Lgg - summatim



Second, updated edition
Maija Saxelin, Ph.D.
Published by
Valio Ltd, R&D
P.O. Box 30,
FIN-00039
Helsinki, Finland
Tel. +358 10 381 121
Fax +358 10 381 3019
http://www.valio.com
Lay-out by
Imageneering
Printed in Finland by
Hämeen Kirjapaino Oy
2002
Valio Ltd 2002

Table of contents
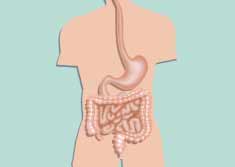
Duodenum <103Jejunum
Large intestine 1010—1012
Stool 1010—1012
Figure 1. Bacterial levels in different sections of the gastrointes-
tinal tract (cfu/g).
Bacterial concentrations at different sites of the gastrointestinal tract vary greatly
(Fig. 1). The mucous membrane of the mouth and the surfaces of the teeth have
high concentrations of bacteria, which pass, with saliva and chewed food, into the
oesophagus and thereafter into the stomach, where the food is mixed with gastric
juices and fl uidised. The acidity of the gastric juice effectively destroys most of the
bacteria that come into contact with it. Food stays in the stomach for around four
hours and is gradually released into the small intestine.
The proximal part of the small intestine is also acidic due to the acid entering
from the stomach. In addition, bile acids secreted into the proximal part of the
small intestine destroy bacteria, so the bacteria level is relatively low. As acidity
decreases and the bile acids are diluted, the bacteria level in the terminal part of the
small intestine rises. The small intestine, several metres long, is densely proliferated
with microvilli, which increase the internal surface area of the mucous membrane
so much so that, if it were spread out, the small intestine would cover the area
of a tennis court. The large surface area enables the effi cient breakdown of food
and the subsequent absorption of nutrients through the mucous membrane into
growth of external
• systemic effects
production, low pH)
Immune – non-pathogenic boost,
formation, lowers
Lowers gasproduction
Sulphate reducers
Anaerobic gram pos. cocci
decomposition andabsorption of food
Synthesis ofvitamins
Bacteria quantity log cfu/g of stool
Figure 2. The most important microbe groups, their quantities, and rough division according
to their potential for harmful and benefi cial effects (1).
the blood stream. Most of the system's immunological tissue is connected with
the small intestine and can be found immediately under the epithelial cells of the
mucous membrane.
The digestive tract pushes food and chyme forward by powerful peristaltic con-
tractions. Moving from the small intestine to the large intestine, peristalsis slows
down and sodium and chloride ions are absorbed with water into the blood stream.
As a result, the contents of the bowel become more solid. At the same time the
bacteria level also rises very sharply. The large intestine has an extensive bacterial
metabolism. Bacteria break down the nutrients remaining in the food, such as par-
tially digested proteins and fi bre components. Around half of the bulk of stools
consists of bacterial mass. Between 400 and 500 species of bacteria have been r ec-
L. rhamnosus
L. rhamnosus GG
• Group of L. rhamnosus
• Can be distinguished
Lactobacillus species
strains in whose total
from other strains of the
same species by fenotype
homology is >70%
or genotype methods
- common morphology
- typical fermentation
- similar biochemical
profile (API50CH)
- homo- or hetero-
- genome analysis
- probiotic characteristics:
- catalase-negative
adhesion, colonisation,
immunological effects etc.
G+C%= the proportion of guanine and cytosine in DNA
Table 1. The common and distinguishing characteristics of bacteria genera, species and
strains using the Lactobacillus rhamnosus GG strain as an example. The lactic acid bacteria
include 15 bacteria genera for which either homofermentative or heterofermentative lactic
acid production is typical. They are gram-positive cocci, rods or coccobacilli which are non-
spore forming and do not form catalase. Lactobacillus is one of the genera forming the lactic
acid bacteria group.
ognised in the large intestine and in stools. Moreover, between 100 and 1,000 times
more anaerobic bacteria are present than aerobic. Genome-based research meth-
ods have shown that human intestines have numerous, though as yet unidentifi ed,
species of bacteria, which do not grow in the culture media currently in use. Fig. 2
presents the most common bacteria genera or groups and their main infl uence on
the bacterial metabolism of the bowel.
Lactobacilli are part of the normal intestinal fl ora. They can be found in the
stomach and in the proximal part of the small intestine, because lactobacilli are spe-
cies that tolerate acidity relatively well. The most common species recognised on
the mucous membrane of the bowel are the Lactobacillus acidophilus group (L.
acidophilus, L. gasseri, L. jenseni, L. crispatus), L. casei, L. paracasei, L. rhamnosus,
L. agilis, L. salivarius, L. plantarum, L. pseudoplantarum, L. buchneri and L. reu-
teri. It has not been possible to identify all intestinal Lactobacillus species (2, 3).
Lactobacillus rhamnosus GG (ATCC 53103), or more briefl y Lactobacillus GG or
LGG, is a probiotic strain that has been isolated from a healthy human intestinal
fl ora. Its probiotic effects on human well-being have been widely researched and
documented in scientifi c journals. The term probiotic means live microorganisms
which, when administered in adequate amounts, confer health benefi ts on the host
(4). A probiotic must always be a certain bacterial strain (cf. Table 1) or a combina-
tion of known strains whose composition remains stable and whose effects have
been demonstrated in studies performed on humans and documented in scientifi c
journals. There is a great deal of research data about Lactobacillus GG in the care
and preventive treatment of different intestinal symptoms. This summary describes
the effects of Lactobacillus GG in healthy intestines, its known clinical uses, and
the mechanisms underlying these effects.

1.1 Colonises temporarily
LGG Colony
Figure 3. A) Lactobacillus GG colonies among other stool lactobacilli fl ora in an
MRS-agar dish. B) Lactobacillus GG bacteria (light microscopy, gram staining).
In healthy intestines so-called colonisation resistance prevails. This naturally prevents
exogenous microbes, both harmful and harmless to the intestine, from establishing
themselves permanently in the digestive tract. Colonisation resistance depends
on chemical (e.g. gastric acid, bile acids, enzymes), physical-biological (adhesion,
prevention/elimination of harmful bacteria, peristalsis) and immunological factors.
Anaerobic bacteria in particular are involved in maintaining colonisation resistance.
Resistance easily breaks down, for example, as a consequence of antibiotic or other
medical treatment, and this can cause diarrhoea and other intestinal disorders.
Lactobacillus GG tolerates intestinal conditions, such as stomach acidity and
bile acids, better than ordinary yoghurt bacteria (5). It adheres both to the intestinal
mucus (6-8) and to epithelial cells and tissues in vitro (9) and ex vivo (10, 11).
Lactobacillus GG also produces antimicrobial material (12 – 14). Because of its typi-
cal colony morphology and other characteristic features, it is possible to analyse
Lactobacillus GG from stool and biopsy samples, even though these contain a great
many other lactobacilli (Fig. 3). In addition to the colony morphology genetic rec-
ognition of the strain is needed to confi rm its identity (15). The capacity of Lacto-
bacillus GG to stay alive within the digestive tract has been shown in many studies
both in healthy people and in cases of illness (5, 16 – 19).
The attachment of Lactobacillus GG to the mucous membrane of the intestine
has been shown by taking biopsy samples from the surface of the large intestine
and by identifying Lactobacillus GG in them (20, 21). These studies demonstrated
that the Lactobacillus GG strain adheres temporarily to the mucous membrane and
stays there for about a week. The colonisation is not permanent because Lactoba-
cillus GG triggers an immune response in the mucous membrane, which prevents
permanent colonisation (22, 23). Lactobacillus GG, given immediately after birth,
was still present in stools in approximately half of premature (24) and full-term
(25) babies 2-4 weeks after the dosage ended. Administration of Lactobacillus GG
to mothers at the time of delivery yielded a long-lasting colonisation (26). Thus, the
permanent colonisation of the intestines of newly born babies may be possible.
1.2 Adapts to healthy intestinal fl ora
The composition of human intestinal microfl ora appears fairly constant. Conven-
tional culture methods only measure bacterial groups or genera and it is evidently
not yet possible to cultivate some of the intestinal bacteria. Changes in the compo-
sition of healthy intestinal microfl ora may occur on a species and strain level and
cannot be measured by conventional culture methods (27).
Lactobacillus GG enhanced the adhesion of bifi dobacteria in vitro (28). Milk
products fermented with Lactobacillus GG, or Lactobacillus GG given in powder
form, have been shown either to increase signifi cantly the quantity of bifi dobacte-
ria and lactobacilli (29, 30) or else showed no changes in the composition of
the fl ora (5, 31). However, in a recent study using the FISH method,
mentation of Lactobacillus GG capsules increased the level of total anaerobic fl ora,
especially bifi dobacteria, bacteroides and clostridia (32), but the level of lactobacilli/
enterococci did not increase. Although Lactobacillus GG becomes part of the bow-
el's microbial fl ora, it does not displace all other lactobacilli. The relative proportion
varies from individual to individual but it usually accounts for less than a quarter of
the total quantity of lactobacilli (33, 34). However, the overall proportion of Lactoba-
cillus GG may be greater on the mucous membrane of the intestine (20, 21).
1.3 Improves colonisation resistance
Mortality %
Colonized with LGG
Days after infection
Figure 4. The mortality of mice with germ-free intestines vs.
Lactobacillus GG colonised intestines following infection with
Salmonella typhimurium (41).
In addition to the prevention of the adhesion and the colonisation of pathogens,
colonisation resistance also means that intestinal bacteria are not translocated to
the blood circulation and other sterile body sites. Animal experiments have shown
that the addition of Lactobacillus GG to animal feed improves colonisation resist-
ance and protects the intestine from harmful bacteria. Salmonella levels were con-
siderably lower in the intestines of mice that received Lactobacillus GG than in
the placebo group. Furthermore, the life spans of Salmonella-infected ex-germ-free
mice were considerably extended by Lactobacillus GG (Fig. 4). Lactobacillus GG
also protected the mice from Candida albicans infection, reduced the growth of
yeast and prolonged the life of the mice. The protective infl uence was based on
both immunological and non-immunological factors (35, 36). Lactobacillus GG was
also shown to prevent the attachment of Clostridium diffi cile onto the wall of ham-
sters' intestines and, in combination with xylitol, to protect hamsters from death
caused by C. diffi cile (37).
Lethally irradiated mice died of bacteraemia of intestinal origin, but no cases of
lactobacilli or Lactobacillus GG bacteraemia were observed. Rather, oral Lactoba-
cillus GG intake was reported to prolong the survival of the mice (38). The infl u-
ence of different probiotics on the extent of liver injury, bacterial translocation and
intestinal fl ora in an acute liver injury model with rats was studied (39). The liver
injury was induced by intraperitoneal injection of the rats with D-galactosamine.
The bacteria were administered rectally eight days before the liver injury. Lacto-
bacillus GG, which was one of the studied strains, reduced signifi cantly the bacte-
rial translocation to portal and arterial blood, and the liver and mesenteric lymph
nodes. The liver injury, measured as alanine aminotransferase, was less serious in
the Lactobacillus GG group compared to the control group (39). In another experi-
mental study, liver injury was caused by chronic alcohol consumption in rats. The
blood of the rats had a lower level of endotoxin and a less injured liver when they
received Lactobacillus GG in their diet (40).
In a study with mice, the translocation rate of Salmonella to several organs was
signifi cantly reduced by the administration of Lactobacillus GG (41). In a study
with neonatal rabbits, Lactobacillus GG was shown to signifi cantly reduce small-
bowel colonisation by Escherichia coli. It also reduced the frequency of intestinal
bacterial translocation in the mesenteric lymph nodes and in the spleen (42).
