Healthnz.co.nz
S⫽1 9/27/10 8:21 Art: 2010-0409
Experimental and Clinical Psychopharmacology
2010 American Psychological Association
2010, Vol. 18, No. 5, 385–394
DOI: 10.1037/a0020834
Pulmonary Delivery of Nicotine Pyruvate:
Sensory and Pharmacokinetic Characteristics
Jed E. Rose, James E. Turner,
Thangaraju Murugesan, and
Health New Zealand Ltd., Christchurch, New Zealand
Fre´de´rique M. Behm
Duke University Medical Center
The aim of this study was to evaluate pharmacokinetic and subjective responses to a prototypenicotine pyruvate (NP) aerosol generation system. In nine healthy adult daily cigarettesmokers, plasma nicotine levels and subjective responses were assessed after double-blindadministration of 10 inhalations of: NP (10 g/puff, 20 g/puff, and 30 g/puff); Nicotrol/Nicorette nicotine vapor inhaler (NV) cartridge; and placebo (room air). Plasma nicotineconcentrations increased to a significantly greater extent after inhalations of 20 g/puff or 30
g/puff NP (by 5.0 ⫾ 3.4 ng/ml and 8.3 ⫾ 3.1 ng/ml) than after placebo and NV conditions.
Satisfaction ratings were higher for all NP conditions than for placebo, and harshness/irritation was lower for the NP 20 condition than for the NV control condition. Pulmonaryfunction showed no adverse changes. These results demonstrate that NP inhalations producerapid increases in plasma nicotine concentrations, provide satisfaction and are well tolerated.
At the 20 g/puff dose, peak nicotine concentrations were higher than with the Nicotrol/Nicorette nicotine vapor inhaler cartridge. Further trials of this promising nicotine inhalationtechnology are warranted to assess its safety and efficacy in smoking cessation treatment orharm reduction approaches.
Keywords: nicotine, smoking cessation, tobacco, aerosol, pyruvic acid
The annual death toll from diseases caused by smoking is
However, current smoking cessation treatments have lim-
estimated to be 440,000/year in the United States (
Smoking-
ited effectiveness. Long-term (⬎1 year) abstinence rates
attributable mortality, 2008) and 5 million/year worldwide
are often less than 25%, despite the advent of new phar-
(Ezzati & Lopez, 2003). Smoking cessation leads to a
macotherapies such as varenicline and bupropion (Fant,
substantial reversal of the risks borne by smokers (Kenfield,
Buchhalter, Buchman, & Henningfield, 2009; Fiore et al.,
Stampfer, Rosner, & Colditz, 2008; Papathanasiou et al.,
2008). Nicotine replacement therapy (NRT) continues to
2007), and thus, smoking cessation treatment assumes a
be one of the mainstays of smoking cessation treatment
primary role in the prevention of smoking related disease.
(Schnoll et al., 2009), and is the only pharmacotherapy
Jed E. Rose, James E. Turner, Thangaraju Murugesan, and
results. JER, TM, and JET are named as inventors on patent
Fre´de´rique M. Behm, Department of Psychiatry and Behavioral
applications filed by Duke University pertaining to the nicotine
Sciences, Duke University Medical Center; Murray Laugesen,
inhalation technology; (2) ML has had no relationships with Philip
Health New Zealand Ltd., Lyttelton, Christchurch, New Zealand.
Morris U.S.A. that might have an interest in the submitted work;
Jed E. Rose contributed to the study design, data analysis, and
(3) their spouses, partners, or children have no financial relation-
manuscript writing/editing; James E. Turner contributed to the
ships that may be relevant to the submitted work, other than those
study design and manuscript drafting/editing; Thangaraju Murug-
disclosed in (1); and (4) Authors have no nonfinancial interests that
esan contributed to the study design, fabrication of the inhalation
may be relevant to the submitted work. CJW and ML received
system, training technical staff, and manuscript drafting/editing;
funding to conduct this work from the Duke University Depart-
Frederique M. Behm contributed to the study design, data analysis,
ment of Psychiatry. Within the last 3 years, JER has received
and manuscript drafting/editing; Murray Laugesen contributed to
consulting payments for work unrelated to this study, from the
the study design, supervision of study protocol, data analysis,
following entities: NIDA, GlaxoSmithKline, Novartis, Philip Mor-
manuscript drafting/editing, and the guarantor ensuring scientific
ris International, Targacept, Catalyst Pharmaceutical Partners,
integrity. All authors, external and internal, had full access to all of
Lorillard, University of Kentucky, Medacorp, Pharmalink, and the
the data (including statistical reports and tables) in the study and
Noble Medical Consulting Group.
can take responsibility for the integrity of the data and the accuracy
We thank Chris J. Wynne for preparation of the application for
of the data analysis. All authors have completed the Unified
ethics committee approval and the supervision of study protocol;
Competing Interest form at www.icmje.org/coi_disclosure.pdf
and Chris M. A. Frampton for biostatistical support.
(available on request from the corresponding author) and declare
Correspondence concerning this article should be addressed to
that (1) JER, JET, TM, and FMB have support from Philip Morris
Jed E. Rose, Center for Nicotine and Smoking Cessation Research,
U.S.A. for the submitted work. The company had no role in the
Duke University Medical Center, 2424 Erwin Road, Suite 201,
design or execution of the study, data analysis or publication of the
Durham, NC 27705. E-mail:
[email protected]
S⫽1 9/27/10 8:21 Art: 2010-0409
ROSE, TURNER, MURUGESAN, BEHM, AND LAUGESEN
approved for over-the-counter (OTC) sales. Nonetheless,
amount of nicotine can be delivered in standard 35-ml puffs
long term success rates in an OTC setting remain low,
than with nicotine vapor alone. Additionally, it was antici-
typically less than 10% (Hughes, Shiffman, Callas, &
pated that NP aerosol would avoid the irritation associated
Zhang, 2003).
with inhalation of pure nicotine base (Caldwell et al., 2009;
To overcome the limited effectiveness of NRT, it will be
Lee, Gerhardstein, Wang, & Burki, 1993), for two reasons:
necessary to recognize and address a major shortcoming of
(1) the near-neutral pH of NP was expected to attenuate the
current NRT products; that is, these products do not provide
irritating effect of pure nicotine base (Armitage & Turner,
smokers with rapid absorption of nicotine in conjunction
1970; Lux & Frecker, 1988); and (2) the small diameter of
with the unique respiratory tract sensory cues accompany-
the NP particles should result in their deposition over the
ing nicotine inhalation. These inhalational cues, along with
large surface area of the lung rather than being concentrated
the delivery of nicotine, are of primary importance in ef-
in a small region of the trachea, which can elicit irritation
fectively relieving smokers' craving for cigarettes (Rose,
and cough (Huchon, 1990; Katz, Schroeter, & Martonen,
1988). Indeed, nicotine administered without airway sen-
2001; Usmani, Biddiscombe, & Barnes, 2005). Lung dep-
sory cues only slightly suppresses craving for cigarettes
osition is difficult to achieve using metered dose inhalers or
(Rose, Behm, Westman, & Johnson, 2000; Rose, Behm,
dry powder delivery systems, having particle sizes well
Westman, Bates, & Salley, 2003). Moreover, blockade of
above 1 m. To gain alveolar entry and deposition of
airway sensations during cigarettes smoking markedly re-
particles with consequent rapid absorption, we speculated
duces the relief of craving after inhaling cigarette smoke
particles would need to be similar in size to cigarette smoke
(Rose, Tashkin, Ertle, Zinser, & Lafer, 1985; Rose, West-
particles (⬍1 m). Particles of the NP aerosol have an
man, Behm, Johnson, & Goldberg, 1999). Conversely, pre-
average mass median aerodynamic diameter (MMAD)
sentation of airway sensory cues has been shown to reduce
of 0.62 m, as estimated by cascade impactor measure-
craving for cigarettes and facilitate smoking cessation (Rose
ments (unpublished data), small enough to be almost totally
& Hickman, 1987; Westman, Behm, & Rose, 1995).
inhaled deep into the lung, ensuring rapid absorption.
