Nfdx.smu.edu.cn
Secretion of glucagon-like peptide-1 (GLP-1) in type 2diabetes: what is up, what is down?
M. A. Nauck & I. Vardarli & C. F. Deacon & J. J. Holst &J. J. Meier
Received: 3 April 2010 / Accepted: 30 July 2010
# Springer-Verlag 2010
Abstract The incretin hormones gastric inhibitory poly-
the variations in published findings of group differences in
peptide and especially glucagon-like peptide (GLP) have an
GLP-1 responses to nutrient intake.
important physiological function in augmenting post-prandial insulin secretion. Since GLP-1 may play a role in
Keywords Glucagon-like peptide-1 . Gut hormones .
the pathophysiology and treatment of type 2 diabetes,
Incretin hormones . L-cell . Meta-analysis .
assessment of meal-related GLP-1 secretory responses in
Pathophysiology . Review
type 2 diabetic patients vs healthy individuals is of greatinterest. A common view states that GLP-1 secretion in
patients with type 2 diabetes is deficient and that this
Dipeptidyl peptidase
applies to a lesser degree in individuals with impaired
Gastric inhibitory polypeptide
glucose tolerance. Such a deficiency is the rationale for
Glucagon-like peptide
replacing endogenous incretins with GLP-1 receptor agonists
or re-normalising active GLP-1 concentrations with dipep-tidyl peptidase-4 inhibitors. This review summarises theliterature on this topic, including a meta-analysis of published
studies on GLP-1 secretion in individuals with and withoutdiabetes after oral glucose and mixed meals. Our analysis
Incretins are gut-derived hormones that can stimulate insulin
does not support the contention of a generalised defect in
secretion and make a significant contribution to overall
nutrient-related GLP-1 secretory responses in type 2 diabetes
postprandial insulin release One of them, glucagon-
patients. Rather, factors are identified that may determine
like peptide (GLP)-1, has glucose-lowering properties [
individual incretin secretory responses and explain some of
and has been the basis of two novel classes of glucose-lowering agents, incretin mimetics (i.e. GLP-1 receptoragonists) and inhibitors of protease dipeptidyl peptidase
M. A. Nauck (*) I. Vardarli
(DPP)-4 (incretin enhancers) []. Since the latter are
Diabeteszentrum Bad Lauterberg,
thought to exert their glucose-lowering activity by prevent-
ing the degradation and inactivation (with regard to
37431 Bad Lauterberg im Harz, Germanye-mail:
[email protected]
insulinotropic effects) of endogenously released intact,biologically active GLP-1 [], their pharmacological action
C. F. Deacon : J. J. Holst
should critically depend on availability of the major
Department of Medical Physiology, Panum Institute,
substrate, GLP-1. Against this background, reports that
University of Copenhagen,Copenhagen, Denmark
GLP-1 secretion from L-cells is reduced in patients withtype 2 diabetes [] have prompted several hypotheses
about how this affects the estimated clinical effectiveness of
Medizinische Klinik I, St Josef-Hospital Bochum,
these novel drugs. They have mainly been based on a study
Ruhr-University Bochum,Bochum, Germany
by Toft-Nielsen et al. [which reported an increase in
‘total' GLP-1 (all forms including intact GLP-1 [7-36
The main meal components that act as potent stimulants of
amide] and the DPP-4-degraded, non-insulinotropic form
GLP-1 secretion are glucose [and triacylglycerol [
GLP-1 [9-36 amide]) from basal levels of around 5 pmol/l
but fructose and some proteins are also effective. Oral
to mean values of about 16 to 17 pmol/l in 33 healthy
glucose is a good stimulus for the release of GLP-1,
participants and 13 to 14 pmol/l in 54 type 2 diabetic
whereas plasma GLP-1 concentrations do not change when
patients, both after 60 min. In the second hour, peak GLP-
glucose is administered intravenously, i.e. by bypassing
1 concentrations were maintained in healthy participants,
absorptive processes in the gut , ]. Typically, ‘total'
but returned to lower levels in type 2 diabetic patients.
GLP-1 concentrations are between 5 and 15 pmol/l in the
Overall, this resulted in a 53% reduction in integrated
basal state, rising to between 20 and 60 pmol/l after oral
incremental GLP-1 concentrations (AUC above baseline)
glucose or meals [].
in type 2 diabetic patients relative to healthy controls and a
The temporal pattern of nutrient intake-related increments
19% reduction in the total AUC (above 0 pmol/l). These
in plasma GLP-1 begins with a rather early rise (starting
differences were highly significant. Participants with
approximately 10 to 15 min after eating , peaks during
impaired oral glucose tolerance ranked between healthy
the second hour and then slowly declines to baseline over
volunteers and type 2 diabetic patients and thus had an
several hours. Some studies suggest a biphasic pattern, with
intermediate GLP-1 response. Based on this largest
an early peak followed by a nadir and a second rise in GLP-1
available cross-sectional study comparing GLP-1 release
concentrations but other studies in humans tend to
in type 2 diabetic patients and healthy controls, it has
describe monophasic secretory responses , ].
specifically been pointed out that:
The intracellular events leading to L-cell secretion have
been reviewed in detail ]. Novel components of L-cells
1. Slightly reduced GLP-1 concentrations after a meal in
that are involved in nutrient sensing are sweet taste
participants with impaired oral glucose tolerance and
receptors and the G-protein gustducin [as well as
more severely impaired GLP-1 secretion in type 2
NEFA receptors or their modifications such as G-protein-
diabetic patients, as demonstrated in cross-sectional
coupled receptor (GPR) 119 [GPR 120 [and
analyses, may translate into a progressive loss of the
perhaps GPR 40 [GPR 119 and 120 are rather specific
ability to secrete GLP-1 with advancing type 2 diabetes
for long-chain monounsaturated NEFA such as α-linolenic
as part of the process of disease progression.
