Vetadv.millcreeksoftware.net


Diagnosis, Treatment and Monitoring
of Hyperadrenocorticism
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. As with all drugs, side effects may occur. In field studies and post-
approval experience, the most common side effects reported were: anorexia, lethargy/depression, vomiting, diarrhea, elevated liver enzymes, elevated potassium with or
without elevated sodium, elevated BUN, decreased Na/K ratio, hypoadrenocorticism, weakness, elevated creatinine, shaking, and renal insufficiency. In some cases, death
has been reported as an outcome of these adverse events. VETORYL Capsules are not for use in dogs with primary hepatic or renal disease, or in pregnant dogs. Refer to
the prescribing information for complete details or visit www.Dechra-US.com.


Confirming the diagnosis of hyperadrenocorticism (HAC)
No test for HAC has 100% diagnostic accuracy. The diagnostic value of all endocrine tests will be significantly enhanced by performing them only when clinical signs consistent with HAC are present in the patient. Three endocrine diagnostic tests are available, al with particular advantages and disadvantages:
Sensitivity & Specificity
Urinary Cortisol to
• Highest sensitivity of all three tests
• To avoid false-positive results, urine samples should
Creatinine Ratio (UCCR)
makes it a great screening test
be collected at home at least two days after a visit to a
veterinary clinic
• Highest confidence in a negative test
• Collect first urine sample from patient in the morning
• Lacks specificity
• Specificity and sensitivity can be increased when
urine from 2-3 days is pooled and collectively tested
• False positives are relatively common
and when the test is performed on dogs showing
symptoms consistent with HAC
• High sensitivity
• Long test (8 hours)
• High confidence in a negative test result
• In some cases may differentiate between PDH and
• Moderate specificity
• False positives can occur
• Considered the screening test of choice unless
latrogenic HAC is suspected
• Highest specificity of all three tests
• Relatively short test (1 hour)
• Highest confidence in a positive test
• Test of choice if there is a history of exogenous steroid
• Lacks sensitivity• False negatives are relatively common
For detailed information on performing and interpreting these tests, please contact Dechra Veterinary Technical Services at
(866) 933-2472 or your reference laboratory consult line.
Differentiating between types
It is necessary to differentiate between Pituitary Dependent
Hyperadrenocorticism (PDH) and Adrenal Dependent Hyperadrenocorticism (ADH) to provide a more accurate prognosis and enable the full range of possible treatments to be discussed with the dog's owner.
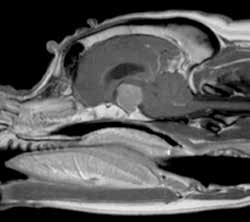
Discriminatory tests available to differentiate between PDH and ADH include the low- and high-dose dexamethasone suppression tests, ultrasonography, and advanced imaging such as MRI and CT and measurement of endogenous ACTH.
MRI image from a Boxer dog with a pituitary macroadenoma (image courtesy of Ruth Dennis, The Animal Health Trust, UK)
A confident diagnosis requires consistent endocrine confirmatory test results in a dog
with clinical signs compatible with hyperadrenocorticism.


Treatment and Monitoring of Hyperadrenocorticism
Start VETORYL® Capsules at approximately 1mg/lb (2.2mg/kg) once daily as per
prescribing information
Give daily, by mouth, with food, in the morning.
DAY 10 -14
History, physical examination, serum biochemistry, with electrolytes
Perform ACTH stim test 4-6 hours after morning capsule
Ensure morning capsule was given with food
Post-ACTH serum cortisol
Post-ACTH serum cortisol >1.45 µg/dL
Showing clinical signs consistent with:
<1.45 µg/dL (<40 nmol/L)
(>40 nmol/L) and clinically well
1. Corticosteroid withdrawal syndrome ("relative"
and clinically well
cortisol deficiency characterized by weakness,
lethargy, stiff gait, anorexia, fever during first 10 days
Continue treatment at current dose
It is not recommended to increase dose
2. Hypoadrenocorticism (e.g., anorexia, lethargy/
yet, even if cortisol is >9.1 µg/dL
depression, weakness, shaking/shivering, vomiting,
diarrhea, bradycardia, collapse)
Stop VETORYL Capsules for
approximately 7 days
≥30 DAYS FROM INITIATION OF
RETURN TO DAY 1 and
administer a LOWER DOSE
History, physical examination, serum
STOP VETORYL TREATMENT
biochemistry, with electrolytes
Repeat ACTH Stim test in 10-14
Confirm whether clinical signs are due to
days after restarting lower dose
ACTH stim test 4-6 hours after morning
hypoadrenocorticism with ACTH stim test and analysis
capsule given with food
of serum electrolytes (in particular Na+ and K+)
Treat symptomatically as required, e.g.
