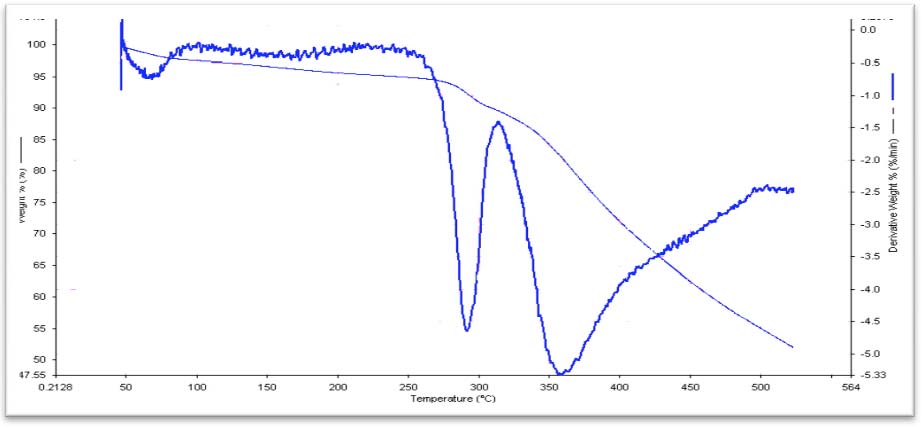
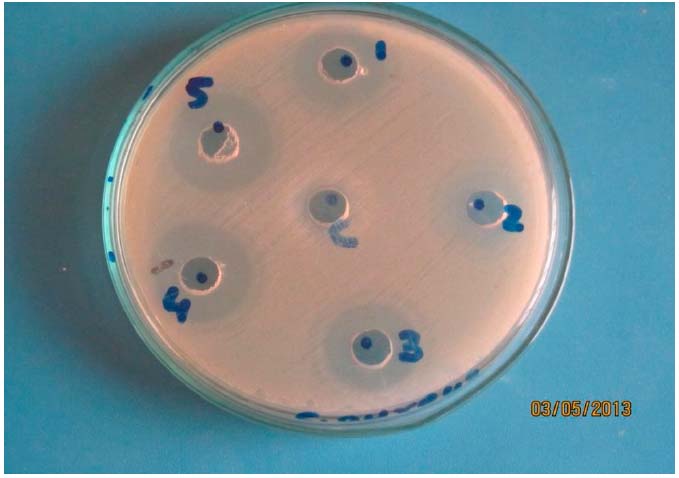
econ.biu.ac.il
Voter Turnout and Fiscal Policy Raphael Godefroy and Emeric Henry Though a large literature on causes of voter turnout has flour- ished, there is scant evidence on consequences of turnout on policiesimplemented in practice. Using data on French municipalities, andinstrumental variables for turnout based on rainfall and influenza in-cidence, we estimate that a 1 percent increase in turnout decreasesthe municipal budget by more than 2 percent. This effect is medi-ated by a decrease in sales and purchases of physical assets. Witha model of electoral competition, we show that a party with a lowbudget platform has a numerical advantage causing its win whenturnout is high.