acid. It is not known whether GPR 119 and/or 120 are
2. This loss of GLP-1 secretion is part of the pathophysio-
located to the luminal (brush border) membrane, which is
logical explanation for a lost incretin effect [], a
primarily involved in sensing NEFA in chymus, or to the
phenomenon typically observed in patients with type 2
baso-lateral membrane, where they would be exposed to
interstitial fluid more similar in composition to plasma
3. The above point (2) provides evidence that loss of GLP-1
secretion with immediate pathophysiological consequen-ces (reduced incretin effect as part of impaired postpran-dial beta cell function) calls for a replacement therapy
Where in the gut is GLP-1 released?
with incretin-based glucose-lowering medications
4. Based on such reasoning, DPP-4 inhibitors cannot be
Especially the predominant location of L-cells in the distal
expected [, ] to lower blood glucose in later stages of
parts of the gut [has prompted hypotheses about the
type 2 diabetes (because of lost ability to secrete GLP-1).
early increase in GLP-1 concentrations immediately after
This review aims to put these hypotheses into perspective
starting a meal [, ]. It has been difficult to
with the available data on GLP-1 release in healthy and type
understand how L-cells in the distal ileum can be exposed
2 diabetic participants, and to address common differences of
to nutrients minutes after their ingestion [Measurement
opinion and some potential misconceptions in the inter-
of plasma concentration profiles after nutrient ingestion (e.g.
pretation of commonly quoted landmark publications.
Fig. with the early phase being the consequence ofpotential indirect mechanisms, followed by a later phase ofdirect stimulation, when gut contents have travelled to
Mechanisms of L-cell GLP-1 secretion
distal parts of the intestines) have not accumulated muchevidence in favour of a biphasic GLP-1 response in human
Physiologically, L-cells, which are most common in the
participants. Nevertheless, some indirect mechanisms have
distal parts of the intestines ], are stimulated to secrete
been proposed. These potential pathways include ‘upper-
all their secretory products (GLP-1, GLP-2, gut-derived
gut signals', i.e. stimulation of the autonomous nervous
glucagon [also called glicentin], peptide tyrosine tyrosine,
system –as well as endocrine transmission through
oxyntomodulin) in response to nutrient ingestion [].
gastrointestinal hormones , ], both of which may
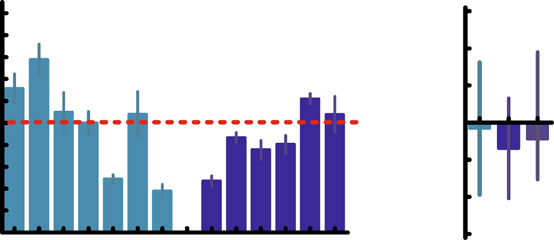
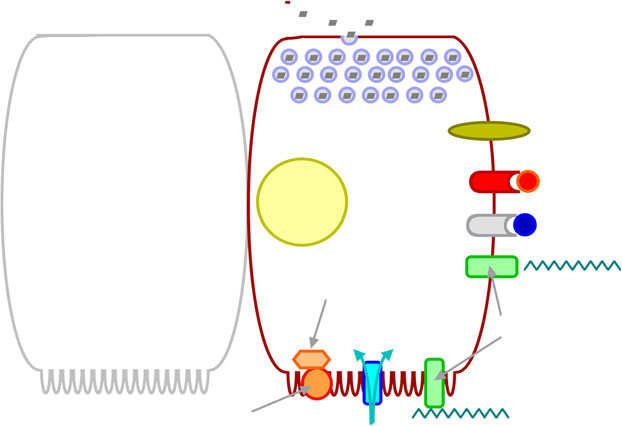
Fig. 1 The L-cell with compo-
nents that may be involved intriggering or modulating GLP-1secretion. The primary process
leading to release of preformedsecretory granules may start
with the absorption of glucose
neuropeptides (e.g. GRP)
through glucose transporters inmicrovilli of the luminal
(brush border) membrane.
Additional signals are sweet
taste receptors, receptors forNEFA, neurotransmitters
peptide, others), gastrointestinalhormones (GIP) and glucagon.
GPR 120 (GPR 119, GPR 40;
For details, see text. Glc, glucose
Long-chain unsaturated NEFA(e.g. α-linolenic acid)
Sweet taste receptor
Glc (binding?) Na+ Glc
be activated when appropriate nutrients enter the duodenum
a rise in GLP-1 concentrations in human participants with
or upper jejunum. Along these lines, gastric inhibitory
] and without [] type 2 diabetes. Neurotransmitters
polypeptide (GIP) triggers release of GLP-1 from cell lines
such as acetylcholine , and gastrin-releasing
resembling L-cells and in animal experiments (rodents) ].
peptide ] also trigger GLP-1 release, suggesting the
However, even high doses of exogenous GIP do not lead to
intramural enteric nervous system is involved in mediating the
85 104 189 Type 2 diabetes
Integrated incremental GLP-1
-60 134 83 217 Healthy controls
[44] [44] [14] [72] [54] [25] [70]
[12] [24] [68] [68] [69] [25]
Studies [reference number]
Fig. 2 Integrated responses of ‘total' GLP to oral glucose or mixed
tolerance test (75 g) and/or after a standardised mixed meal. Two
meals based on individual studies (a) reporting integrated incremental
independent authors (M. Nauck and I. Vardarli) examined the studies
‘total' GLP-1 responses in patients with type 2 diabetes and an
and extracted data. We included nine studies with 13 datasets and 406
appropriate control group (weight-matched, non-diabetic participants)
participants (189 patients with type 2 diabetes, 217 healthy controls) in
and using non-specific assays that measured intact and DPP-4-degraded
the meta-analysis. Data from studies using early, non-specific assays
forms of GLP-1. The response in type 2 diabetic patients (mean ± SEM)
that would cross-react with the major proglucagon fragment ]
is expressed as percentage of the mean value in the control group.
were excluded. As effect size, we used the mean difference. Pooled
*p<0.05 vs control. Numbers in bars indicate number of type 2 diabetic
estimates were obtained using the DerSimonian and Laird random
patients (upper row) and control participants (lower row) studied.
effects model. The pooled estimates of the mean difference of integrated
Studies are indicated by reference number, i.e. , ,
incremental plasma concentrations of GLP-1 between patients with type
72]. a0–20 min, b20–120 min, c0–30 min, d30–180 min. b For
2 diabetes and healthy controls are shown. After an oral glucose
the meta-analysis, electronic databases (PubMed and EMBASE) were
challenge as well as after a mixed meal test, the integrated incremental
searched (until 28 February 2010) for studies dealing with the secretion
plasma concentrations of GLP-1 were not significantly different
and providing integrated incremental responses of GLP-1 in healthy
between patients with type 2 diabetes and controls (p=0.44). Light
participants and patients with type 2 diabetes after an oral glucose
blue, oral glucose; dark blue, mixed meal
‘upper gut signal'. In a recent study, GLP-1 release triggered
probably related to the use of side-viewing antibodies,
by oleate was attenuated by a cholecystokinin type 1 receptor
which also detect the major proglucagon fragment [
More recent studies have described either no abnormalities
However, gut resections at any level of the small or large
at all in oral glucose-induced GLP-1 secretion or GLP-1
intestines do not typically lead to reduced GLP-1 secretion
hyposecretion (Fig. Meal-induced GLP-1 responses
]. This may indicate that the release of GLP-1 occurs
were significantly reduced in the above-mentioned largest
primarily from L-cells immediately exposed to ingested
cross-sectional study [by Toft-Nielsen et al. and more
nutrients (i.e. L-cells in the upper parts of the small intestine),
similar to those found in healthy participants in some other,
rather than from those parts of the gut where the majority of
smaller studies (Fig. ). The second-largest recent study, by
L-cells are located (i.e. the ileum and large intestine)
Vollmer et al. ], found no impairment in mixed meal-
In fact, L-cells have also been identified in the duodenum
induced GLP-1 secretion. The bottom line suggests that there
and certainly in the jejunum with the respective
is some variation in GLP-1 secretion and that in some cohorts,
numbers increasing aborally towards the ileum and colon/
on balance, the GLP-1 response is somewhat reduced, whereas
rectum []. It has been estimated that the amount of GLP-1
in other studies such differences are not as apparent (Fig. ).
secreted after ingestion of a single nutrient load (calculated
Since in some of the studies analysed type 2 diabetic
as integrated incremental response multiplied by the known
patients were treated with metformin, which has been
metabolic clearance rate for GLP-1) accounts for about 10 %
shown to enhance GLP-1 responses [any potential
of the GLP-1 content of jejunal L-cells [A second
difference in GLP-1 secretory responses between type 2
argument in favour of direct stimulation of upper-gut L-cells
diabetic patients and healthy participants may have been
is the observation that the release of GIP is closely related to
modified by this mechanism.
that of GLP-1, even at an intra-individual level, indicating
In the absence of a more general difference in GLP-1
that some individuals have a poor response of GIP and
secretory responses between type 2 diabetic patients and
GLP-1, and others have average or higher release of both
healthy participants, we have attempted to identify participant/
incretin hormones Consistent with this observation, a
patient characteristics that would determine individual GLP-1
subset of entero-endocrine cells has been shown to co-
responses: Fig. shows univariate regression analysis for
express GIP and the proglucagon gene, suggesting concom-
GLP-1 secretory responses after oral glucose ingestion, from
itant secretion of both incretin hormones from such cells [].
which responses appear to be predicted by baseline
Further evidence for immediate secretion of GLP-1 from
characteristics such as age, body weight (obesity), fasting
L-cells in the proximal gut comes from work by Schirra et
NEFA concentrations and fasting glucagon concentrations.
al. [in which GIP and GLP-1 secretion was determined
Similar relationships can be found for mixed meal-induced
in relation to the gastro-duodenal transit of nutrients. These
GLP-1 secretory responses and for GIP secretion (details
studies revealed a rapid onset of GIP and GLP-1 secretion
not shown) in relation to baseline characteristics. As shown
after ingestion of oral glucose, along with high rates of
in Table , most of the individual factors found to be
nutrients passing into the duodenum. Interestingly, the
significantly related to GLP-1 secretory responses in uni-
nutrient flow rates required to sustain stimulated secretion
variate analyses remained significant in a multivariate
of incretin hormones were far higher for GLP-1 (6 kJ/min)
analysis, with similar results for GLP-1 responses induced
than for GIP (<1 kJ/min) [This suggests that the
by oral glucose or mixed meal ingestion. Some relationship
velocity of transpyloric gastric content movement required
between obesity and GLP-1 secretion has been noted before
to stimulate K-cells in the duodenum is lower than that
, Exogenous glucagon has been found to be
required to stimulate L-cells at a more distal location (i.e. at
associated with suppressed GLP-1 secretion although
least the proximal jejunum or further down), where
in the isolated, perfused gut no such interaction was apparent
significant numbers of L-cells can be found ]. On the
The fact that variables related to obesity no longer
basis of the evidence available, we hypothesise that direct
showed a strongly significant correlation in the multivariate
stimulation of the more proximal L-cells is the predominant
analysis (Table may be related to the influence of
mechanism. However, the relative contributions of direct
associated factors, e.g. elevated NEFA concentrations.
and indirect mechanisms prompting secretion of GLP-1 in
Certainly, hyperglycaemia (or oral glucose tolerance) did
humans remain to be fully elucidated.
not turn out to be a strong predictor of GLP-1 secretion,suggesting that other characteristics of type 2 diabeticpatients not represented by elevated glucose concentrations
GLP-1 secretion in patients with type 2 diabetes
in the fasting or postprandial state may determine GLP-1secretion, although opinions differ ]; insulin resistance,
GLP-1 hypersecretion was described in some early studies
moreover, has also been described as a factor impairing
, ] in response to the ingestion of oral glucose,
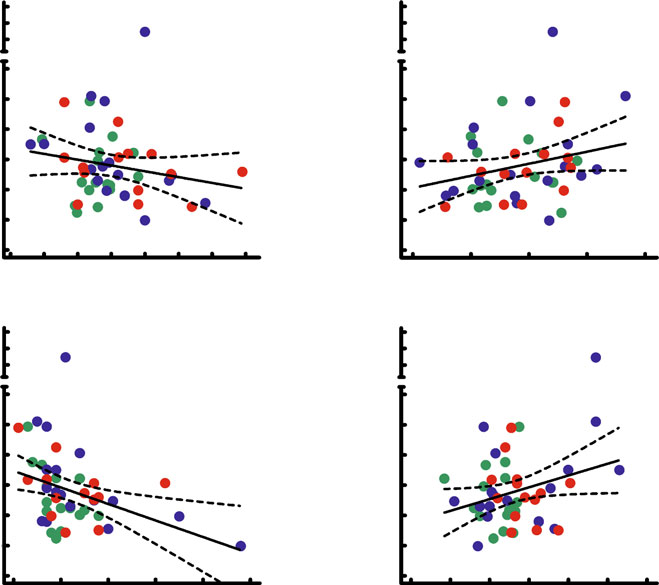
Fig. 3 Univariate correlation of
BMI (a), age (b), fasting
glucagon (c) and NEFA (d) to
integrated incremental responses
of GLP-1 following ingestion of
a mixed meal in participants
with normal oral glucosetolerance (green symbols,
n=14), impaired oral glucose
tolerance (blue symbols, n=17)
and type 2 diabetes (red
symbols, n=17). Data were
re-analysed from Vollmer et al.
Integrated incremental GLP-1
Integrated incremental GLP-1
[]. a r2=0.037, p=0.19;
b r2=0.066, p=0.079; c r2=
0.142, p=0.0082; d r2=0.081,
Integrated incremental GLP-1
Integrated incremental GLP-1
Fasting glucagon (pmol/l)
Fasting NEFA (mmol/l)
Factors that determine GLP-1 secretion give an indication
described by multivariate regression analysis (Table
why some studies found differences in GLP-1 secretory
These associations, however, may only be valid in relatively
responses between type 2 diabetic patients and healthy
well controlled patients with type 2 diabetes, in whom
controls, while others did not. These interdependences are
glucose-lowering treatment is administered as usual when
illustrated in Table Type 2 diabetic cohorts, especially if
the nutrient stimulation to trigger GLP-1 release is performed.
not perfectly matched to healthy participants, will tend to be
The mechanism driving glucagon-induced suppression
older, more obese and exhibit hyperglucagonaemia as well
of GLP-1 secretion has yet to be determined. Since GLP-1
as elevated fasting NEFA levels. Some of these factors
is known to reduce glucagon [a reverse association
predict lower GLP-1 responses (higher weight or BMI, and
may apply. Alternatively, a third, unknown factor may
high glucagon concentrations), while others are determi-
influence GLP-1 secretion and glucagon, explaining this
nants of enhanced GLP-1 secretion (older age, higher
association. A link between glucagon and GLP-1 secretion
fasting NEFA concentrations). Therefore, the ability to
has recently been confirmed []. Nevertheless, open
predict ‘normal' or impaired GLP-1 response in patients
questions remain. Can, for example, a direct effect be
with type 2 diabetes will depend on the individual balance
mediated by glucagon receptors on L-cells? Do motility
of these factors as well as the rate of gastric emptying. The
effects contribute to a reduction of GLP-1 secretion, e.g. by
same reasoning can be applied to reported studies. Thus
slowing antro-duodenal transit of nutrients? Since approx-
Toft-Nielsen et al. [studied healthy and type 2 diabetic
imately 40% of type 2 diabetic patients can be expected to
participants with similar fasting NEFA concentrations, but
have delayed gastric emptying, how does this affect GLP-1
differences in glucagon levels. Otherwise (age, BMI) their
secretion? Does autonomous neuropathy ] play a role in
participants were well matched, meaning that the overall
this context? Is the influence of NEFA or similar ligands
balance was shifted towards reduced GLP-1 secretion. In
mediated by GPR 119, 120 or 40? Does this indicate that
our recent study, type 2 diabetic patients had high NEFA
these receptors are located on the basolateral membrane,
concentrations as well as hyperglucagonaemia, the influen-
thereby being accessible and responsive to changes in
ces of which may have balanced each other out, resulting in
circulating concentrations of NEFA? Are the NEFA that
no net difference in GLP-1 secretory responses ]. These
activate GPR 120 receptors the same ones that stimulate
analyses further strengthen the credibility of the associations
GLP-1 secretion, e.g. long-chain monounsaturated NEFA?
Table 1 Multiple regression analysis of potential determinants of
inhibitors to prevent rapid digestion of starch and complex
GLP-1 secretion after oral glucose loads or mixed meals
carbohydrates, thereby moving intestinal contents downwards
and resulting in preferential digestion and absorption in the
lower jejunum or ileum, where L-cells are most prevalent. Inhealthy participants receiving test meals composed of sucrose
GLP-1 after oral glucosea
and acarbose or voglibose, this approach was highly effective,
Glucose toleranceb
whereas in studies more realistically copying the ingestion of
meals in patients with type 2 diabetes, the effects were
rather small or completely negligible [This may be
Glucagon (pmol/l)
different with second-generation α-glucosidase inhibitors
such as voglibose and miglitol ].
Recently, metformin was seen to lead to higher meal-
GLP-1 after a mixed mealc
related increments in total and intact GLP-1, indicating a
Glucose tolerancea
stimulation of GLP-1 secretion. Moreover, when metformin
was combined with a DPP-4 inhibitor, concentrations of
intact GLP-1 were increased beyond those observed with
Glucagon (pmol/l)
the DPP-4 inhibitor alone ]. The effects of metformin
are compatible with previous results from animal studies
with metformin and other biguanide drugs Themechanism by which metformin augments GLP-1 res-
Integrated incremental GLP-1 responses were determined using methods
ponses is not entirely clear.
for determining ‘total' GLP-1 concentrations (including DPP-4 break-down products)
Certainly, there still is a lack of strategies to augment
secretion of endogenous GLP-1 through dietary measures
B and ß are the non-standardised and standardised regressioncoefficients, respectively, both displayed with their respective standard
or with drugs specifically designed to stimulate L-cell
error (SE); data were re-analysed from Vollmer et al. []
secretion. We expect this to be an active area of research in
Calculations: Statistica 5.0, StatSoft, Hamburg, Germany
the near future.
a For the analysis of GLP-1 after oral glucose, the correlation coefficientsquared (R2) was 0.784, the corrected R2 was 0.753 and the F value with6 and 42 degrees of freedom was 25.4, for a p value <0.0001b
Glucose tolerance was entered as the continuous variable glucose
concentration 120 min after 75 g oral glucosec
First, it is too early to conclude that advancing type 2
For the analysis of GLP-1 after oral glucose, the correlation coefficient
squared (R2) was 0.767, the corrected R2 was 0.734 and the F value with
diabetes is characterised by a progressive loss of the
6 and 42 degrees of freedom was 23.1, for a p value <0.0001
potential to secrete GLP-1 as part of the process of diseaseprogression. To draw such a conclusion, we need studies
Can dietary manipulations be derived from such knowledge
that longitudinally assess GLP-1 secretion in cohorts of
that would help sustain meal-related endogenous release of
prediabetic participants followed until diagnosis of type 2
GLP-1, especially in patients treated with DPP-4 inhibitors?
Table 2 Typical characteristics in type 2 diabetic patients and theirconsequences for GLP-1 secretion based on univariate and multivar-
Augmenting L-cell GLP-1 secretion
iate regression analysis of factors determining GLP-1 responsesassessed from previously reported dataa
By doubling meal-related responses of intact, biologically
Typical consequences
active GLP-1, DPP-4 inhibitors exert profound effects onglycaemic control in patients with type 2 diabetes; indeed,
In type 2 diabetes
For GLP-1 secretion
any augmentation of GLP-1 responses would probably be
welcome and could help control glycaemia in patients with
type 2 diabetes.
Single dietary interventions have met with partial success.
For example, protein preloads ] or large quantities of
Overall individual balance
≈ or ↓ or ↑
single amino acids (glutamine) ] have been used to elicithigher GLP-1 responses by enhancing GLP-1 secretion.
a Vollmer et al. []
The fact that the majority of L-cells are located distally in
b Influence of body weight was significant upon univariate (Fig. ),
the small intestine has prompted attempts to use α-glucosidase
but not multiple regression analysis (Table
diabetes and through progression over the following years.
4. Meier JJ, Nauck MA (2004) The potential role of glucagon-like
peptide 1 in diabetes. Curr Opin Investig Drugs 5:402–410
Current data allow hypotheses, but not predictions of the
5. Nauck MA, Meier JJ, Creutzfeldt W (2003) Incretins and their
results of such a study.