• dexamethasone to treat hypocortisolemia
Assess degree of clinical improvement
• IV 0.9% NaCl to resolve hyperkalemia
CLINICAL SIGNS NOT FULLY CONTROLLED
Rule out concurrent illness
If clinical signs are
cortisol >5.4 µg/dL
not controlled for a full
24 hour period, twice
daily dosing may be
indicated or a dosage
Continue current
dose and recheck
RETURN TO DAY 1
in 1-3 months OR
To change to twice daily dosing, use combinations
of capsule sizes to split the current daily dose into
Continue monitoring history, physical examination,
If Post-ACTH serum cortisol >9.1 μg/dL
electrolytes and ACTH stim test every 90 days.
(>250 nmol/L), total daily dose can be slowly
increased and split into two doses
If dose is altered always recheck ACTH stim again
10-14 days later
RETURN TO
Continue to monitor as per approved label
If you have questions at any point during patient
management, contact Dechra Veterinary Technical
Perform ACTH stim test 4-6 hours post morning
Services at (866) 933-2472
Two dogs developed hypoadrenocorticism during the study. These two dogs had clinical signs consistent with hypoadrenocorticism
(lethargy, anorexia, collapse) and post-ACTH cortisol levels ≤ 0.3 µg/dL. Both dogs responded to trilostane discontinuation and
supportive care, and one dog required continued treatment for hypoadrenocorticism (glucocorticoids and mineralocorticoids) after
the acute presentation.
Additional adverse reactions were observed in 93 dogs. The most common of these included diarrhea (31 dogs), lethargy (30 dogs),
inappetence/anorexia (27 dogs), vomiting (28 dogs), musculoskeletal signs (lameness, worsening of degenerative joint disease)
(25 dogs), urinary tract infection (UTI)/hematuria (17 dogs), shaking/shivering (10 dogs), otitis externa (8 dogs), respiratory signs
(coughing, congestion) (7 dogs), and skin/coat abnormality (seborrhea, pruritus) (8 dogs).
Five dogs died or were euthanized during the study (one dog secondary to adrenal necrosis, discussed above, two dogs due to
progression of pre-existing congestive heart failure, one dog due to progressive central nervous system signs, and one dog due to
Adrenocortical suppressant for oral use in dogs only.
cognitive decline leading to inappropriate elimination). In addition to the two dogs with adrenal necrosis/rupture and the two dogs
with hypoadrenocorticism, an additional four dogs were removed from the study as a result of possible trilostane-related adverse
reactions, including collapse, lethargy, inappetence, and trembling.
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Complete blood counts conducted pre- and post-treatment revealed a statistically significant (p <0.005) reduction in red cell
DESCRIPTION: VETORYL Capsules are available in 5 sizes (5, 10, 30, 60 and
variables (HCT, HGB, and RBC), but the mean values remained within the normal range. Additionally, approximately 10% of the dogs
120 mg) for oral administration based on body weight. Trilostane (4α,5α-epoxy-
had elevated BUN values (≥ 40 mg/dL) in the absence of concurrent creatinine elevations. In general, these dogs were clinically
17β-hydroxy-3-oxoandrostane-2α-carbonitrile) is an orally active synthetic
normal at the time of the elevated BUN.
steroid analogue that selectively inhibits 3 β-hydroxysteroid dehydrogenase in the
adrenal cortex, thereby inhibiting the conversion of pregnenolone to progesterone.
In a long term follow-up study of dogs in the US effectiveness study, the adverse reactions were similar to the short term study.
This inhibition blocks production of glucocorticoids and to a lesser extent,
Vomiting, diarrhea and general gastrointestinal signs were most commonly observed. Lethargy, inappetance/anorexia, heart murmur
mineralocorticoids and sex hormones while steroid precursor levels increase. The
or cardiopulmonary signs, inappropriate urination/incontinence, urinary tract infections or genitourinary disease, and neurological
structural formula is:
signs were reported. Included in the US follow-up study were 14 deaths, three of which were possibly related to trilostane. Eleven
dogs died or were euthanized during the study for a variety of conditions considered to be unrelated to or to have an unknown
INDICATIONS: VETORYL Capsules are indicated for the treatment of pituitary-dependent hyperadrenocorticism in dogs.
relationship with administration of trilostane.