Current forms of NRT, however, fail to provide these
Pyruvate, as sodium pyruvate, has been inhaled at a dose
important inhalational components of cigarette smoking,
of 0.65 mg pyruvate per day to treat chronic obstructive
thereby limiting their efficacy as cessation therapies. For
pulmonary disease (Votto, Bowen, Barton, & Thrall, 2008),
example, the nicotine nasal spray, although it is a rapid-
and in this study 0.76 mg in total was used per subject.
acting NRT, lacks the inhalational cues of cigarette smoke,
Pyruvate is rapidly metabolized, and it was unlikely that
and has aversive irritating properties; these deficiencies,
inhalation in such doses would alter the physiological level
along with restrictions in access because of prescription
of 0.44 mg/100 ml blood (Landon, Fawcett, & Wynn,
requirements, have impeded widespread acceptance by
1962). The acute risk profile of NP was thus assumed to be
smokers. Similarly, the nicotine vapor inhaler, while simu-
similar to that of nicotine alone.
lating the behavioral components of smoking and presenting
This study was an initial investigation in which 9 smokers
some sensory cues resembling those of cigarette smoke,
were exposed to several doses of NP aerosol administered
provides a much lower dose of nicotine, which is slowly
by inhalation, and an active control condition, the Nicotrol/
absorbed through the buccal mucosa without reaching the
Nicorette nicotine vapor (NV) inhaler cartridge, as well as
lung in significant amounts (Lunell, Bergstrom, Antoni,
an inactive placebo (air). Venous plasma nicotine levels and
Langstrom, & Nordberg, 1996; Lunell, Molander, Ekberg,
subjective responses were assessed. The study had three
& Wahren, 2000; Schneider, Olmstead, Franzon, & Lunell,
main aims: (1) to determine whether NP inhalation would
produce higher and more rapid boosts in plasma nicotine
Thus, there is a gap in the field of current NRT products
concentrations than the control conditions; (2) to evaluate
that could be filled with a rapid-acting, lung delivery nico-
the efficacy of NP inhalations relative to the control condi-
tine inhaler having acceptable sensory properties. Such an
tions in providing satisfaction and alleviating subjective
inhaler, which does not deliver other toxic smoke constitu-
withdrawal symptoms (e.g., craving reduction); and (3) to
ents, could hold great promise for assisting smokers in
evaluate the safety and tolerability of acute NP administra-
breaking their addiction to cigarettes, as well as reducing the
tion, based on subjective reports or airway irritation and
harm associated with cigarette smoking. This study reports
objective measurement of pulmonary function using spi-
the completion of the first step in establishing the feasibility
of a novel approach to nicotine replacement therapy thatdelivers nicotine to the lung by inhalation (Rose, Rose,
Materials and Methods
Turner, & Murugesan, 2008). This approach stems from our
Study Design
discovery that nicotine vapor when combined with the va-por of pyruvic acid, a weak organic acid normally present in
This was a preliminary, double-blind, placebo controlled,
all living cells, forms a stream of submicron airborne par-
crossover evaluation of the effects of inhaling NP aerosol on
ticles consisting of nicotine pyruvate (NP) salt. Unlike other
plasma nicotine concentrations, subjective relief of smoking
existing approaches to generating a nicotine aerosol, no
withdrawal symptoms and indices of tolerability and safety.
combustion, heat or propellant is necessary to produce the
Because the primary pharmacokinetic end point was defined
nicotine containing particles. Measurements conducted in
as the
increase in plasma nicotine in the first 5 min, imme-
our laboratory prior to human testing showed that a greater
diately after the tenth puff, one condition could feasibly be
S⫽1 9/27/10 8:21 Art: 2010-0409
PULMONARY DELIVERY OF NICOTINE PYRUVATE
studied every 50 min. This design was consistent with the
room air, followed by a 5-s breath hold before exhaling into
distributional half life of nicotine (9 min; Feyerabend, Ings,
the room air. A total of 10 puffs were taken at 30-s intervals,
& Russell, 1985), and with the expectation that maximum
requiring a total of 5 min.
plasma levels per dose inhaled would be relatively low,
The motorized syringe system and the known dose in
minimizing carryover effects.
each prototype were first tested without human subjects to
A prototype aerosol delivery system was assembled,
measure the average nicotine delivery per puff over 10 puffs
loaded, and calibrated to deliver successively to each par-
of 35 ml. In these tests, particulate matter or nicotine vapor
ticipant five different conditions during the morning of the
was collected by filtration through a Cambridge 44-mm
study day, a different condition administered every 50 min.
diameter high-efficiency filter contained in a holder. These
Three of the conditions involved administering the NP test
samples were shipped on ice and analyzed at Duke CNSCRusing Gas Chromatography (Agilent GC-HP6890 series
medications: either 10 g, 20 g, or 30 g of NP per 35 ml
with Nitrogen Phosphorus Detector (Agilent Technologies
puff. In the remaining two conditions, approximately 10 g
Inc. Santa Clara, CA). Based on five determinations for each
of nicotine per puff was supplied from a Nicotrol/Nicorette
condition, the three NP aerosol conditions were found to
inhaler cartridge (the active control), or room air was sup-
deliver an average of 10.3 g/puff (
SD ⫽ 1.66), 23.7
plied (the inactive control or placebo). To minimize the
g/puff (
SD ⫽ 3.39), and 33.0 g/puff (
SD ⫽ 0.63) of
likelihood of adverse events, the NP doses were presented in
nicotine. These conditions will be referred to below as
ascending order from 10 g to 20 g to 30 g per puff,
"NP 10," "NP 20," and "NP 30," respectively. Based on
whereas the control conditions were arranged in a counter-
three determinations, the nicotine vapor condition ("NV")
balanced sequence alternating with the 3-sequence block of
delivered 9.1 g/puff (
SD ⫽ 0.64), which agrees to within
NP conditions.
10% of the value reported previously (Schneider et al.,2001).
Pyruvic acid of 97% purity was obtained from Sigma-
Aerosol Generation Apparatus
Aldrich Inc. (St. Louis, MO/USA) and stored at 4 °C.
