H4120002994p
Am J Physiol Heart Circ Physiol279: H2994–H3002, 2000.
Effects of exercise training on cardiac function,gene expression, and apoptosis in rats
HONGKUI JIN,1 RENHUI YANG,1 WEI LI,1 HSIENWIE LU,1 ANNE M. RYAN,2ANNIE K. OGASAWARA,1 JOHN VAN PEBORGH,1 AND NICHOLAS F. PAONI11
Department of Cardiovascular Research and 2
Department of Pathology,Genentech Incorporated, South San Francisco, California 94080
Received 1 December 1999; accepted in final form 21 June 2000
Jin, Hongkui, Renhui Yang, Wei Li, Hsienwie Lu,
thermore, systematic studies on the effects of exercise
Anne M. Ryan, Annie K. Ogasawara, John Van Pe-
on hemodynamics and cardiac function assessed in
borgh, and Nicholas F. Paoni. Effects of exercise training
conscious rats are limited. In this study, the effects of
on cardiac function, gene expression, and apoptosis in rats.
treadmill training (for 13 wk) on cardiac function and
Am J Physiol Heart Circ Physiol 279: H2994–H3002,
hemodynamics were assessed by comparison of two
2000.—This study determined the effects of exercise training
sets of control animals: sedentary rats of the same age
on cardiac function, gene expression, and apoptosis. Rats
and others of the same BW as the exercised cohort.
exposed to a regimen of treadmill exercise for 13 wk had asignificant increase in cardiac index and stroke volume index
Hemodynamic and cardiac function measurements
and a concomitant decrease in systemic vascular resistance
were made while the animals were conscious and un-
compared with both age-matched and body weight-matched
sedentary controls in the conscious state at rest. In exercise-
Second, to evaluate the molecular effects of exercise
trained animals, there was no change in the expression of
on the heart, real-time RT-PCR was used to study
several marker genes known to be associated with patholog-
the relative expression of several cardiac muscle
ical cardiac adaptation, including atrial natriuretic factor,
and extracellular matrix genes in the left ventricle
-myosin heavy chain, ␣-skeletal and smooth muscle actins,
(LV) of the exercised rats compared with sedentary
and collagens I and III. Exercise training, however, produced
controls. These results were compared and contrasted
a significant induction of ␣-myosin heavy chain, which was
to changes in gene expression induced by adapta-
not observed in rats with myocardial infarction. No histolog-
tion to the pathological stimulus of myocardial infarc-
ical features of cardiac apoptosis were observed in the tread-
mill-trained rats. In contrast, apoptotic myocytes were de-
Finally, exercise has been reported to produce apo-
tected in animals with myocardial infarction. In summary,exercise training improves cardiac function without evidence
ptosis in the thymocytes of rats (12) and in the skeletal
of cardiac apoptosis and produces a pattern of cardiac gene
muscle of mice (40). It is also known that cardiac
expression distinct from pathological cardiac adaptation.
adaptation to myocardial infarction and chronic pres-sure overload is accompanied by programmed cell
treadmill; hemodynamics; physiological loads; pathological
death (27, 50). The effect of exercise training on cardiac
loads; myocardial infarction
apoptosis, however, has not been investigated. Thehearts of exercised-trained rats were examined forevidence of apoptotic cell death at 4 days, 10 days, and
THE LABORATORY RAT has been used by many investiga-
13 wk after exercise training was initiated, and the
tors to study the adaptation of cardiac function to
results were compared with what was observed at
chronic exercise (3, 4, 6, 8, 14, 15, 17, 21, 24, 25, 29 –31,
similar time points after myocardial infarction.
33, 41, 45, 47, 52, 57, 61, 62), and much useful infor-mation has emerged from these studies. The purpose of
MATERIALS AND METHODS
this investigation was to extend previous findings inseveral important ways. First, exercise training in this
All experimental procedures conformed to the guiding
principles of the American Physiology Society and were ap-
model system can have a significant impact on rodent
proved by the Institutional Animal Care and Use Committee
body weight (BW), and there is a direct relationship
of Genentech. The animals used in this study were male
between BW and hemodynamic parameters, including
Sprague-Dawley rats (6–8 wk of age, Charles River Breeding
blood volume, cardiac output, stroke volume, and pe-
Laboratories). The animals were acclimated to the facility for
ripheral vascular resistance in rats (10). There are no
at least 1 wk before the initiation of the study, fed a pelleted
observations of cardiac function, however, where exer-
rat chow and water ad libitum, and housed in a light- and
cise-trained rats were compared with both BW-
matched and age-matched sedentary controls. Fur-
The costs of publication of this article were defrayed in part by the
Address for reprint requests and other correspondence: H. Jin,
payment of page charges. The article must therefore be hereby
Dept. of Cardiovascular Research, Genentech, Inc., 1 DNA Way, S.
marked ‘‘
advertisement'' in accordance with 18 U.S.C. Section 1734
San Francisco, CA 94080.
solely to indicate this fact.
0363-6135/00 $5.00 Copyright 2000 the American Physiological Society
CARDIAC EFFECTS OF EXERCISE TRAINING
Exercise Training
diac index. Hemodynamic measurements were performed in11 exercise-trained, 10 age-matched, and 6 BW-matched
Rats of approximately the same age were randomly di-
rats, and cardiac output was not successfully measured in 2
vided into two groups: the exercise group (
n ⫽ 31) and the
rats (1 in the exercise group and 1 in the age-matched group)
age-matched sedentary controls (
n ⫽ 19). These groups were
because the thermodilution curve was not reliable.
age-matched in the sense that the average ages of the two
At the conclusion of the experiments, the rats were anes-
groups were almost identical. The rats in the exercise group
thetized with pentobarbital sodium (60 mg/kg). The hearts
trained on a rodent treadmill (model CT-2, Columbus Instru-
were removed, dissected, and weighed in 14 exercise-trained,
ments International) according to the training protocol de-
14 age-matched, and 12 BW-matched rats.
scribed previously (32, 39). An electric grid at the rear of thebelt was used as the running stimulus. The animals trained
5 days/wk for 13 wk, with speed, grade, and duration pro-gressively increased. The rats began training at 10 m/min
Echocardiograms were performed in eight exercise-trained
and 5% grade for 15 min/day. The speed and grade were
rats and eight age-matched controls before catheterization.
gradually increased such that by the end of the second week,
The rats were anesthetized with ketamine and xylazine as
the animals ran at 15 m/min, 15% grade, for 60 min/day.