analogues as new antidiabetic agents. Drug News Perspect
Second, small differences in GLP-1 concentrations
within physiological limits are not sufficient to affect
6. Nauck MA, Meier JJ (2005) Glucagon-like peptide 1 (GLP-1) and
its derivatives in the treatment of diabetes. Regul Pept 124
insulin secretion. Physiological replacement of GLP-1 by
exogenous administration had little effect, but this may
7. Deacon CF, Holst JJ (2006) Dipeptidyl peptidase IV inhibitors: a
underestimate the role of endogenous GLP-1 for insulin
promising new therapeutic approach for the management of type 2
secretion, because intravenous administration bypasses
diabetes. Int J Biochem Cell Biol 38:831–844
important hepato-portal sensors for GLP-1 [Certainly,
8. Åhren B (2007) DPP-4 inhibitors. Best Pract Res Clin Endocrinol
Metab 21:517–533
differences during the second and third hour after meal
9. Holst JJ, Deacon CF (2005) Glucagon-like peptide-1 mediates the
stimulation as reported by Toft-Nielsen et al. ] do not
therapeutic actions of DPP-IV inhibitors. Diabetologia 48:612–
help to explain any of the important differences in early-
phase insulin secretion that are typical of type 2 diabetes
10. Toft-Nielsen MB, Madsbad S, Holst JJ (2001) Determinants of the
effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin
(and are found in the first 30 to 60 min after meal ingestion
Endocrinol Metab 86:3853–3860
begins). The reduced incretin effect in type 2 diabetic
11. Lugari R, Dei Cas A, Ugolotti D et al (2002) Evidence for early
patients is much more closely related to the losses in
impairment of glucagon-like peptide 1-induced insulin secretion in
insulinotropic activity of GIP, which are typical of this
human type 2 (non insulin-dependent) diabetes. Horm Metab Res34:150–154
condition, and less so to those related to GLP-1 ].
12. Toft-Nielsen MB, Damholt MB, Madsbad S et al (2001)
Third, and for the same reason(s), any observed deficit in
Determinants of the impaired secretion of glucagon-like peptide-
GLP-1 responses after meals does not simply call for a
1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–
‘true' replacement therapy with incretin-based glucose-
13. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W (1986) Reduced
lowering medications. Incretin mimetics provide a pharma-
incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia
cological stimulus to insulin secretion and elicit other GLP-
1-receptor-mediated activity as drugs, but are typically
14. Knop FK, Vilsbøll T, Højberg PV et al (2007) Reduced incretin
introduced at much higher concentrations compared with
effect in type 2 diabetes: cause or consequence of the diabeticstate? Diabetes 56:1951–1959
physiological levels of GLP-1 ,
15. Eissele R, Göke R, Willemer S et al (1992) Glucagon-like
Finally, and as a consequence of the above, DPP-4
peptide-1 cells in the gastrointestinal tract and pancreas of rat,
inhibitors can exert their clinical effects even in advanced
pig and man. Eur J Clin Invest 22:283–291
stages of type 2 diabetes. This condition is not in itself
16. Kreymann B, Williams G, Ghatei MA, Bloom SR (1987)
Glucagon-like peptide-1 [7-36]: a physiological incretin in man.
characterised by reduced meal-induced GLP-1 concentra-
Lancet 2:1300–1304
tions that are too low to be effective, as supported by recent
17. Ørskov C, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV,
Holst JJ (1986) Glucagon-like peptides GLP-1 and GLP-2,
In conclusion, while reduced GLP-1 levels have been
predicted products of the glucagon gene, are secreted separatelyfrom pig small intestine but not pancreas. Endocrinology
described individually in some participants as well as in
groups with type 2 diabetes mellitus, this does not seem to be
18. Ørskov C, Jeppesen J, Madsbad S, Holst JJ (1991) Proglucagon
a universal characteristic that is representative of all patients.
products in plasma of noninsulin-dependent diabetics and non-diabetic controls in the fasting state and after oral glucose andintravenous arginine. J Clin Invest 87:415–423
We thank K. Vollmer, Ruhr-University
19. D'Alessio D, Thirlby R, Laschansky E, Zebroski H, Ensinck J
Bochum, Germany, for providing original data from her study
(1993) Response of tGLP-1 to nutrients in humans. Digestion
(Vollmer et al. []) for further analysis.
20. Nauck MA, El-Ouaghlidi A, Gabrys B et al (2004) Secretion of
Duality of interest
The authors declare that there is no duality of
incretin hormones (GIP and GLP-1) and incretin effect after oral
interest associated with this manuscript.
glucose in first-degree relatives of patients with type 2 diabetes.
Regul Pept 122:209–217
21. Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ (1994)
Tissue and plasma concentrations of amidated and glycine-
extended glucagon-like peptide 1 in humans. Diabetes 43:535–539
1. Creutzfeldt W, Nauck M (1992) Gut hormones and diabetes
22. Åhren B, Holst JJ (2001) The cephalic insulin response to meal
mellitus. Diabetes/Metab Rev 8:149–177
ingestion in humans is dependent on both cholinergic and
2. Holst JJ, Ørskov C (2001) Incretin hormones—an update. Scand J
noncholinergic mechanisms and is important for postprandial
Clin Lab Invest 234(suppl):75–85
glycemia. Diabetes 50:1030–1038
3. Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like
23. Rask E, Olsson T, Soderberg S et al (2001) Impaired incretin
peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors
response after a mixed meal is associated with insulin resistance in
in type 2 diabetes. Lancet 368:1696–1705
nondiabetic men. Diabetes Care 24:1640–1645
24. Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ (2001)
GLP-1 and GIP. Am J Physiol (Endocrinol Metab) 290:E 550–
Reduced postprandial concentrations of intact biologically active
glucagon-like peptide 1 in type 2 diabetic patients. Diabetes
45. Mortensen K, Petersen LL, Orskov C (2000) Colocalization of
GLP-1 and GIP in human and porcine intestine. Ann N Y Acad
25. Vollmer K, Holst JJ, Baller B et al (2008) Predictors of incretin
Sci 921:469–472
concentrations in subjects with normal, impaired, and diabetic
46. Nauck MA, Holst JJ, Willms B, Schmiegel W (1997) Glucagon-
glucose tolerance. Diabetes 57:678–687
like peptide 1 (GLP-1) as a new therapeutic approach for type 2-
26. Reimann F (2006) GLP-1. Diabetes 5(suppl 1):S 78–85
diabetes. Exp Clin Endocrinol Diabetes 105:187–195
27. Jang HJ, Kokrashvili Z, Theodorakis MJ et al (2007) Gut-expressed
47. Schirra J, Katschinski M, Weidmann C et al (1996) Gastric
gustducin and taste receptors regulate secretion of glucagon-like
emptying and release of incretin hormones after glucose ingestion
peptide-1. Proc Natl Acad Sci USA 104:15069–15074
in humans. J Clin Invest 97:92–103
28. Lauffer LM, Iakoubov R, Brubaker PL (2009) GPR119 is essential
48. Miholic J, Ørskov C, Holst JJ, Kotzerke J, Meyer HJ (1991)
for oleoylethanolamide-induced glucagon-like peptide-1 secretion
Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1),
from the intestinal enteroendocrine L-cell. Diabetes 58:1058–1066
and reactive hypoglycemia after total gastrectomy. Dig Dis Sci
29. Hirasawa A, Tsumaya K, Awaji T et al (2005) Free fatty acids
regulate gut incretin glucagon-like peptide-1 secretion through
49. Fukase N, Takahashi H, Manaka H et al (1992) Differences in
GPR120. Nat Med 11:90–94
glucagon-like peptide-1 and GIP responses following sucrose
30. Zhao X, Cui YM, Zhou Y et al (2008) Exenatide pharmacokinetics
ingestion. Diabetes Res Clin Pract 15:187–195
in healthy Chinese subjects. Int J Clin Pharmacol Ther 46:459–465
50. Patzelt C, Schiltz E (1984) Conversion of proglucagon in
31. Gray GM (1970) Carbohydrate digestion and absorption. Gastro-
pancreatic alpha cells: the major endproducts are glucagon and a
enterol 58:96–107
single peptide, the major proglucagon fragment, that contains two
32. Herrmann-Rinke C, Vöge A, Hess M, Göke B (1995) Regulation
glucagon-like sequences. Proc Natl Acad Sci USA 81:5007–5011
of glucagon-like peptide-1 secretion from rat ileum by neuro-
51. Migoya E, Miller J, Luo W-L et al (2010) Sitagliptin and metformin
transmitters and peptides. J Endocrinol 147:25–31
increase active GLP-1 by complementary mechanisms in treatment-
33. Brubaker PL (1991) Regulation of intestinal proglucagon-derived
naïve patients with type 2 diabetes. Diabetes 59(suppl 1):A 156
peptide secretion by intestinal regulatory peptides. Endocrinol
52. Ranganath LR, Beety LM, Morgan LM, Wright JW, Howland R,
Marks V (1996) Attenuated GLP-1 secretion in obesity: cause or
34. Rocca AS, Brubaker PL (1999) Role of the vagus nerve in
consequence? Gut 38:916–919
mediating proximal nutrient-induced glucagon-like peptide-1
53. Hansen L, Hartmann B, Mineo H, Holst JJ (2004) Glucagon-like
secretion. Endocrinology 140:1687–1694
peptide-1 secretion is influenced by perfusate glucose concentra-
35. Anini Y, Brubaker PL (2003) Muscarinic receptors control
tion and by a feedback mechanism involving somatostatin in
glucagon-like peptide 1 secretion by human endocrine L cells.
isolated perfused porcine ileum. Regul Pept 118:11–18
54. Muscelli E, Mari A, Casolaro A et al (2008) Separate impact of
36. Roberge JN, Brubaker PL (1993) Regulation of intestinal
obesity and glucose tolerance on the incretin effect in normal
proglucagon-derived peptide secretion by glucose-dependent
subjects and type 2 diabetic patients. Diabetes 57:1340–1348
insulinotropic peptide in a novel enteroendocrine loop. Endocri-
55. Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W
nology 133:233–240
(1993) Normalization of fasting hyperglycaemia by exogenous
37. Roberge JN, Gronau KA, Brubaker PL (1996) Gastrin-releasing
glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-
peptide is a novel mediator of proximal nutrient-induced proglucagon-
dependent) diabetic patients. Diabetologia 36:741–744
derived peptide secretion from the distal gut. Endocrinology 137:
56. Meier JJ, Ritter PR, Jacob A et al (2010) Impact of exogenous
hyperglucagonemia on postprandial concentrations of gastric
38. Nauck MA, Heimesaat MM, Ørskov C, Holst JJ, Ebert R,
inhibitory polypeptide and glucagon-like peptide-1 in humans.
Creutzfeldt W (1993) Preserved incretin activity of glucagon-
J Clin Endocrinol Metab 95:4061–4065
like peptide 1 [7-36 amide] but not of synthetic human gastric
57. Ma J, Stevens JE, Cukier K et al (2009) Effects of a protein
inhibitory polypeptide in patients with type-2 diabetes mellitus.
preload on gastric emptying, glycemia, and gut hormones after a
J Clin Invest 91:301–307
carbohydrate meal in diet-controlled type 2 diabetes. Diabetes
39. Fieseler P, Bridenbaugh S, Nustede R et al (1995) Physiological
Care 32:1600–1602
augmentation of amino acid-induced insulin secretion by GIP and
58. Greenfield JR, Farooqi IS, Keogh JM et al (2009) Oral glutamine
GLP-I but not by CCK-8. Am J Physiol (Endocrinol Metab) 268:
increases circulating glucagon-like peptide 1, glucagon, and
insulin concentrations in lean, obese, and type 2 diabetic subjects.
40. Balks HJ, Holst JJ, von zur Mühlen A, Brabant G (1997) Rapid
Am J Clin Nutr 89:106–113
oscillations in plasma glucagon-like peptide-1 (GLP-1) in
59. Seifarth C, Bergmann J, Holst JJ, Ritzel R, Schmiegel W, Nauck MA
humans: cholinergic control of GLP-1 secretion via muscarinic
(1998) Prolonged and enhanced secretion of glucagon-like peptide 1
receptors. J Clin Endocrinol Metab 82:786–790
(7-36 amide) after oral sucrose due to alpha-glucosidase inhibi-
41. Beglinger S, Drewe J, Schirra J, Göke B, D'Amato M, Beglinger C
tion (acarbose) in type 2 diabetic patients. Diabet Med 15:485–
(2010) Role of fat hydrolysis in regulating glucagon-like peptide-1
secretion. J Clin Endocrinol Metab 95:879–886
60. Göke B, Fuder H, Wieckhorst G et al (1995) Voglibose (AO-128)
42. Nauck MA, Siemsglüß J, Ørskov C, Holst JJ (1996) Release of
is an efficient alpha-glucosidase inhibitor and mobilizes the
glucagon-like peptide 1 (GLP-1 [7-36 amide]), gastric inhibitory
endogenous GLP-1 reserve. Digestion 56:493–501
polypeptide (GIP) and insulin in response to oral glucose after
61. Lee A, Patrick P, Wishart J, Horowitz M, Morley JE (2002) The
upper and lower intestinal resections. Z Gastroenterol 34:159–166
effects of miglitol on glucagon-like peptide-1 secretion and
43. Hansen L, Lampert S, Mineo H, Holst JJ (2004) Neural regulation
appetite sensations in obese type 2 diabetics. Diabetes Obes
of glucagon-like peptide-1 secretion in pigs. Am J Physiol
Metab 4:329–335
(Endocrinol Metab) 287:E 939–947
62. Yasuda N, Inoue T, Nagakura T et al (2002) Enhanced secretion of
44. Theodorakis MJ, Carlson O, Michopoulos S et al (2006) Human
glucagon-like peptide 1 by biguanide compounds. Biochem
duodenal enteroendocrine cells: source of both incretin peptides,
Biophys Res Commun 298:779–784
63. Burcelin R, Da Costa A, Drucker D, Thorens B (2001) Glucose
vildagliptin added to insulin in patients with type 2 diabetes
competence of the hepatoportal vein sensor requires the presence
mellitus. Horm Metab Res 40:427–430
of an activated glucagon-like peptide-1 receptor. Diabetes
68. Vilsbøll T, Krarup T, Sonne J et al (2003) Incretin secretion in
relation to meal size and body weight in healthy subjects and
64. Kjems LL, Holst JJ, Vølund A, Madsbad S (2003) The influence
people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol
of GLP-1 on glucose-stimulated insulin secretion: effects on beta-
Metab 88:2706–2713
cell sensitivity in type 2 and nondiabetic subjects. Diabetes
69. Ryskjaer J, Deacon CF, Carr RD et al (2006) Plasma dipeptidyl
peptidase-IV activity in patients with type-2 diabetes mellitus
65. Kolterman OG, Kim DD, Shen L et al (2005) Pharmacoki-
correlates positively with HbA1c levels, but is not acutely affected
netics, pharmacodynamics, and safety of exenatide in patients
by food intake. Eur J Endocrinol 155:485–493
with type 2 diabetes mellitus. Am Health Syst Pharm 62:173–
70. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M,
Mingrone G (2009) First-phase insulin secretion restoration and
66. Elbrønd B, Jakobsen G, Larsen S et al (2002) Pharmacokinetics,
differential response to glucose load depending on the route of
pharmacodynamics, safety, and tolerability of a single-dose of
administration in type 2 diabetic subjects after bariatric surgery.
NN2211, a long-acting glucagon-like peptide 1 derivative, in
Diabetes Care 32:375–380
healthy male subjects. Diabetes Care 25:1398–1404
71. Fukase N, Igarashi M, Takahashi H et al (1993) Hypersecretion of
67. Fonseca V, Baron M, Shao Q, Dejager S (2008) Sustained efficacy
truncated glucagon-like peptide-1 and gastric inhibitory polypep-
and reduced hypoglycemia during one year of treatment with
tide in obese patients. Diabet Med 10:44–49
Source: http://nfdx.smu.edu.cn/uploadfile/201010/19/182477249.pdf
Anticipated Preferred Drug List (PDL) - January 1, 2011 Drug classes not included on this list are not managed through a Preferred Drug List (PDL). HOWEVER, THIS EXCLUSION IS NOT A GUARANTEE OF PAYMENT OR COVERAGE. Dosage limits and other requirements may apply. Please refer to the Additional Therapeutic Criteria Chart, Dosage Limitation List, Epocrates, and the Wyoming EqualityCare Provider Manual at http://wyequalitycare.org for
Sankt Marien-Hospital Buer Liebe Patientin, lieber Patient Wichtiger Hinweis zu Medikamenten Kleidung, die nutzt Sie haben eine ausgeprägte Arthrose, das heißt einen Lesen Sie bitte den dieser Information beigefügten Bitte bringen Sie zur stationären Aufnahme festes starken Gelenkverschleiß! Auch nach Ausschöp-