The delivery system used to generate the NP aerosol
Nicotine free base USP with a claimed 99.5% purity, re-
(Figure 1) consisted of a cylindrical translucent Teflon tube
F1
tested at the Center for Nicotine and Smoking Cessation
outer housing (11 cm long, 11-mm inside diameter [ID], and
Research (CNSCR) at Duke University, was also stored at 4
13-mm outside diameter [OD]) containing three elements
°C. For operation of the NP delivery system, all constituents
arranged in a series. The first component, where the air-
needed to be at room temperature. The prototype compo-
stream entered (distal to the mouth), consisted of a porous
nents were loaded with NP 10, 20, or 30 or with the NV
polypropylene/polyethylene filter loaded with 140 l of
cartridge at the CNSCR and tested for stability before
pyruvic acid. The second component consisted of a Teflon
shipping for assembly at the study site at Christchurch
washer (11-mm OD), having one, two, or three cylindrical
Clinical Studies Trust (CCST), Christchurch, New Zealand.
openings (3.5-mm ID) that allowed air to flow through the
The NV cartridges were purchased in the United States and
washer. Depending on the nicotine dose condition, the num-
shipped to New Zealand. When assembled before use, the
ber of openings that contained a nicotine-loaded membrane
seal around the cartridge was punctured in order to allow
(60 l Nicotine base) versus empty openings were either: 0
nicotine vapor to be released. CCST staff was trained in the
loaded/3 empty (placebo), 1 loaded/1 empty (NP 10), 1
inhalation procedures by means of detailed training dia-
loaded/0 empty (NP 20), or all three loaded with nicotine
grams and instructions, as well as a site visit from CNSCR
(NP 30). These configurations were derived empirically
(by coauthor TM).
based on benchtop measurements of nicotine deliveriesfrom the system. The loading was accomplished by insert-
Puff Delivery Apparatus
ing a piece of nicotine-loaded membrane (nonwoven poly-ester membrane with an expanded PTFE membrane back-
Measured dose inhalations were delivered by an electric
ing, 6 cm long and 8 mm wide) into a 3-mm ID, 3.5-mm OD
powered motorized syringe pump and 60-ml syringe con-
and 6 cm long, thin walled, clear Teflon tube (a support to
nected to a 3-way valve. The pump was timed to draw in
hold the membranes in place), which was then inserted into
35-ml room air over 2 s and then expel the air out through
the designated openings. The ID of the tube with inserted
the aerosol delivery system for each condition and every
membrane was approximately 2 mm and 60 l of nicotine
puff. The subject inhaled through the same mouthpiece for
base was added to the membrane. The third component
all puffs and conditions. In the placebo condition, when the
(proximal to the mouth end) consisted of a 2 cm long
participant puffed on the mouthpiece, air was inhaled
segment of a charcoal cigarette filter. The first and second
through an empty tube, which contained a cigarette filter to
components were spaced 1 cm apart and the second and
provide draw resistance. Before each inhalation, while the
third components were separated by 1 cm. As air entered the
motorized syringe pump drew in air, the subject exhaled
distal end of the device, pyruvic acid vapor was entrained by
into room air. When the pump expelled air into the delivery
the airstream. Subsequently, the air stream passed through
system the subject inhaled the measured 35-ml volume
the nicotine loaded membrane and the nicotine vapor re-
through the mouthpiece, and completed the inspiration from
acted with the pyruvic acid vapor to form NP particles. As
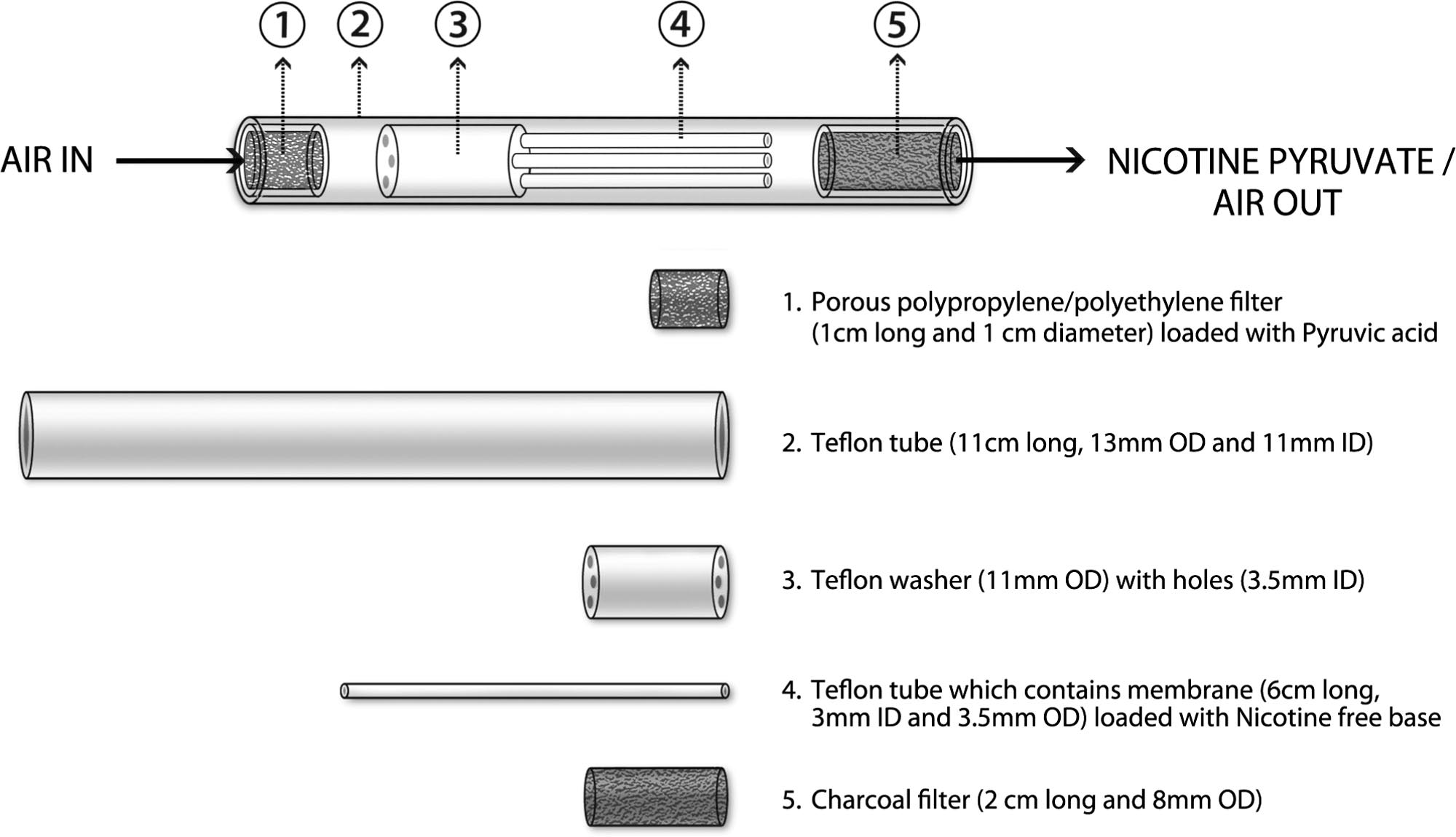
S⫽1 9/27/10 8:21 Art: 2010-0409
ROSE, TURNER, MURUGESAN, BEHM, AND LAUGESEN
Nicotine pyruvate aerosol generation system.
these particles passed through the charcoal filter, excess
blood pressure, urinary evidence of illegal drugs or excess
vapor of pyruvic acid or nicotine was adsorbed or absorbed,
breath alcohol, and recent or current use of other nicotine or
along with approximately 20% of the particles. The remain-
tobacco products.
ing particle stream exited the filter for inhalation by theresearch participant.