described above and examined in the lateral decubitus posi-
Thereafter, the grade and duration were maintained but
tion. An annular array echocardiographic system (Apogee
speed was increased 2–3 m/min each wk. By 10 wk, the rats
CX, ATR Interspec, Bothell, WA) with a 7.5-MHz transducer
ran at 36 m/min and 15% grade for 60 min/day, and this
was used for two-dimensional and M-mode imaging. With the
exercise program was maintained until the end of the study.
use of the two-dimensional parasternal short-axis imaging
Because the exercise training significantly decreased the
plane as a guide to the level of the papillary muscles, a
poststudy BW, the age-matched sedentary controls could not
M-mode tracing of the LV was obtained. The LV anterior and
serve as BW controls. Thus a younger group of sedentary rats
posterior wall thickness at end diastole, LV end-diastolic
(
n ⫽ 12) was established to serve as BW controls. With the
internal diameter, and LV end-systolic internal diameter
use of the knowledge of the BW-versus-age relationship for
were measured according to standard procedures. The LV
both sedentary and exercise-trained rats, we determined that
mass was calculated with the standard cube formula as
rats ⬃2.5 wk younger than the exercise group should emerge
follows: LVM ⫽ 1.04[(AWT ⫹ PWT ⫹ EDD)3 ⫺ EDD3], where
from the study with average BW roughly the same as that of
LVM is LV mass, AWT and PWT are anterior and posterior
the exercise group. Note that the average initial BW will
wall thickness, respectively, and EDD is LV end-diastolic
necessarily be smaller in this BW-matched group than in the
internal diameter. Relative wall thickness was calculated as
older, exercise group.
the ratio of 2PWT to 1EDD.
Assessments of Cardiac Growth and Cardiac Function
Studies on Cardiac Gene Expression
Catheterization. At the end of 13 wk of exercise training,
Animal model and sample preparation. The hearts from
rats in the three experimental groups were anesthetized with
the exercise-trained rats (
n ⫽ 5) and age-matched controls
ketamine hydrochloride (100 mg/kg ip) and xylazine (10
(
n ⫽ 5) were removed and dissected, and the LV were fast-
mg/kg ip). A catheter [polyethylene (PE)-10 fused with PE-
frozen in liquid nitrogen and stored at ⫺70°C for subsequent
50] filled with heparin-saline solution (50 U/ml) was im-
RNA analysis. Cardiac gene expression analysis was also
planted into the abdominal aorta through the left femoral
performed in four rats 13 wk after myocardial infarction
artery. This catheter was used to measure arterial pressure
induced by ligation of the left coronary artery and four
and heart rate. A second catheter (PE-50) was implanted into
sham-operated control rats to allow comparison with cardiac
the right atrium, through the right jugular vein, for measure-
adaptation to a pathological load. The procedure used for left
ment of left atrial pressure and for saline injection. A ther-
coronary ligation has been described in detail elsewhere (18,
mistor catheter (Lyons Medical Instrument, Sylmar, CA) was
26, 38, 63). In brief, the rats were anesthetized with ket-
inserted into the aortic arch from the right femoral artery for
amine hydrochloride and xylazine as described above, intu-
measurement of cardiac output by the thermodilution
bated via tracheotomy, and ventilated by a respirator (model
method (10, 13, 26, 63). The catheters were exteriorized at
683, Harvard Apparatus). After a left-sided thoracotomy, we
the back of the neck with the aid of a stainless steel wire.
ligated the left coronary artery ⬃2 mm from its origin with a
After the catheters were implanted, all rats were housed
7–0 silk suture. Electrocardiograms were obtained under
light metofane anesthesia 1 wk after surgery to document the
Hemodynamic measurements. Mean arterial pressure and
development of infarcts (26, 63). The rats without evident
heart rate were measured in conscious, unrestrained rats
pathological Q waves across the precardial leads were ex-
1 day after catheterization by connecting the catheters to
cluded. Our previous studies (26, 63) have shown that rats
a pressure transducer (model P23 XL, Viggo-Spectramed,
selected by electrocardiogram have myocardial infarcts aver-
Oxnard, CA) coupled to a polygraph (model 7, Grass In-
aging 32–35% of the LV, which led to ventricular hypertro-
struments, West Warwick, RI). For measurement of cardiac
phy and cardiac dysfunction 6–14 wk after ligation.
output, the thermistor catheter was connected to a microcom-
Cardiac RNA analysis. Total RNA was isolated from the
puter system (Lyons Medical Instrument) (26, 63). Isotonic
ventricular samples using the RNeasy Maxi Kit (Qiagen)
saline (0.1 ml) at room temperature was injected as a bolus
according to the manufacturer's instructions. Gene expres-
via the jugular vein catheter. The thermodilution curve was
sion analysis was performed using real-time RT-PCR (Taq-
monitored by VR-16 Simultrace recorders (Honeywell, NY),
Man) technology. RT-PCR was performed on 1 ng of total
and cardiac output was digitally obtained by the microcom-
RNA per reaction using the TaqMan sequence detector
puter. Cardiac indexes were calculated as follows: stroke
(model 7700, ABI-Perkin Elmer) (19). Amplification reaction
volume ⫽ cardiac output/heart rate; cardiac index ⫽ cardiac
conditions (for 50 l) were 1⫻ TaqMan
buffer A, 300 M
output/BW; stroke volume index ⫽ stroke volume/BW; and
dATP, 300 M dCTP, 300 M dGTP, 600 M dUTP, 10%
systemic vascular resistance ⫽ mean arterial pressure/car-
glycerol, 5.5 mM MgCl , 50 U murine leukemia virus reverse
CARDIAC EFFECTS OF EXERCISE TRAINING
transcriptase, 20 U RNase Inhibitor, 1.25 U AmpliTaq Gold,
100 nM forward and reverse primers, and 100 nM fluorogenicprobe. RT-PCR reagents and glycerol were purchased from
Effects of Exercise on BW and Cardiac Growth
Perkin Elmer and Sigma, respectively. Reactions were per-
Because chronic exercise generally induces a signif-
formed in MicroAmp optical tubes and caps (ABI-Perkin
icant reduction in BW, we compared the exercised
Elmer). TaqMan primers and probes were designed accord-
animals to not only age-matched but also BW-matched
ing to guidelines determined by Perkin Elmer and synthe-sized at Genentech except for those for rodent GAPDH, which
sedentary controls. After 13 wk of treadmill training,
were a generous gift from Perkin Elmer. Reverse transcrip-
the BW of the exercised group was ⬃17% lower than
tion was performed at 48°C for 30 min followed by heat
the age-matched sedentary controls (
P ⬍ 0.01) and the
activation of AmpliTaq Gold at 95°C for 10 min. Thermal
same as the BW-matched group, which contained ani-
cycling was at 95°C for 30 s and 60°C for 1.5 min for 40 cycles.
