Research.uchc.edu
HAZARDOUS DRUG SAFETY AND HEALTH PLAN FOR HANDLING ANTINEOPLASTIC
OTHER HAZARDOUS DRUGS IN CLINICAL ENVIRONMENTS
Introduction
Drugs have a successful history of use in treating diseases and are responsible for many medical
advances over the past century. However, virtually every drug has side effects associated with their
use in treating patient illnesses. It follows that both patients and Health Care Workers (HCW) are at
risk of developing these side effects. Side effects to patients are due to administration of the drugs and
effects in workers are due to incidental exposure in preparation, handling, administration and disposal
of drug residues. Drugs are classified as hazardous if studies in animals and/or humans indicate that
exposures to them have a potential for causing cancer, developmental or reproduction toxicity or harm
to body organs. It should be realized that the therapeutic benefits of hazardous drugs administered to
ill patients outweigh the risks of side effects.
However, HCW's exposed during handling of these drugs are at risk for the side effects without any
therapeutic benefit. HCWs may be exposed to a drug throughout the life cycle from
manufacture/preparation, transport, distribution, administration and disposal.
The Occupational Safety and Health Administration (OSHA) requires that employees potentially
exposed to hazardous drugs (chemicals) be informed of the risks and protective measures to be taken
to avoid exposure. This is detailed in the Hazardous Communication Standard 29 CFR
1910.1200. The OSHA Technical Manual, Section VI, Chapter 2, "Controlling Occupational Exposure
to Hazardous Drugs" also provides guidance for controlling such exposures. This Hazardous Drug
Safety and Health Plan was developed utilizing the information provided in the following publications:
OSHA Technical Manual "Controlling Occupational Exposures to Hazardous Drugs"
NIOSH Alert "Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in
Health Care Settings, 2004"
The Online Journal of Issues in Nursing, "Safe Handling of Hazardous Drugs", American Society of
Health System Pharmacists, "ASHP Technical Assistance Bulletin on Handling Cytotoxic and
Hazardous Drugs"
Oncology Nursing Forum, Vol. 36, No. 6, November, 2009, "American Society of Clinical
Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards"
Responsibilities
Individuals are responsible for following these procedures to ensure their risks from exposure to
hazardous drugs are kept to a minimum
Program Directors, Nurse Managers and Supervisors are responsible for implementation and
enforcement of this plan and ensure that all staff are trained and obtain a medical exam as required by
this policy. In addition, they must ensure that any procedures that could produce an aerosol be pre-
approved by the Office of Research Safety.
Office of Research Safety will provide waste containers, pickup hazardous drug waste and provide
chemo spill kits to appropriate areas. The Office of Research Safety will provide safety information and
be available for emergencies. The Office of Research Safety will develop a specialized training
program for those HCWs potentially exposed to hazardous drugs. The Office of Research Safety staff
will train "trainers" to provide training.
Employee Health Service will provide medical examinations of HCW's covered by this program and
provide follow up exams as necessary or in the event of personal contamination with a hazardous drug.
Pharmacy Director will ensure this policy is implemented in the pharmacy as appropriate and maintain
a central file of Safety Data Sheets (SDS's) for hazardous drugs in-use and that all chemo and other
hazardous drugs are clearly labeled. The Pharmacy Director shall ensure potentially exposed staff
shall obtain medical exams as provided in this policy.
Hazardous Drug Toxicity
Exposure to a hazardous drug may have side effects whether it is administered to a patient or if the
HCW is inadvertently exposed during handling and delivering the drug. A patient would receive the
highest concentration of the drug and would exhibit the most severe side effects. HCWs may be
exposed on a daily basis to a very small amount of a hazardous drug the effects of which require time
to develop, if at all. Information concerning hazardous drugs used in the John Dempsey Hospital may
be obtained by contacting the Pharmacy (x4221) or the Office of Research Safety (x2723).
Chemotherapy and other hazardous drugs may pose one or all of the following risks to the HCW if
ingested, inhaled, injected or absorbed through the skin:
Carcinogenicity
Teratogenicity or Developmental Effects In a Fetus
Reproductive Toxicity or Adverse Pregnancy Outcomes
Organ Toxicity at Low Doses
Genotoxicity or Chromosome Alteration
In addition, immediate effects may be noticed such as reddening of the area of the skin contaminated
with the drug, irritation and/or itching. Other effects of exposure include internal effects such as
lightheadedness, headache, nausea, allergic reactions and abdominal distress. The SDS or package
insert for a drug may be useful in learning the symptoms of an exposure. Skin contact/absorption is the
most likely mode of HCW exposure to a drug. If an exposure is suspected, the HCW must visit the
Employee Health Service for evaluation and provide the name of the drug and how the exposure
occurred.