VETORYL Capsules are indicated for the treatment of hyperadrenocorticism due to adrenocortical tumor in dogs.
In two UK field studies with 75 dogs, the most common adverse reactions seen were vomiting, lethargy, diarrhea/loose stools, and
DOSAGE AND ADMINISTRATION: Always provide the Client Information Sheet with prescription (see INFORMATION FOR
anorexia. Other adverse reactions included: nocturia, corneal ulcer, cough, persistent estrus, vaginal discharge and vulvar swelling in
a spayed female, hypoadrenocorticism, electrolyte imbalance (elevated potassium with or without decreased sodium), collapse and
1. Starting dose. The starting dose for the treatment of hyperadrenocorticism in dogs is 1-3 mg/lb (2.2-6.7 mg/kg) once a
seizure, shaking, muscle tremors, constipation, scratching, weight gain, and weight loss. One dog died of congestive heart failure and
day. Start with the lowest possible dose based on body weight and available combinations of capsule sizes. VETORYL Capsules
another died of pulmonary thromboembolism. Three dogs were euthanized during the study. Two dogs had renal failure and another
should be administered with food.
had worsening arthritis and deterioration of appetite.
2. Action at 10-14 day evaluation (Table 1). After approximately 10-14 days at this dose, re-examine the dog and conduct a
In a long term follow-up of dogs included in the UK field studies, the following adverse reactions were seen: hypoadrenocortical
4-6 hour post-dosing ACTH stimulation test and serum biochemical tests (with particular attention to electrolytes, and renal and
episode (including syncope, tremor, weakness, and vomiting), hypoadrenocortical crisis or renal failure (including azotemia, vomiting,
hepatic function). If physical examination is acceptable, take action according to Table 1.
dehydration, and collapse), chronic intermittent vaginal discharge, hemorrhagic diarrhea, occasional vomiting, and distal limb edema.
Signs of hypoadrenocorticism were usually reversible after withdrawal of the drug, but may be permanent. One dog discontinued
Owners should be instructed to stop therapy and contact their veterinarian immediately in the event of adverse
VETORYL Capsules and continued to have hypoadrenocorticism when evaluated a year later. Included in the follow-up were reports
reactions such as vomiting, diarrhea, lethargy, poor/reduced appetite, weakness, collapse or any other unusual
of deaths, at least 5 of which were possibly related to use of VETORYL Capsules. These included dogs that died or were euthanized
developments. If these clinical signs are observed, conduct an ACTH stimulation test and serum biochemical tests
because of renal failure, hypoadrenocortical crisis, hemorrhagic diarrhea, and hemorrhagic gastroenteritis.
(with particular attention to electrolytes, and renal and hepatic function).
Foreign Market Experience: The following events were reported voluntarily during post-approval use of VETORYL Capsules in foreign
Table 1: Action at 10-14 day evaluation
markets. The most serious adverse events were death, adrenal necrosis, hypoadrenocorticism (electrolyte alterations, weakness,
Post-ACTH serum cortisol
collapse, anorexia, lethargy, vomiting, diarrhea, and azotemia), and corticosteroid withdrawal syndrome (weakness, lethargy, anorexia,
and weight loss). Additional adverse events included: renal failure, diabetes mellitus, pancreatitis, autoimmune hemolytic anemia,
vomiting, diarrhea, anorexia, skin reactions (rash, erythematous skin eruptions), hind limb paresis, seizures, neurological signs from
Stop treatment. Re-start at a decreased dose
growth of macroadenomas, oral ulceration, and muscle tremors.
Continue on same dose
POST-APPROVAL EXPERIENCE: As of June 2013, the following adverse events are based on post-approval adverse drug
EITHER: Continue on current dose if clinical signs are well controlled
experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse
OR: Increase dose if clinical signs of hyperadrenocorticism are still evident*
event frequency or establish a causal relationship to product exposure using this data. The following adverse events are listed
Increase initial dose
in decreasing order of reporting frequency: anorexia, lethargy/depression, vomiting, diarrhea, elevated liver enzymes, elevated
*Combinations of capsule sizes should be used to slowly increase the once daily dose.
potassium with or without decreased sodium, elevated BUN, decreased Na/K ratio, hypoadrenocorticism, weakness, elevated
creatinine, shaking, renal insufficiency. In some cases, death has been reported as an outcome of the adverse events listed
3. Individual dose adjustments and close monitoring are essential. Re-examine and conduct an ACTH stimulation test and serum
biochemical tests (with particular attention to electrolytes, and renal and hepatic function) 10-14 days after every dose alteration. Care
For a cumulative listing of adverse reactions for trilostane reported to the CVM see: http://www.fda.gov/ADEreports
must be taken during dose increases to monitor the dog's clinical signs.