In vitro studies support the theory of improvement of colonisation resistance
by Lactobacillus GG. Although adhesion of Salmonella typhimurium on intesti-
nal mucus was enhanced by Lactobacillus GG (43), the invasion to the cells was
reduced (41). Furthermore, Lactobacillus GG reduced the adhesion of enterpatho-
genic Escherichia coli on intestinal mucus (43) and on intestinal cells (44). The
translocation of E. coli through Caco-2 enterocyte monolayer was also reduced by
pre-incubation of the monolayer with the probiotic (45). These results show that
Lactobacillus GG is not an invasive organism. It strengthens the barrier mechanisms
in the intestine either directly or via the modifi cation of intestinal microecology.
1.4 Reduces harmful
metabolism in the colon
• inhibits the invasion
of harmful microbes
• prevents the growth of harmful bacteria
Boosting immune response
• reinforces the mucosal barrier
• promotes recovery in infections
• promotes the immunological
Effect on large intestine enzyme activity
• lowers activity of carcinogen-
activating enzymes
• decreases secretion of toxic compounds• increases quantity of short chain fatty acids
Binding mycotoxins and
bacterial toxins
Figure 5. The effects of Lactobacillus GG on intestinal microecology.
Intestinal bacteria break down the components of food into a more easily digest-
ible form, affect the local immune response of the mucous membrane and promote
colonisation resistance against pathogens. The intestinal microfl ora have also been
shown to participate in the metabolism of harmful compounds in the human diet
and in breaking down drugs as well as toxins (46). The Western diet, with its high
fat and low fi bre content, supposedly increases the risk of colon cancer. Colonic
microfl ora have been shown to be linked to this risk. The hydrolytic enzymes of
the bacteria change pre-carcinogenic compounds in food into carcinogenic com-
pounds. Epidemiological studies have demonstrated that a high consumption of fer-
mented milk products may reduce the risk of colon cancer. Those in the risk group
had less lactic acid bacteria in their intestines than those in the low-risk group (47).
The mechanism may be the protective effect of calcium or conjugated linolic acid,
the low activity of hydrolytic enzymes in lactic acid bacteria (48), and the effect of
lowering the pH of bowel contents. A diet containing Lactobacillus GG-fermented
milk has been shown to lower the activity of hydrolytic enzymes (β-glucuronidase, glycocholic acid hydrolase, nitroreductase) and tryptic activity in the colon con-
tents, and the urinary secretion of toxic compounds. Some of these studies have
also found a lowering of the pH of stools and a decrease in the amount of ammonia
(29, 30, 49-52). All these factors together (Fig. 5) suggest that Lactobacillus GG,
and particularly the fermented milk products that contain it, change the bowel
contents so as to lower the risk of tumour formation.
Further support for the idea has been obtained from experimental studies. In
one study (53) intestinal tumours were chemically induced in rats that were being
fed on a high-fat diet. When the rats' diet contained Lactobacillus GG, signifi cantly
fewer tumours formed in their large intestines, and the number of tumours per
tumour-bearing rat was signifi cantly lower than those in the placebo group (Fig.
6). This work showed that the initiation of tumour formation can be reduced or
delayed by Lactobacillus GG, but that lactobacilli have no effect on the advance of
tumours that have already begun (53).
In another experimental study, bladder cancer cells were transferred to mice
and the effect of oral administration of Lactobacillus GG on the development of
tumour formations was studied (54). The administration of Lactobacillus GG, or
saline as a placebo, was started immediately after implantation of the tumour cells
or one week later. Early administration of Lactobacillus GG reduced the size of
the tumours signifi cantly or totally inhibited their formation. The levels of spleen
T-lymphocytes (CD3, CD4, CD8a) and natural killer cells were signifi cantly higher
in the Lactobacillus GG group compared to the placebo group. The levels of lym-
focytes and granulocytes were also higher in the tumours of the animals in the
Lactobacillus GG group. The conclusion was that Lactobacillus GG may inhibit the
growth of tumours via an immune response (54).
Afl atoxins (AF's) are a group of structurally similar toxins produced by the
common moulds Aspergillus fl avus and A. nomius. The toxins are potentially car-
cinogenic and harmful in food and feed. They can be produced in conditions con-
ductive to the growth of the mould. The risk of the growth of fungus is higher in
conditions with high relative humidity and temperature,
and without competing
Small bowel
Small bowel
Figure 6. The effect of Lactobacillicus GG on the formation of chemically induced
tumours in rats (53).
microfl ora. In laboratory tests Lactobacillus GG has been shown to bind AFB (55,
56) and AFM (57). The binding of AF's seems to be mainly extra-cellular and stable,
removing about 80% of the AFB from a liquid growth medium (58, 59). AFM is the
main form found in the milk of lactating animals, indicating the contamination of
feed by AFB . Pierides et al (57) demonstrated that lactobacilli and lactococci can
potentially be used for the binding and removal of afl atoxin M from milk.
In an in vivo study AFB was injected with Lactobacillus GG bacteria into
chicken duodena, and the concentration of AFB in the luminal fl uid and tissues of
sacrifi ced animals was analysed after one minute. Half the toxin concentration was
removed from the luminal fl uid. The complex was stable under the luminal condi-
tions for a one-hour test period and it reduced the uptake by the intestinal tissue by
74% (60). Based on these studies, is would seem possible to remove AF's from the
intestine by Lactobacillus GG on a signifi cant level and to reduce the toxic load of
the intestine via excreted bacteria.
Most alcohol is metabolised in the liver but recently the role of oro-gastroin-
testinal bacteria has also been realised. Many intestinal facultative anaerobic and
aerobic bacteria can oxidise ethanol to acetaldehyde, which itself is harmful to the
mucous membrane. Nosova et al. (61) studied the capacity of intestinal bifi dobac-
teria, lactobacilli and Lactobacillus GG to oxidise ethanol to acetaldehyde. In gen-
er al, lactobacilli had weak oxidation potential, the most active being Lactobacillus
GG in anaerobic conditions. It also had the highest ability to degrade acetaldehyde
to acetate. The degradation of acetaldehyde by bacteria was generally inhibited by
ethanol, but Lactobacillus GG was not very sensitive to that chemical. The circum-
stances on the colon mucous membrane to oxidise acetaldehyde are favourable but
the level of probiotics should be fairly high, and the relevance of the results remains
to be shown in human trials.
1.5 Does it alleviate constipation?
Lactic acid bacteria are generally considered to alleviate constipation. However,
there is little clinical proof of their effi cacy in severe constipation, and many stud-
ies have been conducted without good research procedures or statistical analysis.
Lactobacillus GG-fermented milk products have been seen to slightly increase the
water content of stools but they have had no effect on the frequency in defecation
of healthy volunteers (29, 51), nor did they have any effect on transit time in those
suffering from constipation (62). However, Lactobacillus GG enhanced the laxative
effects of rye fi bre and had a tendency to reduce intestinal symptoms caused by
the fi bre (62). Suggestions of an increase in bowel activity were obtained in one
Lactobacillus GG study with and without lactulose (63).
In Japanese studies, the daily consumption of an Lactobacillus GG-fermented
milk product was shown to signifi cantly increase the level and ratio of faecal bifi do-
bacteria and lactobacilli and to reduce the level of lecitinase negative clostridia. The
consumption of the product also increased signifi cantly defecation frequency and
relieved discomfort after the bowel movement. There was a tendency to increase
the faecal moisture and decrease pH and ammonia content (30, 64). These results
indicate that not all studies are necessarily applicable to other cultures with a
totally different diet composition from that of the West.
The innate and adaptive immune systems are the two compartments traditionally
described as important for the immune response. Macrophages, neutrophils, natu-
ral killer (NK) cells and a serum complement represent the main components of
the innate system, in charge of the fi rst line of defence against many microorgan-
isms. However, there are many agents that this system is unable to recognize. The
adaptive system (B and T cells) provides an additional means of defence, while
cells of the innate system modulate the beginning and subsequent direction of
adaptive immune responses. Several soluble compounds (cytokines, interleukins,
interferons) are involved in the modulation of the immune system.
In vitro Lactobacillus GG induced the expression and production of the proin-
fl ammatory Th-1-type cytokines TNF-α, IL-1β, IL-6 and IL-18 in peripheral blood mononuclear cells, but not the Th-2 type cytokine IL-4 and relatively little IL-10
(65, 66). Lactobacillus GG also activated the transcription factor NF-κB, which is the central activator of innate immune response, and the Toll-like receptors TLR1
and TLR2, which mediate bacterial recognition and cellular signalling (67, 68). The
results suggest that Lactobacillus GG is able to activate innate immune responses.
In an animal study, orally administered Lactobacillus GG bacteria had dose- and
duration-dependent immunomodulatory effects on the proliferative activity of B
and T murine spleen lymphocytes ex vivo. A dose relevant to human nutrition
enhanced T-cell proliferation at the optimal concanavalin A concentration and B-cell
proliferation at the optimal and supraoptimal concentrations of lipopolysaccharide
(69). In a human intervention, Lactobacillus GG enhanced signifi cantly the forma-
tion of the phagocytic receptors CR1, CR3, FcγRIII and FcαR in neutrophile blood cells in healthy humans but suppressed the response of milk-hypersensitive human
volunteers during a milk challenge. The conclusion was that probiotic bacteria
appear to modulate the non-specifi c immune response differently in healthy sub-
jects and hypersensitive subjects by immunostimulation in healthy and by down-
r egulation in hypersensitive ones (70).
Human administration of Lactobacillus GG combined with an oral rotavirus vac-
cine enhanced the formation of rotavirus specifi c IgM-secreting cells and rotavi-
rus specifi c IgA in sera (71). There was also a trend towards a greater increase in
antigen-specifi c IgA response when Lactobacillus GG was given with an oral Sal-
monella typhi Ty21 vaccine (72). In another study with Salmonella typhi vaccine,
Lactobacillus GG enhanced signifi cantly the IgG and IgA response to the vaccine
(73). In children with rotavirus infection, Lactobacillus GG increased the formation
of immunoglobulin secreting cells in all immunoglobulin classes and in rotavirus
specifi c antibody-secreting IgA cells (74-76).
These studies show that Lactobacillus GG both activates the innate immune
response and enhances adaptive immunity, especially during infections.
3.1 Respiratory infections
Otitis media
Figure 7. The effect of Lactobacillicus GG on the prevalence of respiratory infections and
frequency of antibiotic treatment in children. The children drank either LGG milk or ordinary
milk during daily meals for a period of seven months (77).
Day care centres expose children to infections, especially of the upper respiratory
tract. Overall, more than 90% of child absenteeism from day care is caused by infec-
tious diseases. In addition to discomfort to the children and inconvenience to their
families, illnesses are costly to society. The greatest costs result from the parents'
absence from work because of a child's illness.
A long-term study was made to see if consumption of Lactobacillus GG had an
effect on infections in children (77). A total of 571 children from 18 day care cen-
tres in Helsinki, Finland, participated in the study. During the seven-month research
period, half the children were given pasteurised milk that contained Lactobacillus
GG (5-10x105 cfu/ml) to drink with all meals, and the other half were given ordi-
nary milk. The average milk consumption was 260 ml/day. The children's health
w as carefully monitored: symptoms in the respiratory and digestive tract, as well as
absences from the day care centres, were recorded daily by the parents. Doctors'
diagnoses and antibiotic treatments were also reported. Children in the Lactobacil-
lus GG group had fewer days of absence from day care because of illness (4.9 vs.
5.8 days, p=0.03), an 11% difference. There was also a relative reduction of 17% in
the number of children who suffered from respiratory tract infections with com-
plications, especially ear infections, in the Lactobacillus GG group (Fig. 7). The
number of children who received antibiotic treatment for respiratory infections
was 19% lower in the Lactobacillus GG group than in the placebo group. The con-
clusion was that Lactobacillus GG may reduce children's respiratory infections
and their severity.
Risk of
dental caries (%)
Figure 8. The effect of Lactobacillus GG on
the risk of dental caries. The children drank
either LGG milk or ordinary milk during daily
meals for a period of seven months (79).