Hazardous Drug Safety Information
NIOSH (National Institute for Occupational Safety and Health) has published a list of drugs that are
considered hazardous at this time. Information concerning handling hazardous chemicals may be
found in the UCHC Chemical Hygiene Plan/Hazard Communication Plan that can be obtained by
contacting the Office of Research Safety (x2723) or visiting The primary source of
safety information, biological effects of hazardous chemicals (drugs) and chemical information may be
obtained using a Safety Data Sheet (SDS). SDSs may be obtained by visiting the Office of Research
Safety website noted above, contacting the Pharmacy or the JDH Nursing website
or by "Googling" the name of the chemical/drug followed by sds. The UCHC
Pharmacy maintains SDS sheets for drugs used and should be contacted for more detailed
information. The package insert for the drug is also a source of safety information. Call the Office of
Research Safety (x2723) for information concerning any hazardous material or safety concern. All
chemo therapy and other hazardous drug containers will be labeled by Pharmacy staff to clearly identify
the contents.
Medical Surveillance of HCWs
The OSHA Technical Manual, Section VI, Chapter 2, recommends that HCW's that are potentially
exposed to chemical hazards should be monitored in a systematic program of medical surveillance
intended to prevent occupational injury and disease. This manual recommends exams at the following
times:
1. Before Job Placement 2. Following an Acute Exposure 3. Periodically 4. Termination of Chemically related Duties
HCW medical surveillance is provided by the UCHC Employee Health Service (x2893). OSHA recommends that a pre-employment exam consist of
• Determination of HCW's Duties
• Anticipated Exposure Levels
• Previous Medical Exams/Exposures
• Drug Types and frequency of Drug Handling
New employees with the potential for exposure to hazardous drugs will be offered this specialized
screening during their pre-employment physical exam. It is recommended that the physical exam be
complete with emphasis on the skin, mucous membranes, cardiopulmonary and lymphatic
systems. Laboratory tests may include a complete blood count, liver function, blood urea nitrogen,
creatinine and urine dip stick. The above mentioned components of a medical surveillance program
are recommendations, and actual information gathered will be at the discretion of the Occupational
Medicine Physician. Follow up exams shall be at the discretion of the Occupational Medicine
Physician, based on working and medical history. The Occupational Medicine Physician shall direct all
medical evaluations following an acute exposure to a hazardous drug. Employees may request a
medical evaluation at any time with no personal cost.
Personal Protective Equipment (PPE)
It is imperative that the HCW be protected from exposure to hazardous drugs. The use of personal
protective equipment provides protection from skin contamination, contamination of clothes, eye
contamination and inhalation of hazardous drugs. In addition to PPE, engineering controls such as the
use of a biological safety cabinet (tissue culture hood), fume hood or local exhaust ventilation may be
required. The use of PPE and engineering controls is dictated by the type of work required. Working
with powders and/or liquids that may become airborne requires engineering controls, and if necessary,
respiratory protection. Based on the preparation, distribution and administration of hazardous drugs at
the John Dempsey Hospital protection from inhalation of a hazardous drug would only be required in
the Pharmacy. In areas where drugs are administered, the primary risk would be contamination of
hands and clothing. The types of personal protective equipment recommended for use at the UCHC
include nitrile disposable gloves (or other glove type labeled as chemo-glove), protective gowns,
protective glasses or face shields when splashes are possible and appropriate respiratory equipment
such as an N-95 respirator. For detailed information regarding PPE refer to the UCHC Personal
Protective Equipment Plan on the Office of Research Safety websitor contact the
Office of Research Safety at x2723.
Protective Nitrile Gloves
It is required that nitrile protective gloves be worn for all tasks with know or potential contact with
hazardous drugs. All brands of nitrile protective gloves must have a certification on the outside of the
box indicating they have been testing for chemotherapy agent breakthrough. "Breakthrough" is the time
required for a chemotherapy drug to penetrate to the inside of the glove and is usually stated in
minutes. The use of latex gloves must be avoided. A brand of nitrile glove used presently for handling
chemotherapy agents in the Cancer Center is the Esteem Stretchy Nitrile glove (chemo-block latex free
glove used in pharmacy). This is a blue nitrile exam glove and is powder free. This glove has been
tested for chemotherapy agent penetration and the breakthrough times for several drugs are given in
the following table. Any glove designated as a chemo glove is acceptable.
Hazardous Drug Breakthrough Time, Minutes Carmustine 30 min
Cisplatin >480 min
Cyclophosphamide (Cytoxan)
Dacarbazine >480 min Doxorubicin Hydrochloride >480 min Etoposide >480 min Fluorouacil >480 min Ifosfamide >480 min Mitoxantrone >480 min Paclitaxel >480 min Thiotepa >480 min Vincristine >480 min The above breakthrough times are typical of other drugs tested for different manufacturers of nitrile gloves. Penetration will depend on the thickness of the glove among other factors. See the UCHC Chemical Hygiene Plan, Section 5.1.3. B, for breakthrough times of non-medical hazardous chemicals It should be noted that protective gloves are not intended for immersion in a hazardous chemical solution. The HCW wearing protective gloves should remove them immediately when a hazardous drug contacts the glove. In situations likely to involve hand contamination it is recommended that double gloves be worn. Nursing requires that double glove must be worn during the administration of hazardous drugs. Hand washing is required before and after donning of gloves.
Protective Gowns Gowns should be utilized for incidental exposure to hazardous drugs such as administration in the clinics. Disposable chemotherapy gowns, made of fabric that is resistant to drug penetration if splashed, with a closed front and elastic cuffs must be used. As with disposable gloves, gowns must be immediately removed when contamination occurs. The potential for contamination will determine the type of protective gown/covering to be used.
Protective Glasses/Goggles Protective glasses, goggles or face shields must be used in situations where splashes of a hazardous drug are likely. This would be a more likely event in the Pharmacy area than in the clinical areas where individual doses are delivered and are ready to administer. The HCW needs to exercise judgment and error on the side of safety when selecting PPE. Protective eyewear must be certified by the American National Standards Institute (ANSI).
Respiratory Protection Preparation of hazardous drugs outside of a biological safety cabinet or fume hood may require respiratory protection. Respiratory protection is required when the potential for powders and/or liquids to become airborne. Depending on the engineering controls (Biological safety cabinet or hood) a respirator use may be required in the Pharmacy. Respiratory protection in the clinical areas is not required as drug delivery systems are primed for administration. The proper type of respirator must be selected. If you are performing a task outside the hood, call the Office of Research Safety to determine the proper level of respiratory protection. A basic respirator for particulates would be a paper N95 NIOSH certified respirator. For better protection an air supplied Positive Air Purifying Respirator (PAPR) may be used. N95 respirators provide no protection from chemical exposures or from an oxygen deficient atmosphere. A PAPR unit can be fitted with a filter that will absorb chemicals and


particulates. The use of a biological safety cabinet is the preferred method of respiratory
protection. The use of a respirator requires a medical clearance, training and a fit test annually. Refer
to the Office of Research Safety's home page for a full explanation of the UCHC Respiratory Protection
program of call the Office of Research Safety (x2723).
Disposal of Hazardous Drugs/Contaminated Items
Certain essential drugs used in medical practice are regulated under RCRA (or EPA) as acutely
hazardous waste or P-waste. In order to be in compliance with the Environmental Protection Agency
and Connecticut Department of Energy and Environmental Protection (DEEP) regulations these drug
residues must not be disposed in regulated medical waste containers or in the general trash. The
Office of Research Safety provides "chemo therapy waste containers" for disposal of unused hazardous
drugs that are no P-wastes, drug residues and items contaminated with hazardous drugs. These
containers are yellow and only items potentially contaminated with a hazardous drug (that is not a P-
listed waste) should be placed into them. Once a container is ¾ full, the Office of Research Safety
should be called for a pickup and container replacement. Periodic scheduled pick-up of waste can be
scheduled by contacting the Office of Research Safety. Contaminated syringes/needles that have been
used to deliver a non P-listed hazardous drug or that may contain a residue must be placed in a red
sharps container and disposed as regulated medical waste. Patient excreta and body fluids are not
hazardous waste and should be disposed into the sanitary sewerage system although care should be
taken to avoid splashing and skin/eye contamination. The list of EPA P-listed drugs is provided below.
P-listed waste must be placed into a black waste container. In areas where the possibility of non-P-
listed and P-listed drugs are used they should have a black waste container. In some situations it may
be useful to use a black container only.
Chemo Spill Kit
All Chemo Waste No "P" Waste
Operations That Could Expose the HCW
Clinical and nonclinical HCWs may be exposed to hazardous drugs when they create aerosols,
generate dust, clean up spills, touch contaminated surfaces during preparation, administration or
disposal of hazardous drugs. Exposure to hazardous drugs may result from inhalation of
particles/aerosols, direct skin contact with a hazardous drug, accidently ingesting a hazardous drug or
by direct injection via a needle stick or open cut.
The following table summarized the various activities that could possibly expose a HCW. The table
also indicates, based on practices used at the John Dempsey Hospital, HCW staff that have a potential
for exposure due to the activity. Included staff are pharmacy (PH), transport (T), nursing (N) and waste
removal personnel (RSO). The safety procedures for each group are based on the potential for
exposure as provided in the table below. The controls column indicates the type of personal protective
equipment required for the operation (NG=nitrile/chemo glove, G=gown E=protective eyewear
M=respirator EC=engineered control).
Activity HCW Potentially Exposed
Controls
Reconstituting powered or lyophilized
Drugs, further diluting the powder or Liquid forms of the drug Expelling air from syringes
Administrating hazardous drugs by
Intramuscular, subcutaneous or IV Counting out individual, uncoated oral
doses and tablets from multi-dose bottles Unit-Dosing uncoated tablets in a unit
dose machine Crushing Tablets to make oral liquid dose
Compounding powders into capsules
Contacting drug residue present on drug
vial exteriors, work surfaces, floors and final drug products such as bags, as bags, syringes, etc. Generating aerosols during administration PH, N
by direct IV push or by IV infusion Priming the IV set
Handling body fluids or contaminated items PH, N, RSO
Handling waste products
Performing Specialized procedures in the OR
Handling unused hazardous drugs and
Contaminated lines, bags, etc. Decontaminating drug preparation and use areas N, RSO, PH
Transporting hazardous waste containers
Removing and disposing of contaminated
Personal protective equipment (PPE) Spill Response and Cleanup
Recommended Personal Protective Equipment and Procedures
Pharmacy
The following procedures must be followed when preparing hazardous drugs. It should be noted that
these procedures are in addition to and not a replacement for other procedures regarding
sterility, etc, established by Pharmacy management.
1. Any preparation procedure that could release a powder or aerosol must be done in a certified
biological safety cabinet.
2. Biological safety cabinets must be recertified every six months. 3. Powder free nitrile gloves should be used, unless the manufacturer of the product indicates another
type of glove. Double gloves are highly recommended. Any certified chemotherapy glove may be used.
4. A closed front gown with elastic cuffs must be worn. For drug preparation, the gown must be
resistant to penetration of the drug.
5. Safety glasses, goggles or full face shields should be worn, depending on the potential for eye/face
6. Wash hands prior to donning protective gloves and after removing the gloves. 7. In order to minimize contamination of the interior of the biological safety cabinet, the use of lint free
plastic backed absorbent disposable deck liner should be used. This is a matter of personal judgment – will use of a liner increase the risk of a spill?
8. A respirator is not required in this situation. If a respirator is needed contact the Office of Research
Safety (x2723). All activities with the potential to generate airborne contamination must be done in a biological safety cabinet. The Office of Research Safety must approve any alternative work practices.
9. After the dose is prepared, the outside of the bag, IV sets, etc. must be wiped completely to remove
any surface contamination. These items must be placed in a secondary container that is leak proof and sealed for transport to the clinical area. Wipe must be disposed as waste. All chemo agents and other hazardous drugs must be properly labeled to identify the hazard.
10. When preparation of the dose is completed, all associated items must be placed in the black chemo
waste bucket. Protective gloves must be placed in the waste container. Gowns, if there is no possibility of contamination, may be labeled with your name and hung up in the drug preparation area. A fresh gown must be used each day. If there is any question about a gown being contaminated, it must be placed in the black waste container.
11. The area in which the dose was prepared must be wiped down with a detergent solution if a spill
occurred and periodically as required by Pharmacy management.
12. Care must be taken to ensure transport containers are contamination free. The outside of these
containers must be wiped down prior to allowing an individual who is not trained (a volunteer) to handle them. Place a label on the outside of the transport container.