Study Day Procedure
In accordance with the tenets of the Declaration of Hel-
sinki, and following receipt of approval from the New
Participants were recruited from the Christchurch, New
Zealand Ministry of Health, from its Upper South B Re-
Zealand area by word of mouth advertising and from a
gional Ethics Committee and its Standing Committee on
database of volunteers. Eighteen subjects were screened by
Therapeutic Trials, informed consent was obtained from
a medical practitioner and 10 met requirements for study
nine healthy volunteers, who were admitted to the clinic
participation, after blood tests, ECG, and smoking history.
overnight, abstaining from tobacco or nicotine while at the
Nine of these were enrolled in the study. The Fagerström
clinic. On the study day, a baseline expired air carbon
test for Nicotine Dependence (FTND; Heatherton, Kozlow-
monoxide reading ⬍15 ppm confirmed abstinence from
ski, Frecker, & Fagerström, 1991) was also administered.
smoking. Volunteers were studied during a 5 1/2 hour
Subjects, male or female, age 18 to 65
morning session presenting five conditions at 50-min inter-
years of age, had to be current smokers of at least 10 regular
vals, taking 10 puffs of 35 ml after baseline measurements
brand cigarettes daily, and with an exhaled CO of 10 ppm or
at the start of each condition. Prior to these five experimen-
more, to confirm current smoker status, with pulmonary
tal conditions, a practice condition presented air inhalations.
function (KoKo Digidoser spirometer Model 323200, Pul-
Plasma nicotine responses.
In each condition, 5 ml of
monary Data Services, Louisville, CO) values Forced Ex-
blood was withdrawn 5 min before inhalation, and at 0, 1, 2,
piratory Volume ((FEV1) and Forced Expiratory Flow
5, 10, 20, and 30 min after the tenth puff. Each sample was
(FEF) 25–75) at least 75% of the normal values predicted
immediately placed on ice, centrifuged within 30 min at 4 °C
for that individual based on age, gender, and height. None
and aliquoted into separate plasma samples, stored at ⫺70 °C,
smoked menthol brands.
and later shipped on dry ice for analysis at Environmental
Pregnant women and nursing moth-
Science and Research, Porirua, New Zealand. Laboratory
ers were excluded, along with subjects with serious cardiac,
staff at ESR Porirua were blinded to the sequence of med-
respiratory, psychiatric, or other serious disease, particu-
ication codes. Plasma nicotine concentrations were quanti-
larly cardiac rhythm disorders and abnormally high or low
fied by gas chromatography coupled with mass spectropho-
S⫽1 9/27/10 8:21 Art: 2010-0409
PULMONARY DELIVERY OF NICOTINE PYRUVATE
tometer (GC/MS). Values below the limit of quantification
Because the placebo was known to contain no nicotine,
(2 ng/ml) were estimated as half of that limit (Duval &
one-tailed paired tests were used in these comparisons. As
Karlsson, 2002). For one subject, over 50% of the samples
reported below, the NP 20 and NP 30 dose conditions were
had insufficient volume, and therefore this subject was
the only doses found to be superior to placebo. Thus, in
omitted from data analysis of plasma nicotine concentra-
follow-up analyses, only these two doses were compared to
tions. Among the remaining eight subjects, four samples
the active NV condition, using two-tailed tests with Holm's
could not be assayed because of insufficient volume, and for
p value correction and ␣ ⫽ .05.
these samples nicotine concentrations were estimated by
Three outlying values were identified in the pre-to-post
linear interpolation based on values at adjacent time points.
inhalation nicotine boost: all three showed decreases in
Responses were elicited on a Lik-
nicotine concentrations after inhalation, one subject's value
ert-type scale with responses ranging from 1 to 7 (1 ⫽ not
decreasing by 6.9 ng/ml (5.6 SDs from the mean of the other
at all, 7 ⫽ extremely). The following questionnaires were
subjects) in the NP 10 condition, one decrease of 2.5 ng/ml
(3.4 SDs from the mean) in the NP 30 condition, and one
The smoking withdrawal symptom reports (9 questions
on an abbreviated version of the Shiffman-Jarvik with-
decrease of 3.3 ng/ml in the NV condition (6.2 SDs from the
drawal questionnaire (Shiffman & Jarvik, 1976) were elic-
mean). The results will be presented excluding these outliers
ited 5 min before inhalations began, and again 5 to 10 min
from the analysis of plasma nicotine values; however, a
after the last (tenth inhalation) in each condition. The an-
sensitivity analysis showed that exclusion of these values
swers were scored to give composite answers for craving
did not affect the conclusions regarding the statistical sig-
(urges to smoke, miss a cigarette, and crave a cigarette),
nificance of comparisons between conditions.
negative affect (calm vs. tense, irritable), arousal (wide
For subjective measures related to efficacy, including
awake, able to concentrate), and hunger.
satisfaction and relief of withdrawal symptoms, paired t
An inhalation evaluation questionnaire (19 questions)
tests were first conducted comparing each of the three active
was completed 5 to 10 min after the tenth inhalation, and the
NP dose conditions with the placebo control group. Two-
answers scored for ratings of satisfaction ("satisfying,"
tailed tests (with Holm's correction) were used in these
"taste good"), psychological reward ("calm you down,"
comparisons as it was not known in which direction any
"help you concentrate," "more awake," "reduce your hun-
detectable differences between NP and other conditions
ger," and "less irritable"), nausea/dizziness, enjoyment of
would be found. For example, an aversive taste conceivably
sensations in the throat and chest, and craving reduction,
would result in the NP aerosol being rated worse than
which was based on "Do (the puffs) immediately reduce
placebo. In follow-up analyses, NP doses showing superi-
your craving for cigarettes?" (Cappelleri et al., 2007) The
ority over placebo were compared to the active NV control
strength of sensations were scored separately for the
condition. For measures of tolerability (e.g., harshness/irri-
strength of puffs on tongue, in nose, back of throat, wind-
tation), only those doses of NP that showed evidence of
pipe, and chest, estimated nicotine yield and similarity to
efficacy relative to placebo (NP 20 and NP 30) were com-
the usual brand of cigarettes. Spirometry (FEV1 and FEF
pared against the NV active control condition, again using a
25–75, best of 3 readings) was repeated after all conditions
two-tailed alpha criterion of 0.05 with a Holm correction.
had been completed.
When presenting the outcomes of hypothesis testing we
will first present corrected p values, which take into account
the Holm correction (uncorrected p values will follow inparentheses). Unless explicitly noted, all p values refer to
The original analysis plan in the protocol specified a
two-tailed tests. A number of other secondary variables not
small number of a priori comparisons of interest and no
directly related to hypotheses about efficacy or tolerability
correction for multiple comparisons. However, to achieve a
(e.g., strength of respiratory tract sensations, estimated nic-
more comprehensive understanding of the results, we con-
otine yield) were tabulated for descriptive purposes (paired
ducted a more complete set of comparisons that incorpo-
t tests with uncorrected p values only are reported for these
rated a correction for multiple testing to limit Type I error.
The Holm's correction procedure for multiple testing atthe 0.05 alpha significance level was adopted, which hasbeen shown to be more powerful than the traditional Bon-
ferroni adjustment, while still providing equivalent protec-
tion against Type I error (Aickin & Gensler, 1996). To limitthe number of statistical comparisons, a series of condition-
Nine smokers (7 men, 2 women) participated in the study;
alized comparisons (Keppel, 1982) were conducted to eval-
they reported smoking an average of 13.5 cigarettes/day
uate efficacy and tolerability of the NP conditions relative to
(SD ⫽ 2.85) and had a mean FTND score of 5.2 (SD ⫽ 1.3),
the control conditions. For the analysis of plasma nicotine
indicating moderate nicotine dependence. Participants'
concentrations, the immediate increase in nicotine concen-
mean age was 27.4 years (SD ⫽ 7.99), and they reported
tration from the preinhalation baseline to the first postinha-
having smoked an average of 8.9 years (SD ⫽ 7.8). Expired
lation time point (5 min) was first compared between pla-
air carbon monoxide at the screening session averaged 20.3
cebo and each of the three active NP dose conditions.
ppm (SD ⫽ 13.4). After overnight abstinence, at the begin-
S⫽1 9/27/10 8:21 Art: 2010-0409
ROSE, TURNER, MURUGESAN, BEHM, AND LAUGESEN
ning of the experimental session, expired air CO aver-
as significantly more satisfying than placebo: for the composite
aged 7.2 ppm (SD ⫽ 2.9). Mean body weight was 78.1 kg
scale, t(8) ⫽ 3.01, p ⫽ .017 (uncorrected p ⫽ .017) for NP 10;
(SD ⫽ 21.8).
t(8) ⫽ 3.19, p ⫽ .026 (uncorrected p ⫽ .013) for NP 20;t(8) ⫽ 3.25, p ⫽ .036 (uncorrected p ⫽ .012) for NP 30.