Teeth are in continuous interaction with the surrounding world, mainly saliva and
whatever you put in your mouth. Milk provides calcium and phosphates in the
mouth, which causes remineralisation of places demineralised by caries. Milk and
dairy products are important elements in children's nutr
ition and dental health,
since teeth at this point are particularly vulnerable to attack from caries, having
just begun the mineralisation process. Lactobacilli are common bacteria in the oral
cavity, but they are generally regarded as potentially cariogenic, growing together with
streptococcus mutans. However, in in vitro studies, Lactobacillus GG showed slow or
no fermentation of sucrose and lactose (34), and suppressed the growth of the mutans-
group streptococci, which are the indicator bacteria of dental caries (78).
The long-term effect of Lactobacillus GG on the risk of caries was studied in 18
day care centres in Helsinki, Finland (79). In a randomised, placebo-controlled study
children were given pasteurised milk that contained Lactobacillus GG (5-10x105
cfu/ml) or standard milk as a placebo, fi ve days a week for seven months with their
day care meals. The children's oral health was recorded at baseline and at the end,
and mutans-group streptococci were cultivated from saliva-dental plaque samples.
The risk was classifi ed as high if the child had a score of decayed/missed/fi lled
teeth (dmft) or initial caries of >0 and a mutans streptococci count >105 cfu/ml,
as moderate if either of these was detected, and as no risk if dmft was 0 and the
mutans streptococci count <105 cfu/ml. The results showed less dental caries in
the Lactobacillus GG group at the end of the study and lower mutans streptococci
counts. The risk of dental caries was 44% lower in the Lactobacillus GG compared
to the placebo (OR=0.56, p=0.01; Fig. 8). The conclusion was that the milk contain-
ing the probiotic Lactobacillus GG bacteria may have benefi cial effects on chil-
dren's dental health beyond the effect of standard milk.
LGG and diarrhoea 4.
4.1 Preventive treatment
4.1.1 Acute diarrhoea in children
Figure 9. The effect of Lactobacillus GG
on the occurrence of acute diarrhoea. Chil-
dren hospitalised for non-diarrhoea rea-
sons were given either Lactobacillus GG or
placebo throughout their stay (81).
A fi fteen-month study surveyed the incidence of diarrhoea among under-nourished
Peruvian children living in poor conditions (80). One half of a group of 204 chil-
dren received a Lactobacillus GG dose six times a week at home and the other
half, a placebo. Altogether, 954 diarrhoea episodes were recorded and the infectious
agent was determined in 58% of the cases. Pathogenic bacteria were isolated in
about one half of the cases, parasites in one half, and viruses in one third. Mixed
infections were therefore very common. The Lactobacillus GG group was found
to have signifi cantly fewer diarrhoea episodes caused by the adenovirus;
ference was found in the incidence of other pathogens. Looking at the data as a
whole, the incidence of diarrhoea in the Lactobacillus GG group was 5.2 episodes
per child per year, compared with 6.0 episodes in the placebo group (p=0.028).
Diarrhoea prevention was most effective in children aged 18-29 months (4.9 epi-
sodes LGG vs. 6.2 episodes placebo, p=0.004) and was primarily of benefi t to chil-
dren who were not breastfed. Lactobacillus GG had no effect on the duration of
diarrhoea in this study (80).
Another, short-term clinical study, to evaluate the reduction of the risk of diar-
rhoea by Lactobacillus GG, was made in a Polish hospital (81). Children hospital-
ised for reasons other than diarrhoea were given Lactobacillus GG or a placebo
twice daily during their hospital stay. The risk of diarrhoea was reduced signifi -
cantly in the Lactobacillus GG group compared to the placebo group (6.7% vs.
33.3%, RR 0.2, p=0.002). Surprisingly, there was an equal prevalence of rotavirus
infection in both groups, but the administration of Lactobacillus GG signifi cantly
reduced the risk of rotavirus gastroenteritis (1/45 vs. 6/36, RR 0.13, p=0.02; Fig. 9).
This result poses an interesting question as to the potential of Lactobacillus GG to
protect against rotavirus after a non-diarrhoeal infection.
4.1.2 Antibiotic-associated side effects
Possibly the most common indication for the clinical use of probiotics is their abil-
ity to prevent the side effects of antibiotics, such as diarrhoea and abdominal pain.
Antibiotics change the composition of the bowel microfl ora, allowing the possibil-
ity for opportunistic pathogens such as Clostridium diffi cile to proliferate. Antibiot-
ics also interfere with the metabolism of the microfl ora, for instance, by impeding
the formation of short-chain fatty acids in the colon. Probiotics are therefore well
suited for maintaining or restoring the balance of the bacterial fl ora.
The effect of Lactobacillus GG taken in a capsule form has been proved to
reduce the side effects of antibiotics in children. In a randomised, double-blind,
placebo-controlled study, common acute infections in 188 children were treated
by commonly used antibiotics, and under the care of family physicians (82). Half
the patients received 1 – 2 Lactobacillus GG capsules (1x1010 cfu) once a day, the
Symptoms (%)
Figure 10. The effect of Lactobacillus GG on
intestinal symptoms caused by antibiotics (82).
other half received identical placebo capsules without the bacteria (one capsule
for children <12 kg, two capsules for those >12 kg). Any gastrointestinal complaints
were monitored via telephone interviews. Signifi cantly less diarrhoea and daily def-
ecations were reported in the Lactobacillus GG group than in the control group.
Furthermore, the stools were more solid and the study group had less abdominal
pain than the placebo group (Fig. 10). Lactobacillus GG did not cause any side
effects in this or in other studies.
Another study was conducted in Finland with children prescribed oral antibiot-
ics for the treatment of acute respiratory infections (83). The children were ran-
domised to receive either one placebo (n=58) or one Lactobacillus GG (n=61)
capsule twice a day (2x1010 cfu). The parents kept a daily symptom diary at home
and recorded stool frequency and consistency. In cases of diarrhoea, stool samples
were analysed for adenovirus, rotavirus, calicivirus and astrovirus as well as for
Salmonella, Shigella, Yersinia, Campylobacter, Clostridia diffi cile, Staphylococcus
aureus and yeasts. Within two weeks of antimicrobial treatment the incidence of diar-
rhoea was 5% in the Lactobacillus GG group and 16% in the placebo group (p=0.05).
In diarrhoeal episodes two cases of C. diffi cile were found (one in each group) and
three cases of Norwalk-like calicivirus were positive (one in the Lactobacillus GG
group, two in the placebo group). No other pathogens were recovered (83).
In a small study with adult volunteers Lactobacillus GG reduced signifi cantl y
diarrhoea caused by erythromycin and somewhat reduced abdominal pain (84). In
the study, volunteers took a Lactobacillus GG-fermented milk product or a placebo
yoghurt (post-pasteurised yoghurt without the living bacteria) in the morning and
evening, half an hour after they had taken an antibiotic.
Armuzzi et al. studied the effect of Lactobacillus GG on gastrointestinal discom-
fort caused by the antibiotic treatment of Helicobacter pylori (85, 86). In a pilot
study (86) 120 asymptomatic volunteers carrying H. pylori were randomised to the
eradication therapy with pantoprazole, clarithromycin and tinidazole for one week
or the same regimen supplemented with Lactobacillus GG (6x109 cfu/sachet) for
two weeks. Lactobacillus GG was taken 2 h after breakfast and dinner, mixed
with water. Bloating, diarrhoea and taste disturbances were the most frequent
side effects during the eradication week and were signifi cantly reduced in the
Lactobacillus GG group. The same pattern was observed throughout the follow-up
period. The overall assessment of treatment tolerability showed a signifi cant trend
in favour of the Lactobacillus GG-supplemented group (p=0.03).
In another, double-blinded, placebo-controlled study, 60 healthy asymptomatic H.
pylori positive volunteers were randomised to one week therapy with rebeprazole,
clarithromycin, tinidazole and Lactobacillus GG (6x109 cfu/sachet) for two weeks,
or to the same regimen with a placebo preparation (85). Again, diarrhoea, nausea and
taste disturbances were signifi cantly reduced in the Lactobacillus GG group com-
pared to the placebo (RR=0.1, 0.3 and 0.5 respectively). An overall assessment of
treatment tolerability showed a signifi cant difference in favour of the Lactobacillus
GG group (p=0.04). There was no difference between the groups in the success of
the eradication of H. pylori (in both studies it was about 80%), but supplementation
with Lactobacillus GG helped to improve the tolerability of the antibiotics.
A randomised, double-blinded, placebo-controlled study was performed with 267
initially hospitalised adult patients treated with intravenous or oral antibiotics for
a presumed or proven infection (cellulites, pneumonia, urinary tract infection and
pyelonephritis) (87). The main groups of antibiotics were β-lactams (cephalosporins 60%, penicillin 27%) and fl uoroquinolones (39%). Lactobacillus GG (1x1010 cfu) or
placebo capsules were given twice a day. The Lactobacillus GG intervention had no
effect either on the incidence or on the duration of mild or severe diarrhoea.
Broad-spectrum antibiotics, especially for immunocompromised patients, can
Ref. 90, MD plate
E-test, AB Biodisc
for gram posit., Radiometer
Amoxycillin / Clavulanate
Trimethoprim / Sulphamethoxazole
Table 2. The antibiotic sensitivity of Lactobacillus GG in MIC (minimum inhibitory concentra-
tion) values.
cause serious D-lactic acidosis due to the intestinal lactobacilli producing D-lactic
acid. Lactobacillus GG produces L-lactic acid and has been successfully used to
treat one such case (88).
The susceptibility of LGG to antibiotics
Although Lactobacillus GG is susceptible to the most common antibiotics (89, 90)
(Table 2), it has been shown to survive in the intestines during antibiotic treatment
in most test subjects. Lactobacillus GG was isolated in stools in 75, 76 and 57% of
the test subjects being treated with erythromycin, ampicillin and penicillin respec-
tively (5, 33, 84). The survival of Lactobacillus GG can be explained by the antibi-
otic and bacterial preparations being taken at different times, and possibly by the
lower antibiotic level in the bowel than in the blood stream.
Some species of lacto-
bacilli are naturally resistant to vancomycin, including all strains of the species L.
rhamnosus, L. casei, L. plantarum and L. reuteri. It is also pertinent to ask whether
the genes responsible for vancomycin resistance can be transferred to other bacte-
ria. Vancomycin-resistance genes in Lactobacillus GG were shown to differ from
the van genes in enterococci, and were not transferred to enterococci (90, 91).
Antibiotic-resistance genes can sometimes be transferred via plasmids. Lactobacil-
lus GG does not carry plasmids and is safe in that sense, too (91).
4.1.3 Traveller's diarrhoea
Intestinal troubles are a common complaint among those travelling from cold or
cool climates to warm and tropical countries. Lactic acid bacteria are often used
to prevent intestinal troubles while travelling, even though few studies have been
conducted on their effi cacy. The fi rst Lactobacillus GG study was conducted on
Finnish tourists (n=756) who visited two resorts in Turkey (92). An average of 43.8%
of the travellers had diarrhoea. Lactobacillus GG taken twice a day signifi cantly
reduced the incidence of diarrhoea in those staying one week in one of the resorts
but not in the other. No explanation for the difference in effectiveness between
the resorts was found, but it is possible that the dose (1x109 cfu twide daily) of
Lactobacillus GG used in the study was too low.
The second study was carried out with American tourists (n=245) whose desti-
nations were primarily in Asia, East Africa, South America, India and Central America
(93). One Lactobacillus GG capsule per day provided statistically signifi cant pro-
tection. In the Lactobacillus GG group the average incidence of diarrhoea was
3.9%, whereas in the placebo group it was 7.4% (p=0.05), i.e. a protection factor
of 47%. Travellers who had previously suffered from tourist diarrhoea benefi ted the
most. The best protection against traveller's diarrhoea is still good personal hygiene
such as hand washing, drinking bottled water and drinks without ice cubes, and the
consumption of adequately cooked, hot food. However, Lactobacillus GG provides
extra protection.
4.2 Treatment studies
4.2.1 Rotavirus diarrhoea
Duration of
diarrhoea,days
Figure 11. The effect of Lactobacillus GG on the duration of acute
diarrhoea. Ambulatory children were given Lactobacillus GG mixed in
milk or mother milk substitute for a maximum of fi ve days (101).
Lactobacillus GG accelerates recovery in acute diarrhoea. Studies have been pri-
marily conducted on children with rotavirus, which is the most common cause of
diarrhoea in western countries. Lactobacillus GG accelerated by about one day the
recovery of children hospitalised with acute diarrhoea (Table 3). The acceleration
of recovery was generally noted on the second day: children treated with Lactoba-
cillus GG defecated less often and their stools were more solid than those in the
placebo group (94). Children treated at home, with Lactobacillus GG administra-
tion starting on the second day after the onset of diarrhoea, suffered symptoms
for approximately half as long as the placebo group (Fig. 11). In addition, these
children spread the virus for a shorter time than those in the placebo group, since
after six days signifi cantly fewer of them excreted rotavir
us in their stools than in
Duration of diarrhoea
days or hours (SD)
LGG group
39 Fermented milk
2.8 (1.2) Lactophilus(1
2.6 (1.4) Yoghurt starter 0.04
2.6 (1.3) Placebo
Inactivated powder
after 2 days treatment after 2 days treatment
123 Within ORS: single or
17.7 (12.2–25.6) h
30.4 (23.6–39.3) h
multiple dose, after
(after ORS or placebo)
Rotavirus posit.
1) Other L.rhamnosus strain2) Ambulatory
Table 3. Lactobacillus GG in the treatment of acute diarrhoea. Hospitalised patients were
given LGG twice a day after oral rehydration (ORS), if not otherwise stated.
the placebo group. The effect of Lactobacillus GG in the treatment of rotavirus diar-
rhoea has been confi rmed through a multi-centre study carried out by the ‘diarrhoea
working group' of the European Society of Paediatric Gastroenterology, Hepatology
and Nutrition (95). In a recent systematic review of published studies, Lactobacillus
GG was shown to be, so far, the only probiotic strain with a consistent effect on the
duration of diarrhoea and on the risk of diarrhoea lasting >3 days (96).
The best recovery is obtained when Lactobacillus GG is administered as early as
possible after the symptoms of diarrhoea have appeared. If rehydration is needed,
then Lactobacillus GG treatment is best started at the same time as oral rehydra-
tion (95, 97). The effect of the treatment was the same whether Lactobacillus GG
was administered in powder form (capsule/opened) or in the form of a fermented
dairy product (cf. Table 3). It is also worth mentioning that heat-inactivated Lacto-
bacillus GG accelerated the recovery in acute diarrhoea as effectively as the living
bacteria (75) but the immune effect differed.
4.2.2 Other types of acute diarrhoea
Studies carried out in Thailand and Pakistan using Lactobacillus GG in the treat-
ment of acute diarrhoea showed that recovery from watery diarrhoea was acceler-
ated, but not from diarrhoea with bloody stools (98, 99). Nor did Lactobacillus GG
succeed in removing Klebsiella oxytoca from the intestines of premature babies
(100). On the other hand, an Italian study (101) and the European multicenter study
(95) showed a signifi cant effect of Lactobacillus GG both in rotavirus infections
and in cases where the cause of the diarrhoea was unknown. Similarly, in a study
performed in Petroskoi (Russia), the difference was signifi cantly in favour of the
Lactobacillus GG group, even though only 27% of the patients had rotavirus diar-
rhoea. About a fi fth had diarrhoea caused by known bacteria and in about half the
cases the aetiology was unknown (102). Therefore, it seems that Lactobacillus GG
is effective not only in rotavirus diarrhoea but also in some infections where the
aetiology is unknown. If the mucous membrane is profoundly infl amed or even
destroyed, the effectiveness of Lactobacillus GG remains unclear.
4.2.3 Are all lactobacilli effective?
Lactobacillus GG was compared with another L. rhamnosus strain (Lactophilus®,
Laboratoires Lyocentre, France), traditionally used in the prevention and treatment of
children's diarrhoea in Finland, and with a common yoghurt starter culture powder
(76). Only Lactobacillus GG was found to accelerate recovery from diarrhoea. This
suggests that different bacterial strains within the same species have signifi cant
ferences in their effect. The clinical effi cacy of every single strain must therefore be
proved in carefully conducted studies, preferably made by several study groups.
4.3 Mechanisms behind the effects
4.3.1 Infections - enhancing immune response and
balancing intestinal microfl ora
Figure 12. The effect of Lactobacillus GG on immune
response. A) Total number of immunoglobulin secreting
cells (ICS) in the acute phase of rotavirus diarrhoea and
B) total number of rotavirus specifi c IgA secreting cells
(sASC) three weeks after infection. Points represent indi-
vidual patients, horizontal lines the means (75).
Several studies have shown that the administration of Lactobacillus GG enhances
immune response during rotavirus diarrhoea. Lactobacillus GG signifi cantly
increased both non-specifi c immune response (in immunoglobulin classes IgG, IgA,
IgM) in the acute stage of diarrhoea and in the quantity of rotavirus-specifi c anti-
body-secreting cells in the follow-up stage. Three weeks after the infection, 90% of
those who received Lactobacillus GG had a rotavirus-specifi c IgA response, com-
pared with only 46% in the placebo group (Fig.12). It seems, therefore, that bacte-
rial treatment gives additional protection against re-infection. This and later studies
(74-76) show that the infl uence of Lactobacillus GG is specifi cally mediated
through an enhanced immune response. Lactobacillus GG has also been found
to induce an enhanced response with an oral rotavirus vaccine (71). Not all lacto-
bacilli, however, increase the immune response, which in part explains the dif-
ferences in their effects (76). The effect of Lactobacillus GG on innate defence
systems might also contribute to the accelerated recovery from diarrhoea, e.g.
enhanced production of induced nitric oxide (103), mucin production (44) and
increased rate of enterocyte proliferation (104).
Another possible contributing factor in shortening the duration of diarrhoea is
the balancing of intestinal microfl ora. Acute osmotic diarrhoea may be followed by
bacterial imbalance and the overgrowth of specifi cally urease-producing bacteria.
These may release ammonia, which is toxic to the intestinal mucous membrane.
However, urease activity was not elevated in those subjects treated with Lactoba-
cillus GG (105). Lactobacillus GG adheres to intestinal mucus (106) and is able to
survive in the bowel even during acute diarrhoea, making it suitable for balancing
intestinal microfl ora (19, 105).
4.3.2 Antibiotics and balancing intestinal fl ora
Data has been obtained on the effi cacy of antimicrobial medication and Lactobacillus
GG in the treatment of shigellosis and on their infl uence on the composition of bowel
microfl ora (107). After ten days of treatment, 79% of the children had recovered in the
group that received Lactobacillus GG and 67% in the group that only received medici-
nal treatment (p<0.05). Due to the paucity of the material (n=31) no far-reaching con-
clusions can be drawn about the clinical signifi cance of the treatment.
At the start of treatment, the subjects' intestinal microfl ora was completely
unbalanced, i.e. the quantity of aerobic bacteria was greater than that of anaerobic
bacteria (Fig. 13). Furthermore, there were hardly any lactobacilli at all and the rela-
tive proportion of subordinate bacteria had risen considerably. After fi ve days of
treatment, the quantity of lactobacilli had increased and the microfl ora had par-
tially normalised in both groups that received Lactobacillus GG. After ten da
Log cfu/g
Treatment day 1
Log cfu/g
Treatment day 5
Log cfu/g
Treatment day 10
Anaerobic bacteria
Treatment groups
LGG = Lactobacillus GG twice day
LGG+TS = combined treatmentTS = trimethoprim-sulfa, 36 mg/kg/day
Figure 13. The effect of Lactobacillus GG on the quantity of faecal aerobic and
anaerobic bacteria as well as total lactobacilli during the antimicrobial treatment of
shigellosis (108).
treatment, the level of anaerobes was normal in the Lactobacillus GG group, was
slightly normalised in those who received the combined lactobacilli and medicinal
treatment, and was still low in those who had only received the medicinal treat-
ment. Moreover, lactobacilli were still absent from the intestines of those who had
only received the medicinal treatment (Fig.13). An intestinal microfl ora imbalance,
and particularly a defi ciency of anaerobic bacteria, increases the translocation of
intestinal bacteria from the lumen to the tissues and increases the risk of infections
and bacteraemia (37). Lactobacillus GG is resistant to trimethoprim-sulfamethoxa-
zole, so it is well able to balance the intestinal fl ora during the treatment.
Changes in the intestinal microbe population can also be measured as changes
in its metabolic activity. Bacterial metabolism produces short-chain fatty acids from
carbohydrates and proteins, particularly acetate, propionate and butyrate. Most of
these are absorbed by the mucous membrane as an energy source for colonocytes.
Short-chain fatty acids lower the pH of the bowel contents, and butyrate in particu-
lar is considered to have a protective infl uence on the mucous membrane (108).
In premature babies who received antibiotic treatment, Lactobacillus GG did not
cause any signifi cant changes in the production of short-chain fatty acids (109).
However, with medicinal treatment against Salmonella and Shigella, Lactobacillus
GG normalised the production of short-chain fatty acids, which points to a normali-
sation of intestinal microfl ora (110).
4.4 Indications in
Clostridium diffi cile treatment
C. diffi cile is an opportunistic pathogen which can also be found in normal human
microfl ora. It does not usually cause any symptoms. However, when the microfl ora
balance is disturbed - for example, as a consequence of antibiotic treatment - the C.
diffi cile population can increase considerably and the toxin it produces can cause
varying degrees of chronic diarrhoea and even pseudomembraneous colitis. C. dif-
fi cile diarrhoea recurs in about 10-20% of subjects treated with antibiotics (van-
comycin or metronidazole), and more effective treatments are scarce. The use of
Lactobacillus GG in the treatment of recurrent C. diffi cile diarrhoea has been
reported in around 40 subjects (111-113). A positive treatment response was
achieved with a single treatment in 84% of the cases, and with repeat treatment in
94%. In the preliminary results from a placebo-controlled pilot study, a signifi cant
effect was obtained in those who had C. diffi cile colitis for the fi rst time, but not in
cases which had recurred often (114, 115). Further studies are underway.
The histopathology of C. diffi cile colitis and its effect on the intestinal microfl ora
has been studied in an animal experiment (37). C. diffi cile combined with an anti-
biotic (ampicillin) is inevitably fatal in hamsters. As it was known that Lactobacillus
GG maintains normal intestinal microfl ora and that xylitol prevents the adhesion of
C. diffi cile, the effectiveness of the combination treatment was tested in hamsters. It
was found that this could prevent both the development of enter
ocolitis in animals
(in 4 animals out of 5) and their death. Animals not undergoing the combination
treatment died within 2.5 days. In the hamsters with enterocolitis, the anaerobic
microfl ora of the epithelium of the bowel was almost completely destroyed and
coliform, facultative bacteria had become the dominant microfl ora in the contents
of the bowel. In those hamsters that survived without enterocolitis, the dominant
microfl ora were anaerobic bacteria, and C. diffi cile was found in only low concen-
trations in the bowel lumen of two animals (37).
permeability of the 5.
Transport of
intact protein
Without Peyer's patches
With Peyer's patches
P<0.002 P<0.001
Figure 14. The effect of Lactobacillus GG on mucosal
permeability. Fourteen-day old rats were gavaged daily
with cow milk +/- Lactobacillus GG, and the jejunal
permeability was analysed when the rats were twenty-
one days old (118).
When intestinal infl ammation and microfl ora imbalance occur, the permeability of
the mucous membrane increases, and large antigen molecules (116) and intestinal
bacteria (37) can migrate across the mucous membrane into the system. Similarly,
it has been shown that sensitivity to food antigens increases after acute diarrhoea,
because antigens are abnormally transported across the intestinal mucous mem-
brane (117). Furthermore, experimental studies with rat pups show that both for-
eign antigens in the diet or rotavirus infection increase the permeability of the
immature mucous membrane, with no antigen-specifi c local response. When test
animals received Lactobacillus GG in their diet, the maturation of the mucous
membrane occurred normally: the transport of antigens was strongly reduced and
occurred in a controlled route via Peyer's patches (Fig. 14). The result w
enhancement of a local, antigen-specifi c IgA response (116, 118). It has also been
shown in humans that Lactobacillus GG enhances a local, antigen-specifi c IgA
response to food antigens (31). Such an enhanced response is important as regards
the tolerance of food antigens.