This listing includes Adverse Events reported to CVM for products, such as VETORYL Capsules, that contain the active ingredient
Once daily administration is recommended. However, if clinical signs are not controlled for the full day, twice daily dosing may be needed.
trilostane. Listings by active ingredient may represent more than one brand name.
To switch from a once daily dose to a twice daily dose, the total daily dose should be divided into 2 portions given 12 hours apart. It is not
necessary for the portions to be equal. If applicable, the larger dose should be administered in the morning and the smaller dose in the
To report suspected adverse events and/or obtain a copy of the MSDS or for technical assistance, call Dechra Veterinary Products
evening. For example, a dog receiving 90 mg would receive 60 mg in the morning, and 30 mg in evening.
at (866) 933-2472.
4. Long term monitoring. Once an optimum dose of VETORYL Capsules has been reached, re-examine the dog at 30 days, 90 days
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at:
and every 3 months thereafter. At a minimum, this monitoring should include: • A thorough history and physical examination.
• An ACTH stimulation test (conducted 4-6 hours after VETORYL Capsule administration) - a post-ACTH stimulation test resulting in
INFORMATION FOR DOG OWNERS: Owners should be aware that the most common adverse reactions may include: an
a cortisol of < 1.45 μg/dL (< 40 nmol/L), with or without electrolyte abnormalities, may precede the development of clinical signs of
unexpected decrease in appetite, vomiting, diarrhea, or lethargy and should receive the Client Information Sheet with the prescription.
hypoadrenocorticism.
Owners should be informed that control of hyperadrenocorticism should result in resolution of polyphagia, polyuria and polydipsia.
• Serum biochemical tests (with particular attention to electrolytes, and renal and hepatic function).
Serious adverse reactions associated with this drug can occur without warning and in some cases result in death (see
Good control is indicated by favorable clinical signs as well as post-ACTH serum cortisol of 1.45-9.1 μg/dL (40-250 nmol/L).
ADVERSE REACTIONS and POST-APPROVAL EXPERIENCE).
If the ACTH stimulation test is < 1.45 μg/dL (< 40 nmol/L) and/or if electrolyte imbalances characteristic of
hypoadrenocorticism (hyperkalemia and hyponatremia) are found, VETORYL Capsules should be temporarily discontinued
Owners should be advised to discontinue VETORYL Capsules and contact their veterinarian immediately if signs of
until recurrence of clinical signs consistent with hyperadrenocorticism and ACTH stimulation test results return to normal
intolerance such as vomiting, diarrhea, lethargy, poor/reduced appetite, weakness, or collapse are observed. Owners
(1.45-9.1 μg/dL or 40-250 nmol/L). VETORYL Capsules may then be re-introduced at a lower dose.
should be advised of the importance of periodic follow-up for all dogs during administration of VETORYL Capsules.
CONTRAINDICATIONS:
CLINICAL PHARMACOLOGY: Trilostane absorption is enhanced by administration with food. In healthy dogs, maximal plasma levels
The use of VETORYL Capsules is contraindicated in dogs that have demonstrated hypersensitivity to trilostane. Do
of trilostane occur within 1.5 hours, returning to baseline levels within twelve hours, although large inter-dog variation occurs. There
not use VETORYL Capsules in animals with primary hepatic disease or renal insufficiency (See WARNINGS and PRECAUTIONS). Do not
is no accumulation of trilostane or its metabolites over time.
use in pregnant dogs. Studies conducted with trilostane in laboratory animals have shown teratogenic effects and early pregnancy loss.
WARNINGS: Hypoadrenocorticism can develop at any dose of VETORYL Capsules. In some cases, it may take months for
EFFECTIVENESS: Eighty-three dogs with hyperadrenocorticism were enrolled in a multi-center US field study. Additionally, 30
adrenal function to return and some dogs never regain adequate adrenal function.
dogs with hyperadrenocorticism were enrolled in two UK field studies. Results from these studies demonstrated that treatment with
VETORYL Capsules resulted in an improvement in clinical signs (decreased thirst, decreased frequency of urination, decreased
All dogs should undergo a thorough history and physical examination before initiation of therapy with VETORYL Capsules.
panting, and improvement of appetite and activity). Improvement in post-ACTH cortisol levels occurred in most cases within 14 days
Other conditions, such as primary hepatic and/or renal disease should be considered when the patient is exhibiting signs of
of starting VETORYL Capsules therapy.
illness in addition to signs of hyperadrenocorticism (e.g. vomiting, diarrhea, poor/reduced appetite, weight loss, and lethargy).
Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during,
In these three studies, there were a total of 10 dogs diagnosed with hyperadrenocorticism due to an adrenal tumor or due to
administration of VETORYL Capsules should be considered.
concurrent pituitary and adrenal tumors. Evaluation of these cases failed to demonstrate a difference in clinical, endocrine, or
biochemical response when compared to cases of pituitary-dependent hyperadrenocorticism.
Owners should be advised to discontinue therapy immediately and contact their veterinarian if signs of potential drug toxicity
are observed (see INFORMATION FOR DOG OWNERS, DOSAGE AND ADMINISTRATION, PRECAUTIONS, ADVERSE REACTIONS,
ANIMAL SAFETY: In a laboratory study, VETORYL Capsules were administered to 8 healthy 6 month old Beagles per group at 0X
ANIMAL SAFETY and POST-APPROVAL EXPERIENCE).
(empty capsules), 1X, 3X, and 5X the maximum starting dose of 6.7 mg/kg twice daily for 90 days. Three animals in the 3X group
(receiving 20.1 mg/kg twice daily) and five animals in the 5X group (receiving 33.5 mg/kg twice daily) died between Days 23 and
In case of overdosage, symptomatic treatment of hypoadrenocorticism with corticosteroids, mineralocorticoids and intravenous fluids
46. They showed one or more of the following clinical signs: decreased appetite, decreased activity, weight loss, dehydration, soft
may be required.
stool, slight muscle tremors, diarrhea, lateral recumbency, and staggering gait. Bloodwork showed hyponatremia, hyperkalemia, and
azotemia, consistent with hypoadrenocortical crisis. Post-mortem findings included epithelial necrosis or cystic dilation of duodenal
Angiotensin converting enzyme (ACE) inhibitors should be used with caution with VETORYL Capsules, as both drugs have aldosterone-
mucosal crypts, gastric mucosal or thymic hemorrhage, atrial thrombosis, pyelitis and cystitis, and inflammation of the lungs.
lowering effects which may be additive, impairing the patient's ability to maintain normal electrolytes, blood volume and renal perfusion.
Potassium sparing diuretics (e.g. spironolactone) should not be used with VETORYL Capsules as both drugs have the potential to inhibit
ACTH stimulated cortisol release was reduced in all dogs treated with VETORYL Capsules. The dogs in the 3X and 5X groups had
aldosterone, increasing the likelihood of hyperkalemia.
decreased activity. The 5X dogs had less weight gain than the other groups. The 3X and 5X dogs had lower sodium, albumin, total
protein, and cholesterol compared to the control dogs. The 5X dogs had lower mean corpuscular volume than the controls. There
HUMAN WARNINGS: Keep out of reach of children. Not for human use.
was a dose dependent increase in amylase. Post-mortem findings included dose dependent adrenal cortical hypertrophy.
Wash hands after use. Do not empty capsule contents and do not attempt to divide the capsules. Do not handle the capsules if pregnant
STORAGE INFORMATION: Store at controlled room temperature 25°C (77°F) with excursions between 15°-30°C (59°-86°F)
or if trying to conceive. Trilostane is associated with teratogenic effects and early pregnancy loss in laboratory animals. In the event of
accidental ingestion/overdose, seek medical advice immediately and take the labeled container with you.
HOW SUPPLIED: VETORYL Capsules are available in 5, 10, 30, 60 and 120 mg strengths, packaged in aluminum foil blister cards
PRECAUTIONS: Mitotane (o,p'-DDD) treatment will reduce adrenal function. Experience in foreign markets suggests that when mitotane
of 10 capsules, with 3 cards per carton.
therapy is stopped, an interval of at least one month should elapse before the introduction of VETORYL Capsules. It is important to wait for
VETORYL Capsules 5 mg
NDC 17033-105-30
both the recurrence of clinical signs consistent with hyperadrenocorticism, and a post-ACTH cortisol level of > 9.1 μg/dL (> 250 nmol/L)
VETORYL Capsules 10 mg
NDC 17033-110-30
before treatment with VETORYL Capsules is initiated. Close monitoring of adrenal function is advised, as dogs previously treated with
VETORYL Capsules 30 mg
NDC 17033-130-30
mitotane may be more responsive to the effects of VETORYL Capsules.