mals that were ⬃2.5 wk younger (Table 1). The ratios
Quantitation of the TaqMan results was performed as
of heart and ventricular weights to BW were the same
described by Heid et al. (23) with modifications. Briefly,
in the two sedentary groups despite the difference in
standard curves (1:5 serial dilution) for each target gene of
BW and age, indicating that the heart and body grew
interest were run in duplicate. The threshold cycle (C ) was
proportionally in these animals. The BW-normalized
plotted on the
y-axis versus the log of the total RNA concen-
heart and ventricular weights of the exercised group
tration (
x-axis), and the equation describing the line was
were significantly greater than the two sedentary con-
determined. Experimental samples were analyzed using 3–5
trol groups, however (Table 1).
replicates each, and the quantity of the mRNA for each targetgene was determined from the appropriate standard curve by
LV Geometry Measured by Echocardiography
entering the C (
y value) and solving for the input mRNA (
x
value). The value for the target gene was then normalized to
There was a close correlation between echocardio-
GAPDH by solving the following equation: 10
x1/10
x2, where
x
gram-derived LV mass and actual LV wet weight in a
is the target gene and
x is GAPDH.
combined group of exercise-trained rats and age-
matched controls (
r ⫽ 0.84,
P ⬍ 0.0001,
n ⫽ 16) indi-
Studies on Cardiac Myocyte Apoptosis
cating the accuracy of echocardiographic measurements.
Programmed cell death in the heart has been demon-
strated during the first 1–2 wk, with a peak at several days,
Table 1.
Effects of exercise training on BW, HW,
after the onset of pressure overload or myocardial infarction
MAP, and HR
in rats (27, 50). Cardiac apoptosis was evaluated by exami-nation of morphological features under light microscopy and
by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling reaction (TUNEL) labeling of the 3⬘
307.8 ⫾ 4.6 (14)
306.2 ⫾ 5.8 (14)
252.7 ⫾ 4.7† (12)
508.8 ⫾ 11.1 (14)
612.6 ⫾ 15.1† (14) 502.3 ⫾ 7.6 (12)
OH ends of DNA in myocardial tissue sections after 4 days,
1.351 ⫾ 0.041 (14)
1.374 ⫾ 0.043 (14) 1.082 ⫾ 0.086† (12)
10 days, or 13 wk of exercise training (
n ⫽ 4 for each time
1.281 ⫾ 0.040 (14)
1.277 ⫾ 0.039 (14) 1.015 ⫾ 0.086† (12)
point) or after myocardial infarction induced by coronary
1.019 ⫾ 0.034 (11)
1.001 ⫾ 0.037 (11) 0.803 ⫾ 0.013† (12)
ligation (
n ⫽ 3 for each time point) as described above.
0.241 ⫾ 0.012 (11)
0.244 ⫾ 0.010 (11) 0.213 ⫾ 0.008* (12)
The hearts were removed from the rats under anesthesia,
fixed in 10% neutral-buffered Formalin, processed routinely,
2.668 ⫾ 0.096† (14) 2.247 ⫾ 0.056 (14) 2.151 ⫾ 0.167 (12)
embedded in paraffin, and sectioned at 5 m. Replicate
2.531 ⫾ 0.095† (14) 2.107 ⫾ 0.055 (14) 2.017 ⫾ 0.166 (12)
sections were stained with hematoxylin and eosin for light
microscopic analysis; apoptotic cells were identified by posi-
2.037 ⫾ 0.079† (11) 1.636 ⫾ 0.050 (11) 1.601 ⫾ 0.021 (12)
tive staining with the digoxigenin-dUTP terminal de-
oxytransferase method (ApoTag kit, Oncor, Gaithersburg,
0.482 ⫾ 0.024† (11) 0.400 ⫾ 0.014 (11) 0.408 ⫾ 0.014 (12)
MD). Twelve sections were evaluated on each heart. Forma-
1.69 ⫾ 0.07 (8)
1.68 ⫾ 0.08 (8)
lin-fixed thymus from 4-wk-old C57BL/6 mice treated with 50
1.74 ⫾ 0.07 (8)
1.67 ⫾ 0.07 (8)
3.84 ⫾ 0.20 (8)
4.40 ⫾ 0.22 (8)
g of cortisone acetate for 12 h (to induce thymic involution)
7.85 ⫾ 0.18 (8)
8.45 ⫾ 0.27 (8)
and embryonic
day 14 (E14) mouse embryos were used as
0.445 ⫾ 0.016‡ (8)
0.396 ⫾ 0.016 (8)
positive controls for apoptotic staining. With this method,
apoptotic cells were identified in the thymic cortex and in the
112.6 ⫾ 1.9 (11)
109.9 ⫾ 3.8 (10)
117.1 ⫾ 4.0 (6)
embryonic heart of the control tissues.
360.0 ⫾ 7.1 (11)
370.1 ⫾ 9.2 (10)
368.3 ⫾ 12.5 (6)
Data are expressed as means ⫾ SE; the number in parentheses
Results are expressed as means ⫾ SE. One-way analysis of
represents the number of rats. BW , body weight before the initiation
variance (ANOVA) was performed to assess differences in
of exercise training; BW, body weight 13 weeks after exercise train-
parameters between groups. Significant differences were
ing; HW, heart weight; VW, ventricular weight; LVW, left ventricu-
then subjected to post hoc analysis using the Newman-Keuls
lar (LV) weight; RVW, right ventricular weight; AWT, anterior wallthickness; PWT, posterior wall thickness; ESD, LV end-systolic in-
method. For analysis of gene expression, parameters be-
ternal diameter; EDD, LV end-diastolic internal diameter; RWT,
tween the exercise or infarct group and the respective control
relative wall thickness; MAP, mean arterial pressure; HR, heart
group were compared by an unpaired Student's
t-test.
P ⬍
rate. *
P ⬍ 0.05 and †
P ⬍ 0.01 compared to other two groups. ‡
P ⬍
0.05 was considered significant.
0.05 compared with the age-matched group.
CARDIAC EFFECTS OF EXERCISE TRAINING
No significant difference in LV anterior and posteriorwall thickness was observed between the exercisegroup and age-matched group (Table 1). LV end-sys-tolic and end-diastolic internal diameters tended to bedecreased in the exercise-trained animals comparedwith the age-matched sedentary controls, but the dif-ference was not statistically significant. However,there was a significant increase in relative wall thick-ness, an index of cardiac geometry, in the exercisegroup compared with the age-matched sedentary con-trols (Table 1), indicating that treadmill running wasassociated with alterations in cardiac morphology.
Effects of Exercise on Cardiac Function
Mean arterial pressure and heart rate at rest were
similar in the three experimental groups (Table 1). Thecardiac index and stroke volume index of the treadmill-trained rats were significantly higher (
P ⬍ 0.01) thanthose of either sedentary control group (Fig. 1). Exer-cise also significantly reduced systemic vascular resis-tance (
P ⬍ 0.01). No differences in cardiac index, strokevolume index, and systemic vascular resistance werefound between the two sedentary control groups.
Effects of Exercise and Myocardial Infarction onCardiac Gene Expression
LV expression levels of 11 genes were used to com-
pare the molecular phenotypes of cardiac adaptation tostress induced by exercise training versus myocardialinfarction (Table 2). Treadmill training for 13 wk re-sulted in a significant increase in the expression of onlyone measured gene, ␣-myosin heavy chain (
P ⬍ 0.05),which was unchanged in animals after myocardial in-farction. In contrast, the mRNA abundance of six geneswere significantly increased 13 wk after myocardialinfarction. mRNA levels of atrial natriuretic factor,
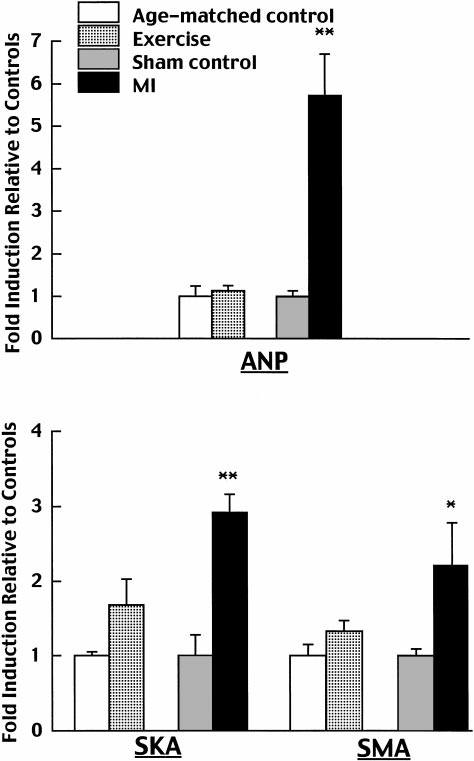
␣-skeletal actin, and ␣-smooth muscle actin were in-creased by 5.7-, 2.9-, and 2.2-fold, respectively (Fig. 2).
The -myosin heavy chain isoform was induced, andmRNA levels of the extracellular matrix proteins col-lagen I and III increased by 2.3- and 2.6-fold, respec-tively (Fig. 3).
Effects of Exercise and Myocardial Infarction on
Fig. 1. Effects of exercise training on cardiac index (CI), stroke
Cardiac Myocyte Apoptosis
volume index (SVI), and systemic vascular resistance (SVR) in con-scious rats. BW, body weight. Data expressed as means ⫾ SE. **
P ⬍
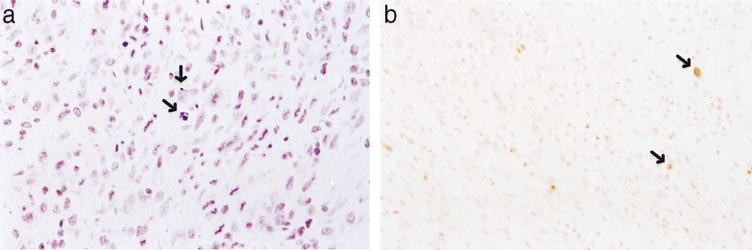
Apoptotic myocytes, ⬃3–5 apoptotic cells/high-power
0.01 compared with the exercise group.
field, were detected adjacent to the myocardial infarct4 days after left coronary artery ligation (Fig. 4). In
stroke volume index at rest in the conscious state
contrast, no apoptotic cells were detected in the hearts
compared with both age-matched and BW-matched
of exercise-trained animals. No histological features of
sedentary controls, indicating that exercise training
apoptosis (nuclear pyknosis and karyorrhexis) were
enhances cardiac function. Second, mRNA levels for
observed in either hematoxylin and eosin-stained or
atrial natriuretic factor, -myosin heavy chain, ␣-skel-
ApoTag-stained myocardial sections after 4 days, 10
etal actin, ␣-smooth muscle actin, collagen I, and col-
days, or 13 wk of treadmill training.
lagen III in the LV were significantly elevated in rats13 wk after myocardial infarction but not in the exer-
cise-trained animals. In contrast, there was a signifi-
There are three major findings in the present study.
cant induction of ␣-myosin heavy chain in the exercise
First, rats subjected to chronic treadmill exercise for 13
group but not in the infarct group. This suggests a
wk exhibited a significant increase in cardiac index and
distinct pattern of cardiac gene expression induced by
CARDIAC EFFECTS OF EXERCISE TRAINING
Table 2. Effects of exercise training vs. MI on cardiac
stroke volume in healthy humans and pigs (7, 48, 59).
gene expression
However, our finding of increased resting cardiac indexin exercise-trained rats is not in agreement with the
observation that there is no significant increase in
Natriuretic factors
resting cardiac index after exercise training in humans
Atrial natriuretic factor
and pigs. This discrepancy may be due mainly to the
change in resting heart rate after exercise training.
␣-Cardiac actin
Humans receiving exercise training exhibit bradycar-
␣-Skeletal actin
dia at rest that offsets the increase in resting stroke
␣-Smooth muscle actin
volume, leading to an insignificant change in cardiac
␣-Myosin heavy chain
output or cardiac index at rest (7, 48). In pigs, exercise
-Myosin heavy chain
training tends to reduce resting heart rate, which is
Myosin light chain 2
associated with a tendency to increase resting cardiac
Sarco(endo)plasmic reticulum Ca2⫹-ATPase
output (59). In the present study, however, resting
heart rate did not change after exercise training in
Extracellular matrix
conscious rats, which is consistent with previous re-
ports (8, 11, 24, 31, 33–35, 62) by the majority of other
investigators who observed little or no change in rest-
n ⫽ 5 rats in each exercise and age-matched group, and n ⫽ 4
rats in each myocardial infarction (MI) and sham group. Results areas compared with the respective control: NS, no significant change;
1, significant increase (P ⬍ 0.05); and 11, very significant increase(P ⬍ 0.01).
the physiological load versus pathological load. Third,myocardial apoptosis was detected in rats 4 days aftermyocardial infarction but not in the exercise-trainedanimals at 4 days, 10 days, and 13 wk. This is the firstdemonstration that myocardial adaptation to exercisetraining was not associated with cardiac apoptosis.