13. If you believe you have been exposed to a hazardous drug, decontaminate the skin area (if needed)
as soon as possible with soap and water and visit the Employee Health Service or the Emergency Department if after hours. Flush eyes immediately if contaminated.
Clinical Areas The potential for HCW exposure to hazardous drugs in the clinics is essentially limited to contamination of surfaces/delivery devices and patient excreta. Preparation and priming of all drug delivery systems is done in the Pharmacy. The following procedure must be followed when administering hazardous drugs in the clinics. 1. Certified chemo gloves shall be worn when administering all chemotherapy agents or when handling
any item potentially contaminated with a chemo agent. Two pairs of gloves are required provided the additional pair of gloves does not inhibit drug delivery.
2. Protective eyewear such as goggles or a disposable face shield are available for use when there is a
potential for a splash of a hazardous drug.
3. Protective closed front gowns are required for connecting chemotherapy to an IV line or
disconnecting an IV line. Gowns are also required any time a nurse is administering chemotherapy via the INM, SQ, or intraperitoneal route
4. Nitrile gloves or other approved chemo glove must be worn when administering oral chemotherapy
5. The use of gowns, gloves and face protection should be determined, in addition to the instances
noted above, by the patient's general condition.
6. Refer to the spill section of this policy for spills of chemo agents. 7. Place all items in direct contact with the chemo agent carefully into the yellow chemo waste
containers provided. In addition, gowns should be placed into the chemo waste if there is a possibility of or known contamination.
8. Wearing gloves, clean chemo administration areas with detergent and water solutions as required by
management. Clean up debris must be placed in a yellow waste container.
9. Change out any personal protective equipment that may be contaminated. 10. When procedure is completed and all contaminated materials are placed in the waste container,
remove gloves and place them in the waste container. Wash hands thoroughly with soap and water.
11. Do not place a P-listed waste into a yellow waste container.
Volunteers UCHC volunteers may be utilized for transporting chemo agents and other hazardous drugs to the clinical areas. These agents will be placed in a secondary closed container that is labeled with instructions to be followed should the container be dropped, it leaks or any other problem develops during transport. Volunteers are provided basic instructions concerning the response they should take in the event an accident occurs. Volunteer exposure to hazardous drugs is not a concern if handled properly. As a minimum, volunteers should be provided with the following information: See appendix III. 1. Do not open transport container for any reason. 2. Should an event occur such as dropping the container, it leaks or you have other concerns call the
Pharmacy (x7627) or the Office of Research Safety (x2723) immediately. Do not touch the container or attempt to place materials back into the container. Prevent individuals from walking through spill area. If skin is contaminated, wash the area thoroughly with soap and water. Wait for trained staff to assist you.
3. Transport the container directly to the destination clinical area. Do not make any stops en-route. 4. After the clinical staff has removed the hazardous drug, return the empty container directly to the
5. Wash hands with soap and water.
Handling Spills of Hazardous Drugs
Spills may occur in the preparation, transport, administration and cleanup/disposal of hazardous
chemicals. Publications have shown that the main risk to the HCW is personal contamination. Small
spills are defined as a small volume spill that may readily cleaned up with little disruption of activities ( ie:
volumes on the order of 5 ml or less). Larger spills posing the possibility of disruption of activities and/or
contaminating other patients or staff (ie: shoe contamination, hand contamination) will require
assistance. During normal working hours the Office of Research Safety (x2723) is available for
immediate response. After hours, the fire department should be called (x7777). Patient excreta needs to
be considered a source of personnel contamination.
Spill Kits
The Office of Research Safety provides spill kits to hospital areas that have the potential for hazardous
drug spills. These areas include the Pharmacy, Oncology 6 and the Neag Cancer Center and
PACU. The Pharmacy is provided an adequate number of small and large Chemo Spill Kits and other
areas a small kit. Other hospital areas will be provided kits as needed. As suggested in the OSHA
Technical Manual concerning Occupational exposure to hazardous drugs, spill kits contain the following
items:
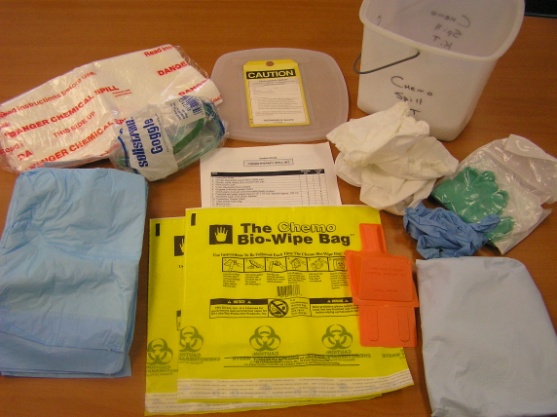
SMALL SPILL KIT CONTENTS
• A Label on the Container "CHEMO SPILL KIT"
• Label Outside of Container "Hazardous Waste"
• Protective Eyewear
• Two Pairs if Nitrile Gloves (Chemo gloves)
• Utility Gloves
• Two Chemo Bio-Wipe Bags
• Two Absorbent Pads
• Instructions on Use
• All Items in Plastic Leak Proof Container
Large spill kits are provided to the Pharmacy as drug preparation is performed in this area. The contents of a large spill kit include: LARGE SPILL KIT CONTENTS • A Label on the Container "CHEMO SPILL KIT"
• Label Outside of Container "Hazardous Waste"
• Protective Eyewear
• Two Pairs of Nitrile Gloves (Chemo gloves)
• Utility Gloves
• Two Chemo Bio-Wipe Bags
• 2 Spill Control Pillows
• Two Absorbent Pads
• Shoe Coverss
• Instructions on Use
• All Items in Large Plastic Leak Proof Container
Small Spills Typically, hazardous drug spills in areas where drugs are administered to patients would be considered small spills. Patient excreta would be considered a large spill requiring assistance from the Office of Research Safety (x2723). A small spill could also occur in the Pharmacy during drug preparation procedures. Response to a small spill should be as follows: 1. Be careful not to step into the spill or touch the immediate spill area. 2. Direct the patient to prevent tracking or touching the spill. 3. Remove any contaminated clothing, shoes, protective gloves and place near immediate spill
area. Immediately wash areas of skin that may be contaminated with soap and water.
4. Obtain a Chemo Spill Kit and remove all items on a clean surface. 5. If the spill is a liquid, obtain spill pad or Chemo Bio-Wipe Bag from spill kit. If broken glass or other
sharp objects are involved, do not clean up with your hands-Use the scoop located in the spill kit. DO NOT USE THE CHEMO BIO-WIPE BAG TO CLEAN UP SHARPS INVOLVEMENT!!
6. Put on two pairs of nitrile chemo gloves and the safety glasses. You should already be wearing a
protective gown.
7. If sharp objects are not involved, place your gloved hand into the Chemo Bio-Wipe Bag and gently
blot the spill area. Follow instructions on bag and turn it inside out and seal. Place bag into spill kit container. Using paper towels wetted with detergent and water, wipe over cleaned spill area three separate times. Place wetted towels into the chemo spill container, place gloves in container and seal.
8. If sharp objects are involved (broken glass, etc), place Bio-Wipe Bag inside of spill kit with absorbent
side up. Obtain a spill pad and place adjacent to spill area.
9. Put on two pairs of nitrile chemo gloves and protective glasses, and using the scoop, scrape the spill
material and glass (or other sharp) onto a spill pad.
10. Carefully place the spill pad into the spill kit container, on top of the Chemo Bio-Wipe Bag.
11. Using paper towels wetted with water and detergent, clean area of spill three different times. DO
THIS ONLY AFTER ALL SHARP OBJECTS HAVE BEEN REOMVED!!!!
12. Place towels into chemo kit container, then you gloves. Seal spill-kit container. 13. Call the Office of Research Safety (x2723) for a pick-up of the spill-kit. 14. For spills involving powders, follow similar procedures as above but use a wetted gauze pad or paper
towel instead of the Chemo Bio-Wipe Bag. The object in this case is to avoid dispersing a powder into the air.
15. If you were contaminated, or believe you were contaminated, visit the Employee Health Service. Large Spills Large spills would usually occur in the Pharmacy but not exclusively in the Pharmacy. As stated previously, contaminated patient excreta would need to be considered as a large spill. The main objective in responding to a large spill is to ensure staff and patients are protected from exposure due to contamination spread by restricting access to the immediate spill area until cleanup has occurred. Response to a large spill should be as follows: 1. Immediately isolate the spill perimeter to avoid tracking/spreading the hazardous drug. 2. Examine yourself, and patient if applicable, for contamination with the drug. Remove contaminated
clothing and shoes immediately and wash contaminated areas of the skin with soap and water. Place contaminated clothing and shoes near the immediate spill area. If needed, ask a co-worker to control access to the immediate spill area.
3. Escort patients and/or staff from the immediate spill area. 4. Obtain the Chemo Spill Kit (in the Pharmacy Large Chemo Spill Kit) and put on two pairs of nitrile
chemo gloves and protective eyewear. Spill pads and spill pillows are located in the spill kit and they can be used to control the spill spread. In addition, blue pads, towels, etc. can be placed on and around the perimeter of the spill to control its movement and size.
5. Avoid disturbing the spill area and call the Office of Research Safety (x2723) for assistance. If during
off-hours, call the UConn Fire Department (x7777),
6. Office of Research Safety staff will clean up the spill and remove the residue for waste disposal. 7. If you were contaminated, or believe you were contaminated, visit the Employee Health Service for
Spills in Biological Safety Cabinets
Spills of hazardous drugs in biological safety cabinets would occur only in the Pharmacy and should be
contained and of little risk of spreading or exposing others in the immediate area. Spill cleanup
procedures would be similar as noted above. There should be little chance of personal contamination but
prudent safety practices need to be followed such as working on absorbent surfaces to absorb any spill
that occurs.
HCW Training
All clinical staff must attend a training session prior to working with a hazardous drug. It should be noted
that this training is designed to inform you of the risks associated with handling hazardous drugs and is in
addition to and not a replacement of any Clinical training requirements. Refresher training is
recommended on a yearly basis. Management is responsible for ensuring all staff are properly trained
prior to working with a hazardous drug. Training information is available by contacting the Office of
Research Safety (x2723) or by contacting a clinical staff member. Training must be documented.
APPENDIX I
NIOSH Sample List of Drugs that Should be Handled as Hazardous
(*drugs that have been used at the Health Center)
AHFS Pharmalocologic – Therapeutic Classification
10:00 Antineoplastic agents
84:92 Miscellaneous skin and mucous membrane agents
10:00 Antineoplastic agents
84:36 Miscellaneous skin and mucous membrane agents (retinoid)
10:00 Antineoplastic agents
Not in AHFS (antineoplastic agent)
10:00 Antineoplastic agents
Arsenic trioxide*
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
92:44 Unclassified therapeutic agents (immunosuppressant)
Bacillus Calmette-Guerin*
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
24:12.92 Vasodilating agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Cetrorelix acetate
92:40 Unclassified therapeutic agents (GnRH antagonist)
10:00 Antineoplastic agents
Chloramphenicol*
8:12.08 Antibacterials
Choriogonadotropin alfa
68:18 Gonadotropins
8:18.32 Antiviral nucleoside
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
92:16 Unclassified therapeutic agents (antigout agents)
Cyclophosphamide*
10:00 Antineoplastic agents
92:00 Immunosuppressive agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Daunorubicin HCl*
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
68:16.04 Estrogens
Diethylstilbestrol
Not in AHFS (nonsteroidal synthetic estrogen)
10:00 Antineoplastic agents
10:00 Antineoplastic agents
92:08 Unclassified therapeutic agents (5-alpha reductase inhibitor)
8:18.32 Antiviral nucleoside
10:00 Antineoplastic
Ergonovine/methylergon
76:00 Oxytocics agents
68:16.04 Estrogens
Estramustine phosphate
10:00 Antineoplastic agents
Estrogen-progestin combinations
68:12 Contraceptives
Estrogens, conjugated*
68:16.04 Estrogens
Estrogens, esterified
68:16.04 Estrogens
68:16.04 Estrogens
68:16.04 Estrogens
10:00 Antineoplastic agents
10:00 Antineoplastic agents
92:08 Unclassified therapeutic agents (5-alpha reductase inhibitor)
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
8:18.32 Antiviral nucleoside
Ganirelix acetate
92:40 Unclassified therapeutic agents (GnRH antagonist)
10:00 Antineoplastic agents
Gemtuzumab ozogamicin
10:00 Antineoplastic agents
Gonadotropin, chorionic
68:18 Gonadotropins
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Ibritumomab tiuxetan
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Imatinib mesylate*
10:00 Antineoplastic agents
Interferon alfa-2a
10:00 Antineoplastic agents
Interferon alfa-2b*
10:00 Antineoplastic agents
Interferon alfa-n1
10:00 Antineoplastic agents
Interferon alfa-n3
10:00 Antineoplastic agents
10:00 Antineoplastic agents
92:36 Unclassified therapeutic agents (antineoplastic agent)
92:20 Unclassified therapeutic agents (biologic response modifiers)
10:00 Antineoplastic agents
Leuprolide acetate*
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Medroxyprogesterone* acetate
68:32 Progestins
10:00 Antineoplastic agents
10:00 Antineoplastic agents
68:18 Gonadotropins
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Methyltestosterone
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Mitoxantrone HCl*
10:00 Antineoplastic agents
Mycophenolate mofetil*
92:44 Unclassified therapeutic agents (immunosuppressive agents)
68:18 Gonadotropins
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
84:16 Cell stimulants
28:16.04.20 Selective seretonin uptake inhibitors
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Pentamidine isethionate*
8:40 Miscellaneous anti-infectives
Pentetate calcium trisodium
10:00 Antineoplastic agents
Not in AHFS (antineoplastic agent)
Not in AHFS (antineoplastic agent)
Piritrexim isethionate
Not in AHFS (antineoplastic agent)
Not in AHFS (antineoplastic agent)
84:92 Miscellaneous skin and mucous membrane agents (mitotic inhibitor)
Podophyllum resin*
84:92 Miscellaneous skin and mucousmembrane agents (mitotic inhibitor)
Not in AHFS (antineoplastic agent)
10:00 Antineoplastic agents
68:32 Progestins
68:12 Contraceptives
68:16.12 Estrogen agonists-antagonists
Not in AHFS (antineoplastic agent)
Rasagiline mesylate
28:36 Antiparkinsonian agents
8:18.32 Antiviral nucleoside
28:16.08.04 Atypical antipsychotics
92:00 Immunosuppressive agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Sunitinib malate
10:00 Antineoplastic agents
92:44 Unclassified therapeutic agents (immunosuppressant)
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
92:20 Unclassified therapeutic agents (biologic response modifier)
10:00 Antineoplastic agents
10:00 Antineoplastic agents
10:00 Antineoplastic agents
Toremifene citrate
10:00 Antineoplastic agents
10:00 Antineoplastic agents
84:16 Cell stimulants and proliferants (retinoid)
52:04.06 Antivirals
Trimetrexate glucuronate
8:30.92 Miscellaneous antiprotozoals
10:00 Antineoplastic agents
Not in AHFS (antineoplastic agent)
8:18.32 Antiviral nucleoside
10:00 Antineoplastic agents
Vinblastine sulfate*
10:00 Antineoplastic agents
Vincristine sulfate*
10:00 Antineoplastic agents
Not in AHFS (antineoplastic agent)
Vinorelbine tartrate*
10:00 Antineoplastic agents
10:00 Antineoplastic agents
8:18:08 Antiretroviral agents
28:12.92 Anticonvulsant
Source: http://research.uchc.edu/wp-content/uploads/sites/1137/2015/09/hazardous_drug_safety2013.pdf
Pesq. Vet. Bras. 31(5):407-412, maio 2011 Equine leukoencephalomalacia (ELEM) due to fumonisins B1 and B2 in Argentina1 Federico Giannitti2* , Santiago Sain Diab3, Ana Maria Pacin4,5, Maria Barrandeguy6, Carlos Larrere7, Joaquin Ortega3 and Francisco Alejandro Uzal3 ABSTRACT.- Giannitti F., Diab S.S., Pacin A.M., Barrandeguy M., Larrere C., Ortega J. &Uzal F.A. 2011. Equine leukoencephalomalacia (ELEM) due to fumonisins B1 andB2 in Argentina. Pesquisa Veterinária Brasileira 31(5):407-412. Laboratorio de DiagnósticoVeterinario, calle 25 de Mayo 139, Bahía Blanca (8000), Buenos Aires, Argentina. E-mail:[email protected]
Clinical MCQs Assessment – Sample Questions The fol owing 20 clinical MCQs are representative of the style and format of MCQs that candidates wil receive as part of the AACP Stage 2 Clinical MCQ Assessment. The answers and explanatory notes are provided at the end of this document. SQ1. Which ONE of the following patients has the HIGHEST calculated creatinine clearance?