Chronic non-steroidal anti-infl ammatory drugs destroy gastrointestinal mucosa,
leading to ulceration. The protective effect of fermented milk drinks on indomet-
acin-induced alterations of mucosal permeability has been studied (119). The fer-
mented milk drinks contained active or heat-inactivated strains of Lactobacillus
GG, L. helveticus and L. acidophilus (>107 cfu/g each). Four gastrointestinal perme-
ability tests were carried out in randomized order on 16 healthy adults: 1) basal, 2)
after indometacin, 3) after indometacin when the fermented milk drink with living
bacteria was consumed for fi ve days, 4) after indometacin when the fermented milk
drink with heat-inactivated bacteria was consumed for fi ve days. Gastric perme-
ability was measured by sucrose urinary excretion, and intestinal permeability
by lactulose/mannitol excretion. Indometacin signifi cantly increased both gastric
and intestinal permeability. The fermented milk with living bacteria signifi cantly
reduced abnormal gastric permeability, but not the intestinal permeability induced
by indometacin. The drink with the heat-inactivated bacteria had no effect.
6.1 Speeds recovery in allergy
Score of atopic
symptoms
Formula + LGG
Figure 15. The effect of Lactobacillus GG on the
atopic dermatitis of milk allergic children, during a
milk elimination diet (121).
Allergies have increased and are still increasing in western countries. In Finland
approximately 2.5% of small children suffer from allergy caused by cow's-milk pro-
tein. In recent years there has been intensive research into how this trend could
be altered through bacterial treatment. Studies on the treatment of atopic and food
allergies have suggested that by restoring the permeability of the intestinal mucous
membrane, by modulating the local immune response and by using bacteria that
suitably alter the food antigens, an immune response that has gone awry can be
guided back in the right direction (120).
A randomised, placebo-controlled study on children with an atopic eczema with
allergy to milk showed that the intensity and extension of the rash and subjective
symptoms decreased signifi cantly faster when their milk elimination diet contained
Lactobacillus GG (Fig. 15). The intestinal infl ammation was measured using the
cytokine content of their stools. Tumour necrosis factor-α was found to fall signifi -
cantly more rapidly in the Lactobacillus GG group compared to the placebo, indi-
cating a faster recovery from infl ammation. Lactobacillus GG also helped those
children who were only fed on mother's milk and where the bacteria were admin-
istered to the mothers (121).
In another clinical study, Lactobacillus GG was given to infants who manifested
atopic eczema during exclusive breastfeeding, and who had no exposure to any
infant food or substitute formula (122). They were weaned to a probiotic (Lactoba-
cillus GG or bifi dobacteria) -supplemented extensively hydrolysed whey protein
formula or to the same formula without probiotics. The skin condition, the growth
and concentrations of circulating cytokines and chemokines as well as soluble cell
surface adhesion molecules in serum and methyl-histamine and eosinophilic pro-
tein X in the urine were determined. According to results after two months, the
atopic eczema was signifi cantly improved in the probiotic groups compared to the
placebo. The median score of atopic dermatitis during breastfeeding was 16 (7-25)
and decreased in the Lactobacillus GG group to 1 (0.1-8.7), vs. 13.4 (4.5-18.2) in
the placebo group (p=0.01). The concentrations of serum soluble CD4 decreased
in the same period in the probiotic groups but not in the placebo group, and the
serum tumour growth factor-β tended to increase in the Lactobacillus GG group. Before intervention, the urine eosinophilic protein X correlated signifi cantly with
the clinical score of atopic symptoms. Its concentration decreased signifi cantly in
the Lactobacillus GG group during supplementation, which supports the clinical
results. In conclusion, the data confi rmed that Lactobacillus GG supplementation
during the weaning period counteracted infl ammatory responses and helped to
tolerate new dietary antigens.
6.2 Prevents the risk of allergy in infancy
An interesting question is whether the development of allergic diseases can be
prevented in early infancy by modulating the intestinal microfl ora with probiotic
bacteria. To evaluate this, a group of families at high risk of allergy was selected
(123). The only inclusion criterion was a family history of atopic disease: one or
more family members with atopic eczema, allergic rhinitis or asthma. In all, 159
eczema (%)
Figure 16. The effect of Lactobacillus
GG on the incidence of atopic eczema in
infants. Pregnent mothers took Lactoba-cillus GG or identical placebo capsules daily for 2 – 4 weeks before the delivery.
After the birth it was given to the new-born baby or alternatively to the breast-
feeding mother (123).
mothers were randomised to receive two Lactobacillus GG (1010 cfu) or placebo
capsules daily for 2-4 weeks before the expected date of the birth. After the
birth, either the breastfeeding mother or the infant consumed the bacteria for
six months. The children were clinically examined until they were two years old
and the incidence of atopic diseases calculated. Parents reported any symptoms
observed in their children which might be related to atopic disease. Sensitisation to
common dietary and respiratory antigens was measured by the skin prick test and
total and antigen-specifi c IgE assays. Altogether, 132 families with atopic diseases
completed the study. Atopic eczema was found in 46 out of 132 children (35%) at the
age of two years, asthma in six and allergic rhinitis in one child. Almost every other
baby in the placebo group developed an atopic disease, but only one in four in the
Lactobacillus GG group (Fig. 16). The mean duration of breastfeeding was as long
in both the atopic (7 mo) and the non-atopic (6.7 mo) group. Surprisingly, there was
no difference in the effect, no matter whether Lactobacillus GG was given directly to
the infant or to the breast-feeding mothers. Concentration of total IgE as well as fre-
quencies of increased antigen-specifi c IgE concentrations and of positive skin-prick
tests were similar between the Lactobacillus GG group and the placebo group.
It is possible that the risk of allergy in infants can be reduced by maintaining
a good bacterial balance in pregnant mothers. The addition of probiotics to the
diet of the nursing mothers enhanced the protective effect of breast milk. In a
randomised, placebo-controlled study (124) with 62 mother-child pairs,
lus GG increased the level of anti-infl ammatory TGF-β2 in breast milk signifi cantly, compared to the placebo group. The risk of developing atopic eczema during the
fi rst two years of life of the infants was signifi cantly reduced in the probiotic group
compared to the placebo group (15% vs. 47%; relative risk 0.32, p=0.0098).
6.3 Mechanisms behind the effects
The mechanisms by which probiotics have an effect in the prevention and allevia-
tion of allergy are not yet fully understood but many factors have been found (125).
Microbial fl ora has an effect on the development of immune response and the bal-
ance of T-helper cell types (Th1/Th2). The balance in turn determines the develop-
ment of oral tolerance. Th-2 type immune cells produce interleukin (IL)-4, which is
essential for B-cell differentiation into IgE-producing cells, and IL-5, which is impor-
tant for the activity of eosinophil lymphocytes. Intestinal permeability also is dis-
turbed, allowing the absorption of antigenic macromolecules (126).
Food antigens, like caseins, enhanced the mitogen-induced proliferation of lym-
phocytes of atopic children, but caseins degraded by Lactobacillus GG had a mod-
erating effect (127). Caseins degraded by Lactobacillus GG also down-regulated
the IL-4 production of lymphocytes compared to the control (128, 129). T-cell acti-
vation was suppressed in vitro by Lactobacillus GG-degraded caseins, production
of IL-2 mRNA was suppressed and the production of IL-2 protein reduced. At the
same time, the levels of IL-4 and IFN-γ were reduced. The mechanism was based on the inhibition of the translocation of protein kinase C (one of the markers of cell
activation) in the peripheral blood mononuclear cells of healthy children (130).
Oral administration of Lactobacillus GG reduced the soluble CD4+, a marker
of T-cell activation (122) and the secretion of IL-10, which is associated with
the Th1/Th2 balance in a concentration-dependent manner (130). Not only the
degraded caseins but also the cell-free homogenates of probiotic bacteria are
shown to affect cell proliferation (131), indicating that the degradation compo-
nent of bacteria may possibly play a role in the modifi cation of immune response.
Since it degrades milk proteins, Lactobacillus GG may also form bioactive peptides,
which may in turn have an infl uence on the digestive tract (132).
Normal immune response
Figure 17. The effect of Lactobacillus GG on immune response of healthy persons, during
gastrointestinal infection and on milk-hypersensitised persons.
Milk allergy is widely believed to be exclusive to young children. However, the
latest studies have shown that a clear immune response can be observed in lactose-
tolerant adults who show or feel symptoms during exposure to milk (133). This
manifested itself as the boosting of a non-specifi c immune response (increasing
of phagocyte receptors and boosting of phagocytoses). Lactobacillus GG adminis-
tered in conjunction with milk exposure reduced the infl ammation response sig-
nifi cantly. In the healthy control group, milk did not cause a phagocyte response;
milk with Lactobacillus GG, however, increased the non-specifi c immune response
instead of lowering it (70). This refl ects the balancing effect of Lactobacillus
GG with regard to immune responses. On one hand, it increases immunological
defences and boosts immune responses in healthy subjects and in those with infec-
tions (see chapter 2); and on the other, it reduces the hyperactive immune response
in allergies (Fig. 17).
The bacteria are transferred from a mother to her child at birth. There are indi-
cations that the intestinal fl ora of atopic infants differs from the fl ora of healthy
infants. At three weeks of age infants who later developed an atopic disease had a
lower level of intestinal bifi dobacteria than non-atopic ones (134). Lactobacillus
GG has been shown to enhance the growth of bifi dobacteria in newborn babies
(25) and in milk-hypersensitive adults (32).
LGG and promising
7.1 Rheumatoid arthritis
In children with chronic arthritis, Lactobacillus GG has been proved to enhance
the IgA class local immune response, increase the specifi c IgA response to food
antigens, and normalise high urease enzyme activity in stools. High urease activity
indicates an imbalance in the intestinal microfl ora. All changes were transient and
related to the short-term (10 days) use of Lactobacillus GG (135, 136). These results
suggest that Lactobacillus GG has the ability to strengthen the intestinal immune
barrier of the mucous membrane in chronic arthritis. In a double-blind, placebo-
controlled, randomised study Lactobacillus GG or placebo capsules were taken by
21 patients with rheumatoid arthritis (137). Clinical examinations were made and
blood samples taken fi ve times during the one-year study. The activity of the arthri-
tis was evaluated by laboratory tests, functioning ability, the number of swollen and
tender joints, a physician's assessment and subjective evaluation by the patient. At
the end of the study the number of swollen and tender joints tended to be reduced
in the Lactobacillus GG group compared to the placebo group. The activity of the
arthritis tended to decrease more in the Lactobacillus GG group compared to pla-
cebo, and the patients in the Lactobacillus GG group also needed less medication
for rheumatoid arthritis. Due to the limited number of patients, the results were not
statistically signifi cant but the tendency towards a benefi cial effect was clear (137).
7.2 Infl ammatory bowel diseases
There are several chronic intestinal diseases without known aetiology, such as
Crohn's disease, ulcerative colitis and pouchitis. They are collectively called infl amma-
tory bowel diseases (IBD). In addition to the genetic background and autoimmune
nature of the disease, the role of intestinal microfl ora in the development of these dis-
eases is also speculated (138). IBD is thought to be caused by an aggressive immune
response to luminal bacteria and is characterised by a Th-1 type cytokine pattern.
Crohn's disease can appear in any section of the digestive tract but is most often
found in the bowel. The clinical description includes increased permeability of
the intestinal mucous membrane and disturbed processing and transport of food
antigens. Because Lactobacillus GG is known to restore the permeability of the
mucous membrane, its effect was studied in patients with Crohn's disease. The
study confi rmed that Lactobacillus GG increased local, antigen-specifi c immune
response in the mucous membrane and in this way corrected the permeability
disturbance of the membrane (135, 136). In a small, open-label pilot study Lacto-
bacillus GG was given in enterocoated tablets to four children with mildly to
moderately active Crohn's disease for six months. The results showed a signifi cant
improvement in clinical activity and improved intestinal permeability (139). There
is still a dearth of randomised, double-blind, placebo-controlled trials.
Human in vivo administration of Lactobacillus GG led to a decrease in the ini-
tially strong proliferative response of peripheral blood CD4+ T-lymphocytes towards
foreign intestinal fl ora and their bacterial components (Bacteroides fragilis and E.
coli). The secretion of IL-10 (Th-2 type cytokine) by peripheral blood CD4+ T-lym-
phocytes increased and the level of IFN-γ and TNF-α (Th-1 type cytokines) was reduced (140, 141). These results indicate that adjunct administration of Lactobacil-
lus GG might have a benefi cial effect in the treatment of IBD.
Preliminary results from an open-label pilot study in the treatment of refractory
"pouchitis" with capsules fi lled with Lactobacillus GG and fructooligosaccharide
report a benefi cial effect as an adjunct therapy to antibiotics (142). Placebo-control-
led studies are in progress.
To study experimentally the potential effect of Lactobacillus GG on colon
infl ammation, this was given to rats with acetic acid-induced colitis, without signifi -
cant health improvement. The need of host-specifi c lactobacilli strains to protect
the colon is still an open question, since another, rat-specifi c lactobacilli strain had
benefi cial effects (143). Theoretically, Lactobacillus GG might suppress infl amma-
tion via the induction of nitrogen oxide (NO) production in enterocytes (103).
NO is an important part of the defence system in the enter
ocytes of the mucosa.
Compounds that induce the epithelial cells to produce NO are known to help the
epithelial cell defence systems and to suppress infl ammation. However, they have
short-term effects, and if NO is induced by intestinal fl ora, the effect might be
more long-term and might support the normal cell functions and defences. NO also
enhances mucin formation, which characteristic has also been demonstrated to take
place with Lactobacillus GG (44).
7.3 Irritable bowel syndrome
Irritable bowel syndrome (IBS) is a widespread functional disorder of the diges-
tive tract. Among the symptoms are bloating, abdominal pain, constipation, faecal
urgency and diarrhoea. Its aetiology is unknown and therapeutic options are lim-
ited. There are only a few trials, which have studied the potential benefi ts of pro-
biotics in improving the symptoms caused by IBS. A pilot study was made with
enterocoated Lactobacillus GG tablets (1010 cfu). The study was a randomised dou-
ble-blinded placebo-controlled and crossover setting with 24 volunteers. The inter-
vention was a two-week run-in with the placebo, followed by 8-wk interventions
with Lactobacillus GG or placebo, a two-week wash-out, and an 8-wk cross-over,
changing the products. IBS medication (used by 83%) was discontinued at the
beginning of the trial. Symptoms were recorded in daily diaries and by periodic
questionnaires. The effi cacy of the placebo (during the run-in period) varied from
0% (nausea) to 29% (constipation and bloating). Lactobacillus GG intake did not
have any signifi cant effects on the symptoms. The study group consisted of patients
with bloating as the main symptom. It was noted, however, that there tended to
be a reduction in the number of unformed bowel motions with Lactobacillus GG
treatment for patients with diarrhoea (144).
In preliminary open-label studies the capsules fi lled with Lactobacillus GG and
fructooligosaccharides relieved the gas-production in patients with IBS (145) and
lactose malabsorption (146). Placebo-controlled studies are in progress.
7.4 Cystic fi brosis
One interesting area of application for bacterial therapy is in the treatment of cystic
fi brosis. In a preliminary report, an Italian research group (147) has shown that
taking Lactobacillus GG bacteria daily for six months signifi cantly reduced the
number of pulmonary infections and abdominal pains, and particularly improved
weight gain in children suffering from Pseudomonas infection. Further study con-
fi rmed the benefi ts for Pseudomonas-infected patients. The incidence and duration
of their infections were signifi cantly reduced, pulmonary function improved and
weight gain increased compared to the placebo group (148). Final reports of the
results are still missing.
LGG quantity
log cfu
Buttermilk,
Capsule,
drink, 2 dl
Figure 18. Lactobacillus GG doses obtained from Gefi lus® products and Lactoba-
cillus GG levels in stools, when the products are taken daily.
We are traditionally accustomed to thinking that food is food and medicine is medi-
cine with no overlap between the two. At the end of the 1980s and particularly
during the 1990s interest in this ‘grey area' increased greatly. Nowadays such
products are termed functional, i.e. foods that have an effect on health beyond
their nutritional value. Their development has aroused wide interest and there are
already hundreds of foods on the market that, in addition to nutrition, also have
health-maintaining or even therapeutic effects. The effi cacy of the active ingredient
used in a functional food or of a product that contains it has to be demonstrated in
humans. There has to be a suffi cient quantity of the active ingredient in the food.
The quantity of Lactobacillus GG varies according to the type of product and the
manufacturer. Finnish Lactobacillus GG products (Gefi lus®) have been shown to con-
tain suffi cient Lactobacillus GG to colonise the bowel (16-18, 33, 77)(Fig. 18). It has
been observed that milk and apparently other protective compounds in food improve
the survival of Lactobacillus GG through the stomach, i.e. there is a buffering effect.
Consequently the quantity of Lactobacillus GG in powder form or in capsules has to
be greater ( 1010 cfu/day) than in milk-based products (108 – 109 cfu/day).
The lowest dose, with which the clinical effi cacy of Lactobacillus GG in powder
form has been documented, was 3x109 cfu twice a day, in the prevention and treat-
ment of acute diarrhoea (81, 101). On the other hand, clinical effi cacy was achieved
with dairy products, which had a corresponding quantity of Lactobacillus GG (see
Table 3). In healthy children even a lower level ( 108 cfu/day) of Lactobacillus GG
in milk reduced the risk of respiratory infection (77) and dental caries (79). It is a
matter of individual preference whether one chooses to consume probiotic bacte-
ria in everyday food or in a more pharmaceutical form.
1. Gibson GR. Dietary modulation of the human gut microfl ora using prebiotics. Br J Nutr
1998;80:S209–12.
2. Molin G, Jeppsson B, Johansson ML, Ahrne S, Nobaek S, Stahl M, Bengmark S.
Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of
the human intestines. J Appl Bacteriol 1993;74:314–23.
3. Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal Lacto-
bacillus fl ora of healthy human rectal and oral mucosa. J Appl Microbiol 1998;85:88–94.
4. Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics–approaching a defi ni-
tion. Am J Clin Nutr 2001;73:361S–4S.
5. Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of
Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 1992;37:121–8.
6. Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. The ability of probiotic bac-
teria to bind to human intestinal mucus. FEMS Microbiol Lett 1998;167:185–9.
7. Tuomola EM, Ouwehand AC, Salminen SJ. Human ileostomy glycoproteins as a
model for small intestinal mucus to investigate adhesion of probiotics. Lett Appl Microbiol
1999;28:159–63.
8. Ouwehand AC, Niemi P, Salminen SJ. The normal faecal microfl ora does not affect the
adhesion of probiotic bacteria in vitro. FEMS Microbiol Lett 1999;177:35–8.
9. Elo S, Saxelin M, Salminen S. Attachment of Lactobacillus casei strain GG to human
colon carcinoma cell line Caco-2: comparison with other dairy strains. Lett Appl Microbiol
1991;13:154–6.
10. Ouwehand AC, Salminen S, Tölkkö S, Roberts P, Ovaska J, Salminen E. Recent
human colonic tissue: new model for characterizing adhesion of lactic acid bacteria. Clin
Diagn Lab Immunol 2002;9:184–6.
11. Sarem-Damerdji L, Sarem F, Marchal L, Nicolas JP. In vitro colonization
ability of human colon mucosa by exogenous Lactobacillus strains. FEMS Microbiol Lett
1995;131:133–7.
12. Aureli A. State of the art concerning Lactobacillus spp. potential for stabilizing intestinal
microfl ora and preventing gastrointestinal infections. Gastroent Int 1998;11:22–6.
13. Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human
Lactobacillus strain. Antimicrob Agents Chemother 1987;31:1231–3.
14. Yang Z, Suomalainen T, Mäyrä-Mäkinen A, Huttunen E. Antimicrobial activity
of 2-pyrrolidone-5-carboxylic acid produced by lactic acid bacteria. J Food Protect
1997;60:786–90.
15. Tynkkynen S, Satokari R, Saarela M, Mattila-Sandholm T, Saxelin M. Comparison
of ribotyping, randomly amplifi ed polymorphic DNA analysis, and pulsed-fi eld gel electro-
phoresis in typing of Lactobacillus rhamnosus and L. casei strains. Appl Environ Microbiol
1999;65:3908–14.
16. Saxelin M, Elo S, Salminen S, Vapaatalo H. Dose response colonization of faeces after
oral administration of Lactobacillus casei strain GG. Microb Ecol Health Dis 1991;4:209–14.
17. Saxelin M, Pessi T, Salminen S. Fecal recovery following oral administration of Lacto-
bacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int J Food Micro-
biol 1995;25:199–203.
18. Saxelin M, Ahokas M, Salminen S. Dose response on the faecal colonization of Lac-
tobacillus strain GG administered in two different formulations. Microb Ecol Health Dis
1993;6:119–22.
19. Kaila M, Isolauri E, Sepp E, Mikelsaar M, Salminen S. Fecal recovery of a human
Lactobacillus strain (ATCC 53103) during dietary therapy of rotavirus diarrhea in infants.
Biosci Microfl ora 1998;17:149–51.
20. Alander M, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von
Wright A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett Appl
Microbiol 1997;24:361–4.
21. Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sand-
holm T, von Wright A. Persistence of colonization of human colonic mucosa by a pro-
biotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol
1999;65:351–4.
22. Marini A, Clerici-Bagozzi D, Maglia T, Casetta P, Negretti F. Microbiological and
immunological observations in the stools of preterm neonates orally treated with probiotic
products. Note III: treatment with Lactobacillus GG. Dev Physiopath Clin 1997;7:87–94.
23. Negretti F, Casetta P, Clerici-Bagozzi D, Marini A. Researches on the intestinal and
systemic immunoresponses after oral treatment with Lactobacillus GG in the rabbit. Dev
Physiophatol Clin 1997;7:15–21.
24. Millar MR, Bacon C, Smith SL, Walker V, Hall MA. Enteral feeding of premature
infants with Lactobacillus GG. Arch Dis Child 1993;69:483–7.
25. Sepp E, Mikelsaar M, Salminen S. Effect of administration of Lactobacillus casei strain
GG on the gastrointestinal microbiota of newborns. Microb Ecol Health Dis 1993;6:309–14.
26. Schultz M, Young RJ, Iwen P, Bilyeu DV, Vanderhoof JA. Maternal administration
of probiotic during pregnancy results in infantile colonization. J Pediatr Gastroenterol Nutr
2001;33:403 (A137).
27. O'Sullivan DJ. Methods for analysis of the intestinal microfl ora. Curr Issues Intest Micro-
biol 2000;1:39-50.
28. Ouwehand AC, Isolauri E, Kirjavainen PV, Tölkkö S, Salminen SJ. The mucus bind-
ing of Bifi dobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact.
delbrueckii subsp. bulgaricus. Lett Appl Microbiol 2000;30:10–3.
29. Benno Y, He F, Hosoda M, Hashimoto H, Kojima T, Yamazaki K, Iino H, Myk-
känen H, Salminen S. Effect of Lactobacillus GG yoghurt on human intestinal microecol-
ogy in Japanese subjects. Nutrition Today 1996;31:9S–11S.
30. Hosoda M, He F, Hiramatu M, Hashimoto H, Benno Y. Effects of Lactobacillus GG
strain intake on fecal microfl ora and defecation in healthy volunteers. Bifi dus (Japan Bifi dus
Foundation) 1994;8:21–2.
31. Malin M, Verronen P, Korhonen H, Syväoja E-L, Salminen S, Mykkänen H,
Arvilommi H, Eerola E, Isolauri E. Dietary therapy with Lactobacillus GG, bovine colos-
trum or bovine immune colostrum in patients with juvenile chronic arthritis: evaluation of
effect on gut defence mechanisms. Infl ammopharmacol 1997;5:219–36.
32. Apostolou E, Pelto L, Kirjavainen PV, Isolauri E, Salminen SJ, Gibson GR. Dif-
ferences in the gut bacterial fl ora of healthy and milk-hypersensitive adults, as measured by
fl uorescence in situ hybridization. FEMS Immunol Med Microbiol 2001;30:217–21.
33. Saxelin M. Development of dietary probiotics: estimation of optimal Lactobacillus GG con-
centrations. PhD Thesis 1995. Turku University Department of Biochemistry and Food Chemistry.
34. Saxelin M. Lactobacillus GG – a human probiotic strain with thorough clinical documen-
tation. Food Rev Int 1997;13:293–313.
35. Wagner RD, Pierson C, Warner T, Dohnalek M, Far
mer J, Roberts L, Hilty M,
Balish E. Biotherapeutic effects of probiotic bacteria on candidiasis in immunodefi cient
mice. Infect Immun 1997;65:4165–72.
36. Wagner RD, Warner T, Roberts L, Farmer J, Balish E. Colonization of congenitally
immunodefi cient mice with probiotic bacteria. Infect Immun 1997;65:3345–51.
37. Naaber P, Mikelsaar RH, Salminen S, Mikelsaar M. Bacterial translocation, intestinal
microfl ora and morphological changes of intestinal mucosa in experimental models of Clos-
tridium diffi cile infection. J Med Microbiol 1998;47:591–8.
38. Dong MY, Chang TW, Gorbach SL. Effects of feeding Lactobacillus GG on lethal irra-
diation in mice. Diagn Microbiol Infect Dis 1987;7:1–7.
39. Adawi D, Ahrné S, Molin G. Effects of different probiotic strains of Lactobacillus and
Bifi dobacterium on bacterial translocation and liver injury in an acute liver injury model. Int
J Food Microbiol 2001;70:213–20.
40. Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and
severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med 1994;205:243–7.
41. Hudault S, Lievin V, Bernet-Camard MF, Servin AL. Antagonistic activity exerted in
vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infec-
tion. Appl Environ Microbiol 1997;63:513–8.
42. Lee DJ, Drongowski RA, Coran AG, Harmon CM. Evaluation of probiotic treatment
in a neonatal animal model. Pediatr Surg Int 2000;16:237–42.
43. Tuomola E. In vitro adhesion of probiotic lactic acid bacteria. PhD Thesis 1999. Turku
University Department of Biochemistry and Food Chemistry.
44. Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit
enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression.
Am J Physiol 1999;276:G941–50.
45. Mattar AF, Drongowski RA, Coran AG, Harmon CM. Effect of probiotics on entero-
cyte bacterial translocation in vitro. Pediatr Surg Int 2001;17:265–8.
46. Goldin B. The metabolic activity of the intestinal microfl ora and its role in colon
cancer: Lactobacillus and other factors that alter intestinal metabolic activity. Nutrition Today
1996;31:24–7S.
47. Lidbeck A, Nord CE, Gustafsson JA, Rafter J. Lactobacilli, anticarcinogenic activities
and human intestinal microfl ora. Eur J Cancer Prev 1992;1:341–53.
48. Ling WH, Saxelin M, Hänninen O, Salminen S. Enzyme profi le of Lactobacillus strain
GG by a rapid API ZYM system: a comparison of intestinal bacterial strains. Microb Ecol Health
Dis 1994;7:99–104.
49. Korpela R. Role of rye fi bre and Lactobacillus GG in colonic metabolism. PhD Thesis 1995.
Kuopio University Publications D Medical Sciences 65.
50. Ling WH. Effect of lactobacilli-containing vegan diet and Lactobacillus GG on colonic
chemical loading in man. PhD Thesis 1992. Kuopio University Publications D Medical Sciences.
51. Ling WH, Hänninen O, Mykkänen H, Heikura M, Salminen S, Von Wright A. Colo-
nization and fecal enzyme activities after oral Lactobacillus GG administration in elderly nurs-
ing home residents. Ann Nutr Metab 1992;36:162–6.
52. Ling WH, Korpela R, Mykkänen H, Salminen S, Hänninen O. Lactobacillus strain
GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy
female adults. J Nutr 1994;124:18–23.
53. Goldin BR, Gualtieri LJ, Moore RP. The effect of Lactobacillus GG on the initiation and
promotion of DMH-induced intestinal tumors in the rat. Nutr Cancer 1996;25:197–204.
54. Lim BK, Mahendran R, Lee YK, Bay BH. Chemopreventive effect of Lactobacillus
rhamnosus on growth of a subcutaneously implanted bladder cancer cell line in the mouse.
Jpn J Cancer Res 2002;93:36–41.
55. El-Nezami H, Kankaanpää P, Salminen S, Ahokas J. Physicochemical alterations
enhance the ability of dairy strains of lactic acid bacteria to remove afl atoxin from contami-
nated media. J Food Prot 1998;61:466–8.
56. El-Nezami H, Kankaanpää P, Salminen S, Ahokas J. Ability of dairy strains of
lactic acid bacteria to bind a common food carcinogen, afl atoxin B1. Food Chem Toxicol
1998;36:321–6.
57. Pierides M, El-Nezami H, Peltonen K, Salminen S, Ahokas J. Ability of dairy strains
of lactic acid bacteria to bind afl atoxin M1 in a food model. J Food Protect 2000;63:645–50.
58. Haskard C, Binnion C, Ahokas J. Factors affecting the sequestration of afl atoxin by
Lactobacillus rhamnosus strain GG. Chem Biol Interact 2000;128:39–49.
59. Haskard CA, El-Nezami HS, Kankaanpää PE, Salminen S, Ahokas JT. Surface bind-
ing of afl atoxin B(1) by lactic acid bacteria. Appl Environ Microbiol 2001;67:3086–91.
60. El-Nezami H, Mykkänen H, Kankaanpää P, Salminen S, Ahokas J. Ability of Lacto-
bacillus and Propionibacterium strains to remove afl atoxin B, from the chicken duodenum. J
Food Prot 2000;63:549–52.
61. Nosova T, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. Acetaldehyde
production and metabolism by human indigenous and probiotic Lactobacillus and Bifi dobac-
terium strains. Alcohol Alcohol 2000;35:561–8.
62. Niemi SM, Saxelin M, Korpela R. Effects of fi ber-rich rye bread and yoghurt with Lac-
tobacillus GG on bowel movement. Am J Clin Nutr 2001;73:490–1S.
63. Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and
mucosal protection. Scand J Gastroenterol 1997;32:45–8.
64. Hosoda M, He F, Kojima T, Hashimoto H, Iino H. Effects of fermented milk with Lac-
tobacillus rhamnosus GG strain administration on defecation, putrefactive metabolites and
fecal microfl ora of healthy volunteers. J Nutritional Food 1998;1:1–9.
65. Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis
factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun
1996;64:5403–5.
66. Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto
M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and
gamma interferon production in human peripheral blood mononuclear cells. Infect Immun
1998;66:6058–62.
67. Miettinen M, Lehtonen A, Julkunen I, Matikainen S. Lactobacilli and Streptococci
activate NF-kappa B and STAT signaling pathways in human macrophages. J Immunol
2000;164:3733–40.
68. Miettinen M. Regulation of cytokine gene expression by Gram-positive bacteria. PhD Thesis
2000. Publications of the National Public Health Institute A14/2000, Helsinki, Finland.
69. Kirjavainen PV, El-Nezami HS, Salminen SJ, Ahokas JT, Wright PF. Effects of
orally administered viable Lactobacillus rhamnosus GG and Propionibacterium freuden-
reichii subsp. shermanii JS on mouse lymphocyte proliferation. Clin Diagn Lab Immunol
1999;6:799–802.
70. Pelto L, Isolauri E, Lilius EM, Nuutila J, Salminen S. Probiotic bacteria down-reg-
ulate the milk-induced infl ammatory response in milk-hypersensitive subjects but have an
immunostimulatory effect in healthy subjects. Clin Exp Allergy 1998;28:1474–9.
71. Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immuno-
genicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine
1995;13:310–2.
72. He F, Tuomola E, Arvilommi H, Salminen S. Modulation of humoral immune
response through probiotic intake. FEMS Immunol Med Microbiol 2000;29:47–52.
73. Jung LK. Lactobacillus GG augments the immune response to typhoid vaccination: A
double-blinded, placebo-controlled study. IASEB J 1999;13:A872.
74. Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of
the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus
strain. Pediatr Res 1992;32:141–4.
75. Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari T. Viable versus inactivated
Lactobacillus strain GG in acute rotavirus diarrhoea. Arch Dis Child 1995;72:51–3.
76. Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of
acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr 1995;20:333–8.
77. Hatakka K, Savilahti E, Pönkä A, Meurman JH, Poussa T, Näse L, Saxelin M, Kor-
pela R. Effect of long term consumption of probiotic milk on infections in children attend-
ing day care centres: double blind, randomised trial. Bmj 2001;322:1327 – 9.
78. Meurman JH, Antila H, Korhonen A, Salminen S. Effect of Lactobacillus rhamnosus
strain GG (ATCC 53103) on the growth of Streptococcus sobrinus in vitro. Eur J Oral Sci
1995;103:253–8.
79. Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, Korpela R, Meur-
man JH. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus
GG, in milk on dental caries and caries risk in children. Caries Res 2001;35:412–20.
80. Oberhelman RA, Gilman RH, Sheen P, Taylor DN, Black RE, Cabrera L, Lescano
AG, Meza R, Madico G. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea
in undernourished Peruvian children. J Pediatr 1999;134:15–20.
81. Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Effi cacy of
Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr 2001;138:361–5.
82. Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ.
Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr
1999;135:564–8.
83. Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, Isolauri E.
Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respi-
ratory infections: a randomized study. Pediatrics 1999;104:e64.
84. Siitonen S, Vapaatalo H, Salminen S, Gordin A, Saxelin M, Wikberg R, Kirkkola
AL. Effect of Lactobacillus GG yoghurt in prevention of antibiotic associated diarrhoea. Ann
Med 1990;22:57–9.
85. Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cam-
marota G, Anti M, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. The effect of
oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects
during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2001;15:163–9.
86. Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, Santarelli
L, Cammarota G, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. Effect of Lactoba-
cillus GG supplementation on antibiotic-associated gastrointestinal side effects during Heli-
cobacter pylori eradication therapy: a pilot study. Digestion 2001;63:1–7.
87. Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect
of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial.
Mayo Clin Proc 2001;76:883–9.
88. Gavazzi C, Stacchiotti S, Cavalletti R, Lodi R. Confusion after antibiotics. Lancet
2001;357:1410.
89.Charteris WP, Kelly PM, Morelli L, Collins JK. Gradient diffusion antibiotic suscepti-
bility testing of potentially probiotic lactobacilli. J Food Prot 2001;64:2007–14.
90. Klein G, Hallmann C, Casas IA, Abad J, Louwers J, Reuter G. Exclusion of vanA,
vanB and vanC type glycopeptide resistance in strains of Lactobacillus reuteri and Lactobacil-
lus rhamnosus used as probiotics by polymerase chain reaction and hybridization methods.
J Appl Microbiol 2000;89:815–24.
91. Tynkkynen S, Singh KV, Varmanen P. Vancomycin resistance factor of Lactobacillus
rhamnosus GG in relation to enterococcal vancomycin resistance (van) genes. Int J Food
Microbiol 1998;41:195–204.
92. Oksanen PJ, Salminen S, Saxelin M, Hämäläinen P, Ihantola-Vormisto A, Muuras-
niemi-Isoviita L, Nikkari S, Oksanen T, Pörsti I, Salminen E, et al. Prevention of travel-
lers' diarrhoea by Lactobacillus GG. Ann Med 1990;22:53–6.
93. Hilton E, Kolakowski P, Singer C, Smith M. Effi cacy of Lactobacillus GG as a diar-
rheal preventive in travelers. J Travel Med 1997;4:41–3.
94. Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacil-
lus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in chil-
dren. Pediatrics 1991;88:90–7.
95. Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek
S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H,
Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with
acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr 2000;30:54–60.
96. Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infec-
tious diarrhea in infants and children: a systematic review of published randomized, double-
blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 2001;33:S17–25.
97. Rautanen T, Isolauri E, Salo E, Vesikari T. Management of acute diarrhoea with
low osmolarity oral rehydration solutions and Lactobacillus strain GG. Arch Dis Child
1998;79:157–60.
98. Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, Hart CA.
Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr
1996;42:162–5.
99. Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus
GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J
1995;14:107–11.
100. Grönlund MM, Lehtonen OP, Kero P, Saxelin M, Salminen S. Lactobacillus
GG supplementation does not reduce faecal colonization of Klebsiella oxytoca in preterm
infants. Acta Paediatr 1997;86:785–6.
101. Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial
therapy reduces the duration of symptoms and of viral excretion in children with mild diar-
rhea. J Pediatr Gastroenterol Nutr 1997;25:516–9.
102. Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari T. A trial in the
Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea.
Acta Paediatr 1997;86:460–5.
103. Korhonen R, Korpela R, Saxelin M, Mäki M, Kankaanranta H, Moilanen E.
Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macro-
phages and human T84 intestinal epithelial cells. Infl ammation 2001;25:223–32.
104. Banasaz M, Holma R, Norin E, Midtvedt T. Intestinal cell kinetics in ex-germfree rats
monoassociated with Lactobacillus rhamnosus GG. XVI International Congress on Microbial
Ecology and Disease, The Netherlands, October 3–6, 2001.
105. Isolauri E, Kaila M, Mykkänen H, Ling WH, Salminen S. Oral bacteriotherapy f or
viral gastroenteritis. Dig Dis Sci 1994;39:2595–600.
106. Juntunen M, Kirjavainen PV, Ouwehand AC, Salminen SJ, Isolauri E. Adherence
of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infec-
tion. Clin Diagn Lab Immunol 2001;8:293–6.
107. Sepp E, Tamm E, Torm S, Lutsar I, Mikelsaar M, Salminen S. Impact of a Lacto-
bacillus probiotic on the faecal microfl ora in children with shigellosis. Microecol Therapy
1995;23:74–80.
108. Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR,
Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastroin-
testinal physiology and function. Br J Nutr 1998;80:S147–71.
109. Stansbridge EM, Walker V, Hall MA, Smith SL, Millar MR, Bacon C, Chen S.
Effects of feeding premature infants with Lactobacillus GG on gut fermentation. Arch Dis
Child 1993;69:488–92.
110. Siigur U, Tamm E, Torm S, Lutsar I, Salminen S, Midtvedt T. Effect of bacterial
infection and administration of a probiotic on faecal short-chain fatty acids. Microbial Ecol
Health Dis 1996;9:271–7.
111. Bennet RG, Gorbach SL, Goldin BR, Chang T-W, Laughon BE, Greenough III
WB, Bartlett JG. Treatment of relapsing Clostridium diffi cile diarrhea with Lactobacillus GG.
Nutrition Today 1996;31:35S–8S.
112. Biller JA, Katz AJ, Flores AF, Buie TM, Gorbach SL. Treatment of C. diffi cile colitis
with Lactobacillus GG. J Ped Gastroent Nutr 1995;21:224–6.
113. Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium dif-
fi cile colitis with Lactobacillus GG. Lancet 1987;2:1519.
114. Pochapin M, Oltikar A, Pringe-Smith R, C. S. A prospective randomised placebo-
controlled trial of Lactobacillus GG in combination with standard antibiotics for the treat-
ment of Clostridium diffi cile infection. Am J Gastroenterol 1998;93:1697.
115. Pochapin M. The effect of probiotics on Clostridium diffi cile diarrhea. Am J Gastroen-
terol 2000;95:S11–3.
116. Isolauri E, Kaila M, Arvola T, Majamaa H, Rantala I, Virtanen E, Arvilommi H.
Diet during rotavirus enteritis affects jejunal permeability to macromolecules in suckling
rats. Pediatr Res 1993;33:548–53.
117. Kaila M. Immune response evoked by cow milk products in health and during rotavi-
rus diarrhoea: MD Thesis 1993. Acta Universitatis Tamperensis. Ser A.
118. Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacil-
lus casei strain GG reverses increased intestinal permeability induced by cow milk in suck-
ling rats. Gastroenterology 1993;105:1643–50.
119. Gotteland M, Cruchet S, Verbeke S. Effect of Lactobacillus ingestion on the gastroin-
testinal mucosal barrier alterations induced by indometacin in humans. Aliment Pharmacol
Ther 2001;15:11–7.
120. Majamaa H. Gut mucosal barrier – target for probiotic therapy. MD Thesis 1996. Acta
Universitatis Tamperensis Ser A;Vol. 476.
121. Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food
allergy. J Allergy Clin Immunol 1997;99:179–85.
122. Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S. Probiotics in the manage-
ment of atopic eczema. Clin Exp Allergy 2000;30:1604–10.
123. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probi-
otics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet
2001;357:1076–9.
124. Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding
might confer immunomodulatory protection against atopic disease in the infant. J Allergy
Clin Immunol 2002;109:119–21.
125. Murch SH. Toll of allergy reduced by probiotics. Lancet 2001;357:1057–9.
126. Kirjavainen PV, Apostolou E, Salminen SJ, Isolauri E. New aspects of probiotics--a
novel approach in the management of food allergy. Allergy 1999;54:909–15.
127. Sütas Y, Soppi E, Korhonen H, Syväoja EL, Saxelin M, Rokka T, Isolauri E. Sup-
pression of lymphocyte proliferation in vitro by bovine caseins hydrolyzed with Lactobacil-
lus casei GG-derived enzymes. J Allergy Clin Immunol 1996;98:216–24.
128. Sütas Y. Food allergy and atopic dermatitis in children. Studies on nutrition and immu-
nologic treatments. MD Thesis 1996. Acta Universitatis Tamperensis Ser A;Vol. 506.
129. Sütas Y, Hurme M, Isolauri E. Down-regulation of anti-CD3 antibody-induced IL-4
production by bovine caseins hydrolysed with Lactobacillus GG-derived enzymes. Scand J
Immunol 1996;43:687–9.
130. Pessi T, Isolauri E, Sütas Y, Kankaanranta H, Moilanen E, Hurme M. Suppression
of T-cell activation by Lactobacillus rhamnosus GG-degraded bovine casein. Int Immunophar-
macol 2001;1:211–8.
131. Pessi T, Sütas Y, Saxelin M, Kallioinen H, Isolauri E. Antiproliferative effects of
homogenates derived from fi ve strains of candidate probiotic bacteria. Appl Environ Micro-
biol 1999;65:4725–8.
132. Rokka T, Syväoja EL, Tuominen J, Korhonen H. Release of bioactive peptides
by enzymatic proteolysis of Lactobacillus GG fermented UHT milk. Milchwissenschaft
1997;52:675–8.
133. Pelto L, Salminen S, Lilius EM, Nuutila J, Isolauri E. Milk hypersensitivity--key to
poorly defi ned gastrointestinal symptoms in adults. Allergy 1998;53:307–10.
134. Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct
pattern of neonatal gut microfl ora in infants in whom atopy was and was not developing. J
Allergy Clin Immunol 2001;107:129–34.
135. Malin M. Evaluation of the intestinal mucosal barrier in Crohn's disease and juvenile
chronic arthritis. MD Thesis 1997. Acta Universitatis Tamperensis Ser A;Vol 582.
136. Malin M, Verronen P, Mykkänen H, Salminen S, Isolauri E. Increased bacterial
urease activity in faeces in juvenile chronic arthritis: evidence of altered intestinal microfl ora?
Br J Rheumatol 1996;35:689–94.
137. Hatakka K, Martio J, Korpela M, Laasanen T, Herranen M, Saxelin M, Korpela
R. Double blind comparison of probiotic therapy and placebo in patients with rheumatoid
arthritis. Probiotics, Prebiotics and New Foods, Rome, September 2–4 2001.
138. Panes J. Infl ammatory bowel disease: pathogenesis and targets for therapeutic inter-
ventions. Acta Physiol Scand 2001;173:159–65.
139. Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in
children with Crohn's disease? Results of a preliminary, open-label study. J Pediatr Gastroen-
terol Nutr 2000;31:453–7.
140. Schultz M, Gunningham-Rundles S, Lehn N, Falk W, Scholmerich J. Oral therapy
with Lactobacillus GG (LGG) alters the proliferative response of PBMC and CD4 T-lympho-
cytes towards Bacteroides sp. Gastroenterology 1999;116:G3537.
141. Schultz M, Linde HJ, Staudner H, Lehn N, Falk W, Schoelmerich J. Oral admin-
istration of Lactobacillus GG (LGG) induces an antiinfl ammatory, Th-2 mediated systemic
immune response towards intestinal organisms. Gastroenterology 2000;118:A4180.
142. Friedman G. Treatment of refractory "pouchitis" with prebiotic and probiotic therap y.
Gastroenterology 2000;118:Abstract 4167.
143. Holma R, Salmenperä P, Lohi J, Vapaatalo H, Korpela R. Effects of Lactobacillus
rhamnosus GG and Lactobacillus reuteri R2LC on acetic acid-induced colitis in rats. Scand J
Gastroenterol 2001;36:630–5.
144. O'Sullivan MA, O'Morain CA. Bacterial supplementation in the irritable bowel
syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis
2000;32:294–301.
145. Friedman G. Amelioration of "gas syndromes" in the irritable bowel syndrome with
prebiotic and probiotic therapy. Gastroenterology 2000;118:Abstract 4953.
146. Friedman G. Reversal of lactose malabsorption with prebiotic and probiotic therapy.
Gastroenterology 2000;118:Abstract 5179.
147. Guarino A. Effects of probiotics in children with cystic fi brosis. Gastroenterol Int
1998;11:11.
148. Guarino A, Spagnuolo MJ, Valeria R. Probiotics as adjunctive treatment in cystic
fi brosis. Gastroenterology 1999;116:AGA Abstract G 3848.
cfu = colony forming units
log = logarithm
n.s. = not signifi cant
n = quantity
SD = standard deviation
P = statistical signifi cance
Products containing LGG
around the world, spring 2002
Dairy products, juices, capsules
Dairy products, juices, capsules
Infectodiarrstop, LGG
Powders, capsules
Iceland and Greenland
Yoplait everybody
Dicoflor, Floridral, Giflorex
Dairy products, juices
Emmi Aktifit Plus, 4PLUS
Middle East
United Arab Emirates
Onaka-He-GG, LGG Milk
Beautiful Day LGG
Latin America
Chile and Bolivia
Source: http://www.nleducation.co.uk/wp-content/uploads/LGG_Summatim_english.pdf
37412_SpanishCover:37412_SpanishCover 9/17/09 10:37 AM Page 1 Publicado por la American Society for Reproductive Medicine, bajo la dirección del Comité de Educación del Paciente y el Comité de Publicaciones. Ninguna parte en este documento puede ser reproducida en ninguna forma sin permiso por escrito. Este folleto no pretende de ninguna manera sustituir, dictar ni definir
Carnegie acts as financial adviser to investigate the possibilities to raise liquid funds through a new share issue or other financial instruments. Head of Research, Nordic Head of Research, Sweden Head of Research, Denmark Head of Research, Norway Head of Research, Finland Sector HeadsCommercial Services & Supplies















