2004 hormones role of ipgs in mediating is & hyperandrogenism in pcos
HORMONES 2004, 3(4):244-251
A Paradox: The Roles of Inositolphosphoglycans in mediating insulin
sensitivity and Hyperandrogenism in the Polycystic Ovary Syndrome
Kai I. Cheang1, Paulina Essah2, John E. Nestler2,3
1Ph.D., Department of Pharmacy, 2M.D., Department of Internal Medicine, 3M.D., Department ofObstetrics and Gynecology, Medical College of Virginia Campus, Virginia CommonwealthUniversity, Richmond, Virginia
Supported in part by NIH grants to J.E.N.: RO1HD35629 and K24HD40237
Keywords
Inositolphosphoglycans, D-chiro-inositol, Myo-inositol, Polycystic ovary syndrome, Insulin
resistance
Current evidence suggests that insulin resistance contributes to the pathophysiologic features of thepolycystic ovary syndrome (PCOS). The observation that insulin resistance is a central feature ofPCOS leads to studies of the insulin signaling system in this disorder. This article reviews thepossible role of putative insulin mediators, inositolphosphoglycans (IPG) mediators, in PCOS. Wewill focus on how IPGs mediate (a) hyperandrogenism and (b) insulin sensitivity in this disorder.
INSULIN RESISTANCE AND THE PATHOGENESIS OF PCOS
PCOS affects approximately 6-10% of women of reproductive age, and is characterized by chronicanovulation and hyperandrogenism. Insulin resistance, with its compensatory hyperinsulinemia, is avirtually universal feature of PCOS1-3. There is a form of insulin resistance that is intrinsic to the disorderand is present even in lean women with PCOS3. In addition, women with PCOS have an addedcomponent of insulin resistance from adiposity. About 50% to 80% of women with PCOS areobese, and obesity itself induces insulin resistance and hyperinsulinemia4.
Insulin resistance, along with its concomitant hyperinsulinemia, is critical in the pathogenesis ofhyperandrogenism in PCOS. In vitro evidence shows that in isolated thecal cells from PCOSwomen, insulin stimulates ovarian testosterone production5. In vivo evidence also supports the roleof hyperinsulinemia in the elevated circulating levels of testosterone in PCOS. Suppression ofinsulin release by diazoxide decreases both serum total and free testosterone levels in women withPCOS. In addition, when insulin-sensitizing agents such as metformin6-8, troglitazone9,10, and morerecently, rosiglitazone11,12, are administered to women with PCOS, serum testosteroneconcentrations decrease together with serum insulin concentrations. Besides playing a role in theanovulatory infertility of PCOS, insulin resistance in PCOS is associated with an increased risk ofhypertension13,14, dyslipidemia15-18, type 2 diabetes19-23, metabolic syndrome24, andcardiovascular disease25-29.
The above observations suggest that the hyperinsulinemia of PCOS plays an important role in thehyperandrogenism of the disorder. However, these observations also suggest a contradictory tenet tothe insulin resistance hypothesis in PCOS: while PCOS women are "insulin resistant" in terms ofglucose disposal, their ovarian tissues remain sensitive to insulin's stimulation in terms oftestosterone biosynthesis. In an attempt to provide a unifying hypothesis for these phenomena, wepropose that ovarian androgen biosynthesis and glucose homeostasis in PCOS may be mediated viaa signaling system for insulin that is separate from the classical tyrosine phosphate signalingmechanism.
THE INOSITOLPHOSPHOGLYCAN SIGNALING SYSTEM
Some actions of insulin may involve low molecular weight inositolphosphoglycan (IPG) mediators,also known as putative insulin mediators or second messengers30-32. When insulin associates withits receptor, mediators of this class are generated by hydrolysis of glycosylphosphatidylinositollipids and/or proteinated species located at the outer leaflet of the cell membrane. Releasedmediators are then internalized and alter the activities of purified enzymes33,34 and the metabolismof intact cells32,35 in an insulin mimetic fashion. Two different IPG mediators have been extractedfrom rat liver36. One of these is the D-chiro-inositolphosphoglycan mediator (DCI-IPG) whichactivates pyruvate dehydrogenase (PDH) phosphatase. The other IPG mediator is the myo-inositolphosphoglycan mediator (MYO-IPG) which inhibits cyclic AMP-dependent protein kinase(PKA). These IPG mediators may mediate the alternate insulin signaling mechanism.
IPGS AND OVARIAN ANDROGEN BIOSYNTHESIS IN PCOS
Over a decade ago, experiments from our laboratory examined whether IPGs mediate insulin'ssteroidogenic actions in human placental tissue. In studies using placental cytotrophoblasts isolatedfrom human placentae, purified liver extracts containing either the MYO-IPG or the DCI-IPGmimicked insulin's inhibitory action on aromatase37 and stimulatory action on 3â-hydroxysteroiddehydrogenase38 in a concentration dependent fashion39. In this system, it was also shown thatMYO-IPG may be more potent than DCI-IPG39. Preincubation of the cytotrophoblasts with apolyclonal antibody that blocked IPGs completely abolished insulin's ability to either inhibitaromatase or stimulate 3b-hydroxysteroid dehydrogenase activity. These studies suggested that theIPG system may be responsible for transducing insulin's regulation of human placentalsteroidogenesis.
Similarly, Romero et al. demonstrated that IPG mediators mimicked insulin's stimulation ofprogesterone production by swine granulosa cells40. In addition, insulin's action on progesteronesynthesis was abolished when the swine cells were preincubated with an antibody that blocked theIPG mediators40. These observations suggested that IPGs mediate insulin's steroidogenic actions inswine granulosa cells.
In order to assess specifically in PCOS the mediation of insulin stimulated steroidogenic action byIPGs, our laboratory and collaborators in Caracas, Venezuela, obtained ovarian tissue from PCOSwomen undergoing gynecologic surgery41. Thecal cells, the cells responsible for ovariantestosterone biosynthesis, were isolated and cultured. Upon exposure to insulin, testosteronebiosynthesis increased 10- to 12- fold in these primary thecal cultures. Experiments were alsoperformed using a putative chiro-IPG mediator, named "INS-2" (provided by InsmedPharmaceuticals). INS-2 is a synthetic pseudo-disaccharide of pinitol (3-O-methyl D-chiro-inositol)
and galactosamine that is believed to be closely related to the pH 2.0 DCI-IPG putative insulinmediator described by Larner and colleagues41,43. Exposure to the INS-2 mediator led to thecaltestosterone biosynthesis in a dose-dependent fashion. In addition, the INS-2 mediator inmicromolar concentrations stimulated thecal testosterone biosynthesis to a degree at least equal toand sometimes greater than that of insulin in maximal concentrations. These observations suggestedthat the synthetic INS-2 mediator could mimic insulin's action in stimulating thecal testosteronebiosynthesis.
To confirm that insulin stimulated testosterone biosynthesis via the IPG signal transduction system,PCOS thecal cells were preincubated with the polyclonal anti-inositol antibody A23939.
Preincubation with A23939 completely inhibited insulin's ability to stimulate testosteronebiosynthesis. These results were confirmed by using a second anti-inositolglycan antibody, aIPG,that had been shown to block the action of inositolglycan mediators44. Preincubation with aIPGsimilarly abolished the ability of insulin to stimulate thecal testosterone biosynthesis. The inhibitoryeffect of the anti-inositol antibodies was specific for insulin, since preincubation with theseblocking antibodies did not affect stimulation of testosterone biosynthesis by hCG.
The results of these studies demonstrated that a synthetic DCI-IPG (INS-2) mimicked insulin'sstimulation of thecal testosterone biosynthesis in a dose-responsive manner and to a degree at leastequal to that of insulin. These studies also suggested that IPG mediators may serve as the signaltransduction system for insulin's stimulation of human thecal testosterone biosynthesis. It isimportant to note, however, that these studies were not designed to identify which of the many IPGswas primarily responsible for mediating insulin's action on steroidogenesis, but as reportedpreviously, the MYO-IPG was more potent in human placental steroidogenesis than DCI-IPG.
IMPROVEMENT OF INSULIN SENSITIVITY IN PCOS BY D-CHIRO-INOSITOL
Besides IPGs' role in transducing androgen production in PCOS, available data suggest that the IPGsystem also mediates glucose metabolism. Among the different species of IPG mediators that havebeen identified, several lines of evidence support the hypothesis that a deficiency specifically ofDCI-IPG may result in insulin resistance. Although several studies have linked insulin resistance (asdetermined by the hyperinsulinemic-euglycemic clamp technique) with decreased urinary excretionof total DCI45-48, large urinary losses of DCI have been shown in both type 1 and type 2 diabetesusing a more sensitive gas chromatography/mass-spectroscopy technology49. This urinary loss ofDCI was out of proportion to that expected in diabetic glycosuria. Furthermore, significantlydecreased muscle DCI-IPG bioactivity and decreased total DCI content have been noted in needlebiopsies48 and autopsy specimens50 from type 2 diabetic subjects compared with controls. In astudy of rats, intragastric administration of DCI significantly decreased hyperglycemia in low-dosestreptozocin rats and improved glucose tolerance in normal rats51. Administered intravenously tomonkeys with varying degrees of insulin resistance, DCI accelerated glucose disposal and increasedthe rate of decrease of plasma insulin51. These observations suggested that a deficiency in DCI-IPGmay lead to insulin resistance, and that exogenous administration of DCI may enhance insulinsensitivity and improve insulin action in insulin-resistant individuals.
To determine if DCI-IPG is deficient specifically in women with PCOS, a preliminary study wasconducted to compare the total DCI content in urine and blood of women with PCOS to that of age-and weight-matched normal women, and to determine if this difference may be accompanied by (a)decreased release of DCI-IPG (as determined by bioactivity) in blood of women with PCOS duringan oral glucose challenge and (b) decreased whole-body insulin sensitivity52.
As reported in preliminary form, urinary DCI clearance was 5-fold higher in PCOS as comparedwith normal women (p=0.006). Urinary DCI clearance was also correlated inversely with insulinsensitivity as defined by the Bergman minimal model (p=0.0003). Furthermore, DCI-IPG releasewas impaired in response to insulin release in women with PCOS upon oral glucose challenge, asshown by a 3-fold AUC
decrease in PCOS (p<0.0001)52.
These results confirmed a functional deficiency of DCI in women with PCOS, and suggested thatDCI deficiency may be related to increased urinary DCI clearance and may contribute to insulinresistance of the syndrome. These data also corroborated a previous study showing that urinary DCIexcretion was increased in insulin resistant states49. However, these data did not rule out theexistence of either an intra-tissue deficiency of DCI, or a defect in incorporating DCI into the DCI-IPG mediator.
If a functional deficiency of this specific IPG mediator, DCI-IPG, is responsible for insulinresistance in PCOS, then administration of DCI might replenish DCI-IPG stores and improveinsulin sensitivity. To test this hypothesis, 44 obese women (BMI >28 kg/m2) with PCOS weregiven DCI 1200 mg orally or placebo once daily for six to eight weeks53. Fasting serum steroidswere measured and oral glucose tolerance tests were performed before and after the treatmentperiod. Serum progesterone was obtained weekly to monitor for ovulation.
In the women given DCI (n=22), the mean AUC
(±SD) curve after oral glucose challenge
decreased from 13417±2467 to 5158±1431 µU/ml/min (P= 0.007)53. In women who demonstratedimpaired glucose tolerance at baseline, AUC
during the OGTT decreased from 13983±3625
to 10945±2410 mg/dl/min (P=0.03), resulting in normal glucose tolerance curves in the six womengiven DCI, but no change in the women given placebo. Diastolic (P<0.001) and systolic (P=0.05)blood pressures decreased by 4 mmHg, and plasma triglyceride concentrations decreased from184±88 to 110±61 mg/dl (P=0.002 compared to the placebo group). These parameters did notchange substantially in the placebo group. Moreover, ovulation occurred in 19 of the 22 women(86%) who received DCI, but only in 6 of the 22 women (27%) in the placebo group (P<0.001).
Serum free testosterone in all women treated with DCI decreased by 53% from 1.1±0.8 to 0.5±0.5ng/dl and did not change substantially in the placebo group (p=0.006 for the comparison).
The above study showed that administration of DCI enhanced insulin action in PCOS, therebyimproving ovulation and decreasing serum androgen concentrations. DCI administration in PCOSalso ameliorated components of the metabolic syndrome. These results support the idea that theinsulin resistance of PCOS is, at least in part, related to a functional deficiency in DCI, andpresumably DCI-IPG.
To further test the hypothesis that DCI deficiency contributes to the insulin resistance of PCOSindependent of adiposity, a study similar to the above investigation was conducted in lean womenwith PCOS to determine if DCI administration was beneficial in this subset54. In this study, 600 mgDCI or placebo once daily was administered for six to eight weeks. As in the previous study, DCIadministration improved insulin sensitivity, ovulation and serum androgen levels in these leanwomen with PCOS. These findings confirmed that lean women with PCOS also suffered frominsulin resistance and a functional deficiency in DCI may contribute to their insulin resistance.
Since DCI deficiency seems to contribute to insulin resistance in PCOS, we wondered whethersome of the beneficial effects of insulin sensitizing drugs in this disorder may be mediated byimprovements in DCI-IPG release. We utilized stored serum samples from a study where weadministered metformin or placebo for 4 to 8 weeks, and quantified DCI-IPG release from thesestored samples55. Nineteen obese (BMI ³ 27.5 kg/m2) women with PCOS who took metformin (n=10) or placebo (n=9) wereinvestigated. Insulin and DCI-IPG release during an oral glucose tolerance test were assessed.
Treatment with metformin decreased the AUC
(±SE) compared to placebo during oral glucose
challenge (-3574 ± 962 vs. +1367 ± 1021 mIU/min x ml, p=0.003). In addition, after metformintherapy, the bioactive DCI-IPG per unit of insulin (AUC
) increased by 160%. In
comparison, this value decreased by 29% after placebo (p=0.002). This study suggested thepossibility that metformin improved insulin sensitivity in PCOS in part by improving insulin-stimulated release of DCI-IPG mediators.
In summary, these findings support the idea that DCI deficiency contributes to insulin resistance inPCOS. They further suggest that DCI deficiency in PCOS is unrelated to adiposity, since it appearsto be present in both obese and lean women with PCOS. In addition, exogenous DCI administrationin lean and obese women with PCOS can overcome DCI deficiency and alleviate insulin resistancein the disorder. Finally, some of the improvement in insulin sensitivity mediated by metformin maybe related to an improvement in insulin-stimulated release of DCI-IPG mediators.
A UNIFYING HYPOTHESIS: THE ROLE OF IPGS IN BOTH GLUCOSE HOMEOSTASIS ANDHYPERANDROGENEMIA IN PCOS
The above findings present us with an apparent paradox. If IPG mediators are responsible in part formediating insulin sensitivity and also transducing ovarian andgrogen production, then one mightexpect that a deficiency of IPG mediators in PCOS would logically lead to insulin resistance and alow level of circulating androgens. DCI administration would therefore increase circulatingandrogens in women with PCOS. Yet this is not the case since DCI administration to women withPCOS improves insulin sensitivity, improves ovulation and lowers circulating androgens53,54. Howdoes the IPG transduction system explain both the insulin resistance and the hyperandrogenism ofthis disorder?
In this regard, it is important to emphasize that while studies have shown that DCI-IPG deficiencyin PCOS may contribute to the insulin resistance of the syndrome, studies in steroidogenesis havenot identified which of the many IPGs are responsible for transducing insulin's signal in vivo fortestosterone biosynthesis. In human placental cytotrophoblasts in vitro, MYO containing IPG seemsto be more potent than DCI-IPG in mediating steroidogenesis39. We postulate that MYO-IPG, butnot DCI-IPG, may be the primary mediator for testosterone biosynthesis in PCOS, although thistheory awaits validation by further studies. In contrast, evidence suggests that DCI-IPG mediatesinsulin sensitivity in PCOS.
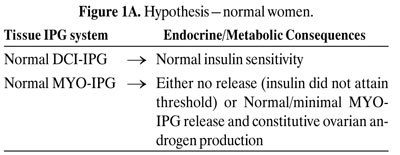
Figures 1 A-C depict our hypothesis. Levels of DCI-IPG and MYO-IPG are normal in the absenceof PCOS (Figure 1A). In PCOS, a functional deficiency in DCI-IPG exists as supported byavailable data, and we hypothesize that there may be an increased level of MYO-IPG due tocompensatory hyperinsulinemia resulting from insulin resistance (Figure 1B). Administration ofDCI restores normal levels of DCI-IPG, and may improve insulin sensitivity. Decreased circulatinginsulin then results in decreased MYO-IPG release, reducing ovarian testosterone biosynthesis(Figure 1C). To date, we have preliminary data supporting a functional deficiency of DCI inPCOS52. Additional studies are underway to assess whether there is an excess of MYO-IPG in thisdisorder.





CONCLUSION AND FUTURE DIRECTIONS
IPGs play a role in both ovarian testosterone biosynthesis and insulin resistance in PCOS.
Deficiency of DCI-IPG may be in part responsible for the insulin resistance in PCOS. DCIadministration ameliorates insulin resistance, hyperandrogenemia and improves ovulation in thisdisorder. We postulate that MYO-IPG may be the primary IPG responsible for ovarian testosteronebiosynthesis, but this awaits validation by future studies. Additional studies should also investigate
the ratio of DCI-IPG to MYO-IPG in ovarian tissues in PCOS women. Therapeutic moieties todecrease MYO-IPG or increase DCI-IPG may be a possible research avenue to correct thehyperandrogenism and insulin resistance of this disorder.
REFERENCES 1. Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A, 1987 Characterization of groups ofhyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/orhyperinsulinemia. J Clin Endocrinol Metab 65: 499-507. 2. Dunaif A, Mandeli J, Fluhr H, Dobrjansky A, 1988 The impact of obesity and chronichyperinsulinemia on gonadotropin release and gonadal steroid secretion in the polycystic ovarysyndrome. J Clin Endocrinol Metab 66: 131-139. 3. Dunaif A, Segal KR, Futterweit W, Dobrjansky A, 1989 Profound peripheral insulin resistance,independent of obesity, in polycystic ovary syndrome. Diabetes 38: 1165-1174. 4. Campbell PJ, Gerich JE, 1990 Impact of obesity on insulin action in volunteers with normalglucose tolerance: demonstration of a threshold for the adverse effect of obesity. J Clin EndocrinolMetab 70: 1114-1118. 5. Nestler JE, Jakubowicz DJ, Falcon de Vargasm A, Brik C, Quinterro N, Medina F, 1998 Insulinstimulates testosterone biosynthesis by huan thecal cells from women with polycystic ovarysyndrome by activating its own receptor and using inositolglycan mediators as the signaltransduction system. J Clin Endocrinol Metab 83: 2001-2005. 6. Lord JM, Flight IHK, Norman RJ, 2003 Metformin in polycystic ovary syndrome: systematicreview and meta-analysis. BMJ 327: 951-960. 7. Nestler JE, Jakubowicz DJ, 1996 Decreases in ovarian cytochrome P450c17 alpha activity andserum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl JMed 335: 617-623. 8. Nestler JE, Jakubowicz DJ, 1997 Lean women with polycystic ovary syndrome respond toinsulin reduction with decreases in ovarian P450c17{alpha} activity and serum androgens. J ClinEndocrinol Metab 82: 4075-4079. 9. Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R, 1996 The insulin-sensitizing agenttroglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. JClin Endocrinol Metab 81: 3299-3306. 10. Ehrmann DA, Schneider DJ, Sobel BE, et al, 1997 Troglitazone improves defects in insulinaction, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovarysyndrome. J Clin Endocrinol Metab 82: 2108-2116. 11. Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW, 2003 Effect of rosiglitazone onspontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome.
Fertil Steril 79: 562-566. 12. Shobokshi A, Shaarawy M, 2003 Correction of insulin resistance and hyperandrogenism inpolycystic ovary syndrome by combined rosiglitazone and clomiphene citrate therapy. J SocGynecol Investig 10: 99-104. 13. Bjorntorp P, 1996 The android woman—a risky condition. J Intern Med 239: 105-110. 14. Rebuffe-Scrive M, Cullberg G, Lundberg PA, Lindstedt G, Bjorntorp P, 1989 Anthropometricvariables and metabolism in polycystic ovarian disease. Horm Metab Res 21: 391-397. 15. Conway GS, Agrawal R, Betteridge DJ, Jacobs HS, 1992 Risk factors for coronary arterydisease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol 37: 119-125. 16. Legro RS, Kunselman AR, Dunaif A, 2001 Prevalence and predictors of dyslipidemia in womenwith polycystic ovary syndrome. Am J Med 111: 607-613. 17. Robinson S, Henderson AD, Gelding SV, et al, 1996 Dyslipidaemia is associated with insulinresistance in women with polycystic ovaries. Clin Endocrinol 44: 277-284. 18. Talbott E, Clerici A, Berga SL, et al, 1998 Adverse lipid and coronary heart disease risk profilesin young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol51: 415-422.
19. Dahlgren E, Johansson S, Lindstedt G, et al, 1992 Women with polycystic ovary syndromewedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulatinghormones. Fertil Steril 57: 505-513. 20. Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A, 1987 Characterization of groups ofhyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/orhyperinsulinemia. J Clin Endocrinol Metab 65: 499-507. 21. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J, 1999 Prevalence ofimpaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care22: 141-146. 22. Legro RS, Kunselman AR, Dodson WC, Dunaif A, 1999 Prevalence and predictors of risk fortype 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: aprospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84: 165-169. 23. Wild S, Pierpoint T, McKeigue P, Jacobs H, 2000 Cardiovascular disease in women withpolycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol52: 595-600. 24. Taponen S, Martikainen H, Jarvelin MR, et al, 2004 Metabolic cardiovascular disease riskfactors in women with self-reported symptoms of oligomenorrhea and/or hirsutism: NorthernFinland Birth Cohort 1966 Study. J Clin Endocrinol Metab 89: 2114-2118. 25. Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, Fitzpatrick LA, 2003Prevalence and predictors of coronary artery calcification in women with polycystic ovarysyndrome. J Clin Endocrinol Metab 88: 2562-2568. 26. Dahlgren E, Janson PO, Johansson S, Lapidus L, Oden A, 1992 Polycystic ovary syndrome andrisk for myocardial infarction. Evaluated from a risk factor model based on a prospective populationstudy of women. Acta Obstet Gynecol Scand 71: 599-604. 27. Legro RS, 2003 Polycystic ovary syndrome and cardiovascular disease: a prematureassociation? Endocr Rev 24: 302-312. 28. Paradisi G, Steinberg HO, Hempfling A, et al, 2001 Polycystic ovary syndrome is associatedwith endothelial dysfunction. Circulation 103: 1410-1415. 29. Talbott EO, Guzick DS, Sutton-Tyrrell K, et al, 2000 Evidence for association betweenpolycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women.
Arterioscler Thromb Vasc Biol 20: 2414-2421. 30. Larner J, 1983 Mediators of postreceptor action of insulin. Am J Med 74: 38-51. 31. Larner J, 1988 Insulin-signaling mechanisms. Lessons from the old testament of glycogenmetabolism and the new testament of molecular biology. Diabetes 37: 262-275. 32. Saltiel AR, 1990 Second messengers of insulin action. Diabetes Care 13: 244-256. 33. Jarett L, Seals JR, 1979 Pyruvate dehydrogenase activation in adipocyte mitochondria by aninsulin-generated mediator from muscle. Science 206: 1407-1408. 34. Larner J, Galasko G, Cheng K, et al, 1979 Generation by insulin of a chemical mediator thatcontrols protein phosphorylation and dephosphorylation. Science 206: 1408-1410. 35. Larner J, Romero G, Kennington AS, Lilley K, Kilgour E, Zhang C et al., 1990 Duality in themechanism of action of insulin. Adv Second Messenger Phosphoprotein Res 24: 290-294. 36. Larner J, Huang LC, Schwartz CF, et al, 1988 Rat liver insulin mediator which stimulatespyruvate dehydrogenase phosphate contains galactosamine and D-chiroinositol. Biochem BiophysRes Commun 151: 1416-1426. 37. Nestler JE, 1987 Modulation of aromatase and P450 cholesterol side-chain cleavage enzymeactivities of human placental cytotrophoblasts by insulin and insulin-like growth factor I.
Endocrinology 121: 1845-1852. 38. Nestler JE, 1989 Insulin and insulin-like growth factor-I stimulate the 3 beta-hydroxysteroiddehydrogenase activity of human placental cytotrophoblasts. Endocrinology 125: 2127-2133. 39. Nestler JE, Romero G, Huang LC, Zhang CG, Larner J, 1991 Insulin mediators are the signaltransduction system responsible for insulin's actions on human placental steroidogenesis.
Endocrinology 129: 2951-2956.
40. Romero G, Garmey JC, Veldhuis JD, 1993 The involvement of inositol phosphoglycanmediators in the modulation of steroidogenesis by insulin and insulin-like growth factor-I.
Endocrinology 132: 1561-1568. 41. Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F, 1998 Insulinstimulates testosterone biosynthesis by human thecal cells from women with polycystic ovarysyndrome by activating its own receptor and using inositolglycan mediators as the signaltransduction system. J Clin Endocrinol Metab 83: 2001-2005. 42. Larner J, Price JD, Heimark D, et al, 2003 Isolation, structure, synthesis, and bioactivity of anovel putative insulin mediator. A galactosamine chiro-inositol pseudo-disaccharide Mn2+ chelatewith insulin-like activity. J Med Chem 46: 3283-3291. 43. Larner J, Allan G, Sleevi M, Price J, Rule G, Piccariello T, 1997 Molecular mechanisms ofinsulin resistance. Structure and formation of a novel pinitol-galactosamine disaccharide—aputative insulin mediator. Program of the 16th International Diabetes Federation Congress 1997.