VETORYL Capsules 60 mg
NDC 17033-160-30
VETORYL Capsules 120 mg
The use of VETORYL Capsules will not affect the adrenal tumor itself. Adrenalectomy should be considered as an option for cases that are
good surgical candidates. The safe use of this drug has not been evaluated in lactating dogs and males intended for breeding.
ADVERSE REACTIONS: The most common adverse reactions reported are poor/reduced appetite, vomiting, lethargy/dullness, diarrhea,
and weakness. Occasionally, more serious reactions, including severe depression, hemorrhagic diarrhea, collapse, hypoadrenocortical
crisis or adrenal necrosis/rupture may occur, and may result in death.
NADA 141-291, Approved by FDA.
In a US field study with 107 dogs, adrenal necrosis/rupture (two dogs) and hypoadrenocorticism (two dogs) were the most severe adverse
reactions in the study. One dog died suddenly of adrenal necrosis, approximately one week after starting trilostane therapy. One dog
Dechra Veterinary Products
developed an adrenal rupture, believed to be secondary to adrenal necrosis, approximately six weeks after starting trilostane therapy. This
7015 College Boulevard, Suite 525
dog responded to trilostane discontinuation and supportive care.
Overland Park, KS 66211
VETORYL is a trademark of Dechra Ltd 2015, Dechra Ltd
Source: http://vetadv.millcreeksoftware.net/media/attachments/2015/09/09/VETORYLTreatmentMonitoringBrochurev6.15.15.pdf
THE NATIONAL TELECOMMUNICATIONS REGULATORY COMMISSION ICT NEWSLETTER NTRC ICT NEWSLETTER ISSUE #68 December 2015 Cyber Tips Realize that you are an attractive target to hackers. Don't ever say "It won't happen to me." - management. Use a strong mix of characters, and don't use the same password for multiple sites.
Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance of job duties. Post exposure prophylaxis (PEP) refers to comprehensive medical management to minimise the risk of infection among Health Care Personnel (HCP) following potential exposure to blood-borne pathogens (HIV, HBV, HCV). This includes counselling, risk assessment, relevant laboratory investigations based on informed consent of the source and exposed person, first aid and depending on the risk assessment, the provision of short term (four weeks) of antiretroviral drugs, with follow up and support. Who is at risk? All Health Care Personnel, including emergency care providers, laboratory personnel, autopsy personnel, hospital employees, interns and medical students, nursing staff and students, physicians, surgeons, dentists, labour and delivery room personnel, laboratory technicians, health facility sanitary staff and clinical waste handlers and health care professionals at all levels. Also at risk are public safety workers, including law enforcement personnel, prison staff, fire-fighters, workers in needle exchange programme and workers in HIV programmes. What is the risk? Health Care Personnel are at risk of blood-borne infection transmission through exposure of a percutaneous injury (e.g. needle-stick or cut with a sharp instrument), contact with the mucous membranes of the eye or mouth of an infected person, contact with non-intact skin (particularly when the exposed skin is chapped, abraded, or afflicted with dermatitis or contact with blood or other potentially infectious body fluids. potentially infectious body fluids Any direct contact (i.e., contact without barrier protection) with concentrated virus in a research laboratory or production facility requires clinical evaluation. Transmission of HIV infection from human bites is rarely reported. The average risk of acquiring HIV infection from different types of occupational exposure is low compared to risk of infection with HBV or HCV. In terms of occupational exposure the important routes are needle stick exposure (0.3% risk for HIV, 9–30% for HBV and 1–10% for HCV) and mucous membrane exposure (0.09% for HIV).e What is infectious and what is not? Exposure to blood, semen, vaginal secretions, cerebrospinal fluid, synovial, pleural, peritoneal, pericardial fluid, amniotic fluid and other body fluids contaminated with visible blood can lead to infection. Exposure to tears, sweat, saliva, urine and faeces is non-infectious unless these secretions contain visible blood. Step 1: First aid in management of exposure For skin — if the skin is broken after a needle-stick or sharp instrument:















