromuscular blocker (required for its
long duration of effect)
Aciclovir eye ointment 3%
Ophthalmic herpes
Azithromycin dihydrate eye drops 1.5%
Trachomatous conjunctivitis
Ciprofloxacin eye drops 0.3% (as HCl)
Corneal ulcers
Econazole eye drops 1%
Fungal keratitis
b) Prednisolone tablet 5mg
Ophthalmic malignancies
Prednisolone eye drops 1% (acetate)
Deeper ocular inflammation Ocular inflammation where steroids are
Ketorolac trometamol eye drops 0.5%
contraindicated
Methylprednisolone PFI 1g vial
Optic neuritis
(as sod. succinate) Triamcinolone acetonide injection
Severe allergic conjunctivitis, diabetic
(aq. suspension) 40mg/1mL amp
retinopathy, intermediate uveitis, post-op in
KEML 2016
Item Added
paediatric eye surgery Open-angle glaucoma, in patients resistant to
Dorzolamide eye drops 2% (as HCl)
beta-blockers Raised intra-ocular pressure in open-angle
Latanoprost eye drops 0.005%
glaucoma; ocular hypertension
Atropine sulfate eye drops 0.5%
Replaces 1% as preferred strength
Tropicamide eye drops 0.5%
Short-term dilation Rapid dilation during eye surgery in non-
Epinephrine eye drops 2% (as HCl)
hypertensive patients Allergic conjunctivitis, vernal kerato-
Sodium cromoglicate eye drops 2%
Bevacizumab injection 25mg/mL
Diabetic retinopathy
(4mL vial) Haemodialysis solutions, parenteral
Haemodialysis
(of appropriate composition)
Especially for geriatrics when smaller doses
b) Chlorpromazine HCl tablet 50mg
Flupentixol decanoate injection
Combined antipsychotic + antidepressant
(oily, depot) 20mg/mL (2mL amp)
effect Paediatric presentation and for clandestine
Haloperidol oral liquid 2mg/mL
administration24 to uncooperative patients 2nd generation antipsychotic; much improved adverse effect profile; useful for
Olanzapine PFI 10mg vial
patients refractory to, or intolerant of, older antipsychotics Improved adverse effect profile compared
Quetiapine tablet 200mg (scored)
with olanzapine and useful antidepressant effects
a) Zuclopenthixol acetate injection (oily) 50mg/1mL (2mL amp)
For calming agitated/aggressive patients
b) Zuclopenthixol acetate injection (depot, oily) 200mg/1mL amp Chlorpromazine HCl injection 25mg/mL
Specialist list when used in children
(2mL amp) [c] a) Clozapine tablet (scored) 25mg
Useful 2nd line option for unresponsive
b) Clozapine tablet (scored) 100mg
a) Haloperidol injection 5mg/1mL amp
Specialist list when used in children
b) Haloperidol tablet (scored) 5mg
Risperidone tablet 1mg
Useful 2nd line option (especially in
24 Only after obtaining written guardian consent
KEML 2016
Item Added
persistently aggressive patients)
Fluoxetine tablet (scored) 20mg [c]
Complementary list when used in children Useful alternative to tricyclics without their
Venlafaxine capsule 75mg
sedative & antimuscarinic side-effects
Lithium carbonate tablet 400mg
Replaces tablet 300mg; improved
(modified release)
adherence/management
a) Valproic acid (sodium valproate)
Increased dosage flexibility
tablet 200mg (enteric-coated)
Bromazepam tablet 3mg (scored)
Anxiety with agitation (replaces diazepam)
Clomipramine HCl capsule 25mg
Obsessive-compulsive disorders
Diazepam tablet 5mg
Alcohol dependency
a) Nicotine chewing gum 2mg
Smoking cessation
b) Nicotine chewing gum 4mg a) B vitamins high potency injection IM
Alcohol dependence
b) B vitamins high potency injection IV a) Buprenorphine + naloxone tablet
(sublingual) 2mg + 500 micrograms
b) Buprenorphine + naloxone tablet
Opioid dependence
(sublingual) 8mg + 2mg
Naltrexone tablet 50mg
Attention deficit hyperactivity disorder
Methylphenidate tablet 10mg
Formoterol fumarate + budesonide dry
powder inhaler 6 micrograms + 200
Refractory chronic asthma
micrograms/metered dose a) Ipratropium bromide inhalation
(aerosol) 20 micrograms/metered dose
COPD and refractory paediatric asthma
b) Ipratropium bromide nebuliser
solution 250 micrograms/1mL unit dose vial
a) Montelukast (as sod. salt) granules
4mg sachet
Stepped management of asthma
b) Montelukast (as sod. salt) tablet
ORS + zinc sulphate co-pack 500mL
Dehydration in children
sachets (4) + zinc sulph tab 20mg (10) Rehydration solution for malnutrition
(WHO formula - ReSoMal) sachet
Dehydration in patients with malnutrition
(powder for making 1L solution)
b) Potassium chloride injectable
Severe hypokalaemia
KEML 2016
Item Added
solution 15% (10mL amp) [c]
Sodium chloride injectable solution 3%
Bronchiolitis in children; hyponatrenia in
(hypertonic) (10mL amp)
renal conditions
Calcium carbonate tablet (chewable)
Calcium deficiency, eg. in chronic renal
disease & rickets
Cholecalciferol (Vit D
Deficiency states
(drops) 400 IU/mL [c] Ergocalciferol (Vit D
2) tablet 1.25mg
Deficiency states
Pyridoxine HCl (Vit B6) tablet 50mg
Replaces 25mg (was in Complementary List)
Cholecalciferol (Vit D
3) injection IM
Severe deficiency states
(oily) 300,000 IU/1mL amp Hydrogen peroxide solution (ear
Wax softening and removal
drops) 5% Ciprofloxacin HCl + betamethasone
Infected otitis externa with inflammation
sodium solution (ear drops) 0.3% + 0.1%
and eczema
Clotrimazole solution (ear drops) 1%
Otitis externa with fungal infection
Liquid paraffin nasal drops 100%
Nasal canal dryness (eg. rhinitis sicca)
Sodium chloride nasal drops 0.9%
Nasal congestion
Budesonide nasal spray
Allergic & vasomotor rhinitis
100 micrograms/metered dose Dexamethasone injection 4mg/mL
In new Section 29.2; for enhancing neonatal
(as sodium phosphate)
Allopurinol tablet 300mg (scored)
Replaces tablet 100mg For use when methotrexate and sulfasalazine
Leflunomide tablet 20mg
cannot be used
Aspirin tablet 300mg (scored)
In new Section 30.3 Juvenile joint disease
Total parenteral nutrition (TPN) (amino acids + lipids + glucose +
Required for providing TPN
electrolytes) infusion (emulsion) (triple-chamber) Ready to use therapeutic food (RUTF)
oral paste standard formula (500 kcal sachet) F-75 therapeutic milk PFOL for approx.
In new Section 32: Preparations For managing
600mL (standard formula 102.5g
Severe Acute Malnutrition
sachet) F-100 therapeutic milk PFOL for
approx. 600mL (standard formula 114g sachet)
KEML 2016
Deletions25
Item Deleted
Bupivacaine injection 0.25%
Not required, use 0.5% (dilute as required)
Lignocaine injection 1%
2% preferred (dilute if 1% required)
Lignocaine HCl topical solution 4%
Replaced by lidocaine HCl topical solution 2%
(in spray bottle)
Lignocaine + adrenaline injection
1% + 1:80,000 Lignocaine dental cartridge 2%
Lignocaine topical solution 10%
Not required (use 2%)
spray Diazepam injection 5mg/mL
Replaced by preferred midazolam
Diazepam tablet 5mg
preparations (faster onset and recovery)
Promethazine oral liquid
Efficacy, obsolete (better alternatives
available, eg. midazolam)
Hyoscine hydrobromide injection
Not required (use atropine)
400 micrograms/mL Diclofenac injection 25mg/mL
Not recommended, increased risk of cardiovascular adverse effects
Diclofenac suppository 100mg
Paracetamol suppository 60mg
Not required (use 125mg)
Morphine oral liquid 10mg/5mL
Not required (use 1mg/mL or 10mg/mL)
Morphine tablet 60mg
Not required (use 30mg)
(prolonged-release)
Replaced by tablet 300mg (scored) in new
Allopurinol tablet 100mg
Cyclizine injection 50mg/mL
Cyclizine 50mg Docusate sodium capsule 100mg
Not used/required (alternatives available)
Docusate sodium oral liquid 50mg/5mL Chlorphenamine inj 10mg/mL
Replaced by cetirizine
Chlorphenamine oral liquid
(better adverse effect profile)
Prednisolone tablet 25mg
Not required for this indication Not recommended (safer & more effective
Aminophylline injection 25mg/mL
alternatives available)
25 Note that deletion of a medicines is specific to a particular section/use and not necessarily the whole list (eg. chlorphenamine injection has been deleted from section 3 but added to section 1.3)
KEML 2016
Item Deleted
Diazepam injection 5mg/mL
Replaced by lorazepam injection
Ethosuximide oral liquid
Ethosuximide tablet 250mg Niclosamide tablet, chewable
Ivermectin tablet, scored 6mg
Not required Replaced by tablet 250mg dispersible, scored
Amoxicillin PFOL 125mg/5mL
(preferred presentation)
Amoxicillin + clavulanic acid PFOL
Replaced by DT, scored 200mg + 28.5mg
250mg + 62.5mg/5mL Amoxicillin + clavulanic acid tablet
Replaced by tablet 875mg + 125mg
Ampicillin PFI 500mg
Minimal use, high levels of resistance
Benzathine penicillin PFI 1.44g
Replaced by 900mg (1.2MU) vial
(2.4MU) in 5mL vial
(preferred presentation)
Benzylpenicillin PFI 3g (5MU) vial
Not required (use 600mg 1MU vial)
Cefuroxime injection 750mg vial
Not recommended, poorly tolerated, high
Cefuroxime tablet 250mg
cost (use amoxicillin + clavulanic acid)
Chloramphenicol capsule 250mg Chloramphenicol injection PFI 1g
Not required for updated protocols
Chloramphenicol oral liquid 125mg/5mL
Erythromycin PFOL 125mg/5mL
Replaced by preferred azithromycin (better tolerated, simpler dose regime, improved
Erythromycin tablet 250mg
Metronidazole tablet 400mg
Use 200mg (improved cost benefit)
Isoniazid tablet (scored) 50mg
Replaced by oral liquid 50mg/5mL
Ethambutol oral liquid 25mg/mL
Not required
Ofloxacin tablet 200mg
Not required (use levofloxacin or moxifloxacin)
Streptomycin PFI 1g vial
Not required for updated TB regimes
Clotrimazole vaginal tablet
Replaced by 500mg (single dose, improved
Abacavir oral liquid 100mg/5mL Didanosine PFOL 100mg packet Didanosine PFOL 167mg packet Didanosine PFOL 250mg packet
Not required in updated treatment protocols
Didanosine capsule 125mg (e/c, unbuffered) Didanosine capsule 200mg (e/c, unbuffered)
KEML 2016
Item Deleted
Didanosine capsule 250mg (e/c, unbuffered) Didanosine tablet 25mg (buffered, chewable, dispersible) Didanosine tablet 50mg (buffered, chewable, dispersible) Didanosine tablet 100mg (buffered, chewable, dispersible) Didanosine tablet 150mg (buffered, chewable, dispersible) Didanosine tablet 200mg (buffered, chewable, dispersible) Stavudine capsule 15mg Stavudine capsule 20mg Stavudine capsule 30mg Tenofovir tablet 300mg Zidovudine capsule 100mg
Not required in updated treatment protocols
Zidovudine oral liquid 50mg/5mL Zidovudine tablet 300mg Efavirenz capsule 50mg
Efavirenz capsule 100mg Efavirenz oral liquid 150mg/5mL Lopinavir + ritonavir capsule 133.3mg + 33.3mg
Lopinavir + ritonavir tablet 200mg + 50mg Efavirenz + emtricitabine + tenofovir tablet 600mg + 200mg + 300mg Lamivudine + nevirapine +
stavudine tablet 150mg + 200mg + 30mg Lamivudine + stavudine tablet 300mg + 300mg Artemether oily injection
Use artesunate as 1st line for severe malaria
No longer required for malaria treatment
Doxycycline tablet 100mg
(use AL) but retained for prophylaxis
Dapsone tablet 100mg
Not used/required (use cotrimoxazole +
Pyrimethamine tablet 25gm
sulfadoxine/pyrimethamine [SP])
Sulphadiazine tablet 500mg
KEML 2016
Item Deleted
Cisplatin injection 1mg/mL,
Not required (use 50mL vial)
10mL vial Dacarbazine PFI 100mg
Replaced by PFI 200mg vial (as citrate)
a) Etoposide capsule 100mg
Not used/required (use injection)
Vincristine PFI 1mg vial
Not used/required
Vincristine PFI 5mg vial Methylprednisolone injection (aq.
Replaced by PFI 500mg vial (as sod.succinate)
supension) 40mg/1mL
Biperiden tablet 2mg (HCl)
Not used/required (benzhexol retained)
Phytomenadione (Vit K1) injection
Replaced by 1mg/1mL amp presentation
10mg/mL in 0.2mL amp
Replaced by preferred polygeline IV infusion
Dextran 70 injection solution 6%
3.5% 500mL pack Replaced by carvedilol tablet 6.25mg and
Atenolol tablet 50mg
12.5mg (better benefit/risk profile)
Verapamil tablet 40mg
Not used/required Replaced by carvedilol tablet 6.25mg and
Atenolol tablet 50mg
12.5mg (better benefit/risk profile)
Verapamil tablet 40mg
Replaced by 80mg (scored) Replaced by carvedilol tablet 6.25mg and
Atenolol tablet 50mg
12.5mg (better benefit/risk profile)
Streptokinase PFI 750,000 IU vial
Not used, not cost-effective Obsolete preparation (use crotamiton cream
Calamine lotion 15%
or appropriate steroid cream) Not available/used
Coal tar solution 5%
(use coal tar 2% + salicylic acid 2% ointment)
Dithranol ointment 1%
Not available/required
Salicylic acid solution 5%
Not available/required
Fluorescein eye drops 1%
Replaced by fluorescein test strips 0.6mg
Mannitol injectable solution 10%
Not required; use 20% No evidence of benefit compared with
Magnesium trisilicate co tablet,
placebo (safe and effective alternative
available - omeprazole)
Ranitidine injection 25mg/mL
Superseded by omeprazole
Ranitidine tablet 150mg Promethazine oral liquid
Not used/required (less effective than
available alternatives)
Sulfasalazine suppository 500mg
Not used/required
Sulfasalazine tablet 500mg
Superseded by mesalazine
Prednisolone tablets 5mg
Not used/required
KEML 2016
Item Deleted
Ethinylestradiol + norethisterone
Use ethinylestradiol + levonorgestrel tablet
tablet 35 micrograms + 1mg
30 micrograms + 150 micrograms
Norethisterone enantate oily
Not used in current family planning protocols
solution 200mg/1mL ampoule Vecuronium bromide PFI 10mg
Replaced by cisatracurium (better adverse
Pilocarpine eye drops 2%
Rarely used (use dorzolamide eye drops)
Timolol eye drops 0.25%
Not useful/effective (use 0.5%)
Atropine sulfate eye drops 1%
Not preferred (use 0.5%)
Carbamazepine tablet 100mg
Not required (use 200mg scored)
Lithium carbonate tablet 300mg
Replaced by 400mg (modified release) Replaced by bromazepam tablet 3mg
Diazepam tablet 5mg
(scored); more effective in managing somatic anxiety
Clonidine tablet 100 micrograms
Beclomethasone inhalation
Not required (use 100 micrograms/dose
(aerosol) 50 micrograms/dose
Salbutamol oral liquid 2mg/5mL
Salbutamol tablet 4mg
Potassium chloride powder for
dilution to make 7.5% oral solution Sodium hydrogen carbonate
(bicarbonate) injectable solution
1.4% (isotonic) Ascorbic acid tablet 50mg
Ergocalciferol oral drops 400
Replaced by cholecalciferol oral liquid (drops)
Nicotinamide (Vit B
Cholecalciferol tablet
Replaced by oral liquid (drops) 400 IU/mL [c]
10 micrograms (400 IU) Pyridoxine HCl (Vit B6) tablet 10mg
Replaced by tablet 50mg (scored)
Pyridoxine HCl (Vit B6) tablet 25mg Acetic acid solution (ear drops) 2%
Not used/required
Polyvidone iodine mouthwash 1%
Aminoacids infusion 3%
Not used/required (other strengths available)
KEML 2016
Kenya Essential Medicines List 2016
Dose-form
1. ANAESTHETICS
1.1 General anaesthetics & oxygen
1.1.1 Inhalational anaesthetics
1.1.1.1
Inhalation (250mL)
Inhalation (250mL)
Inhalation (medicinal gas)
Inhalation (medicinal gas)
Inhalation (medicinal gas)
1.1.2 Injectables
1.1.2.1
Ketamine hydrochloride
50mg/mL (10mL vial)
10mg/mL (20mL vial)
Thiopental sodium
1.2 Local anaesthetics27
a) 0.5% (10mL vial) b) 0.5% (5mg/mL) +
glucose 8% (80mg/mL)
a) 2% (30mL vial)
Topical solution
b) 2% (spray bottle)
Lidocaine HCl + epinephrine
Dental cartridge
(1.8mL cartridge)
Specialist List 1.2.4
Ephedrine HCl29
Injection
30mg/1mL amp
1.3 Pre- and intra-operative medication and sedation for short-term procedures
1.3.1
Atropine sulphate
Chlorphenamine maleate30
a) Injection (as HCl) 1mg/mL (5mL vial)
b) Oral liquid [c]31
7.5mg (as maleate)
26 Thiopental may be used as an alternative where propofol is not available
27 For spinal, epidural, caudal or IV regional anaesthesia, use preservative-free injections
28 Also referred to as ‘heavy spinal'; may also be available with glucose 7.5% (75mg/mL) 29 For specialist use in spinal anaesthesia during caesarean sections for prevention of
30 Use only for managing pre- and intra-operative allergic reactions 31 With graduated oral dispenser; 2.5mg/mL presentation may be available and used as
KEML 2016
Dose-form
(HCl or sulphate)
Specialist List 1.3.5
Fentanyl32
Injection (as citrate)
50 micrograms/mL (2mL amp)
2. MEDICINES for PAIN and PALLIATIVE CARE
2.1 Non-opioids and non-steroidal anti-inflammatory medicines (NSAIMs)
2.1.1
a) Oral liquid [c]
10mg/mL (100mL vial)
(for IV infusion)
b) Oral liquid [c]
c) Suppository [c]
d) Tablet (scored)
2.2 Opioids
2.2.1
Codeine phosphate37
(HCl or sulphate)
(HCl or sulphate)
2.3 Medicines for other common symptoms in palliative care
2.3.1
(as sod. phosphate)
5mg/mL (2mL amp)
c) Gel or rectal
5mg/mL (0.5mL tube)
32 Restricted for intra-operative use only
33 Do not use for children <3 months old 34 Not for anti-inflammatory use (no proven benefit) 35 Use only for post-operative analgesia 36 Alternative strength: 120mg/5mL 37 Only use for adults
38 May also be prescribed by specially trained palliative care professionals
KEML 2016
Dose-form
d) Tablet (scored)
Hyoscine butylbromide
400 micrograms/1mL
Hyoscine hydrobromide [c]
5mg/mL (2mL amp)
2mg/mL (2mL amp)
Ondansetron [c]40
3. ANTIALLERGICS and MEDICINES used in ANAPHYLAXIS
(as disod. phosphate) 1mg/1mL amp41
Epinephrine (adrenaline)
(as HCl or hyd.tartrate) 100mg vial
(as sod. succinate)
a) Oral liquid [c]
Specialist List 3.6
Chlorphenamine maleate42
Injection
10mg/1mL amp
39 If not available, use diazepam injection solution 5mg/mL instead, administered rectally by
syringe – without the needle!
40 Use only in children >1 month old 41 Strength may also be expressed as 1 in 1,000 or 0.1%
42 Use only as premedication for prevention of hypersensitivity reactions to paclitaxel
KEML 2016
Dose-form
4. ANTIDOTES and OTHER SUBSTANCES used in POISONINGS
4.1 Non-specific
4.1.1
Activated charcoal
4.2 Specific
4.2.1
200mg/mL (10mL amp)
Atropine sulphate
100 micrograms/mL
(5mL amp) 400 micrograms/
Naloxone hydrochloride
Protamine sulphate
10mg/mL (5mL amp)
Specialist List 4.2.7
Deferoxamine mesilate
500mg vial
Dimercaprol43
Injection (in oil)
50mg/mL (2mL amp)
a) Injection
100% (10mL amp)45
Ethanol44
b) Oral liquid46
Fomepizole sulphate
Injection
5mg/mL (20mL amp)
Penicillamine47
Pralidoxime chloride
Sodium calcium edetate
Injection
200mg/mL (5mL amp)
Sodium nitrite48
Injection
30mg/mL (10mL amp)
Sodium thiosulphate49
Injection
250mg/mL (50mL amp)
Succimer50
5. ANTICONVULSANTS/ANTIEPILEPTICS
a) Oral liquid [c]
200mg (cross-scored)
43 To be phased out as better alternatives are available 44 Pharmaceutical grade (ie. BP, EP, USP); for use in methanol poisoning 45 Also known as dehydrated or absolute alcohol; for administration as a 10% solution in
glucose 5% IV infusion
46 For dilution (1 part + 4 parts water) before use as a 20% solution; if unavailable use
ethanol 40% solution (eg. vodka)
47 Use with close monitoring 48 Use focused on areas where cyanide poisoning is more prevalent 49 Use focused on areas where cyanide poisoning is more prevalent
50 Also called dimercaptosuccinic acid (DMSA)
KEML 2016
Dose-form
5mg/mL (0.5mL tube)
Magnesium sulphate53
(10mL amp)) a) 30mg/1mL amp [c]54
Phenobarbital sodium
b) 200mg/1mL amp
c) Tablet (scored)
50mg/mL (5mL vial)
Phenytoin sodium
(sodium valproate)
Specialist List
Lamotrigine
6. ANTI-INFECTIVES
6.1 Anthelmintics
6.1.1 Intestinal anthelmintics
6.1.1.1
Tablet (chewable)
6.1.2 Antifilarials
6.1.2.1
Tablet (chewable)
Diethylcarbamazine
dihydrogen citrate (DEC)
6.1.3 Antischistosomals and other antitrematodes
6.1.3.1
51 If not available, use diazepam injection solution 5mg/mL instead, administered rectally by
syringe – without the needle!
52 Use always with close monitoring 53 Use only in management of pre-eclampsia; provides 5g per 10mL amp 54 Use in paediatric emergencies 55 Do not use in 1st trimester
56 Do not use in 1st trimester
KEML 2016
Dose-form
6.2 Antibacterials
6.2.1 Beta Lactams
a) DT57 (scored)
Amoxicillin + clavulanic
875mg + 125mg (1g)
Benzathine benzylpenicillin
900mg (1.2MU) vial
Benzylpenicillin59
600mg (1MU) vial
(sodium or potassium)
400mg (as trihydrate)
Ceftriaxone62, 63
(as sodium salt)
(as sodium salt)
(as potassium salt)
Specialist List
Ceftazidime65
a) 250mg vial
(as pentahydrate)
b) 1g vial a) 250mg + 250mg vial
Imipenem + cilastin66
b) 500mg + 500mg vial
57 Dispersible tablet
58 Also called co-amoxiclav; strength may be expressed as the total of the components 59 Use only at Level 2 facilities in pre-referral management of a very sick child (with
60 Use only in surgical prophylaxis (patients >1mth) 61 Use at Level 2 restricted to syndromic management of STIs only
62 Do not administer with calcium; avoid in infants with hyperbilirubinaemia; only use if
infant is >41 weeks corrected gestational age
63 Use at Level 2 restricted to STI syndrome management only 64 Use only in sickle-cell prophylaxis and as an alternative to benzathine penicillin where
this is not tolerated in prophylaxis of rheumatic heart disease
65 For specialist 2nd line use only where required laboratory diagnostic support and clear
antibiotic use proctocols are available
66 For specialist 2nd line use only in treatment of life-threatening hospital-based
infections due to suspected or proven multi-drug resistant organisms; imipenem present as monohydrate, cilastin as sodium salt
KEML 2016
Dose-form
6.2.2 Other antibacterials
a) Tablet (scored)
250mg (anhydrous)
a) Oral liquid [c]
Clarithromycin68
96mg/mL (10mL amp)
(sulfamethoxazole +
c) Tablet (scored)
100mg (as hyclate)
a) 10mg/mL (2mL vial)71
5mg/mL (100mL vial)
Specialist List
Solution for IV
2mg/mL (50mL bottle)
Ciprofloxacin
infusion [c]
(as lactate)
a) Capsule
150mg (as HCl) 150mg/mL (2mL vial)
Clindamycin74
b) Injection
(as phosphate)
c) Oral liquid [c]
75mg/5mL (as palmitate)
Vancomycin75
250mg vial (as HCl)
67 Use at Level 2 restricted to STI syndromic management and penicillin hypersensitive
patients only
68 Use only in combination drug regimes for treatment of H. pylori infection 69 Use only in treatment of pneumocystis pneumonia (PCP) and toxoplasmosis 70 Use in children <8 years only for life-threatening infections if there is no alternative
71 Use only for pre-referral management of a very sick child (with benzylpenicillin) 72 Use only in 2nd line syndromic management of STI 73 Useful longer-acting alternative to metronidazole in treatment regimes where single
daily doses may be used to improve adherence
74 For specialist use only in bone & joint infections and secondary bacterial infections in
KEML 2016
Dose-form
6.2.3 Antileprotics76
6.2.4 Antituberculosis medicines77
Individual drugs
a) 100mg (dispersible)
Pyrazinamide (Z)
Fixed-dose combinations (FDCs) 6.2.4.6
Isoniazid + ethambutol (HE)
Rifampicin + isoniazid (RH)
b) 75mg + 50mg [c]
Rifampicin + isoniazid +
150mg + 75mg + 275mg
ethambutol + (RHE)
Rifampicin + isoniazid +
pyrazinamide + (RHZ)
b) 75mg + 50mg +
Rifampicin + isoniazid +
pyrazinamide + ethambutol
Specialist List 6.2.4.11
500mg vial (as sulphate)
Bedaquiline
Capreomycin
1g vial (as sulphate)
Cycloserine
Delamanid
Kanamycin
1g vial (as sulphate)
75 Use only in endocarditis & other serious methicillin-resistant staphylococcus aureus
(MRSA) infections
76 Use only in combination, never individually
77 Antituberculosis treatment must always be with FDCs +/- additional individual drugs
KEML 2016
Dose-form
Levofloxacin
Tablet (scored)
Linezolid
Moxifloxacin
p-aminosalicylic acid (PAS)
4g sachet
Prothionamide
Rifabutin (RFB3)
6.3 Antifungals
50mg vial (as sodium
Amphotericin B78
d) Oral liquid [c]
6.4 Antivirals
6.4.1 Antiherpes medicines
6.4.1.1
Specialist List 6.4.1.2
Aciclovir82
250mg vial (as sodium salt)
6.4.2 Antiretrovirals
6.4.2.1 Nucleoside/nucleotide reverse transcriptase inhibitors (NRTI)
6.4.2.1.1
Lamivudine (3TC)
Tenofovir disoproxil
Zidovudine (ZDV or AZT)
78 Only use in invasive fungal infections 79 Use as alternative to fluconazole in pregnancy 80 Use at Level 2 restricted to STI syndromic management only 81 Use only in cryptococcal meningitis
82 Use only in viral encephalitis
KEML 2016
Dose-form
6.4.2.2 Non-Nucleoside/nucleotide reverse transcriptase inhibitors (NNRTI)
(double-scored)
Efavirenz (EFV or EFZ)
Etravirine (ETV)
Nevirapine (NVP)
6.4.2.3 Protease inhibitors
6.4.2.3.1
Atazanavir + ritonavir
a) Oral liquid (susp) 100mg/mL
400mg + 100mg/5mL
Lopinavir + ritonavir (LPV/r)
(heat-stable) a) Oral liquid
6.4.2.3.4 Ritonavir
6.4.2.4 Integrase inhibitor
6.4.2.5 Fixed-dose combinations (FDCs)
a) 60mg (as sulphate)
Abacavir + lamivudine
b) 120mg (as sulphate)
+ 60mg c) 600mg (as sulphate)
+ 60mg a) 400mg + 300mg +
Efavirenz + lamivudine +
b) 600mg + 300mg +
(EFV + 3TC + TDF)
Emtricitabine + tenofovir
83 This strength is being phased out
84 Tenofovir disoproxil fumarate (TDF)
KEML 2016
Dose-form
Lamivudine + tenofovir
(disoproxil fumarate)
Lamivudine + zidovudine
b) 150mg + 300mg
a) 30mg + 50mg +
Lamivudine + nevirapine +
6.4.2.5.6 zidovudine
b) 150mg + 200mg +
(3TC + NVP + ZDV)
6.4.3 Other antivirals
Specialist List
6.4.3.1
Ganciclovir85
500mg vial
Ribavirin86
(film-coated)
6.4.4 Antihepatitis medicines87
6.4.4.1 Hepatitis B medicines
Specialist List
Pegylated interferon
Injection (vial or
6.4.4.1.1
180 micrograms
prefilled syringe)
Tenofovir disoproxil
6.4.4.1.2
fumarate (TDF)
6.4.4.2 Hepatitis C medicines
Specialist List
Pegylated interferon
Injection (vial or
6.4.4.2.1
180 micrograms
prefilled syringe) Tablet
6.4.4.2.2
Ribavirin89
(film-coated)
6.5 Antiprotozoals
6.5.1 Antiamoebic & antigiardia Medicines
6.5.1.1
500mg/100mL vial
85 Use in management of cytomegalovirus retinitis (CMV) 86 Use in treatment of viral haemorrhagic fevers and, in combination with pegylated
interferon, in treatment of hepatitis C
87 This section will be revised following the review and update of national hepatitis
management guidelines in line with new WHO recommendations
88 TDF equivalent to 245mg tenofovir disoproxil 89 Use also in treatment of viral haemorrhagic fevers
90 Use only in patients >25kg
KEML 2016
Dose-form
6.5.2 Antileishmaniasis medicines
50mg vial (as sodium
Injection solution
375mg base/mL (as
sulphate) (2mL amp)
Sodium stibogluconate93
100mg/mL (100mL vial)
6.5.3 Antimalarials
6.5.3.1 Curative
Artemether + lumefantrine94
(dispersible)96 c) Tablet97
Rectal capsule101
Dihydroartemisinin +
piperaquine (DHA+PPQ)102
300mg/mL (2mL amp)
91 Useful for giardia (2g single dose); may also be used for other indications as a longer
acting alternative to metronidazole
92 Use only in combination with sodium stibogluconate; also called aminosidine
93 Use only in combination with paromomycin 94 Do not use in 1st trimester of pregnancy (use oral quinine) 95 Use for patients 24-35kg 96 Use for patients <24kg 97 Use for patients >35kg
98 Always follow artesunate treatment (24 hours minimum) with a 3-day course of
artemether + lumefantrine (once the patient can take oral medication)
99 Co-packed with 0.5mL amp of sodium bicarbonate 5% (50mg/mL) and 2.5mL amp of
sodium chloride 0.9% (9mg/mL) as diluents
100 Co-packed with 1mL amp of sodium bicarbonate 5% (50mg/mL) and 5mL amp of
sodium chloride 0.9% (9mg/mL) as diluents
101 Use for management of severe malaria only if parenteral artesunate is not available
102 Use only as 2nd line medicine for confirmed uncomplicated malaria treatment failure
with 1st line AL
103 Use only for severe malaria when 1st line artesunate injection is not available
KEML 2016
Dose-form
300mg (sulphate or
b) Tablet (f/c)104
bisulphate) 7.5mg
(as diphosphate)
6.5.3.2 Prophylactic
6.5.3.2.1
Proguanil hydrochloride107
6.5.3.3 Intermittent presumptive treatment in pregnancy (IPTp)
6.5.4 Antipneumocystosis and antitoxoplasmosis medicines
96mg/mL (5mL amp)
6.5.5 Antitrypanosomal medicines
6.5.5.1 African trypanosomiasis
a) Treatment of 1st stage
6.5.5.1.1
Pentamidine isetionate
Specialist List 6.5.5.1.2
Suramin sodium
b) Treatment of 2nd stage
Specialist List
3.6% solution (5mL amp)
6.5.5.1.3
Melarsoprol
Injection
(= 180mg)
7. ANTIMIGRAINE MEDICINES
7.1 Acute attack
7.1.1
a) Oral liquid [c]
b) Suppository [c]
c) Tablet (scored)
104 Use only for uncomplicated malaria treatment in 1st trimester of pregnancy
105 Use only for radical cure of P. vivax infection (14 day course) 106 Use only in patients >8 years; for prophylaxis in non-immune visitors to endemic areas 107 Use only for prophylaxis in patients with sickle-cell disease and tropical splenomegaly
108 Do not use in children < 3 months old 109 120mg/5mL is an alternative strength
KEML 2016
Dose-form
7.2 Prophylactics
7.2.1
Propranolol HCl110
8. ANTINEOPLASTICS and IMMUNOSUPPRESSIVES
8.1 Immunosuppressives
Specialist List
100mg vial (sodium salt)
Azathioprine
b) Tablet (scored)
50mg a) 25mg
Ciclosporin
c) Concentrate for
50mg/1mL amp
injection111 PFI (as sod.
succinate)
Mycophenolic acid112
Tablet (e/c)
(as mycophenolate sod.)
Prednisolone
a) Concentrate for
5mg/1mL amp
IV infusion
Tacrolimus
b) 500 micrograms
c) 1mg d) 5mg
8.2 Cytotoxics and adjuvants
Specialist List
8.2.1
Alendronic acid113
70mg a) 100mg
Allopurinol114
Asparaginase115
10,000 IU vial
Bleomycin116
15mg vial (as sulphate)
110 Use only for this indication (ie. do not use as an alternative antihypertensive)
111 Use in organ transplantation
112 Mycophenolate mofetil tablets 250mg & 500mg may be available as a much cheaper
alternative but with a less favourable side-effects profile; the two salts are not interchangeable as they have different pharmacokinetics
113 Use as adjuvant in management of breast & prostate cancers and multiple myeloma 114 Use for prophylaxis of chemotherapy-induced hyperuricaemia 115 Use in acute lymphoblastic leukaemia; anaphylaxis treatment must be available; the
type required is that produced by Erwinia chrysanthemi (also known as crisantaspase)
116 Use in Hodgkin lymphoma, Kaposi sarcoma, ovarian and testicular germ cell tumour
KEML 2016
Dose-form
Injection
a) 10mg/mL (5mL vial)
Calcium folinate117
b) 15mg a) 150mg
Capecitabine118
b) 500mg a) 10mg/mL (15mL vial)
Carboplatin119
Injection
b) 10mg/mL (45mL vial)
Chlorambucil120
Cisplatin121
Injection
1mg/mL (50mL vial) a) 200mg vial
b) 500mg vial
c) Tablet
Cytarabine123
100mg vial
Dacarbazine124
200mg vial (as citrate)
Dactinomycin125
500 micrograms vial
Daunorubicin126
20mg vial (as HCl) a) 20mg
Docetaxel127
Injection128
117 Use in early stage colon & rectal cancers, gestational trophoblastic neoplasia,
metastatic colorectal cancer, osteosarcoma, Burkitt lymphoma; also useful in gynaecological tumours
118 Use in early stage colon & rectal cancers, metastatic breast & colorectal cancers 119 Use in early stage breast cancer, epithelial ovarian cancer, nasopharyngeal cancer,
non‐small cell lung cancer, osteosarcoma, retinoblastoma
120 Use in chronic lymphocytic leukaemia
121 Use in cervical, head & nasopharyngeal cancers - as a radio‐sensitizer; non‐small cell
lung cancer, osteosarcoma, ovarian & testicular germ cell tumours
122 Use in chronic lymphocytic leukaemia, diffuse large B‐cell lymphoma, early stage
breast cancer, gestational trophoblastic neoplasia, Hodgkin & follicular lymphomas, rhabdomyosarcoma, Ewing sarcoma, acute lymphoblastic leukaemia, Burkitt lymphoma, metastatic breast cancer
123 Use in acute myelogenous, lymphoblastic & promyelocytic leukaemias, Burkitt
124 Use in Hodgkin lymphoma
125 Use in gestational trophoblastic neoplasia, rhabdomyosarcoma, Wilms tumour 126 Use in acute lymphoblastic, myelogenous & promyelocytic leukaemias 127 Use in early stage & metastatic breast cancers, metastatic prostate cancer 128 Both strengths may be available (as a concentrate for dilution for infusion) as either a
vial of PFI + diluent or as ready-made injection solution. Selection should be based on relative availability & cost
KEML 2016
Dose-form
a) 10mg vial (as HCl)
Doxorubicin129
b) 50mg vial (as HCl)
Etoposide130
Injection
20mg/mL (5mL vial)
Injection
a) 120 micrograms/0.2mL
Filgrastim131
(prefilled syringe)
b) 300 micrograms/0.5mL
Fluorouracil132
Injection
50mg/mL (5mL vial) a) 200mg vial
Gemicitabine133
b) 1g vial
(hydroxyurea)
b) 500mg a) 1g vial
Ifosfamide135
b) 2g vial
Imatinib136
Tablet (as mesilate)
400mg a) 20mg/mL (2mL vial)
Irinotecan137
Injection
b) 20mg/mL (5mL vial)
Tablet (scored)
Melphalan139
Injection
100mg/mL (2mL amp)
129 Use in diffuse large B‐cell lymphoma, early stage breast cancer, Hodgkin lymphoma,
Kaposi sarcoma, follicular lymphoma, metastatic breast cancer, osteosarcoma, Ewing sarcoma, acute lymphoblastic leukaemia, Wilms tumour, Burkitt lymphoma
130 Use in testicular germ cell tumour, gestational trophoblastic neoplasia, Hodgkin and
Burkitt lymphomas, non‐small cell lung cancer, ovarian germ cell tumour, retinoblastoma, Ewing sarcoma, acute lymphoblastic leukaemia
131 Use as primary prophylaxis in those at high risk for developing febrile neutropenia
associated with myelotoxic chemotherapy; use as secondary prophylaxis for patients who have experienced neutropenia following prior myelotoxic chemotherapy, to facilitate administration of dose dense chemotherapy regimens
132 Use in early stage breast, colon & rectal cancers, metastatic colorectal cancer,
nasopharyngeal cancer
133 Use in epithelial ovarian cancer, non‐small cell lung cancer 134 Use in chronic myeloid leukaemia
135 Use in testicular & ovarian germ cell tumour, osteosarcoma, rhabdomyosarcoma,
136 Use in chronic myeloid leukaemia, gastrointestinal stromal tumour 137 Use as 2nd line agent in metastatic colorectal cancer 138 Use in acute lymphoblastic & promyelocytic leukaemias
139 Use in multiple myeloma
KEML 2016
Dose-form
a) PFI (as sod. salt)
25mg/mL (2mL vial)
Methotrexate141
(preservative-free) b) Tablet
2.5mg (as sodium salt) a) 2mg/mL (25mL vial)
Oxaliplatin142
Injection
b) 2mg/mL (50mL vial) a) 6mg/mL (5mL vial)
Paclitaxel143
Injection
b) 6mg/mL (16.7mL vial)144
Procarbazine
50mg (as HCl) a) 10mg/mL (10mL vial)
Rituximab145
Injection
b) 10mg/mL (50mL vial)
Thalidomide146
100mg a) 150mg vial
Trastuzumab147
b) 440mg vial + diluent
Vinblastine sulphate148
Injection
1mg/mL (10mL vial) a) 10mg/mL (1mL vial)
Vinorelbine149
Injection
b) 10mg/mL (5mL vial) 800 micrograms/mL
Zoledronic acid150
Injection
(5mL vial)151
8.3 Hormones and antihormones
Specialist List
8.3.1
Anastrozole152
Bicalutamide153
140 Use in testicular & ovarian germ cell tumours, osteosarcoma, rhabdomyosarcoma,
141 Use in early stage breast cancer, gestational trophoblastic neoplasia, osteosarcoma,
acute lymphoblastic and promyelocytic leukaemias
142 Use in early stage colon cancer, metastatic colorectal cancer 143 Use in epithelial ovarian cancer, early stage & metastatic breast cancers, Kaposi
sarcoma, nasopharyngeal cancer, non‐small cell lung cancer, ovarian germ cell tumour
144 Providing a total of 100mg/vial 145 Use in diffuse large B‐cell and follicular lymphomas, chronic lymphocytic leukaemia 146 Use (with melphalan & prednisolone) in management of multiple myeloma 147 Use in early stage & metastatic HER2-positive breast cancer
148 Use in Hodgkin lymphoma, Kaposi sarcoma, testicular & ovarian germ cell tumour 149 Use in non‐small cell lung cancer, metastatic breast cancer 150 Use as adjuvant in management of breast & prostate cancers and multiple myeloma 151 Provides a total of 4mg per vial 152 Letrozole tablets 2.5mg may be available and used as a much cheaper alternative
153 Use in metastatic prostate cancer
KEML 2016
Dose-form
4mg/1mL amp
a) Injection
Dexamethasone154
(as sodium phosphate)
b) Tablet
500 micrograms
Diethylstilboestrol (DES)155
a) 3.6mg (as acetate)
Goserelin156
(in syringe
b) 10.8mg (as acetate)
applicator)
100mg vial
(as sodium succinate)
Methylprednisolone158 [c]
500mg vial
a) Oral liquid [c]
Prednisolone159
Tamoxifen160
20mg (as citrate)
8.4 Medicines for benign prostatic hyperplasia (BPH)
Specialist List
8.4.1
Finasteride
Tamsulosin HCL
400 micrograms
9. ANTIPARKINSONISM MEDICINES
9.1
Levodopa + carbidopa
Specialist List
a) 180 micrograms base
Pramipexole
Tablet (scored)
b) 700 micrograms base
10. MEDICINES AFFECTING the BLOOD
10.1 Antianaemics
elemental iron161
154 Use in acute lymphoblastic leukaemia 155 Use in management of prostate cancer 156 Use in early stage & metastatic breast cancers & metastatic prostate cancer
(leuprorelin may be available and used as an alternative)
157 Use in acute lymphoblastic leukaemia 158 Use in acute lymphoblastic leukaemia 159 Use in chronic lymphocytic & acute lymphoblastic leukaemias; diffuse large B‐cell,
Hodgkin, follicular & Burkitt lymphomas
160 Use in early stage & metastatic breast cancers
161 Eg. ferrous sulphate solution 125mg/mL
KEML 2016
Dose-form
elemental iron162 60-65mg elem. iron +
Ferrous salt + folic acid
400 micrograms a) 400 micrograms163
Hydroxocobalamin164
1mg/1mL amp (as HCl,
acetate or sulphate)
10.2 Medicines affecting coagulation
(5mL vial) a) 1mg/1mL amp [c]
Phytomenadione (Vit K1)
Protamine sulphate
10mg/mL (5mL amp)
(5mL amp) a) 1mg (scored)
Specialist List
Injection
a) 40mg/0.4mL
Enoxaparin
(prefilled syringe)
b) 80mg/0.8mL
Heparin sodium [c]
Injection
5,000 IU/mL (5mL vial)
Protamine sulphate [c]
Injection
10mg/mL (5mL amp) a) 1mg (scored)
Warfarin sodium [c]
b) 5mg (scored)
10.3 Other medicines for haemaglobinopathies
Specialist List
Deferasirox
Deferoxamine mesilate
500mg vial
Hydroxycarbamide
(hydroxyurea)165
162 Eg. dried ferrous sulphate tablets 200mg 163 Use periconceptually for prevention of first occurrence of neural tube defects 164 Use cyanocobalamin (vit B12) tablet 1mg as the preferred alternative (if available)
165 Previously called hydroxyurea; use in management of sickle-cell disease
KEML 2016
Dose-form
11. BLOOD PRODUCTS of HUMAN ORIGIN and PLASMA SUBSTITUTES
11.1 Blood and blood components
11.1.1
Plasma, fresh frozen
11.2 Plasma-derived medicines
11.2.1 Human immunoglobulins (Ig)
750 IU/mL (2mL vial)166
Anti-rabies Ig (equine)
200 IU/mL (5mL vial)
Anti-tetanus Ig (human)
Specialist List
Injection (IV)
a) 5% protein solution
Normal Ig167
(100mL vial)
b) 10% protein solution
11.2.2 Blood coagulation factors
Specialist List
11.2.2.1
Coagulation factor VIII
500 IU vial
Coagulation factor IX
500 IU vial
11.3 Plasma substitutes
11.3.1
3.5% (500mL pack)
12. CARDIOVASCULAR MEDICINES
12.1 Antianginals
Glyceryl trinitrate
Tablet (sublingual)
Isosorbide dinitrate
12.2 Antiarrythmics
50 micrograms/mL
a) 62.5 micrograms
b) 250 micrograms
Lidocaine HCl170
20mg/mL (5mL amp)
166 Contains 1,500 IU = 300 micrograms per 2mL vial when reconstituted 167 Use for primary immune deficiency and Kawasaki disease 168 Partially degraded gelatin 169 Measure doses with graduated pipette provided
170 Only for IV use in ICU; preservative-free
KEML 2016
Dose-form
b) 80mg (scored)
Specialist List 12.2.5
Amiodarone HCl171
Injection
50mg/mL (3mL amp) 100 micrograms/mL (10mL
Epinephrine (adrenaline) 172
Injection
amp) (as HCl or acid tartrate)
12.3 Antihypertensives
12.3.1
Hydrochlorthiazide
Specialist List 12.3.8
Sodium nitroprusside
50mg ampoule
12.4 Medicines used in heart failure
50 micrograms/mL
a) 62.5 micrograms
b) 250 micrograms
10mg/mL (2mL amp)
b) Oral liquid [c]
c) Tablet (scored)
Hydrochlorthiazide
Specialist List 12.4.6
Dopamine HCl177
Injection
40mg/mL (5mL vial)
171 Only for IV use in ICU 172 *Alert* Note the strength and presentation for this indication - equivalent to 0.01% or
1 in 10,000 - this is 1/10 of the strength of the other form of epinephrine (adrenaline) listed elsewhere; must be diluted for paediatric use
173 As besylate, maleate or mesylate 174 As hydrogen maleate 175 Only use for hypertension in pregnancy
176 Measure doses with graduated pipette provided
KEML 2016
Dose-form
12.5 Antithrombotics178
12.5.1 Antiplatelets
12.5.1.1
Specialist List 12.5.1.2
Clopidogrel
12.6 Lipid-lowering agents
12.6.1
13. DERMATOLOGICALS (topical)
13.1 Antifungals
13.1.1
Terbinafine HCl180
13.2 Anti-infectives
13.2.1
Silver sulphadiazine182
Specialist List 13.2.4
Mupirocin183
13.3 Anti-inflammatories and antipruritics
13.3.1
Betamethasone184
0.1% (15g) (as valerate)
Hydrocortisone acetate
Mometasone furoate
Specialist List 13.3.2
Clobetasone butyrate
(as monohydrate)
13.4 Astringents
None selected
13.5 Medicines affecting skin differentiation & proliferation
13.5.1
Benzoyl peroxide
177 Only for use in ICU 178 See also Section 10.2 Medicines affecting coagulation
179 Use only in high-risk patients; simvastatin may be used as an alternative 180 Use only for refractory infections 181 Use only for <10 days; sodium fusidate cream 2% may also be available/used 182 Use only in patients >2mths 183 Use only for prevention of local infection in performing dialysis procedures
184 Avoid use in neonates (hydrocortisone cream preferred)
KEML 2016
Dose-form
Coal tar + salicylic acid
Podophyllum resin
benzoin tincture)
13.6 Scabicies and pediculocides
13.6.1
13.7 Medicines for jiggers186
13.7.1
White soft paraffin187
Topical application
13.8 Sunscreen preparation
13.8.1
Sunscreening agent/s188
14. DIAGNOSTIC AGENTS
14.1 Ophthalmic diagnostics
14.1.1
14.2 Radiocontrast media189
Specialist List
Injection (solution
140-420mg iodine/mL (as
Amidotrizoate
for IV infusion)
sodium or meglumine salt)
Barium sulphate
Suspension (aq)
Injection (solution
140-350mg iodine/mL
for IV infusion) Injection (solution
Iopromide
150-370mg iodine/mL
for IV infusion) Injection (solution
Meglumine iotroxate
5g iodine in 100mL bottle190
for IV infusion)
14.3 MRI contrast media191
Specialist List
14.3.1
Gadobutrol
IV injection solution
1 mmol/mL192
Gadodiamide
IV injection solution
0.5 mmol/mL193
185 Adult strength: dilute with equal volume of water to obtain 12.5% for paediatric use 186 Tunga penetrans infestation; use both medicines in combination therapy
187 Also called petroleum jelly or white petrolatum 188 Must protect against both UVA and UVB; various preparations may be available 189 Required presentations to be selected by the radiologist 190 Equivalent to 105mg/mL 191 Required presentations to be selected by the radiologist
192 Equivalent to 604.72mg/mL
KEML 2016
Dose-form
IV injection
Gadopentate dimeglumine
0.5 mmol/mL194
(solution)
15. ANTISEPTICS and DISINFECTANTS
15.1 Antiseptics
5% (digluconate)
4% (as digluconate
(equiv. to iodine 1%)
15.2 Disinfectants
Isopropyl alcohol 75%
Alcohol-based hand rub
(500mL dispenser)
Sodium hypochlorite
4-6% chlorine198
16. DIURETICS
16.1
10mg/mL (2mL amp)
b) Oral liquid [c]
c) Tablet (scored)
Hydrochlorthiazide
Injection solution
Specialist List [c] 16.6
Tablet (scored)
Spironolactone
Injection solution
17. GASTROINTESTINAL MEDICINES
17.1 Antacids and other antiulcer medicines
17.1.1
Lansoprazole [c]
Dispersible tablet
193 Equivalent to 287mg/mL
194 Equivalent to 469.01mg/mL 195 Use only for umbilical cord care; ensure that it is not mistakenly used as an eye
196 Previously called glutaraldehyde 197 Use within 6 months of date of manufacture; use only freshly made dilutions
198 Provides approximately 50,000 ppm available chlorine
KEML 2016
Dose-form
b) PFI (as sod. salt)
17.2 Antiemetics
(as sodium phosphate)
5mg/mL (2mL amp)
Metoclopramide HCl200
2mg/mL (base, as HCl)
b) Tablet (orally
4mg (base equivalent)
disintegrating)
Specialist List 17.2.5
Dexamethasone
500 micrograms
17.3 Anti-inflammatories
Specialist List
17.3.1
Mesalazine
Tablet (e/c)
Prednisolone
17.4 Laxative
17.5 Medicines used in diarrhoea
Oral rehydration solution
WHO low-osmolality
(sachet for 500mL)
formula 500mL sachets + zinc
Co-pack (4 sachets
ORS + zinc sulphate
sulphate tab 20mg
Rehydration solution for
malnutrition (ReSoMal)
Zinc sulphate202 [c]
Tablet (dispersible)
199 Alternative in patients who cannot tolerate metoclopramide and in young children
requiring an oral liquid antiemetic; additional restrictions apply (small increased cardiac toxicity risk)
200 Do not use in neonates 201 Use only in patients >1 month
202 Use with ORS in acute diarrhoea
KEML 2016
Dose-form
18. HORMONES, other ENDOCRINE MEDICINES and CONTRACEPTIVES
18.1 Adrenal hormones and synthetic substitutes
Specialist List
18.1.1
Fludrocortisone acetate
100 micrograms
Hydrocortisone
18.2 Androgens
18.2.1
Testosterone
18.3 Contraceptives
18.3.1 Oral hormonal contraceptives
Ethinylestradiol +
For emergency contraception
18.3.2 Injectable hormonal contraceptives
Estradiol cypionate +
medroxyprogesterone
a) Depot injection
Medroxyprogesterone
(prefilled syringe)
acetate (DMPA)203
b) Depot injection
(prefilled syringe)
18.3.3 Intrauterine devices (IUD)
18.3.3.1
Copper-containing device205
Levonorgestrel (LNG)
Reservoir with 52mg
system (LNG-IUS)
18.3.4 Barrier methods
18.3.4.1
18.3.5 Contraceptive implants206
18.3.5.1
Etonorgestrel-releasing
203 May be used at Level 1 (Community) in areas with community midwife services 204 May be referred to as DMPA-SC 205 Different registered preparations may be available
206 May be used at Level 1 (Community) in areas with community midwife services
KEML 2016
Dose-form
18.3.6 Intravaginal contraceptive
18.3.6.1
2.074g (micronized)
18.4 Estrogens
18.4.1
Conjugated oestrogens
300 micrograms
18.5 Insulins and other antidiabetics
18.5.1
Glibenclamide208
Insulin, intermediate-acting
100 IU/mL (10mL vial)
(human) (70/30) 210
Insulin, soluble (human)
100 IU/mL (10mL vial)
Specialist List [c] 18.5.5
Insulin, rapid acting211
Injection
100 IU/mL (10mL vial)
Metformin HCl
18.6 Ovulation inducer
Specialist List
18.6.1
Clomifene citrate
18.7 Progestogens
Specialist List
18.7.1
5mg (acetate)
18.8 Thyroid hormones and anti-thyroid medicines
18.8.1
(a) 25 micrograms [c]
Levothyroxine sodium
(b) 50 micrograms
(c) 100 micrograms
Specialist List 18.8.3
Lugol's iodine [c]
Oral liquid
130mg total iodine/mL
207 For use in women actively breastfeeding (ie. at least 4 times daily) 208 Do not use for patients >60 years
209 Use only in patients >60 years 210 As biphasic isophane insulin (70% isophane insulin + 30% soluble insulin) 211 Use insulin lispro, insulin aspart or insulin glulisine with selection being made based on
local availability and relative cost
212 Use as drug of choice (ie. rather than carbimazole) during 1st trimester of pregnancy
and in lowest effective dose to control hyperthyroid state
KEML 2016
Dose-form
18.9 Other endocrine medicines
Specialist List
18.9.1
Bromocriptine213
Tablet (scored)
2.5mg (as mesilate)
19. IMMUNOLOGICALS
19.1 Diagnostic agents
Tuberculin, purified protein
Injection (solution)
derivative (PPD)214
(single dose)215
19.2 Sera and immunoglobulins
immunoglobulin216
19.3 Vaccines
Recommended for all
1mL vial (10 doses)217
(live attenuated) DPT + HiB + HepB vaccine
5mL vial (10 doses)
(suspension) Injection
HPV vaccine218 (2-valent)
1mL vial (2 doses)
Measles vaccine219
5mL vial (10 doses)
(live attenuated) Measles + rubella vaccine
5mL vial (10 doses)
Polio vaccine (IPV)
5mL vial (10 doses)
Polio vaccine, oral (OPV)
2mL vial (20 doses)
(live attenuated)
Rotavirus vaccine
1.5mL (single dose)
Tetanus toxoid (adsorbed)
20mL vial (10 doses)220
Recommended for certain high-risk populations 19.3.10
Cholera vaccine221
1.5mL vial (single dose)
213 Use for prevention or suppression of lactation 214 For Mantoux test 215 2TU/0.1mL
216 Minimum of 11 species mixture covering Bitis, Naja, Echis, Dendroaspis spp 217 Dose: adults 0.1mL, child <1 year 0.05mL 218 Human papillomavirus vaccine; for school health programme roll-out 219 Being phased out globally and in Kenya replaced by MR vaccine in the routine
vaccination programme
220 >40 IU/0.5mL dose
KEML 2016
Dose-form
Hepatitis B vaccine (adult)222
1mL vial (single dose)
Meningococcal meningitis
Pneumococcal vaccine
1mL vial (2 doses)
(10-valent ads. conjugate)
Rabies vaccine224
(single dose)225 0.5mL vial
Typhoid vaccine226
Injection (solution)
(single dose) 0.5mL vial
Varicella vaccine227
Yellow fever vaccine228
10mL vial (20 doses)229
Specialist List
Pneumococcal vaccine
Injection
(23-valent adsorbed
0.5mL vial (single dose)
(suspension)
conjugate)230
20. MUSCLE RELAXANTS (PERIPHERALLY-ACTING) and
CHOLINESTERASE INHIBITORS
Atracurium besilate
10mg/mL (5mL amp)
2mg/mL (10mL vial)
Glycopyrronium bromide +
500 micrograms +
neostigmine metilsulfate231
Neostigmine metilsulfate
Pancuronium bromide
2mg/mL (2mL amp)
Suxamethonium chloride
50mg/mL (2mL amp)
221 Use only in management of outbreaks 222 Use mainly for health providers and their dependants; contains 20 micrograms
antigen protein/1mL dose
223 Sero-type specific; use for outbreaks, asplenic patients & travellers to affected areas 224 Human diploid type may also be available & used as a more expensive alternative 225 Rabies antigen ≥ 2.5 IU/0.5 mL dose when reconstituted as a suspension 226 Use reserved for specific at risk patients, ie. nephrotics, immunosuppressed patients,
travellers to typhoid prevalent areas
227 Also called chickenpox vaccine 228 Use only for health-workers during outbreaks & travellers to areas with yellow fever 229 Contains 1,000 LD50 units/0.5mL dose 230 Use for patients with sickle-cell disease
231 Use only for reversal of neuromuscular blockade
KEML 2016
Dose-form
21. OPHTHALMOLOGICALS
21.1 Anti-infectives
21.1.1
0.3% (as sulfate)
Tetracycline HCl
Specialist List 21.1.3
Aciclovir
Eye ointment
Azithromycin dihydrate
Eye drops
Ciprofloxacin
Eye drops
0.3% (as HCl)
Econazole
Eye drops
21.2 Anti-inflammatories
0.5% (sod. phosphate)
Specialist List 21.2.2
Prednisolone
Eye drops
1% (acetate)
Ketorolac trometamol
Eye drops
1g vial (as sodium succinate)
Injection232
Triamcinolone acetonide
40mg/1mL amp
(aq.suspension)
21.3 Local anaesthetic
21.3.1
Tetracaine HCl233
21.4 Miotics and anti-glaucoma medicines
21.4.1
0.5% (as hyd. maleate)
Specialist List 21.4.3
Dorzolamide
Eye drops
2% (as HCl)
Latanoprost
Eye drops
0.005%234
21.5 Cycloplegics and mydriatics
21.5.1
Atropine sulfate235
21.6 Anti-allergics
21.6.1
Sodium cromoglicate
21.7 Anti-vascular endothelial growth factor (VEGF)
Specialist List
21.7.1
Bevacizumab
Injection
25mg/mL (4mL vial)
232 Use as a depot 233 Previously called amethocaine; do not use in pre-term neonates 234 50 micrograms/mL
235 Only use in patients >3 months
KEML 2016
Dose-form
22. OXYTOCICS and ANTIOXYTOCICS
22.1 Oxytocics
(Prostaglandin E2)
200 micrograms/1mL
b) Vaginal tablet239
Specialist List
Mifepristone + misoprostol240
200 micrograms
22.2 Anti-oxytocics (tocolytics)
23. DIALYSIS SOLUTIONS
Specialist List
Intraperitoneal dialysis
solutions (CAPD)241
Parenteral solutions
Of appropriate composition242
Haemodialysis solutions
24. MEDICINES for MENTAL and BEHAVIOURAL DISORDERS
24.1 Antipsychotics
a) 25mg/mL (2mL amp)
Chlorpromazine HCl
Flupentixol decanoate
20mg/mL (2mL amp)
(oily, depot) Injection
Fluphenazine decanoate
(oily, depot) a) Injection
b) Oral liquid243
236 As hydrogen maleate; ensure protection from light (amber ampoules, dark storage) 237 Use with caution due to narrow safety margin
238 May be used at Level 1 (Community) in areas with community midwife services 239 Use only for induction of labour 240 Use only under close supervision 241 Continuous ambulatory peritoneal dialysis (fluid) 242 Various dialysis solutions & systems are available & in use; selection of the most
appropriate presentations will be made by specialists
KEML 2016
Dose-form
c) Tablet (scored)
a) Injection (oily)
50mg/mL (2mL amp)
Zuclopenthixol acetate
(depot, oily)245
Specialist List 24.1.7
Chlorpromazine HCl [c]
Injection
25mg/mL (2mL amp) a) 25mg
Clozapine
Tablet (scored)
a) Injection
5mg/1mL amp
Haloperidol [c]
b) Tablet (scored)
Risperidone
2mg (scored)
24.2 Medicines used in mood disorders
24.2.1 Medicines use in depressive disorders
24.2.1.1
Amitriptyline HCl
Specialist List 24.2.1.3
Fluoxetine246 [c]
Tablet (scored)
20mg (as HCl)
Venlafaxine
75mg (as HCl)
24.2.2 Medicines used in bipolar disorders
24.2.2.1
200mg (cross-scored)
(sodium valproate)
(enteric-coated)
Specialist List 24.2.2.2
Lithium carbonate
400mg (modified release)
24.3 Medicines used in anxiety disorders
24.3.1
24.4 Medicines used in obsessive compulsive disorders
24.4.1
Clomipramine HCl
24.5 Medicine used in disorders due to psychoactive substance abuse
24.5.1
243 Drops with dosing pipette 244 Use only in patients refractory to, or intolerant of, 1st generation antipsychotics 245 Use only in patients refractory to, or unable to tolerate, other antipsychotics 246 Only use in patients >8 years 247 Only use in anxiety with agitation
248 Only use in management of alcohol dependence
KEML 2016
Dose-form
Specialist List250
a) Injection IM
7mL (in 2 amps)251
B vitamins, high potency
b) Injection IV
10mL (2 x 5mL amps)252
Buprenorphine + naloxone
a) 2mg + 500 micrograms
Tablet (sublingual)
(both as HCl)
b) 8mg + 2mg
Methadone HCl
Oral liquid
5mg/mL (concentrate)
Naltrexone HCl
24.6 Medicine used in attention deficit hyperactivity disorder (ADHD)
24.6.1
Methylphenidate253
25. MEDICINES for RESPIRATORY DISORDERS
25.1 Anti-asthmatics and medicines for chronic obstructive pulmonary disease
diproprionate254
Epinephrine (adrenaline)
50 micrograms/mL
Specialist List
Formoterol fumarate +
6 micrograms + 200
Dry powder inhaler
budesonide
micrograms/metered dose
a) Inhalation
20 micrograms/metered dose
(aerosol)
Ipratropium bromide
b) Nebuliser
250 micrograms/1mL unit dose
solution
vial (isotonic)
a) Granules
4mg sachet
Montelukast (as sodium salt)
b) Tablet
249 As polacrilex (polacrilin complex) 250 Use under close supervision within substance dependency treatment programmes 251 Ascorbic acid 500 mg, nicotinamide 160 mg, pyridoxine hydrochloride 50 mg,
riboflavin 4 mg, thiamine hydrochloride 250mg/7mL
252 Ascorbic acid 500 mg, nicotinamide 160 mg, pyridoxine hydrochloride 50 mg,
riboflavin 4 mg, thiamine hydrochloride 250mg/10mL
253 Use should be strictly controlled and actively monitored 254 Budesonide inhalation 100 micrograms/metered dose is an available alternative
255 As hydrochloride or hydrogen tartrate; strength also expressed as 0.1% or 1 in 1,000
KEML 2016
Dose-form
26. SOLUTIONS CORRECTING WATER, ELECTROLYTE and ACID–BASE
26.1 Oral
Oral rehydration solution
osmolality formula) 500mL sachets (4) +
ORS + zinc sulphate
zinc sulphate tablets
(disp.) 20mg (10)
Rehydration solution for
PFOL (to make 1L)
malnutrition (ReSoMal)
26.2 Parenteral
a) 5% (isotonic)
(500mL infusion pack) b) 10% (hypertonic)
Injectable solution
(500mL infusion pack) c) 50% (hypertonic)
11.2% (20mL amp)257
Potassium chloride
15% (10mL amp)258
Injectable solution
Sodium hydrogen carbonate
Injectable solution
8.4% (10mL amp)260
Injectable solution
Sodium lactate compound261
BP formula262 (500 mL)
Specialist List [c]
3% (hypertonic)263
Sodium chloride
Injectable solution
(10mL amp)
256 Use only in dialysis, ICU and other central line fluids enhancement 257 Equivalent to K+ and Cl- 1.5 mmol/mL 258 Equivalent to K+ and Cl- 2 mmol/mL
259 Equivalent to Na+ and Cl- 154 mmol/L 260 Equivalent to Na+ and HCO -3 1,000 mmol/L
261 Ringer's lactate, Hartmann's solution 262 Equivalent to Na+ 131, K+ 5, Ca2+ 2, Cl- 111, HCO -3 (as lactate) 29 mmol/L
263 Equivalent to Na+ and Cl- 513 mmol/L; use in bronchiolitis, and in hyponatremia in
renal conditions in children
KEML 2016
Dose-form
26.3 Other
26.3.1
Water for injection
27. VITAMINS and MINERALS
27.1
Calcium carbonate
Tablet (chewable)
Calcium gluconate
Cholecalciferol (Vit D3)
Oral liq. (drops) [c]
Ergocalciferol (Vit D2)
1.25mg (50,000 IU)
Pyridoxine HCl (Vit B6)266
Thiamine HCl (Vit B1)267
Specialist List [c] 27.8
Cholecalciferol (Vit D3)268
Injection IM (oily)
300,000 IU/1mL amp
28. EAR and NOSE MEDICINES
28.1 Ear medicines
Ciprofloxacin HCl
(ear drops) Solution
Hydrogen peroxide269
Specialist List270
Ciprofloxacin HCl +
Solution (ear drops)
0.3% + 0.1%
betamethasone sodium
Clotrimazole
Solution (ear drops)
28.2 Nose medicines
28.2.1
Specialist List271 28.2.3
Budesonide
Nasal spray
100 micrograms/metered dose
264 Equivalent to calcium (elemental) 500mg (Ca2+ 12.5 mmol ) 265 Equivalent to 10 micrograms/mL 266 Only use in TB patients for isoniazid-induced neuropathy
267 Only use in alcohol withdrawal and properly diagnosed vitamin B deficiency 268 Use only where oral therapy not tolerated or adherence likely to be poor 269 This 3% strength is also expressed as '10-volume'. If the ear drops are unavailable, use
other available forms & strengths and dilute as required to 3% for use as ear drops
270 Specialists here include Clinical Officer specially trained in ENT
271 Specialists here include Clinical Officer specially trained in ENT
KEML 2016
Dose-form
29. SPECIFIC MEDICINES for NEONATAL CARE
29.1 Medicines administered to the neonate [c]
29.1.1
Chlorhexidine gluconate272
Specialist List
a) Injection
20mg/mL273 (3mL vial)
Caffeine citrate
b) Oral liq. (drops)
Ibuprofen
Injection solution
5mg/mL (2mL amp)
Prostaglandin E
500 micrograms/1 mL amp
Injection solution
(alprostadil)
(in alcohol)
Suspension for
25mg/mL274 or
Surfactant
intra-tracheal
80mg/mL275
instillation
29.2 Medicines administered to the mother
Dexamethasone276
(as sodium phosphate)
30. MEDICINES used in JOINT DISEASES
30.1 Medicine for gout
30.1.1
30.2 Disease-modifying agents used in rheumatoid disorders (DMARDs)
150mg (phosphate or
Specialist List 30.2.2
Azathioprine
Leflunomide277
Methotrexate
Sulfasalazine
30.3 Juvenile joint disease
30.3.1
272 Use for umbilical cord care; delivering chlorhexidine 4% (avoid use of other
presentations which resemble eye ointment)
273 Equivalent to 10mg caffeine base/mL 274 Beractant (bovine lung extract) (4mL single-use vial)
275 Poractant alpha (porcine lung phospholipid fraction) (1.5mL vial) 276 At Level 2, only for use by trained midwives in management of pre-term labour in
Focused Antenatal Care (FANC) clinics
277 Use only when methotrexate and sulfasalazine cannot be used 278 Use in treatment of acute or chronic rheumatic fever, juvenile arthritis, Kawasaki
KEML 2016
Dose-form
31. PREPARATIONS for PARENTERAL NUTRITION
Specialist List
a) 5-6% with glucose [c] (1oomL bottle)
Amino acids279
b) 10% (with electrolytes) (500mL bottle) a) 10% (500mL)
Fat (lipid)280
Infusion (emulsion)
b) 20% (100mL [c] & 500mL)
Total parenteral nutrition
Infusion (emulsion)
Composition & size according
(TPN) (amino acids + lipids +
(triple-chamber)
to specialist requirements
glucose + electrolytes)
32. PREPARATIONS for MANAGING SEVERE ACUTE MALNUTRITION
Specialist List
Ready to use therapeutic
Standard formula
Oral paste
food (RUTF)281
(500 kcal sachet) Standard formula
F-75 therapeutic milk282
PFOL (for approx.
(102.5g sachet)
Standard formula
F-100 therapeutic milk283
(114g sachet)
33. MEDICINES for other CONDITIONS
Specialist List
33.1
Levamisole284
50mg (as HCl)
279 Required product/composition to be determined by the specialist 280 Required product/presentation to be determined by the specialist
281 Micronutrient-fortified peanut/milk paste providing 500 kcal per sachet 282 Micronutrient-fortified milk powder for reconstitution with water; also known as
Formula 75, or Phase 1 (stabilisation phase) Therapeutic Milk
283 Micronutrient-fortified milk powder for reconstitution with water; also known as
Formula 100, or Phase 2 (rehabilitation phase) Therapeutic Milk
284 Use in treatment of nephrotic syndrome
KEML 2016
Annex 1: Contributors to KEML 2016
Development
Following is a list of those who contributed to the various stages of
KEML 2016 development as described on pindicating their position or
area of expertise and place of work.
The National Medicines & Therapeutics Committee (NMTC 2014)
MoH, Head of DCRHS (Chair)
MoH, Pharmaceutical Services (Secretary)
Dr Priscilla S Migiro
MoH, Specialised Clinical Services
MoH, General Clinical Services
Dr Josphat Mbuva
MoH, Pharmaceutical Services
Mr Fredrick Omiah
MoH, Nursing Services
Mr Francis Mwalloh
MoH, Medical Laboratory Services
Dr Elizabeth Onyiego
MoH, Oral Health Services
The Technical Working Group on KEML Review & Update
Dr Josphat N Mbuva
Pharmacist (MSS)
MoH/PSU (Secretary)
Dr Chris Forshaw
Sen. Pharm. Adviser
MoH/PSU (Editor)
Dr Oduor Onyango
Pharmacist (MMU)
County HS, Garissa
Dr Bernard Makenzie
Pharmacist (MMU)
County HS, Kwale
Pharmacist (MRA)
Pharmacist (MPU)
Pharmacist (MPU)
Ms Teresia Kimita
County HS, Nairobi
Ms Veronica Wanjohi
Clinical Medicine
County HS, Nairobi
Practitioner (PHC)
Clinical Pharmacist
Dr Beatrice Jakait
Pharmacist (HSCM)
Internal Medicine
Specialist/Lecturer
KEML 2016
Dr Joseph Mbuthia
Paediatric Specialist
Private Clinic, NBI
Prof Grace Irimu
Paediatric Specialist/
Dr David Githanga
Paediatric Specialist
Pharmacist (HCM)
Notes on areas of pharmaceutical expertise: HCM = health commodity
management, HSCM = hospital supply chain management, MPU = medicines
procurement & utilisation, MMU = medicines management & use, MRA =
medicines regulation & assessment, MSS = medicines supply system
Other contributors
Dr Tom Menge
Chief Pharmacist/
Toxicology Specialist
Clinical Pharmacist
Dr Rachel Nyamai
Paediatric Specialist
MoH, NCAHU (Head)
Dr Mariam Tatu Mwanje Neglected Tropical
Diseases Specialist
Clinical Pharmacist
Dr Michael Gichangi
Dr Monicah Bitok
Ophthalmology Spec.
Ophthalmic Nurse
Palliative Care Spec.
KEHPCA (Exec.Dir)
Dr Esther Muinga
Palliative Care Spec.
Dr Asaph Kinyajui
Palliative Care Spec.
Dr Esther Nafula
Palliative Care Spec.
Palliative Care (Nursing) KEHPCA
Dr Simon Njuguna
Mental Health Spec.
Mental Health Spec.
Dr Fredrick Owiti
Mental Health Spec.
Mental Health Spec.
Mathari NTRH, NBI
KEML 2016
Dr Josphine Omondi
Mental Health Spec.
Dr Beatrice Ogola
Clinical Pharmacist
Mathare Hosp, NBI
Dr Dorothy Memusi
Anaesthetic Specialist Nairobi Hosp/KSA
Dr Jacqueline Andhaga
Anaesthetic Specialist Mama Lucy Kibaki
Dr Phenina N Kitili
Anaesthetic Specialist KNH
Dermatology Specialist Upper Hill MC, NBI
Oncology Specialist
Prof William Macharia
Oncology Specialist
AKU Hospital, NBI
Dr Fredrick Chite
Oncology Specialist
Prof Jessie Githanga
Oncology Specialist
Prof Isaac M Macharia
Renal Specialist
Diviner K Nyarera
Renal Spec. (Nursing)
Dr Patrick Mburugu
Urology Specialist
Dr Renson Mukhwana
Gertrude's Children's
Endocrinology Spec.
Internal Medicine Spec. Nakuru L5 Hospital/
Egerton University
Dr Richard Muthoka
Clinical Pharmacist
MoH, TB & Lep. CU
Dr Bartilol Kigen
Gynaecology Spec.
Mr Charles Mburu
Imaging Specialist
MoH, Radiology Unit
Dr Daniel Tewolde
Nutritional Feeding Sp. UNICEF, Kenya
Pharmacist/PH Spec.
Dr Regina Mbindyo
Country Medicines
KEML 2016
Contributors Abbreviations & Acronyms
AKU
Aga Khan University
Clinical Officer
HIV Care & Treatment Programme (under NASCOP)
Division of Child and Adolescent Health
Deputy Chief Pharmacist
Department of Clinical & Rehabilitative Health Services
Division of Family Health
Division of Leprosy, Tuberculosis and Lung Disease
Division of Malaria Control
Department of Standards and Regulatory Services
Gastrointestinal
Health Commodities Management Programme
Kenya Medical Research Institute/Wellcome Trust
Kenya Medical Supplies Agency
Kenya Hospices & Palliative Care Association
Kenya Medical Association
Kenyatta National Hospital
Kenya Paediatric Association
Kenya Society of Anaesthetists
Mission for Essential Drugs and Supplies
Ministry of Health
Management Sciences for Health
Moi Teaching & Referral Hospital, Eldoret
National AIDS & STI Control Programme
KEML 2016
Neonatal, Child & Adolescent Health Unit
Neglected Tropical Diseases Unit
National Teaching & Referral Hospital
Primary Health Care
Pharmacy & Poisons Board
Pharmaceutical Services Unit
Quality Assurance
Reproductive Health
Spec or Sp Specialist Trop
University of Nairobi
World Health Organization
KEML 2016
Annex 2: References
1. The Selection and Use of Essential Medicines 20th Report of the WHO Expert
Committee (including the 19th WHO Model List of Essential Medicines and the 5th WHO Model List of Essential Medicines for Children) WHO Technical Report Series #994, 2015 (available at This is commonly referred to as the WHO Model List and is the prime reference and evidence base for the KEML.
2. British National Formulary (BNF) 71 (March 2016) BMJ Publishing Group and
Royal Pharmaceutical Society (book and eBook) Used to obtain information on available drug presentations and prices.
3. Kenyatta National Hospital Formulary, Kenyatta National Hospital (2013)
Used to obtain information on the medicines presentations currently in use at the national referral hospital in Nairobi.
4. Kenya Drug Index 14th edition (2015-16) ePharmacy.co.ke (available at
http://www.epharmacy.co.ke/product/kenya-drug-index-hard-copy-book-14th-edition/) Used to obtain information on locally available drug presentations and prices.
5. KEMSA Customer Order Form (current version April 2015) Kenya Essential
Medical Supplies Authority, Nairobi Used to obtain information on local (public sector) medicines presentations availability and prices.
6. MEDS Catalogue 2016, Mission for Essential Drugs & Supplies, Nairobi
Used to obtain information on local (FBO sector) medicines presentations availability and prices.
7. National Guidelines for the Diagnosis, Treatment and Prevention of Malaria In
Kenya 5th edition (draft) Ministry of Health (2016)
KEML 2016
8. National Policy Guidelines on Prevention and Control of Jigger Infestations
Division of Environmental Health Division of Environmental Health, Ministry of Health (2014)
9. Algorithms for Managing Common STI Syndromes National AIDS/STI Control
Programme (NASCOP), Ministry of Health (current, undated)
10. Rapid Advice on Syndromic STI Management in Kenya National AIDS/STI
Control Programme, Ministry of Health (Nov 2015)
11. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in
Children with Medical Illnesses WHO 2012 (available at
12. Guidelines for the Treatment of Chronic Hepatitis B and C Viral Infections in
Kenya, The Gastroenterology Society of Kenya (2015) (available at
13. National Palliative Care Guidelines, Ministry of Health (2013) (available at
14. Integrated Management of Childhood Illness (IMCI), A guide for healthcare
workers, Division of Child and Adolescent Health (DCAH), Department of Family Health, Ministry of Public Health & Sanitation (Aug 2012 edition)
15. National Policy Guidelines on Immunization 2013, Division of Vaccines &
Immunization, Ministry of Health (available at
16. National Family Planning Guidelines for Service Providers, Division of
Reproductive Health, Ministry of Public Health and Sanitation (2010) (available at
KEML 2016
17. National Guidelines for Quality Obstetrics and Perinatal Care, Ministry of
Public Health & Sanitation and Ministry of Medical Services (undated) (available at
18. National Guidelines for Cancer Management Kenya (August 2013) Ministry of
Health (available a
19. Guidelines for Management of Tuberculosis and Leprosy in Kenya (July 2013
edition) Division of Leprosy, Tuberculosis & Lung Disease, Ministry of Health (available a
20. National Guidelines on Management of Tuberculosis in Children, 2nd edition
Aug 2013, Division of Leprosy, Tuberculosis & Lung Disease, Ministry of Health (available at
21. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV
Infection: Rapid Advice, National AIDS/STI Control Programme (NASCOP), Ministry of Health (June 2014) (available at
22. Basic Paediatric Protocols, 4th edition (Feb 2016), Ministry of Health (available
KEML 2016
Annex 3: KEML Amendment Proposal Form
Please complete each of the sections and submit the Form together with hard and/or soft copies of supporting evidence and any other relevant documentation to:
The Chief Pharmacist
Afya House, Cathedral Rd
Box 30016-00100, Nairobi, Kenya
Name of Proposer:
1. Type of Amendment Proposed (please tick):
c) Change of Presentation [ ] d) Other [ ]
2. Details of Proposal:
KEML 2016
3. Supporting Arguments/Evidence Base:
4. Supporting References/Relevant Documentation:
5. Signature:
KEML 2016
Annex 4: Terms of Reference for the TWG on
Review & Update of the KEML
(i) Select medicines for listing on the next revised edition of the Kenya
Essential Medicines List
(ii) Apply essential medicines concepts and the principles of rational
selection, affordable pricing and sustainable financing in the review process
(iii) Make reference to the Constitution of Kenya 2010, Vision 2030, Kenya
Health Policy 2014-2030, Kenya National Pharmaceutical Policy (KNPP) and the current World Health Organization (WHO) Model List of Essential Medicines and any other relevant documents in the review process.
(iv) Adhere to the standard operating procedures (SOP) adopted by the
NMTC for the review process including those for managing conflict of interests.
(v) Engage/consult/collaborate with relevant experts and stakeholders in the
KEML 2016
Annex 5: The National Medicines & Therapeutics
Committee (2014)
1. Director of Medical Services (DMS) (Chair) 2. Head, Pharmaceutical Services/Chief Pharmacist (Secretary) 3. Pharmacist (Pharmaceutical Services/Medicines Supply Chain) 4. Head, Nursing Services 5. Head, Laboratory Services 6. Head, Oral Health Services 7. Head, Specialised Clinical Services 8. Head, General Clinical Services 9. Head, Research Unit 10. Head, Administration
Terms of Reference:
1. Coordinate the development and review of policies on clinical
governance and use of medicines & other EHPT286
2. In coordination with the MoH Department of Health Standards,
Quality Assurance & Regulation (DHSQAR) develop standards and guidelines on:
Establishment and operations of Medicines and Therapeutics
Committees at various levels (national, county and institutional)
Appropriate prescribing and dispensing Safe and cost-effective use of medicines and other EHPT, including
use of evidence-based standardized approaches, adverse event monitoring and reporting, medicines information, and quality assurance
Clinical audits and medicines use evaluation studies
3. In coordination with DHSQAR, formulate, review and update all
relevant therapeutics guidelines, including the:
National Clinical Guidelines National Formulary National Essential Medicines & Medical Supplies (Devices) lists
285 All MoH officers
286 Essential Health Products and Technologies
KEML 2016
4. In collaboration with relevant stakeholders, review medicines and
therapeutics research findings and recommend appropriate interventions to inform policy development; identify, propose and commission as appropriate, areas requiring further research
5. Collaborate with the relevant National and County health authorities
to plan and implement mitigation measures in the event of emergency disease outbreaks or health threats, e.g. identification of items for inclusion in buffer stocks of EHPT, coordination and use of emergency donations
6. Collaborate with relevant departments involved in the introduction of
disease-based or vertical programmes in relation to the selection and use of any medicines and/or other EHPT
7. Increase awareness and understanding of the critical role, functions
and activities of the NMTC and advocate for adequate support and funding
8. Provide leadership in improving awareness and education relating to
the safe and appropriate use of medicines & other EHPT amongst health care professionals and consumers
9. Provide technical support to county and facility MTCs through the
development and dissemination of MTC guidelines, training materials, and capacity building
10. Support the development, review and revision as necessary of pre-
service, in-service and CPD training courses in therapeutics and the management and use of medicines and other EHPT
11. Perform any other relevant task as may be assigned by the appointing
See over for the NMTC Organisational Structure
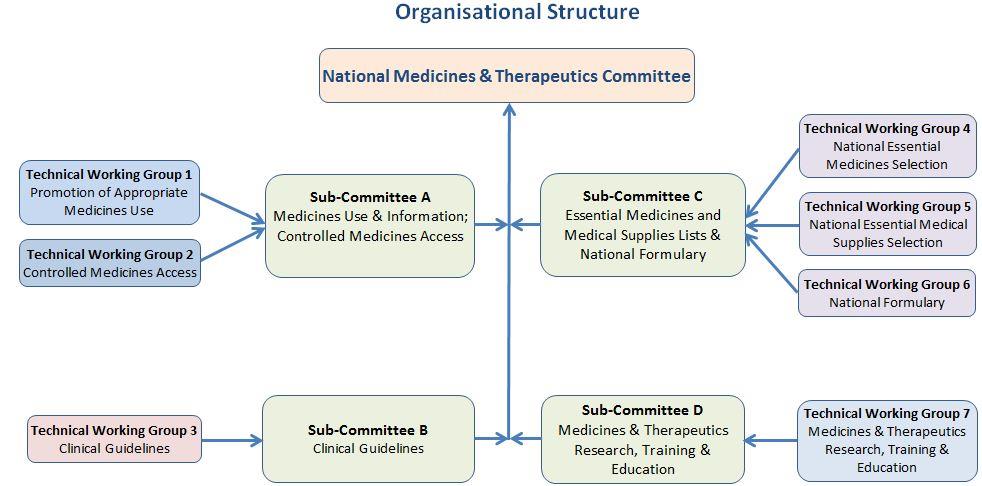
KEML 2016
KEML 2016
Ascorbic acid, 29
Asparaginase, 44 Aspirin, 24, 32, 43, 52, 66
Abacavir (ABC), 26, 39
Atazanavir + ritonavir, 14, 40
Abacavir + lamivudine, 14, 40
Absolute alcohol, 34
Atorvastatin, 19, 52
Acetazolamide, 60
Atracurium, 21, 59
Atropine, 31, 34
Acetylcysteine, 34
Aciclovir, 14, 39
Azathioprine, 44, 66
Azithromycin, 37
eye, 21, 60
eye, 21, 60
Activated charcoal, 12, 34 Adrenaline, 18, 33, 51, 63
Air, medical, 11 Albendazole, 12, 35
B vitamins, 23, 63
Alendronic acid, 44
Barium sulphate, 53
Allopurinol, 24, 25, 44, 66
Alprostadil, 66
Beclometasone inhaler, 63
Beclomethasone inhalation, 29
Amidotrizoate, 53
Bedaquiline, 13, 38
Amikacin, 38
Benzathine benzylpenicillin, 13, 36
Benzathine penicillin, 26
Amino acids, 67
Aminophylline, 25
Benzoyl peroxide, 19, 52
Benzyl benzoate, 53
Amiodarone, 51
Benzylpenicillin, 26, 36
Amitriptyline, 32, 62
Betamethasone (topical), 52
Amoxicillin, 13, 26, 36
Bevacizumab, 22, 60
Amoxicillin + clavulanic acid, 13, 26, 36
Bicalutamide, 17, 47
Amphotericin B, 39, 42
Biperiden, 28
Biphasic isophane insulin, 57
Anastrozole, 17, 47
Bisacodyl, 20, 32, 55
Anti-D immunoglobulin, 50
Bleomycin, 44
Anti-rabies immunoglobulin, 50
Blood (whole), 18, 50
Anti-tetanus immunoglobulin, 50
Bromazepam, 23, 62
Bromocriptine, 58
+ lumefantrine, 15, 42
Budesonide (nose), 24, 65
Artesunate, 15, 42
Budesonide inhalation, 63
KEML 2016
Bupivacaine, 25, 31
Clomipramine, 23, 62
Buprenorphine + naloxone, 23, 63
Clonidine, 29 Clopidogrel, 19, 52
ear, 24, 65
Caffeine citrate, 66
vaginal, 14, 26, 39
Calcium carbonate, 24, 65
Clozapine, 22, 62
Calcium folinate, 45
Coagulation factor IX, 50
Calcium gluconate, 65
Coagulation factor VIII, 50
Capecitabine, 16, 45
Capreomycin, 13, 38
+ salicylic acid, 19, 53
Carbamazepine, 29, 34, 62
Co-amoxiclav, 36
Carboplatin, 16, 45
Condom (female), 56
Carvedilol, 18, 50, 51
Condom (male), 56
Cefazolin, 13, 36
Conjugated oestrogens, 20, 57
Copper-containing device (IUD), 56
Ceftazidime, 36
Cotrimoxazole, 37, 43
Crisantaspase, 44
Crotamiton, 19, 52, 53
Cetirizine, 12, 33
Cyanocobalamin, 49
Charcoal (activated), 12, 34
Chickenpox vaccine, 59
Cyclophosphamide, 16, 45
Chlorambucil, 45
Cycloserine, 13, 38
Chloramphenicol, 26
Cytarabine, 45
Chlorhexidine, 19, 54, 66 Chloroquine, 66
Chlorphenamine, 11, 25, 31, 33 Chlorpromazine, 22, 61, 62
Dacarbazine, 16, 28, 45
Cholecalciferol, 24, 29, 65
Dactinomycin, 45
Cholera vaccine, 21, 58
Ciclosporin, 15, 44
Darunavir, 14, 40
Ciprofloxacin, 13, 37
Daunorubicin, 45
+ betamethasone (ear), 24, 65
Deferasirox, 12, 18, 34, 49
Deferoxamine, 18, 34, 49
eye, 21, 60
Dehydrated alcohol, 34
Cisatracurium, 21, 29, 59
Delamanid, 13, 38
Cisplatin, 45
Desonide, 19, 52
Clarithromycin, 37
Dexamethasone, 17, 20, 24, 32, 33, 48,
Clindamycin, 37
Clobetasone, 19, 52
Clofazamine, 13, 38
Diazepam, 11, 12, 23, 25, 26, 29, 32, 35,
Clomifene, 57
KEML 2016
Ethosuximide, 26
Etonorgestrel implant, 56
Diethylcarbamazine, 13, 35
Etoposide, 28, 46
Diethylstilboestrol, 17, 48
Etravirine (ETV), 40
Digoxin, 50, 51 Dihydroartemisinin + piperaquine, 15,
Fat emulsion, 67
Dimercaprol, 34
Female condom, 56
Dimercaptosuccinic acid, 34
Fentanyl, 11, 32
Dinoprostone, 61
Ferrous salt, 17, 48
+ folic acid, 49
Ferrous sulphate, 48
Filgrastim, 16, 46
Docetaxel, 16, 45
Finasteride, 17, 48
Flucloxacillin, 36
Domperidone, 20, 55
Dopamine, 51
Fludrocortisone, 56
Dorzolamide, 22, 60
Flumazenil, 12, 34
Doxorubicin, 16, 46
Fluorescein, 19, 53
Doxycycline, 27, 37, 43
Fluorouracil, 46
DPT + HiB + HepB vaccine
Fluoxetine, 23, 62
(pentavalent), 58
Flupentixol, 22, 61 Fluphenazine, 61
Folic acid, 17, 49 Fomepizole, 12, 34
Econazole (eye), 21, 60
Food (therapeutic), 24, 67
Efavirenz, 14, 27, 40
Formoterol fumarate + budesonide, 23,
+ emtricitabine + tenofovir, 27
+ lamivudine + tenofovir, 14, 40
Furosemide, 18, 19, 51, 54
Fusidic acid, 52
Enoxaparin, 18, 49 Epinephrine, 18, 33, 51, 63
Ergocalciferol, 24, 29
Gabapentin, 11, 12, 33, 35
Gadobutrol, 53
Erythromycin, 26
Gadodiamide, 53
Estradiol + medroxyprogesterone, 56
Gadopentate dimeglumine, 54
Estradiol + medroxyprogesterone
Ganciclovir, 15, 41
Ethambutol, 26, 38
Gemicitabine, 16, 46
Ethanol, 12, 34, 54
Ethinylestradiol + levonorgestrel, 56
Ethinylestradiol + norethisterone, 29
Glibenclamide, 57
KEML 2016
Gliclazide, 20, 57
biphasic isophane, 57
glulisine, 57
intermediate-acting, 57
Glutaraldehyde, 54
lispro, 57
Glyceryl trinitrate, 50
rapid acting, 20, 57
Glycopyrronium bromide +
neostigmine metilsulfate, 21, 59
Intraperitoneal dialysis solutions, 61
Goserelin, 17, 48
Iohexol, 53
Griseofulvin, 39
Iopromide, 53 Ipratropium, 23, 63
Irinotecan, 16, 46 Isoflurane, 11, 31
Haemodialysis solutions, 22, 61
Isoniazid, 26, 38
Haloperidol, 11, 22, 33, 61, 62
Isoniazid + ethambutol, 38
Isosorbide dinitrate, 50
Hand rub, 19, 54
Ivermectin, 19, 26, 53
Heparin, 49 Hepatitis B vaccine, 59
HPV vaccine, 58 Hydralazine, 51
Kanamycin, 38
Hydrochlorthiazide, 51, 54
Hydrocortisone, 33, 48, 56
Ketorolac, 21, 60
Hydrogen peroxide (ear), 24, 65
Hydroxocobalamin, 49 Hydroxycarbamide, 16, 18, 46, 49
Lactulose, 11, 33
Hydroxyurea, 16, 18, 46, 49
Hyoscine butylbromide, 11, 33
+ nevirapine + stavudine, 27
Hyoscine hydrobromide, 25, 33
+ nevirapine + zidovudine, 41 + stavudine, 27
+ tenofovir, 14, 41 + zidovudine, 14, 41
Ibuprofen, 32, 43, 66
Lamotrigine, 12, 35
Ifosfamide, 16, 46
Lansoprazole, 19, 54
Imatinib, 16, 46
Latanoprost, 22, 60
Imipenem + cilastin, 13, 36
Leflunomide, 24, 66
Immunoglobulin
Levamisole, 67
Levodopa + carbidopa, 17, 48
Levofloxacin, 13, 39
anti-tetanus, 50
Levonorgestrel, 20, 56
normal, 18, 50
snake antivenom, 58
Levothyroxine, 57
Lidocaine, 11, 31, 50
aspart, 57
+ epinephrine, 31
KEML 2016
Mometasone, 19, 52
+ adrenaline, 25
Montelukast, 23, 63
dental, 25
Morphine, 11, 25, 32
Moxifloxacin, 13, 39
Linezolid, 13, 39
Mupirocin, 19, 52
Liquid paraffin (nasal), 24, 65
Mycophenolic acid, 15, 44
Lithium, 23, 29, 62 Loperamide, 11, 33
Lopinavir + ritonavir, 27, 40 Lorazepam, 12, 35
Losartan, 18, 51
Naltrexone, 23, 63
Lugol's iodine, 21, 57
Neostigmine, 59 Nevirapine (NVP), 40
Niclosamide, 26 Nicotinamide, 29
Magnesium sulphate, 35
Nicotine, 23, 63
Magnesium trisilicate, 28
Nitrofurantoin, 37
Mannitol, 28, 54
Nitrous oxide, 31
Measle + rubella vaccine, 58
Norethisterone enantate, 29
Measles vaccine, 58
Normal immunoglobulin, 18, 50
Medical air, 11, 31
Medroxyprogesterone acetate, 20, 21,
Meglumine iotroxate, 53 Melarsoprol, 43
Ofloxacin, 26
Melphalan, 16, 46
Olanzapine, 22, 62
Meningococcal meningitis vaccine, 59
Omeprazole, 19, 54
Mercaptopurine, 46
Ondansetron, 12, 20, 33, 55
Mesalazine, 20, 55
Oral rehydration solution, 55, 64
Mesna, 16, 46
+ zinc sulphate, 20, 23, 55, 64
Oxaliplatin, 16, 47
Methadone, 63
Methotrexate, 47, 66 Methyldopa, 51
Methylphenidate, 23, 63 Methylprednisolone, 15, 17, 28, 44, 48
Paclitaxel, 16, 47
eye, 21, 60
p-aminosalicylic acid, 13, 39
Metoclopramide, 12, 33, 55
Pancuronium, 21, 59
Metronidazole, 26, 37, 41
Paracetamol, 11, 15, 25, 32, 43
Midazolam, 11, 12, 31, 33
Mifepristone + misoprostol, 61
liquid (nasal), 24, 65
Milk (therapeutic), 24, 67
KEML 2016
Paraffin (soft white), 53
Paromomycin, 42 PAS, 39
Quetiapine, 22, 62
Pegylated interferon, 15, 41
Penicillamine, 34 Pentamidine, 43
Petroleum jelly, 53 Phenobarbital, 12, 35
Rabies vaccine, 59
Phenoxymethylpenicillin, 13, 36
Raltegravir, 14, 40
Phytomenadione, 17, 28, 49
Red blood cells, 18, 50
ReSoMal, 20, 23, 55, 64
Plasma (fresh frozen), 18, 50
Platelets, 18, 50
Ribavirin, 15, 41
Pneumococcal vaccine, 21, 59
Rifabutin, 14, 39
Podophyllum resin, 53
Polio vaccine (IPV), 21, 58
Polio vaccine oral (OPV), 58
+ isoniazid + ethambutol, 38
Polygeline, 18, 50
+ isoniazid + pyrazinamide, 13, 38
Polyvidone iodine (mouthwash), 29
+ isoniazid + pyrazinamide +
Potassium chloride, 23, 29, 64
Risperidone, 22, 62
Povidone iodine, 54
Ritonavir, 14, 40
Pralidoxime, 12, 34
Rituximab, 16, 47
Pramipexole, 17, 48
Rotavirus vaccine, 21, 58
Praziquantel, 35
Prednisolone, 15, 17, 20, 21, 25, 28, 33,
eye, 21, 60
Primaquine, 15, 43
Salbutamol, 29, 63
Procarbazine, 47
Salicylic acid, 28
Progesterone (vaginal ring), 20, 57
Silver sulphadiazine, 52
Proguanil, 15, 43
Promethazine, 25, 28
Snake antivenom immunoglobulin, 58
Propofol, 11, 31
Sodium bicarbonate, 29, 64
Sodium calcium edetate, 34
Propylthiouracil, 21, 57
Sodium chloride, 64
Prostaglandin E1, 66
hypertonic, 24, 64
Prostaglandin E2, 61
Protamine, 12, 34, 49
Sodium cromoglicate, 60
Prothionamide, 14, 39
Pyrazinamide, 38
Sodium fusidate, 52
Pyridoxine, 24, 29, 65
Sodium hydrogen carbonate, 29, 64
Pyrimethamine, 27
Sodium lactate compound, 64
KEML 2016
Sodium nitrite, 12, 34
Typhoid vaccine, 59
Sodium nitroprusside, 51 Sodium stibogluconate, 42
Sodium thiosulphate, 12, 34 Sodium valproate, 23, 35, 62
Soft paraffin (white), 53
Spironolactone, 19, 51, 54
Streptokinase, 28
DPT + HiB + HepB (pentavalent), 58
Succimer, 34
Sulfadoxine + pyrimethamine, 15, 43
Sulfamethoxazole + trimethoprim, 37
Sulfasalazine, 28, 66
measles + rubella, 58
Sulphadiazine, 27
meningococcal meningitis, 59
Sunscreening agent, 19, 53
oral polio (OPV), 58
Suramin, 43
pneumococcal, 21, 59
Surfactant, 66
Suxamethonium, 59
polio (IPV), 58 rabies, 59
rotavirus, 21, 58 tetanus, 58
Tacrolimus, 15, 19, 44, 52
Tamoxifen, 17, 48
varicella, 21, 59
Tamsulosin, 17, 48
yellow fever, 59
Tenofovir, 15, 27, 41
Valproic acid, 23, 35, 62
Terbinafine, 19, 52
Vancomycin, 37
Testosterone, 20, 56, 57
Varicella vaccine, 21, 59
Tetanus toxoid (adsorbed), 58
Tetanus vaccine, 58
Venlafaxine, 23, 62
Verapamil, 18, 28, 51
Tetracycline, 60
Vinblastine, 17, 47
Thalidomide, 17, 47
Vincristine, 28
Therapeutic food, 24, 67
Vinorelbine, 17, 47
Therapeutic milk, 24, 67
Tinidazole, 13, 37, 42
Vitamin B6, 24, 29, 65
Tranexamic acid, 18, 49
Vitamin D2, 24, 65
Trastuzumab, 17, 47
Vitamin D3, 24, 65
Triamcinolone (eye), 21, 60
Vitamin K1, 28, 49
Tropicamide, 22, 53, 60
Vitamins B, 23, 63
Tuberculin (purified protein
KEML 2016
Water for injection, 65
Zinc sulphate, 55
White petrolatum, 53
Zoledronic acid, 17, 47
White soft paraffin, 19, 53
Zuclopenthixol, 22, 62
Whole blood, 18, 50
Yellow fever vaccine, 59
The Kenya Essential Medicines List 2016 is an indispensable guide
to the medicines recommended for the management of common conditions in Kenya. It is primarily directed at health care providers and medicines supply managers in the public and non-public health sectors. It should be used together with the current versions of updated national clinical guidelines for those conditions for which such guidelines exist.
As technical and administrative guides for the health sector, these documents should also be of great interest and usefulness to healthcare trainers, trainees, interns and researchers as well others who may have an interest in, or responsibility for, the national health system.
The medicines recommended in the KEML have been carefully and systematically selected using a meticulous process, applying well-defined criteria and based on the latest available and internationally accepted evidence on best therapeutic practice.
Therefore, they are the optimum set of medicines needed to ensure provision of the Kenya Essential Package for Health (KEPH), which is part of the sector's comprehensive approach for health services delivery to the population. The KEML is published in the context of ongoing health reforms aimed at reversing the declining trends in the national health status, by ensuring equitable access to healthcare services.
The KEML is an indispensable guide for ensuring access to Essential Medicines, aimed at stimulating investment in local pharmaceutical production, procurement and supply systems, improved prescribing and dispensing of medicines, as well as strategies for healthcare financing and for Appropriate Medicines Use (AMU). Routine use as recommended can be expected to have a major positive impact on the health status of Kenyans.
Source: http://www.health.go.ke/?wpdmdl=1814
Mr. Sanjay Singh MBBS, MS, FRACS, FRCS (UK) 2 - 4 Charles Street Tel: 02 4474 3774 Fax: 02 4474 3775 Write questions or notes here: Surgery for Ingrowing Toenail (adult) Further Information and Feedback:You can get more information about this procedure at aboutmyhealth.orgTell us how useful you found this document at www.patientfeedback.orgBrochure code: GS16
Towards the Knowledge-based Graduate School of Strategic Management, Copyright © 2016 Nonaka and Toyama P.F. Drucker We need an economic theory that puts knowledge into the center of the wealth-producing process. Such a theory alone can explain the present economy. It alone can explain economic growth. It alone can explain innovation. P. F. Drucker. (1993) "Post Capitalist Society" p.183