44. Romero G, Gamez G, Huang LC, Lilley K, Luttrell L, 1990 Anti-inositolglycan antibodiesselectively block some of the actions of insulin in intact BC3H1 cells. Proc Natl Acad Sci USA 87:1476-1480. 45. Ortmeyer HK, Bodkin NL, Lilley K, Larner J, Hansen BC, 1993 Chiroinositol deficiency andinsulin resistance. I. Urinary excretion rate of chiroinositol is directly associated with insulinresistance in spontaneously diabetic rhesus monkeys. Endocrinology 132: 640-645. 46. Suzuki S, Kawasaki H, Satoh Y, et al, 1994 Urinary chiro-inositol excretion is an index markerof insulin sensitivity in Japanese type II diabetes. Diabetes Care 17: 1465-1468. 47. Craig JW, Larner J, Asplin CM, Chiro-inositol deficiency and insulin resistance. In: Draznin B,LeRoith D, editors. Molecular Biology of Diabetes II. Totowa, N.J.: Humana Press, 1998: 343-362. 48. Kennington AS, Hill CR, Craig J, et al, 1990 Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med 323: 373-378. 49. Ostlund RE Jr, McGill JB, Herskowitz I, Kipnis DM, Santiago JV, Sherman WR, 1993 D-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci USA 90: 9988-9992. 50. Asplin I, Galasko G, Larner J, 1993 chiro-inositol deficiency and insulin resistance: acomparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated fromurine, hemodialysate, and muscle of control and type II diabetic subjects. Proc Natl Acad Sci USA90: 5924-5928. 51. Ortmeyer HK, Huang LC, Zhang L, Hansen BC, Larner J, 1993 Chiroinositol deficiency andinsulin resistance. II. Acute effects of D-chiroinositol administration in streptozotocin-diabetic rats,normal rats given a glucose load, and spontaneously insulin-resistant rhesus monkeys.
Endocrinology 132: 646-651. 52. Baillargeon J-P, Diamanti-Kandarakis E, Apridonidze T, Iuorno MJ, Nestler JE. 2004 D-chiro-inositol clearance, insulin stimulated release of D-chiro-inositol-containing inositolphosphoglycanand insulin sensitivity in women with polycystic ovary syndrome. The Endocrine Society 86thAnnual Meeting, New Orleans, Louisiana OR31-2, 118. 53. Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G, 1999 Ovulatory and metabolic effectsof D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med 340: 1314-1320. 54. Iuorno MJ, Jakubowicz DJ, Baillargeon JP, et al, 2002 Effects of D-chiro-inositol in leanwomen with the polycystic ovary syndrome. Endocr Pract 8: 417-423. 55. Baillargeon JP, Iuorno MJ, Jakubowicz DJ, Apridonidze T, He N, Nestler JE, 2004 Metformintherapy increases insulin-stimulated release of D-chiro-inositol-containing inositolphosphoglycanmediator in women with polycystic ovary syndrome. J Clin Endocrinol Metab 89: 242-249.
Address correspondence and requests for reprints to:John E. Nestler, M.D., Virginia Commonwealth University, MCV Campus, PO Box 980111, Richmond, VA 23298-0111, e-mail: [email protected]
Received 17-08-04, Revised 20-09-04, Accepted 25-09-04
Source: http://mypcos.info/1/wp-content/uploads/2010/01/2004-Hormones-Nestler-Role-of-IPGs-in-mediating-IS-hyperandrogenism-in-PCOS.pdf
FOR WORKERS' COMPENSATION PROFESSIONALS WORKERS' COMPENSATION BILL PASSES Enhanced benefi t, cost containment, housekeeping items The Minnesota Legislature passed a workers' compensation law that contains the most signi icant changes to the workers' compensation Testing begins for new system in almost 20 years. The bill was negotiated by the AFL-CIO
• Editorial June 2013• 25th Pezcoller Symposium: Abstracts of oral presentations Abstracts of posters• Call for 2014 International Award For Cancer Research Pezcoller Foundation World It's with great pleasure that I can report that the re- ler Symposium entitled "Metabolism and Tumorigene- cipient of the 2013 Pezcoller Foundation-AACR Interna-