In the present study, animals receiving exercise
training exhibited a significant enhancement in car-diac index and stroke volume index at rest in theconscious state compared with both age-matched andBW-matched sedentary controls. The improvement incardiac function was associated with a reduction insystemic vascular resistance. The decrease in afterloadmay contribute to the enhanced cardiac function byreducing the impedance of LV ejection. Recent studies(46, 54) in dogs suggest that exercise training is asso-ciated with an increase in nitric oxide formation thatmay mediate endothelium-dependent peripheral vaso-dilation. In addition, another mechanism for enhancedcardiac function observed in exercise-trained ratsmight relate to an increase in myocardial contractility.
It has been reported that exercise training increasescontractile performance of rat hearts in vitro (5, 20, 42,43, 51). A study (51) on the effect of exercise training onexcitation-contraction coupling in the rat myocardiumdemonstrated that treadmill exercise enhances myo-cardial performance by increasing Ca2⫹ availability tothe contractile element. A further study is needed todetermine the effect of exercise training on myocardialcontractility in conscious rats, because it was not fea-
Fig. 2. Effects of exercise training versus myocardial infarction (MI)
sible for us to use a high-fidelity Millar catheter with a
on cardiac gene expression of atrial natriuretic factor (ANF), ␣-skel-
pressure transducer at the tip to obtain these measure-
etal actin (SKA), and ␣-smooth muscle actin (SMA). Expression data
ments in the conscious state.
were first normalized by dividing all values in a particular compar-
The present study demonstrated a significant in-
ison by the average expression in the corresponding control group,
crease in stroke volume index at rest in conscious rats
thus forcing the control average to be 1. Means ⫾ SE of thesenormalized data are displayed. *P ⬍ 0.05 and **P ⬍ 0.01 compared
by exercise training. This is consistent with the finding
with the respective control; n ⫽ 5 rats in each exercise and age-
that exercise training significantly augments resting
matched group, and n ⫽ 4 rats in each MI and sham group.

CARDIAC EFFECTS OF EXERCISE TRAINING
ing heart in conscious and anesthetized normal ratsafter exercise training. With unchanged heart rate, theincreased stroke volume index would elevate cardiacindex in exercise-trained rats.
The incremental load on the heart after myocardial
infarction reflects a blend of pressure and volume over-loading. The adaptation to this pathological load hasbeen shown to be associated with a unique molecularphenotype of altered myocardial gene expression. Thepresent study showed that LV expression of severalgenes, including atrial natriuretic factor, -myosinheavy chain, ␣-skeletal actin, and ␣-smooth muscleactin, were increased 13 wk after myocardial infarc-tion. This is consistent with recent studies (22, 36, 64,65) that demonstrate the increased ventricular expres-sion of these genes coding for the fetal phenotypeduring ventricular remodeling after myocardial infarc-tion in rats. Less is known, however, about ventricularexpression of the fetal genes after chronic physiologicalloads. It has been reported that atrial natriuretic factorgene expression in the rat ventricle is unchanged aftertreadmill training (2) and minimally increased afterswimming training compared with a profound increaseafter chronic pathological loads in rats (9). The presentstudy is the first to demonstrate that cardiac adapta-tion to exercise training was not associated with the LVinduction of mRNA encoding the fetal contractile pro-teins (-myosin heavy chain, ␣-skeletal actin, and
␣-smooth muscle actin) in addition to atrial natriureticfactor.
In the present study, the LV mRNA level of ␣-myosin
heavy chain was significantly increased after exercisetraining but not after myocardial infarction, whereasthere was an induction of LV -myosin heavy chaingene in the infarct group but not in the exercise group.
It is known that the mature adult rat expresses mainly
␣-myosin heavy chain as the major contractile proteinin the LV. Our findings are consistent with the previ-
Fig. 3. Effects of exercise training versus MI on cardiac gene expres-
ous observations that a physiological load (exercise
sion of ␣-myosin heavy chain (aMHC), -myosin heavy chain(bMHC), collagen I (Col I), and collagen III (Col III). Expression data
training) results in a further increase in the V myosin
were first normalized by dividing all values in a particular compar-
isoenzyme (␣-myosin heavy chain) and a pathological
ison by the average expression in the corresponding control group,
load induces a shift in the isoenzyme pattern from the
thus forcing the control average to be 1. Means ⫾ SE of these
V to V isoenzyme (-myosin heavy chain) in rats (37,
normalized data are displayed. *P ⬍ 0.05 and **P ⬍ 0.01 compared
with the respective control; n ⫽ 5 rats in each exercise and age-
␣-Myosin heavy chain is associated with high
matched group, and n ⫽ 4 rats in each MI and sham group.
ATPase activity and increased contractility, whichmight contribute, in part, to the enhanced cardiacindex and stroke volume index observed in the exer-cise-trained rats. In contrast, -myosin heavy chain
Fig. 4. Hematoxylin and eosin-stained(a) and ApoTag-stained (b) sections ofmyocardial adjacent to the infarct atthe 4-day time point after MI. Individ-ual apoptotic cells exhibiting nuclearpyknosis and karryorhexis or labeledvia ApoTag are indicated (arrows).
CARDIAC EFFECTS OF EXERCISE TRAINING
has a fivefold lower ATPase activity, conferring de-
In contrast, there was no evidence of myocardial apo-
creased velocity of shortening, and its expression in the
ptosis 4 days, 10 days, and 13 wk after chronic running
heart after myocardial infarction may be teleologically
exercise. These data suggest that cardiomyocyte apo-
attributable to the more efficient utilization of de-
ptosis may play an important role in the early stage of
creased energy reserves (37, 44).
cardiac adaptation to pathological loads but not to
Experimental and clinical studies have demon-
physiological loads. Accordingly, myocardial apoptosis
strated an increase in interstitial collagens of the LV or
appears to be a new index for distinction between
nonischemic myocardium at a chronic or late stage
cardiac adaptation to physiological loads and patholog-
after myocardial infarction, which may enhance car-
ical loads at the early period.
diac stiffness and result in diastolic dysfunction, finally
It is known that the effect of exercise training on
leading to heart failure (16, 22, 53, 55, 56). In contrast
heart and body growth varies because of differences in
to myocardial infarction, we found exercise training did
species, age, sex, the mode or regimen of exercise
not affect the cardiac mRNA of collagen I and III.