Plasma Nicotine Results
Subsequent comparisons with the NV condition showed
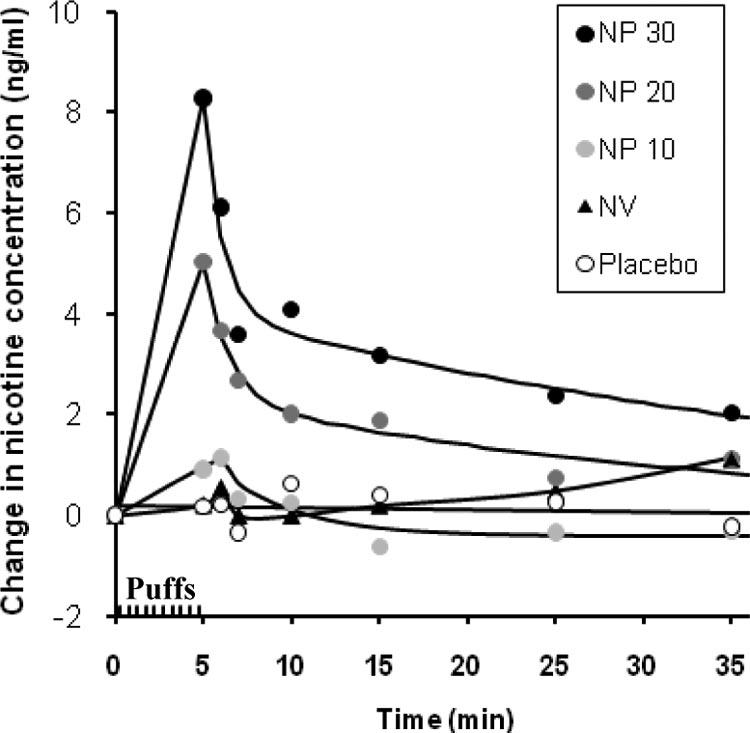
Figure 2 presents the plasma nicotine concentrations in
only nonsignificant trends for the three NP doses to be
all conditions and at all time points. Baseline-corrected
rated higher. Ratings of immediate craving reduction
values are depicted, obtained by subtracting the preinhala-
showed a significant difference between the NP 20 dose
tion baseline values from the values at each postinhalation
condition and placebo: t(8) ⫽ 3.36, p ⫽ .03 (uncorrected
time point (baseline values did not differ across conditions,
p ⫽ .01). A follow-up comparison with the NV condition
mean values ranging from 1.0 –2.7 ng/ml). As the graph
did not reach statistical significance. Comparisons be-
shows, the NP 20 and NP 30 dose conditions yielded rapid
tween active NP conditions and placebo on ratings of
increases in nicotine levels, which were apparent at the 5
psychological reward did not reach statistical signifi-
min time point, immediately after completion of the inha-
lations. Statistical comparisons confirmed that the NP 20
Although not the focus of hypotheses in the present study,
and NP 30 conditions produced higher nicotine concentra-
descriptive statistics for other subjective ratings are also
tions than placebo: For the NP 20 condition, the mean
presented in Table 1 (which lists only uncorrected p values
increase in nicotine concentration was 5.0 (SD ⫽ 3.4) ng/ml
for uniformity). Airway sensations following inhalation of
(difference from placebo ⫽ 4.8 ng/ml), t(7) ⫽ 4.85, p
NP were generally reported throughout the respiratory tract.
(one-tailed) ⫽ .007 (uncorrected p ⫽ .0035) for the com-parison versus placebo. For the NP 30 condition, the mean
Estimated nicotine content also showed higher values in the
increase in nicotine concentration was 8.3 (SD ⫽ 3.1) ng/ml
nicotine conditions relative to placebo.
(difference from placebo ⫽ 8.0 ng/ml), t(6) ⫽ 7.56, p(one-tailed) ⫽ 0.0004 (uncorrected p ⫽ .00015) for the
comparison versus placebo. Follow-up comparisons alsoshowed that the NP 20 and NP 30 dose conditions produced
Table 1 (bottom) also displays the change in the craving
significantly higher plasma peak nicotine concentrations
rating scale of the withdrawal questionnaire from pre- to
than the active NV control condition: for the NP 20 condi-
postinhalations. The NP 20 dose condition showed a
tion, the mean difference from NV in nicotine concentra-
marked reduction in craving relative to placebo: t(8) ⫽ 3.51,
tions was 4.7 ng/ml, t(6) ⫽ 3.31, p ⫽ .016 (uncorrected p ⫽
p ⫽ .024 (uncorrected p ⫽ .008). The NP 10 condition also
.016); for the NP 30 condition, the mean difference from
showed a significant difference from placebo: t(8) ⫽ 2.89,
NV was 6.6 ng/ml, t(5) ⫽ 5.94, p ⫽ .004 (uncorrected p ⫽
p ⫽ .04 (uncorrected p ⫽ .02). The comparisons with the
NV condition, however, did not reach significance. Other
Puff Ratings
withdrawal symptoms scales showed no significant effectsof condition.
Table 1 depicts the subjective ratings of the rewarding
effects of inhalations. All three NP dose conditions were rated
There were no significant adverse reactions to any of the
conditions. Of the nine subjects, one had headache afterexposure to the NP 10 condition; one other subject hadheadache and one subject reported loose stool, but theseinstances preceded exposure to any of the inhalation con-ditions. Out of the 45 sets of 10 inhalations, mild coughoccurred during 19 of the exposures: 15 coughs (among 5subjects) in the NP 10 condition, 5 coughs (2 subjects) in theNP 20 condition, 12 coughs (6 subjects) in the NP 30condition, 8 coughs (5 subjects) in the NV condition, and 1cough (1 subject) after the room air placebo.
Based on assessments of efficacy described above (e.g.,
increases in plasma nicotine), the NP 20 and NP 30 doseconditions were the only conditions demonstrating superi-ority over the NV control condition. Therefore, a statisticalcomparison of harshness/irritation ratings was conductedbetween each of the two NP conditions and NV, which
Change in plasma nicotine concentration over time
showed the NP 20 condition to be significantly less harsh/
relative to preinhalation baseline for the five experimental condi-tions. After baseline, the first measurement was 5 min after the first
irritating than the NV condition: t(8) ⫽ 3.09, p ⫽ .03
puff, at the end of the tenth puff.
(uncorrected p ⫽ .015).
S⫽1 9/27/10 8:21 Art: 2010-0409
PULMONARY DELIVERY OF NICOTINE PYRUVATE
Table 1Subjective Evaluation of Puffs on 7-Point Scale, and p Values (Uncorrected) for Comparisons With Placebo andNicotine Vapor Inhaler Cartridge (NV)
Did you enjoy the sensations in your mouth and throat?
p value vs. placebo
p value vs. NV
Did they immediately reduce your craving for cigarettes?
p value vs. placebo
p value vs. NV
How high in nicotine?
p value vs. placebo
p value vs. NV
How similar to your cigarette?
p value vs. placebo
p value vs. NV
How harsh or irritating?
p value vs. placebo
p value vs. NV
How harsh or irritating on tongue?
p value vs. placebo
p value vs. NV
How harsh or irritating in nose?
p value vs. placebo
p value vs. NV
How harsh or irritating back of mouth and throat?
p value vs. placebo
p value vs. NV
How harsh or irritating in windpipe?
p value vs. placebo
p value vs. NV
How harsh or irritating in chest?
p value vs. placebo
p value vs. NV
Nausea and dizziness
p value vs. placebo
p value vs. NV
Psychological reward
p value vs. placebo
p value vs. NV
p value vs. placebo
p value vs. NV
Change in craving
⫺0.44 (0.87) ⫺0.85 (1.03) ⫺0.29 (0.84) ⫺0.59 (0.86) 0.41 (0.80)
p value vs. placebo
p value vs. NV
Unless indicated, values are M (SD). NP 10 ⫽ 10 g/puff of nicotine pyruvate; NP 20 ⫽ 20 g/puff of nicotine pyruvate; NP
30 ⫽ 30 g/puff of nicotine pyruvate.
Indices of pulmonary function showed no significant
The findings confirm that NP inhalation at the NP 20
changes from the beginning of the experimental session to
and NP 30 dose conditions produced rapid increases in
the end (Table 2).
plasma nicotine concentrations assessed after inhalationsended, relative to the placebo and active nicotine vapor
control conditions. Although blood samples were not
Spirometry Results on Study Day (Best of 3 Readings)
collected during the 10 inhalations of each condition, the
FEF 25 75 (liters/s)
first postinhalation sample showed that blood levels hadalready reached significant levels, averaging over 8 ng/ml
Before inhalations
3.87 (3.2–4.32)
3.89 (2.70–5.65)
in the NP 30 condition, indicative of rapid pulmonary
After inhalations
3.92 (3.23–4.36)
3.98 (2.95–5.5)
1.01 (0.97–1.03)
1.03 (0.90–1.13)
absorption of nicotine. This result is consistent withparticle size measurements showing that the mass median
Values are mean (range). FEV1 ⫽ forced expiratory vol-
ume in one second; FEF ⫽ forced expiratory flow.
aerodynamic diameter is approximately 0.6 m (unpub-
S⫽1 9/27/10 8:21 Art: 2010-0409
ROSE, TURNER, MURUGESAN, BEHM, AND LAUGESEN
lished data), comparable to that of cigarette smoke
plicitly rated, enjoyment of airway sensations received the
(Hinds, 1978), and readily able to reach the alveoli of the
lowest possible median rating of 1 on a 7-point scale
(range ⫽ 1–2) versus a median value of 3 (range ⫽ 1–5) for
The absence of detectable peak nicotine concentrations in
the NP 20 condition, suggesting greater enjoyment of the
the NV and NP 10 dose conditions is not surprising in view
NP 20 condition. The present method is also advantageous
of the low total dose of nicotine (0.1 mg) delivered in these
in not requiring propellants or cumbersome spacer devices.
two conditions. In contrast, a typical cigarette dose of ap-
Although the technology evaluated in this study was not
proximately 1 mg nicotine typically produces venous
presented as a commercial product, in principle the tech-
plasma nicotine peaks of 10 to 15 ng/ml (Benowitz, Porchet,
nology could be incorporated into a device that can provide
& Jacob, 1990). At roughly one tenth of this dose, the NV
a smoker with a supply of nicotine, conveniently accessible
and NP 10 dose conditions would only have yielded peaks
on demand. One can envision two main applications of this
of ⬍2 ng/ml, close to the detection threshold of the assay
type of lung delivery nicotine inhalation system. First, this
method used. Our results thus confirmed the prediction that
technology may have promise as an adjunct to smoking
a nicotine aerosol delivery system can deliver a higher dose
cessation treatment. By providing rapid nicotine delivery
than a vapor delivery system.
along with many of the rewarding effects of nicotine inhaled
Subjective ratings suggested that all doses of NP were
in cigarette smoke, a lung delivery nicotine inhaler could
moderately satisfying to smokers, yielding higher ratings of
prove more effective than current NRT in weaning smokers
satisfaction than placebo. There were trends for ratings to
off of cigarettes. In this application, the ultimate goal would
exceed those of the NV condition, but these did not reach
be to wean smokers gradually off of nicotine altogether.
statistical significance. Craving reduction, assessed by the
While inhaled nicotine is likely to be more addictive than some
prepost puffing change in the craving scale of the Shiffman-
forms of NRT (e.g., patch), it may nonetheless prove to be less
Jarvik questionnaire, was greater for the NP 10 and NP 20
difficult to relinquish than cigarettes, for the following reasons:
conditions than for placebo. The NP 20 condition was also
(1) it would not deliver many of the nonnicotine compounds
rated higher than placebo for ratings of how much puffs
that are contained in cigarette smoke, such as monoamine
"immediately reduced your craving." However, none of the
oxidase inhibitors (Fowler et al., 1996a, 1996b) and acetalde-
NP conditions was significantly different from the NV con-
hyde, which are thought to potentiate the addictive properties
dition. With respect to harshness/irritation, NP at the 20
of nicotine (Cao et al., 2007); (2) the dose could be set to a
g/puff dose was rated significantly less irritating than the
range lower than that obtained from most cigarette brands,
nicotine vapor condition; this finding was likely to be the
and the inhaler could be used to supplement slow-acting
result of the mildly acidic pH of the NP particles along with
forms of NRT. In this scenario, the inhaler would be used as
their small aerodynamic size, resulting in dispersion of
a "rescue" or relapse prevention treatment to address break-
particles over the large surface area of the lung.
through craving occurring during use of standard slower-
Thus, tolerability of the NP aerosol was excellent, and
onset NRT products such as patch, gum or lozenge; and (3)
there were no significant adverse events. Pulmonary func-
the sensory qualities might be engineered to be less appeal-
tion measurements also showed no changes from the begin-
ing than cigarettes, while still sufficiently acceptable to
ning to the end of the session, supporting the acute safety of
promote efficacy.
NP inhalation at the doses used in this study. Future studies
A second application of this nicotine inhalation technol-
will be needed to assess the safety and tolerability of ex-
ogy could be for long term nicotine replacement, to be used
tended use of a NP delivery system, and to evaluate its
by smokers who would otherwise relapse to smoking; this
usefulness in promoting abstinence from cigarettes smok-
approach would be analogous to methadone maintenance,
which has been demonstrated to be an effective treatment of
The results of the present study may also be compared
heroin addiction (Strain, Stitzer, Liebson, & Bigelow,
with those of a recently published pilot study of a metered
1994). In this harm reduction scenario, ex-smokers would
dose inhaler system (Caldwell et al., 2009). In that study,
continue to receive the perceived benefits of nicotine while
smokers were asked to inhale 10 "puffs" from a metered
minimizing the risk of disease from combustion and pyrol-
dose inhaler delivering nicotine in an ethanol/hydrofluroal-
ysis products, including nitrosamines, polycyclic aromatic
kane propellant, using a spacer device attached to facilitate
hydrocarbons, carbon monoxide and numerous other toxic
pulmonary deposition. Two dose conditions, 50 g/puff and
substances contained in tobacco smoke. Accumulating evi-
100 g/puff, were tested, and peak plasma nicotine concen-
dence suggests that some smokers may be using cigarettes
trations averaged 12.5 ng/ml and 9.4 ng/ml, respectively. In
to self medicate a variety of psychiatric disorders, including
contrast to the present study results, there was a slight delay
depression, anxiety, attention-deficit/hyperactivity disorder
of approximately 5 min after inhalations until peak levels
(ADHD) and schizophrenia. A major challenge for future
were reached. Inhalations were "reasonably well tolerated,"
research will be to identify smokers who are good candi-
although one of 10 subjects could not complete the high
dates for long term nicotine maintenance. For these smok-
dose condition because of coughing. Ratings of satisfaction,
ers, long term use of nicotine replacement may indeed be
craving and several other subjective responses were com-
warranted if they will not otherwise quit smoking. If the
parable to those reported after NP inhalation. However,
nicotine inhalation technology evaluated in this study con-
initial inhalations from the metered dose inhaler often trig-
tinues to prove more acceptable and efficacious than current
gered coughing, and while harshness/irritation was not ex-
forms of NRT, it could have enormous potential for improv-
S⫽1 9/27/10 8:21 Art: 2010-0409
PULMONARY DELIVERY OF NICOTINE PYRUVATE
ing public health. The potential for reducing cigarette re-
Duval, V., & Karlsson, M. O. (2002). Impact of omission or
lated harm was noted by Sumner (2003), who stated: "Even
replacement of data below the limit of quantification on param-
if used very broadly, clean inhaled nicotine might reduce
eter estimates in a two-compartment model. Pharmaceutical
public health problems as much as a very successful tobacco
Research, 19, 1835–1840.
Ezzati, M., & Lopez, A. D. (2003). Estimates of global mortality
control programme."
attributable to smoking in 2000. Lancet, 362, 847– 852.
This study had a number of strengths as well as limita-
Fant, R. V., Buchhalter, A. R., Buchman, A. C., & Henningfield,
tions. Strengths included the repeated measures design
J. E. (2009). Pharmacotherapy for tobacco dependence. Hand-
(which provided greater sensitivity than a between-groups
book of Experimental Pharmacology, 487–510.
comparison), subject and experimenter blinding, and precise
Feyerabend, C., Ings, R. M., & Russell, M. A. (1985). Nicotine
control over nicotine dosing using machine-controlled puff
pharmacokinetics and its application to intake from smoking.
volume and timed breath hold. Additional strengths in-
British Journal of Clinical Pharmacology, 19, 239 –247.
cluded the measurement of both pharmacokinetic and sub-
Fiore, M. C., Jaen, C. R., Baker, T. B., Bailey, W. C., Bennett, G.,
jective responses, as well as the use of an active control
Benowitz, N. L., . . Williams, C. (2008). Clinical practice
condition (NV) in addition to the placebo control. Limita-
guideline: Treating tobacco use and dependence: 2008 update.
tions included the small sample size and short duration of
Rockville, MD: Department of Health and Human Services.
Fowler, J. S., Volkow, N. D., Wang, G.-J., Pappas, N., Logan, J.,
exposure. In addition, blood sampling began after the com-
MacGregor, R. R., . . Cliento, R. (1996a). Inhibition of mono-
pletion of inhalations, limiting the precision with which the
amine oxidase B in the brains of smokers. Nature, 379, 732–
rate of rise in plasma nicotine concentrations could be
Fowler, J. S., Volkow, N. D., Wang, G.-J., Pappas, N., Logan, J.,
In summary, the results of this study indicate that the
Shea, C., . . Wolf, A. P. (1996b). Brain monoamine oxidase A
novel technology employed to generate nicotine containing
inhibition in cigarette smokers. Proceedings of the National
particles for inhalation has promise as a potentially more
Academy of Sciences, USA, 93, 14065–14069.
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerström,
effective form of nicotine replacement. The pharmacoki-
K. L. (1991). The Fagerström test for nicotine dependence: A
netic and subjective data demonstrated that this technology
revision of the Fagerström tolerance questionnaire. British Jour-
can be used to administer nicotine by the pulmonary route
nal of Addiction, 86, 1119 –1127.
for rapid absorption, coupled with acceptable sensory qual-
Hinds, W. C. (1978). Size characteristics of cigarette smoke.
ities, to provide subjective satisfaction and relief of craving.
American Industrial Hygiene Association Journal, 44, 720 –726.
Further controlled trials of this technology are warranted to
Huchon, G. (1990). Aerosol deposition in the alveolar space. Lung,
fully assess its safety and efficacy in aiding smokers to
168(Suppl.), 672– 676.
Hughes, J. R., Shiffman, S., Callas, P., & Zhang, J. (2003). A
relinquish cigarettes and thereby avoid the deadly diseases
meta-analysis of the efficacy of over-the-counter nicotine re-
caused by smoking.
placement. Tobacco Control, 12, 21–27.
Katz, I. M., Schroeter, J. D., & Martonen, T. B. (2001). Factors
affecting the deposition of aerosolized insulin. Diabetes Tech-
nology and Therapeutics, 3, 387–397.
Kenfield, S. A., Stampfer, M. J., Rosner, B. A., & Colditz, G. A.
Aickin, M., & Gensler, H. (1996). Adjusting for multiple testing
(2008). Smoking and smoking cessation in relation to mortality
when reporting research results: The Bonferroni vs Holm meth-
in women. Journal of the American Medical Association, 299,
ods. American Journal of Public Health, 86, 726 –728.
Armitage, A. K., & Turner, D. M. (1970). Absorption of nicotine
Keppel, G. (1982). Design and analysis: A researcher's handbook
in cigarette and cigar smoke through the oral mucosa. Nature,
(2nd ed.). Englewood Cliffs, NJ: Prentice Hall, Inc.
Landon, J., Fawcett, J. K., & Wynn, V. (1962). Blood pyruvate
Benowitz, N. L., Porchet, H., & Jacob, P. I. (1990). Pharmacoki-
concentration measured by a specific method in control subjects.
netics, metabolism, and pharmacodynamics of nicotine. In S.
Journal of Clinical Pathology, 15, 579 –584.
Wonnacott, M. A. H. Russell, & I. P. Stolerman (Eds.), Nicotine
Lee, L.-Y., Gerhardstein, D. C., Wang, A. L., & Burki, N. K.
psychopharmacology (pp. 112–157). Oxford: Oxford University
(1993). Nicotine is responsible for the airway irritation evoked
by cigarette smoke inhalation in men. Journal of Applied Phys-
Caldwell, B., Dickson, S., Burgess, C., Siebers, R., Mala, S., Parkes,
iology, 75, 1955–1961.
A., & Crane, J. (2009). A pilot study of nicotine delivery to
Lunell, E., Bergstrom, M., Antoni, G., Langstrom, B., & Nordberg,
smokers from a metered-dose inhaler. Nicotine Tobacco Research,
A. (1996). Nicotine deposition and body distribution from a
11(4), 342–347. doi: Ntp027 [pii] 10.1093/ntr/ntp027
nicotine inhaler and a cigarette studied with positron emission
Cao, J., Belluzzi, J. D., Loughlin, S. E., Keyler, D. E., Pentel, P. R.,
tomography. Clinical Pharmacology and Therapeutics, 59,
& Leslie, F. M. (2007). Acetaldehyde, a major constituent of
593–594. doi: S0009-9236(96)90188 –5 [pii] 10.1016/S0009-
tobacco smoke, enhances behavioral, endocrine, and neuronal
9236(96)90188 –5
responses to nicotine in adolescent and adult rats. Neuropsycho-
Lunell, E., Molander, L., Ekberg, K., & Wahren, J. (2000). Site of
pharmacology, 32(9), 2025–2035. doi: 1301327 [pii] 10.1038/
nicotine absorption from a vapour inhaler– comparison with
cigarette smoking. European Journal of Clinical Pharmacol-
Cappelleri, J. C., Bushmakin, A. G., Baker, C. L., Merikle, E.,
ogy, 55, 737–741. doi: 00550737.228 [pii]
Olufade, A., & Gilbert, D. G. (2007). Confirmatory factor anal-
Lux, J. E., & Frecker, R. C. (1988). Generation of a submicrometre
yses and reliability of the modified cigarette evaluation ques-
nicotine aerosol for inhalation. Medical and Biological Engi-
tionnaire. Addictive Behaviors, 32, 912–923.
neering and Computing, 26, 232–234.
S⫽1 9/27/10 8:21 Art: 2010-0409
ROSE, TURNER, MURUGESAN, BEHM, AND LAUGESEN
Papathanasiou, A., Milionis, H., Toumpoulis, I., Kalantzi, K.,
predicts successful smoking cessation with transdermal nico-
Katsouras, C., Pappas, K., . . Goudevenos, J. (2007). Smoking
tine: A validation study. Pharmacology Biochemistry and Be-
cessation is associated with reduced long-term mortality and the
havior, 92, 6 –11. doi: S0091–3057(08)00345– 6 [pii] 10.1016/
need for repeat interventions after coronary artery bypass graft-
ing. European Journal of Cardiovascular Prevention and Re-
Shiffman, S. M., & Jarvik, M. E. (1976). Smoking withdrawal
habilitation, 14, 448 – 450.
symptoms in two weeks of abstinence. Psychopharmacol-
Rose, J. E. (1988). The role of upper airway stimulation in smok-
ogy, 50, 35–39.
ing. In O. F. Pomerleau & C. S. Pomerleau (Eds.), Nicotine
Smoking-attributable mortality, years of potential life lost, and
replacement: A critical evaluation (Vol. 261, pp. 95–106). New
productivity losses–United States, 2000 –2004. (2008). Morbid-
York: Alan R. Liss, Inc.
ity and Mortality Weekly Report, 57, 1226 –1228.
Rose, J. E., Behm, F. M., Westman, E. C., Bates, J. E., & Salley,
Strain, E. C., Stitzer, M. L., Liebson, I. A., & Bigelow, G. E.
A. (2003). Pharmacologic and sensorimotor components of sa-
(1994). Comparison of buprenorphine and methadone in the
tiation in cigarette smoking. Pharmacology Biochemistry and
treatment of opioid dependence. American Journal of Psychia-
Behavior, 76, 243–250. doi: S0091305703002491 [pii]
try, 151, 1025–1030.
Rose, J. E., Behm, F. M., Westman, E. C., & Johnson, M. (2000).
Sumner, W., 2nd. (2003). Estimating the health consequences of
Dissociating nicotine and non-nicotine components of cigarette
replacing cigarettes with nicotine inhalers. Tobacco Control, 12,
smoking. Pharmacology Biochemistry and Behavior, 67, 71– 81.
Rose, J. E., & Hickman, C. S. (1987). Citric acid aerosol as a
Usmani, O. S., Biddiscombe, M. F., & Barnes, P. J. (2005).
potential smoking cessation aid. Chest, 92, 1005–1008.
Regional lung deposition and bronchodilator response as a func-
Rose, J. E., Rose, S. D., Turner, J. E., & Murugesan, T. (2008).
tion of beta2-agonist particle size. American Journal of Respi-
U.S. Patent Application No. 20080241255. Washington, DC:
ratory and Critical Care Medicine, 172, 1497–1504. doi:
U.S. Patent and Trademark Office.
200410-1414OC [pii] 10.1164/rccm.200410-1414OC
Rose, J. E., Tashkin, D. P., Ertle, A., Zinser, M. C., & Lafer, R.
Votto, J. J., Bowen, J. B., Barton, R. W., & Thrall, R. S. (2008).
(1985). Sensory blockade of smoking satisfaction. Pharmacol-
Inhaled sodium pyruvate improved FEV1 and decreased expired
ogy Biochemistry and Behavior, 23, 289 –293.
breath levels of nitric oxide in patients with chronic obstructive
Rose, J. E., Westman, E. C., Behm, F. M., Johnson, M. P., &
pulmonary disease. Journal of Aerosol Medicine and Pulmonary
Goldberg, J. S. (1999). Blockade of smoking satisfaction using
Drug Delivery, 21, 329 –334. doi: 10.1089/jamp.2007.0678
the peripheral nicotinic antagonist trimethaphan. Pharmacology
Westman, E. C., Behm, F. M., & Rose, J. E. (1995). Airway
Biochemistry and Behavior, 62, 165–172.
sensory replacement combined with nicotine replacement for
Schneider, N. G., Olmstead, R. E., Franzon, M. A., & Lunell, E.
smoking cessation: A randomized, placebo controlled trial using
(2001). The nicotine inhaler: Clinical pharmacokinetics and
a citric acid inhaler. Chest, 107, 1358 –1364.
comparison with other nicotine treatments. Clinical Pharmaco-kinetics, 40, 661– 684.
Received March 7, 2010
Schnoll, R. A., Patterson, F., Wileyto, E. P., Tyndale, R. F.,
Revision received June 23, 2010
Benowitz, N. L., & Lerman, C. (2009). Nicotine metabolic rate
Accepted June 23, 2010 䡲
Online First Publication
APA-published journal articles are now available Online First in the PsycARTICLES database.
Electronic versions of journal articles will be accessible prior to the print publication, expeditingaccess to the latest peer-reviewed research.
All PsycARTICLES institutional customers, individual APA PsycNET威 database package sub-
scribers, and individual journal subscribers may now search these records as an added benefit.
Online First Publication (OFP) records can be released within as little as 30 days of acceptance andtransfer into production, and are marked to indicate the posting status, allowing researchers toquickly and easily discover the latest literature. OFP articles will be the version of record; thearticles have gone through the full production cycle except for assignment to an issue andpagination. After a journal issue's print publication, OFP records will be replaced with the finalpublished article to reflect the final status and bibliographic information.
Source: http://www.healthnz.co.nz/NicotinePyruvate_2010.pdf
Richmond upon Thames LGBT Forum Appendix 3 - Drug Misuse and the LGBT Community Submission to the Richmond upon Thames Drug Misuse Scrutiny Task Group – 31 January 2011 Introduction The Richmond upon Thames Lesbian, Gay, Bisexual and Trans (LGBT) Forum is a voluntary community group providing a voice for LGBT people who live, work, study or visit in the borough. We were established in 2007 with support from the Community Safety Partnership. We now operate as an independent community group. We work collaboratively with statutory service providers such as Richmond upon Thames Council, Metropolitan Police Service, NHS Richmond and a wide range of voluntary and community groups. Our interests include health, community safety, domestic abuse, housing, homelessness, older people, youth and education. We actively participate in many local community engagement and strategic groups and engage with specialist LGBT organisations across London and nationally. This submission has been prepared at quite short notice and is based upon member's knowledge, discussion with community members and service providers and review of published research.
for considering FARMERS® kitchens, bathrooms and furniture. Our children's future is important to us, The sun rises over the famous beaches We are extremely proud of our award That's why we source our timber from sustainable plantation forest owned and operated in Australia. of the Gold Coast, Australia. winning range and look forward to hand finishing one of our unique themes for you.