training, the disease model, etc. In rats, for example,
Consistent with this finding, Burgess et al. (8) showed
treadmill running is often associated with "relative
that total collagen content in the LV is not altered in
hypertrophy" (an increase in heart-to-BW ratio with
exercise-trained rats compared with control rats but is
unchanged absolute heart weight), whereas swimming
significantly greater in the heart subjected to chronic
training may cause "true cardiac hypertrophy" (an
hypertension. In addition, we show that the LV gene
increase in both heart-to-BW ratio and absolute heart
expression of ␣-smooth muscle actin is substantially
weight). The data on rats that have been exercise
increased after myocardial infarction but is unchanged
trained by swimming are confounded, however, by ex-
after exercise training. Recent studies (1, 16) suggest
perimental evidence that swimming produces addi-
that ␣-smooth muscle actin expression by fibroblasts
tional stress in the animals that may also contribute to
and myofibroblasts contributes to collagen remodeling
the induction of cardiac hypertrophy independent of
and may play a role in mediating wound healing in the
the exercise (38). Furthermore, a recent study (66) in
heart after myocardial infarction.
rats after myocardial infarction showed that high-in-
The pathological adaptation to pressure overload has
tensity sprint training improved cardiac function and
also been shown to be associated with an increase in
increased cardiac expression of ␣-myosin heavy chain
the expression of several marker genes, including
but was associated with reduced myocyte hypertrophy.
atrial natriuretic factor, -myosin heavy chain, ␣-skel-
Perrault and Turcotte (38) reviewed animal and hu-
etal actin, and collagens (28, 49, 58, 60), whereas car-
man studies on exercise training over the past three
diac expression of these marker genes were not
decades and found that using cardiac hypertrophy as
changed in exercise-trained animals in the present
an expected adaptation to regular exercise may not be
study. In addition, cardiac expression of ␣-myosin
totally warranted. Although we did not find true car-
heavy chain is decreased in pressure overload (49). In
diac hypertrophy in the treadmill-trained rats com-
contrast, this gene expression was upregulated after
pared with age-matched sedentary controls, it is clear
exercise training. Furthermore, mRNA levels of sarco-
from our data that myocardial adaptation to treadmill
(endo)plasmic reticulum Ca2⫹-ATPase and phospho-
training is characterized by improved function (cardiac
lamban have been reported to be depressed in rats with
index and stroke volume index) and altered cardiac
pressure overload (28, 49, 58, 60), but exercise training
gene expression (induction of ␣-myosin heavy chain)
did not alter cardiac expression of these two calcium
and geometry (increased relative wall thickness).
handling genes. Thus compared with pathological ad-
In summary, treadmill-trained rats displayed im-
aptation to pressure overload, physiological adaptation
proved cardiac function in association with a profile of
to exercise training is also associated with distinct
cardiac gene expression distinct from pathological car-
alterations in cardiac molecular phenotype.
diac adaptation. The cardiac adaptation to exercise
Recent studies have shown that apoptosis may be
training was not associated with myocyte apoptosis,
involved in the pathogenesis of heart remodeling after
which also contrasted to cardiac remodeling in the
pathological loads. With the use of an in situ assay,
early phase after pathological loads.
Teiger et al. (50) found a phase of apoptosis during thefirst 7 days after pressure overload, with a peak at 4
We are grateful to Dr. Michael Ostland for guidance and assis-
tance of statistical analysis.
days, whereas cardiac growth continued for over 30days (50). The apoptosis was mainly observed in car-
diomyocytes. Their findings suggest that cardiac adap-tation to pressure overload is initiated by a wave of
1. Arora PD and McCulloch CA. Dependence of collagen remod-
apoptosis of cardiomyocytes. Furthermore, Kajstura et
eling on alpha-smooth muscle actin expression by fibroblasts.
J Cell Physiol 159: 161–175, 1994.
al. (27) demonstrated that programmed cardiomyocyte
2. Azizi C, Bouissou P, Galen F-X, Lattion A-L, Lartigue M,
death is the major form of myocardial damage at 2–6 h
and Carayon A. Alterations in atrial natriuretic peptide gene
after coronary artery ligation in rats. The apoptosis is
expression during endurance training in rats. Eur J Endocrinol
continuously observed for at least 7 days with gradu-
133: 361–365, 1995.
ally decreasing values. Consistent with these findings,
3. Baldwin KM, Ernst SB, Herrick RE, Hooker AM, and Mul-
lin WJ. Exercise capacity and cardiac function in trained and
we showed apoptosis in cardiomyocytes 4 days after
untrained thyroid-deficient rats. J Appl Physiol 49: 1022–1026,
myocardial infarction in a similar experimental model.
CARDIAC EFFECTS OF EXERCISE TRAINING
4. Baldwin KM, Ernst SB, Herrick RE, and MacIntosh AM.
factor-1 in experimental heart failure in rats treated with
Effects of physical training and thyroxine on rodent cardiac
chronic ACE inhibition. J Cardiovasc Pharmacol 26: 420–425,
function and biochemical properties. Pflu
¨ gers Arch 391: 190–
27. Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH,
5. Bersohn MM and Scheuer J. Effects of physical training on
Krajewski S, Reed JC, Olivetti G, and Anversa P. Apoptosis
end-diastolic volume and myocardial performance of isolated rat
and necrotic myocyte cell deaths are independent contribution
hearts. Circ Res 40: 510–516, 1977.
variables of infarct size in rats. Lab Invest 74: 86–107, 1996.
6. Bowles DK, Farrar PR, and Starnes JW. Exercise training
28. LekanneDeprez RH, van den Hoff MJ, de Boer PA, Ruijter
improves cardiac function after ischemia in the isolated, working
PM, Maas AA, Chamuleau RA, Lamres WH, and Moorman
rat heart. Am J Physiol Heart Circ Physiol 263: H804–H809, 1992.
AF. Changing patterns of gene expression in the pulmonary
7. Braun LT. Exercise physiology and cardiovascular fitness. Nurs
trunk-banded rat heart. J Mol Cell Cardiol 30: 1877–1888, 1998.
Clin North Am 26: 135–147, 1991.
29. Li YX, Lincoln T, Mendelowitz D, Grossman W, and Wei
8. Burgess ML, Buggy J, Price RL, Abet FL, Terracio L,
JY. Age-related differences in effect of exercise training on
Samarel AM, and Borg TK. Exercise- and hypertension-in-
cardiac muscle function in rats. Am J Physiol Heart Circ Physiol
duced collagen changes and related to left ventricular function in
251: H12–H18, 1986.
rat hearts. Am J Physiol Heart Circ Physiol 270: H151–H159,
30. Liang MT, Paulson DJ, Kopp SJ, Glonek T, Meneses P,
Gierke LW, and Schwartz FN. Effects of anabolic steroids and
9. Buttrick PM, Kaplan M, Leinwand LA, and Scheurer J.
endurance exercise on cardiac performance. Int J Sports Med 14:
Alterations in gene expression in the rat heart after chronic
324–329, 1993.
pathological and physiological loads. J Mol Cell Cardiol 26:
31. Lortet S and Verget P. Alteration of cardiovascular function in
61–67, 1994.
trained rats fed with fish oil. Int J Sports Med 16: 519–521, 1995.
10. Carbonell LF, Salom MG, Salazar FJ, Garcia-Estan J,
32. Mazzeo RS, Brooks GA, and Horvath SM. Effects of age on
Ubeda M, and Quesada T. Normal hemodynamic parameters
metabolic responses to endurance training in rats. J Appl
in conscious Wistar rats. Rev Esp Fisiol 41: 437–442, 1985.
Physiol 57: 1369–1374, 1984.
11. Chen CY, DiCarlo SE, and Scislo TJ. Daily spontaneous
33. Musch TL. Effects of spring training on maximal stroke volume
running attenuated the central gain of the arterial baroreflex.
of rats with a chronic myocardial infarction. J Appl Physiol 72:
Am J Physiol Heart Circ Physiol 268: H662–H669, 1995.
1437–1443, 1992.
12. Concordet J-P and Ferry A. Physiological programmed cell
34. Negrao CE, Irigoyen MC, Moreira ED, Brum PC, Freire
death in thymocytes is induced by physical stress (exercise).
PM, and Krieger EM. Effect of exercise training on RSNA,
Am J Physiol Cell Physiol 265: C626–C629, 1993.
baroreflex control, and blood pressure responsiveness. Am J
13. Cooper T, Pinakah T, and Richardson A. The use of the
Physiol Regulatory Integrative Comp Physiol 265: R365–R370,
thermal dilution principal for measurement of cardiac output in
the rat. Med Electron Biol Eng 1: 61–65, 1963.
35. Negrao CE, Moreira ED, SantosVMA Farah MCLM, and
14. Cutilletta AF, Edmiston K, and Dowell RT. Effect of a mild
Krieger EM. Vagal function impairment after exercise taining.
exercise program on myocardial function and the development of
J Appl Physiol 72: 1749–1753, 1992.
hypertrophy. J Appl Physiol 46: 354–360, 1979.
36. Oie E, Bjonerheim R, Grogaard HK, Kongshaug H, Smi-
15. DeBlieux PC, Barbee RW, McDonough KH, and Shepherd
seth OA, and Attramadal H. ET-receptor antagonism, myo-
R. Exercise training improves cardiac performance in diabetic
cardial gene expression, and ventricular remodeling during CHF
rats. Proc Soc Exp Biol Med 203: 209–213, 1993.
in rats. Am J Physiol Heart Circ Physiol 275: H868–H877, 1998.
16. Dixon IMC, Ju H, Jassal DS, and Peterson DJ. Effects of
37. Orenstein TL, Parker TG, Bautany JW, Goodman JM,
ramipril and losartan on collage expression in right and left
Dawood F, Wen W-H, Wee L, Martino T, McLaughlin PR,
heart after myocardial infarction. Mol Cell Bioch 165, 1996.
and Liu PP. Favorable left ventricular remodeling following
17. Friberg P, Hoffmann P, Nordlander M, and Thoren P.
large myocardial infarction by exercise training. J Clin Invest 96:
Effects of voluntary physical exercise on cardiac function and
858–866, 1995.
energetics in spontaneously hypertensive rats. Acta Physiol
38. Pfeffer JM, Pfeffer MA, Fletcher PJ, and Braunwald E.
Scand 133: 495–500, 1988.
Progressive ventricular remodeling in rats with myocardial in-
18. Geenen DL, White TP, and Lampman RM. Papillary me-
farction. Am J Physiol Heart Circ Physiol 260: H1406–H1414,
chanics and cardiac morphology of infarcted rat hearts after
training. J Appl Physiol 63: 92–96, 1987.
39. Raab DM, Smith EL, Crenshaw TD, and Thomas DP. Bone
19. Gibson UEM, Heid CA, and Williams PM. A novel method for
mechanical properties after exercise training in young and old
real time quantitative RT-PCR. Genome Res 6: 995–1001, 1996.
rats. J Appl Physiol 68: 130–134, 1990.
20. Guisti R, Bersohn M, Malhotra A, and Scheuer J. Cardiac
40. Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Ar-
function and actomyosin ATPase activity in hearts of condi-
slan P, Monti D, and Franceschi C. Apoptosis, DNA damage
tioned and deconditioned rats. J Appl Physiol 44: 171–174, 1978.
and ubiquitin expression in normal and mdx muscle fibers after
21. Haddad F, Bodell PW, Mccue SA, Herrick RE, and Bald-
exercise. FEBS Lett 373: 291–295, 1995.
win KM. Food restriction-induced transformations in cardiac
41. Schaible TF, Penpargkui S, and Scheuer J. Cardiac re-
functional and biochemical properties in rats. J Appl Physiol 74:
sponses to exercise training in male and female rats. J Appl
606–612, 1993.
Physiol 50: 112–117, 1981.
22. Hanatani A, Yoshiyama M, Kim S, Omura T, Toda I,
42. Schailble TF and Scheuer J. Effects of physical training by
Akioka K, Teragaki M, Takeuchi K, Iwao H, and Takeda T.
running and swimming on ventricular performance of rat hearts.
Inhibition by angiotensin II type 1 receptor antagonist of cardiac
J Appl Physiol 46: 854–860, 1979.
phenotypic modulation after myocardial infarction. J Mol Cell
43. Scheuer J and Bhan K. Cardiac contractile protein, adenosine
Cardiol 27: 1905–1914, 1995.
triphosphatase activity and physiological function. Circ Res 45:
23. Heid CA, Stevens J, Livak KJ, and Williams PM. Real time
1–12, 1979.
quantitative PCR. Genome Res 6: 986–994, 1996.
44. Scheuer J and Butrick P. The cardiac hypertrophic responses
24. Heller BA, Paulson DJ, Kopp SL, Peace DJ, and Tow JP.
to pathologic and physiologic loads. Circulation 75, Suppl I:
Depressed in vivo myocardial reactivity to dobutamine in stre-
63–68, 1987.
tozotocin diabetic rats: influence of exercise training. Cardiovasc
45. Sharma RV, Tomanek RJ, and Bhalla RC. Effects of swim-
Res 22: 417–424, 1988.
ming training on cardiac function and myosin ATPase activity in
25. Hilty MR, Groth H, Moore RL, and Musch TI. Determina-
SHR. J Appl Physiol 59: 758–765, 1985.
in rats after high-intensity sprint training.
46. Shen W, Zhang X, Zhao G, Wolin MS, Sessa W, and Hintze
J Appl Physiol 66: 195–201, 1989.
TH. Nitric oxide production and NO synthase gene expression
26. Jin H, Yang R, Gillett N, Clark RG, Ko A, and Paoni NF.
contribute to vascular regulation during exercise. Med Sci Sports
Beneficial effects of growth hormone and insulin-like growth
Exerc 27: 1125–1134, 1995.
CARDIAC EFFECTS OF EXERCISE TRAINING
47. Starnes JW, Beyer RE, and Edington DW. Myocardial adap-
57. Wei JY, Li Y, Lincoln T, Grossman W, and Mendelowitz D.
tation to endurance exercise in aged rats. Am J Physiol Heart
Chronic exercise training protects aged cardiac muscle against
Circ Physiol 245: H560–H566, 1983.
hypoxia. J Clin Invest 83: 778–784, 1989.
48. Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, and
58. Weinberg EO, Thienelt CD, Katz SE, Bartunek J, Tajima
Abrass IB. Cardiovascular responses to exercise. Effects of
M, Rohrbach S, and Lorell BH. Gender differences in molec-
aging and exercise training in healthy men. Circulation 89:
ular remodeling in pressure overload hypertrophy. J Am Coll
1648–1655, 1994.
Cardiol 34: 264–273, 1999.
49. Takahashi T, Schunkert H, Isoyama S, Wei JY, Nadal-
59. White FC, McKirnan MD, Breisch EA, Guth BD, Liu Y-M,
Ginard B, Grossman W, and Izumo S. Age-related differences
and Bloor CM. Adaptation of the left ventricle to exercise-
in the expression of proto-oncogene and contractile protein genes
induced hypertrophy. J Appl Physiol 62: 1097–1110, 1987.
in response to pressureoverload in the rat myocardium. J Clin
60. Wong K, Bohler KR, Petrou M, and Yacoub MH. Pharma-
Invest 89: 939–946, 1992.
50. Teiger E, Dam T-V, Richard L, Wisnewsky C, Tea BS,
cological modulation of pressure-overload cardiac hypertrophy:
Gaboury L, Tremblay J, Schwartz K, and Hamet P. Apo-
changes in ventricular function, extracellular matrix, and gene
ptosis in pressure overload-induced heart hypertrophy in the rat.
expression. Circulation 96: 2239–2246, 1997.
J Clin Invest 97: 2891–2897, 1996.
61. Woodiwiss AJ, Kalk WJ, and Norton GR. Habitual exercise
51. Tibbits GF, Barnard RJ, Baldwin KM, Cugalj N, and Rob-
attenuates myocardial stiffness in diabetes mellitus in rats.
erts NK. Influence of exercise on excitation-contraction coupling
Am J Physiol Heart Circ Physiol 271: H2126–H2133, 1996.
in rat myocardium. Am J Physiol Heart Circ Physiol 240: H472–
62. Woodiwiss AJ and Norton GR. Exercise-induced cardiac hy-
pertrophy is associated with an increased myocardial compli-
52. Vogt M, Ott B, Rupp H, and Jacob R. Significance of physical
ance. J Appl Physiol 78: 1303–1311, 1995.
exercise in hypertension. Influence of water temperature and
63. Yang R, Bunting S, Gillett N, Clark RJ, and Jin H. Growth
beta-blockade on blood pressure, degree of cardiac hypertrophy
hormone improves cardiac performance in experimental heart
and cardiac function in swimming training of spontaneously
failure. Circulation 92: 262–267, 1995.
hypertensive rats. Basic Res Cardiol 81: 157–169, 1986.
64. Young RL, Gundlach AL, and Louis WJ. Altered cardiac
53. Volders PGA, Williams IEMG, Cleutjens JPM, Arends J-W,
hormone and contractile protein messenger RNA levels follow-
Havenith MG, and Daemen MJAP. Interstitial collagen is
ing left ventricular myocardial infarction in rats: an in situ
increased in the non-infarcted human myocardium after myo-
hybridization histochemical study. Cardiovasc Res 37: 187 –
cardial infarction. J Mol Cell Cardiol 25: 1317–1323, 1993.
54. Wang J, Wolin MS, and Hintze TH. Chronic exercise en-
65. Yue P, Long CS, Austin R, Chang KC, Simpson PC, and
hances endothelium-mediated dilation of epicardial coronaryartery in conscious dogs. Circ Res 73: 829–838, 1993.
Massie BM. Post-infarction heart failure in the rat is associated
55. Weber KT and Brilla CG. Pathological hypertrophy and car-
with distinct alterations in cardiac myocytes molecular pheno-
diac interstium. Fibrosis and renin-angiotensin-aldosterone sys-
type. J Mol Cell Cardiol 30: 1615–1630, 1998.
tem. Circulation 83: 1849–1865, 1991.
66. Zhang X-Q, Ng Y-C, Musch TI, Moore RL, Zelis R, and
56. Weber KT, Sun Y, Tyagi SC, and Cleutjens JPM. Collagen
Cheung JY. Spring training attenuates myocyte hypertrophy
network of the myocardium: function, structure remodeling and
and improves Ca2⫹ homeostasis in postinfarction myocytes.
regulatory mechanisms. J Mol Cell Cardiol 26: 279–292, 1994.
J Appl Physiol 84: 544–552, 1998.
Source: http://ydrtkxlab.hunnu.edu.cn/ztwxk/left10/21.pdf
Innovations in Pharmaceuticals and Pharmacotherapy eISSN: 2321–323X Original Article Ciprofloxacin, oxacillin, piperacillin and sulfamethoxazole by systemic administration for the control of severe infections: Is dose adjustment required for critical burn patients? 1Cristina Sanches-Giraud, PhD*, 2David S Gomez, MD, 2Marcus C Ferreira, MD, 3Carlindo V Silva Jr,
ORIGINAL ARTICLE COMPARATIVE STUDY OF EFFECTIVENESS AND RESISTANCE PROFILE OF CHLOROQUINE AND SULFADOXINE-PYRIMETHAMINE IN UNCOMPLICATED PLASMODIUM FALCIPARUM MALARIA IN KOLKATA Ayan Basu1,Santanu Saha2, Biswanath Sharma Sarkar3, Somnath Mukherjee4, Subhasish Kamal Guha5, Dyuti Basu6 HOW TO CITE THIS ARTICLE:










