Impact of recipient abh secretor status on outcome in minor aboincompatible hematopoietic stem cell transplantation
Impact of recipient ABH secretor status on outcome
in minor ABO-incompatible hematopoietic
stem cell transplantation
Andreas Holbro,1,2 Martin Stern,1 Laura Infanti,1,2 Alix O'Meara,1 Beatrice Drexler,1 Beat M. Frey,3
Jean-Marie Tiercy,4 Jakob R. Passweg,1 Christoph Gassner,3 Andreas Buser,1,2 and Joerg-Peter Sigle2,5
BACKGROUND: The impact of ABO incompatibility on
hematopoietic stem cell transplantation (HSCT)
tion (HSCT) is a potentially curative treatmentapproach for different malignant and nonma-
outcome is controversial. As ABH substances are
lignant diseases.1 Several given factors such
expressed on tissues and secreted in body fluids, they
as patient age, comorbidities, donor type, and donor-
could drive an immune response in minor ABO-
recipient sex combinations have been shown to affect sur-
incompatible HSCT. The aim of the study was to inves-
vival and major outcomes after HSCT.2 Scoring systems
tigate the prognostic role of the recipients' ABH secretor
integrate these pretransplant determinants into a global
transplant risk assessment.3
STUDY DESIGN AND METHODS: Patients who under-
ABO incompatibility is not considered an obstacle for
went minor ABO-incompatible HSCT were included.
HSCT and occurs in approximately 30% to 50% of trans-
Secretor status was determined either serologically or
plants.4 Different types of donor-recipient ABO incompat-
by molecular genetics.
ibilities exist and are classified as either major, minor, or
RESULTS: Between March 1996 and June 2012, a
bidirectional.5 In major ABO-incompatible HSCT the
total of 176 patients received minor ABO-incompatible
patient has preformed antibodies (i.e. isohemagglutinins)
HSCT and 150 (85%) were secretors. Incidence and
against A and/or B antigens expressed on donor red blood
severity of acute graft-versus-host disease (GVHD) and
cells (RBCs). Minor ABO-incompatible HSCT is character-
chronic GVHD did not differ between secretors and
ized by the transfer of donor isohemagglutinins against
nonsecretors (cumulative incidences ± standard errors:
recipient RBC antigens and of the corresponding immune
acute GVHD on Day 100, 41 ± 11 and 46 ± 5%,
cells (i.e., lymphocytes). A bidirectional blood group
p = 0.59; chronic GVHD at 2 years, 52 ± 13 and56 ± 5%, p = 0.62, for secretors and nonsecretors,
ABBREVIATIONS: HR = hazard ratio; HSCT = hematopoietic
respectively). Additionally, nonrelapse mortality (NRM)
stem cell transplantation; NRM = nonrelapse mortality;
and overall survival (OS) were similar in the two groups
OS = overall survival.
(2-year NRM, 27 ± 9 and 23 ± 3%, p = 0.45; 4-year OS,64 ± 10 and 55 ± 4%, p = 0.28, for secretors and nonse-
From the 1Division of Hematology, University Hospital, and the
2Blood Transfusion Centre, Swiss Red Cross, Basel, Switzerland;
CONCLUSION: The recipients' ABH secretor status in
the 3Blood Transfusion Centre, Swiss Red Cross, Zurich,
minor ABO-incompatible HSCT has no prognostic
Switzerland; the 4National Reference Laboratory for
impact on major transplant outcomes.
Histocompatibility, Department of Internal Medicine,
University Hospitals, Geneva, Switzerland; and the 5Blood
Transfusion Centre, Swiss Red Cross, Aarau, Switzerland.
Address reprint requests to: Joerg-Peter Sigle, MD, Blood
Transfusion Centre, Swiss Red Cross, Kantonsspital Aarau, 5001
Aarau, Switzerland; e-mail:
[email protected].
Received for publication March 14, 2014; revision received
May 19, 2014, and accepted May 22, 2014.
doi: 10.1111/trf.12768
HOLBRO ET AL.
barrier is a combination of major and minor ABO incom-
MATERIALS AND METHODS
patibility. Various specific complications—for example,
All adult patients that underwent minor or bidirectional
pure RBC aplasia in major ABO-incompatible HSCT or
ABO-incompatible allogeneic HSCT at our institution
delayed hemolysis through passenger lymphocyte syn-
between March 1996 and June 2012 were included in this
drome in minor ABO-incompatible HSCT—can occur.
retrospective study. Patients with blood group A1, who
Several approaches to prevent complications after ABO-
received a HSCT from an A2 donor, were also included in
incompatible HSCT have been proposed, including pre-
the analysis. We excluded patients who received more
ventive measures in the recipient and different graft
than one HSCT, cord blood as stem cell source, or highly
processing steps.5
T-cell-depleted haploidentical HSCT. Patient, disease, and
Several studies have addressed the impact of ABO
transplant characteristics were collected by chart review
incompatibility on HSCT outcome. A large study found no
and through the electronic database of our institution. All
difference in overall survival (OS), transplant-related mor-
patients provided written informed consent to have their
tality, and Grade II to IV acute graft-versus-host disease
data on disease, treatment, and outcome reported.
(GVHD) after ABO-identical, major, minor, or bidirec-tional ABO-incompatible HSCT from HLA-identical sib-lings.6 On the other hand, Kanda and coworkers7 found a
ABH secretor status
lower OS in a subgroup of patients after minor ABO-incompatible, unrelated HSCT with bone marrow as stem
According to the Lewis (LE) phenotype and the secretor
cell source. As A and B antigens and their precursor, the
gene (α1,2-l-fucosyltransferase;
FUT2), individuals can be
H glycoprotein, are expressed not only by RBC, but also
classified as secretors and nonsecretors.11 Thus
Se and
se
many other tissues including vascular endothelium
(the two alleles of
FUT2,
Se being dominant over
se) deter-
("histo-blood group"), one may speculate that in the pres-
mine the presence or absence of the ABH substance in
ence of an ABO barrier the tissue expression of ABH anti-
body fluids.
gens can trigger or sustain an inflammatory reaction
The ABH secretor status was assessed through deter-
similar to that occurring in GVHD.8 In particular, in minor
mination of LE phenotype or by Se genotyping. At our
ABO-incompatible HSCT a humoral immune response
institution serologic typing for all clinically relevant blood
mediated by antibodies produced by donor lymphocytes
groups, including LE, is routinely performed before HSCT
against recipient ABH antigens may trigger GVHD by
in all patients by gel test (Gel Test ID-system, Bio-Rad
binding to and thus damaging the recipient's endo-
Laboratories DiaMed GmbH, Cressier, Switzerland) or by
thelium. Data regarding the effect of minor ABO-
conventional agglutination test in tubes (antisera from
incompatible HSCT on rate and severity of GVHD are
Immucor, Inc., Norcross, GA). Se genotyping was per-
conflicting. Some studies have shown an increased risk of
formed in patients where serologic LE phenotyping was
GVHD in minor ABO-incompatible HSCT.5,9 The study by
missing, equivocal (mixed field after recent transfusions),
Stussi and coworkers9 showed similar OS after minor ABO-
or negative for both Lea and Leb.
incompatible HSCT compared to ABO-compatible HSCT,but a higher incidence of acute GVHD (Grade I-IV). Otherstudies failed to demonstrate a significant effect of minor
Molecular determination of secretor status
ABO-incompatible HSCT on either rate or severity of
Genomic DNA was isolated from peripheral blood
mononuclear cells with the use of a DNA isolation
However, the above-mentioned studies did not
kit (MagnaPure LC, Roche Diagnostics, Mannheim,
include the recipients' secretor status, which could
Germany). The classic human secretor locus (Se)
FUT2
explain the conflicting results. Eighty percent of all indi-
encodes α1,2-l-fucosyltransferase and is located on Chro-
viduals, who are defined as secretors, do not only express
mosome 19. A nonsense mutation involving Codon 143
their ABH antigens on tissues, but are also capable of
(numbered from the putative initiator methionine of the
secreting soluble ABH substance in their body fluids,
short FUT2 protein) is responsible for the nonsecretor
including plasma.10
phenotype.12 The nonsense mutation is due to a G-to-A
Soluble A/B antigens in the recipient's plasma poten-
transition at Nucleotide 428. Wild-type (Se, 428G) and
tially neutralize in vivo circulating anti-A and/or anti-B
mutant (se, 428A) alleles of
FUT2 gene were detected by
derived from donor lymphocytes in minor ABO-
polymerase chain reaction using sequence specific
incompatible HSCT and thus mitigate possible immuno-
priming technology in two independent reactions. Het-
logic and/or inflammatory responses. This could affect
erozygous individuals would give positive amplification
incidence and severity of GVHD, disease relapse, and OS.
in both reactions, and homozygous individuals in one
The aim of this retrospective study was to investigate the
reaction only. Primers for the wild-type allele (428G)
prognostic role of the recipients' ABH secretor status after
were FUT2-all+523R (CCGGCTCCCGTTCACCTG-3′) and
minor ABO-incompatible HSCT.
FUT2-Se+428G-F (CCGGCTACCCCTGCTCGTG-3′), and
ABH-SECRETOR STATUS AND TRANSPLANTATION
FUT2-all+523R and FUT-se+428A-F (ACCGGCTACCCCTGCTCGTA-3′) for the mutant allele (428A), respectively.
TABLE 1. Patient, disease, and transplant
Concentrations of the primers in the final reaction volume
characteristics according to the secretor status
were 200 nmol/L, and those of the control primers
90 nmol/L. Sequences of the control primers, reaction,
and cycling conditions have been described previously.13
Mean age at HSCT (years)
Patient, disease, and transplant characteristics were com-
pared between secretors and nonsecretors using Pear-
son's chi-square tests for categorical variables. For acute
and chronic GVHD and nonrelapse mortality (NRM),
competing risks analysis was used. For univariate analysis
of OS, the Kaplan-Meier method was used. Multivariable
Cox analysis was used to adjust for donor type and disease
Conditioning regimen
stage. All comparisons were two-sided, and p values of
less than 0.05 were considered significant. All analyses
were carried out with computer software (Stata, Version
12, StataCorp, College Station, TX).
Mismatched related
Between March 1996 and June 2012, a total of 788 adult
patients underwent allogeneic HSCT at our institution. A
total of 201 patients received a minor ABO-incompatible
HSCT. Patients who received more than one HSCT (n = 19)
or a highly T-cell-depleted haploidentical HSCT (n = 5)
ALL = acute lymphoblastic leukemia; AML = acute myeloid
were excluded from the analysis. One additional patient
leukemia; CMV = cytomegalovirus; CYA = cyclosporine;
had to be excluded because of missing LE phenotyping
D = donor; LPD/PCD = lymphoproliferative disorders/plasma
and no available DNA for molecular testing. Overall, 176
cell diseases; MDS = myelodysplastic syndrome;MMF = mycophenolate; MPN = myeloproliferative disorder;
patients were included in the analysis. ABO donor-
MTX = methotrexate; R = recipient; RIC = reduced-intensity
recipient combinations were as follows: O/A1/2 91; O/AB 3;
O/B 22; A/B 11; B/A 17; A2/A1 19; A2/A1B 3; B/AB 3; andA/AB 7. A total of 112 patients (64%) were male, and64 (36%) were female. Mean age at transplantation was
quent (n = 64; 36%). Diagnosis distribution and disease
40 years and was not different between secretors and
stage at HSCT were not different between secretors and
nonsecretors. Most patients (n = 140; 80%) received amyeloablative conditioning regimen. Ninety-five patients(54%) received a HSCT from an unrelated donor. GVHD
Secretors and nonsecretors
prophylaxis consisted primarily of cyclosporine and
A total of 150 patients (85%) were secretors and 26 (15%)
methotrexate. Table 1 summarizes patient, disease, and
were nonsecretors. Of the nonsecretors, 20 patients (11%)
transplant characteristics, according to the secretor
had their secretor status determined by molecular analy-
sis because of missing LE phenotype (n = 6) or becausethe phenotype was Le(a–b–) (n = 14). Seven of thesepatients were homozygous (
Se/
Se) and 10 heterozygous
(
Se/
se) in the secretor gene locus and three were nonse-
Cumulative incidence of acute (≥Grade II) GVHD on
cretors. Overall, 22 nonsecretors (85%) were male.
Day 100 was 41 ± 11% for secretors and 46 ± 5% for non-secretors (p = 0.59). Incidence of chronic GVHD at 2 yearswas 52 ± 13% for secretors and 56% ± 5% for nonsecretors
Disease and transplant characteristics
(p = 0.62; Fig. 1).
The main indication for HSCT was acute leukemia (n = 93;
Adjusted multivariable Cox analysis confirmed that
53%), with acute myeloid leukemia being the most fre-
secretor status was not predictive of GVHD development:
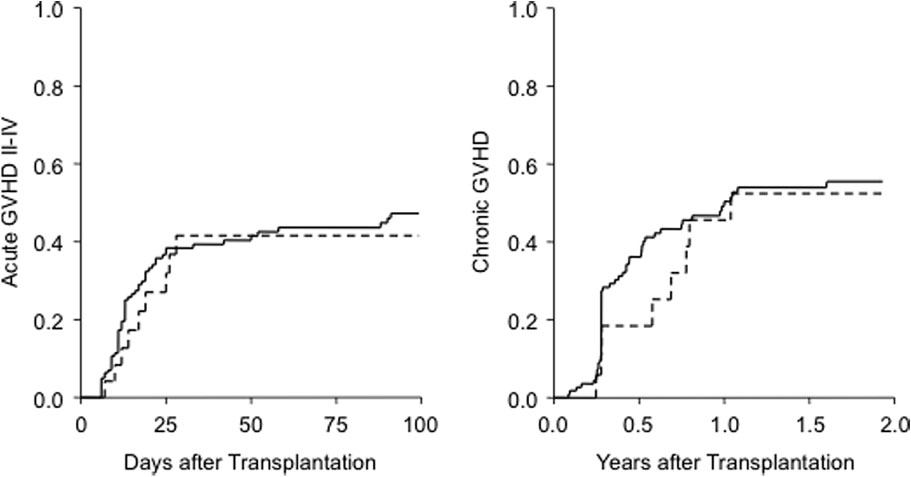
HOLBRO ET AL.
Fig. 1. Acute (≥Grade II) and chronic GVHD according to secretor status. (—) Nonsecretor; (- - -) secretor.
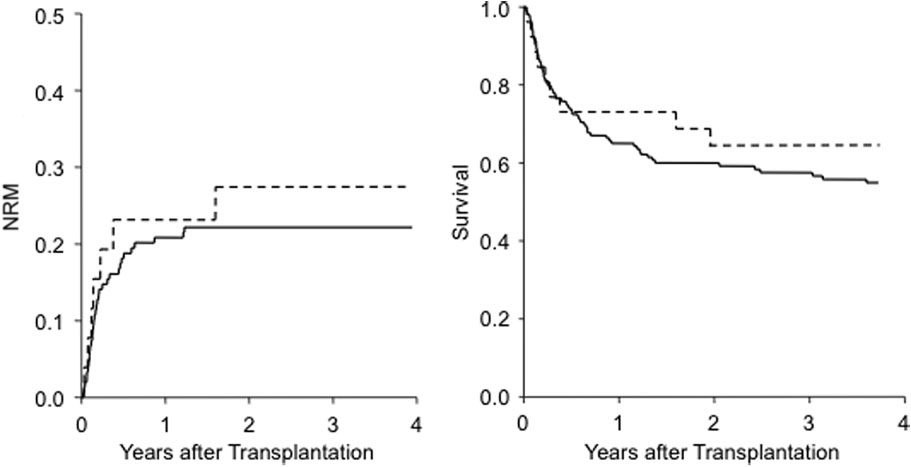
Fig. 2. NRM and OS according to secretor status. (—) Nonsecretor; (- - -) secretor.
hazard ratio (HR) secretor versus nonsecretor for acute
the other hand, strongly affects NRM, incidence and
GVHD 0.85, 95% confidence interval (CI) 0.42-1.72,
severity of GVHD, and OS.14 For several decades, HSCT has
p = 0.64; and HR for chronic GVHD 1.08, 95% CI 0.58-2.01,
been performed—if unavoidable—across the ABO blood
group barrier. While in major ABO-incompatible HSCT,acute hemolysis and pure RBC aplasia are the major short-
NRM and OS
term complications, passenger lymphocyte syndromeis a possible complication in minor ABO-incompatible
Two-year NRM was similar between secretors and nonse-
HSCT, with varying clinical course from asymptomatic
cretors (27 ± 9% and 23 ± 3% for secretors and nonsecre-
laboratory finding to severe and even life-threatening
tors, respectively; p = 0.45).
condition.15,16 Besides the above-mentioned immune-
The same was seen in the 4-year OS, which was
hematologic complications, the data on the impact of
64 ± 10% for secretors and 55 ± 4% for nonsecretors
minor ABO blood group incompatibility on OS as well as
(p = 0.28; Fig. 2). Again, adjusted multivariable Cox analy-
incidence and severity of GVHD is controversial.5 As
sis confirmed that secretor status was not predictive of
ABO blood group antigens are expressed on different
NRM and OS: HR secretor versus nonsecretor for NRM
tissues, the presence of antibodies against these antigens
1.17, 95% CI 0.54-2.55, p = 0.70; and HR for OS 0.79, 95%
could have an impact on the underlying disease, GVHD,
CI 0.41-1.55, p = 0.50.
and OS. In particular, antibodies produced by donorlymphocytes in minor ABO-incompatible HSCT are
directed against recipient ABO antigens and could
In contrast to solid organ transplantation ABO incompat-
induce a humoral immune response. Endothelial cells
ibility is of minor importance for HSCT. HLA matching, on
which express A and/or B substances could be a
ABH-SECRETOR STATUS AND TRANSPLANTATION
possible target of this immune response, as there is a per-
Even though we found no impact of secretor status in
sistence of recipient type endothelium after HSCT.17
minor ABO-incompatible HSCT, the role of secretor status
Recipients, who are ABH secretors could—on the other
in ABO-incompatible solid organ transplants should be
hand—neutralize these donor antibodies ("in vivo"
investigated. It is widely recognized that anti-A and anti-B
adsorption), thus mitigating a possible immunologic
isohemagglutinins can cause hyperacute rejection of
reaction in both directions (graft-versus-leukemia and
incompatible transplants. However, the current organ
graft-versus-host reaction). To the best of our knowledge,
shortage has driven new incentives and strategies, includ-
the impact of ABH secretor status in recipients of
minor ABO-incompatible HSCT has not been investigated
Together with immunosuppression and different prepara-
tive protocols including rituximab and immunoad-
In this study, 85% of the 176 patients with minor ABO-
sorption and/or plasma exchange in ABO-incompatible
incompatible HSCT were ABH secretors, which is consis-
solid organ transplantation, ABH secretion could have an
tent with the known prevalence in a Caucasian
impact on short- and long-term transplant outcomes,
population. We did not find any differences in major trans-
acting as an "in vivo" adsorption mechanism.
plant outcomes, including acute (≥Grade II) and chronic
In conclusion, the role of the recipients' ABH secretor
GVHD, NRM, and OS between secretors and nonsecretors.
status in minor ABO-incompatible HSCT appears to be
Our data therefore do not suggest a clinically significant
not relevant for clinical outcome and is not an explanation
effect of secretor status on a donor-derived humoral
for the discrepant results of the published literature. This
immune response against recipients' ABO antigens. One
may not be the case for ABO-incompatible solid organ
possible explanation for this finding could be the lack
transplantation, an expanding field that deserves further
of a general increase of donor derived anti-A/B after
minor ABO-incompatible HSCT.18 Additionally one mayfurther speculate that transplant-associated micro-
angiopathy as a manifestation of GVHD also representsan antibody-mediated endothelial cell activation and
We thank Sonja Sigurdardottir for development and implemen-
damage. However, in a previous study we did not find ABO
tation of the molecular determination of secretor status. AH, JPS,
incompatibility to be a risk factor for the development of
and AB designed the study and drafted the manuscript; AH and
JPS collected the data; MS performed statistical analysis; CG per-
From a pathophysiologic viewpoint, the transfusion
formed molecular genetic analysis; and all authors contributed to
of ABO-compatible nonidentical plasma can mimic a sce-
data analysis, data interpretation, and writing of the manuscript.
nario similar to the one described above. Soluble ABHsubstance in plasma obtained from secretors can interact
CONFLICT OF INTEREST
with recipients' isohemagglutinins, if transfused in anABO-compatible but nonidentical manner. A large retro-
The authors have disclosed no conflicts of interest.
spective study has described a potentially negative impactof ABO-compatible nonidentical plasma transfusions
on survival.20 The authors hypothesized that immunecomplex formation could be a possible cause. However,
1. Passweg JR, Baldomero H, Bregni M, et al. Hematopoietic
our finding on NRM and OS suggests that immune
SCT in Europe: data and trends in 2011. Bone Marrow
complex formation, which could occur in secretors in
minor ABO-incompatible HSCT has a negligible impact
2. Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality
after allogeneic hematopoietic-cell transplantation. N Engl
Our study has various limitations. These include the
J Med 2010;363:2091-101.
relatively small overall number of nonsecretors, the lack of
3. Gratwohl A, Stern M, Brand R, et al. Risk score for outcome
data regarding recipient isohemagglutinin titers before
after allogeneic hematopoietic stem cell transplantation:
and during the course of HSCT and the lack of additional
a retrospective analysis. Cancer 2009;115:4715-26.
data on immunohematologic complications after minor
4. Rowley SD, Donato ML, Bhattacharyya P. Red blood cell-
ABO-incompatible HSCT, including passenger lympho-
incompatible allogeneic hematopoietic progenitor cell
cyte syndrome in secretors and nonsecretors. An addi-
transplantation. Bone Marrow Transplant 2011;46:1167-85.
tional limitation is the power of our retrospective analysis,
5. Booth GS, Gehrie EA, Bolan CD, et al. Clinical guide to
which would detect differencies in the defined outcome
ABO-incompatible allogeneic stem cell transplantation.
variables with a HR of 2 to 2.5. ABH secretor status was
Biol Blood Marrow Transplant 2013;19:1152-8.
determined either through determination of LE pheno-
6. Seebach JD, Stussi G, Passweg JR, et al.; GVHD Working
type or by genotyping. Therefore, we could not further
Committee of Center for International Blood and
analyze the influence of zygosity of the secretor gene.
Marrow Transplant Research. ABO blood group barrier in
HOLBRO ET AL.
allogeneic bone marrow transplantation revisited. Biol
14. Petersdorf EW. The major histocompatibility complex: a
Blood Marrow Transplant 2005;11:1006-13.
model for understanding graft-versus-host disease. Blood
7. Kanda J, Ichinohe T, Matsuo K, et al. Impact of ABO mis-
matching on the outcomes of allogeneic related and unre-
15. Kimura F, Sato K, Kobayashi S, et al.; Japan Marrow Donor
lated blood and marrow stem cell transplantations for
Program. Impact of AB0-blood group incompatibility on
hematologic malignancies: IPD-based meta-analysis of
the outcome of recipients of bone marrow transplants
cohort studies. Transfusion 2009;49:624-35.
from unrelated donors in the Japan Marrow Donor
8. Socie G, Blazar BR. Acute graft-versus-host disease:
Program. Haematologica 2008;93:1686-93.
from the bench to the bedside. Blood 2009;114:4327-36.
16. Daniel-Johnson J, Schwartz J. How do I approach ABO-
9. Stussi G, Muntwyler J, Passweg JR, et al. Consequences of
incompatible hematopoietic progenitor cell transplanta-
ABO incompatibility in allogeneic hematopoietic stem cell
tion? Transfusion 2011;51:1143-9.
transplantation. Bone Marrow Transplant 2002;30:87-93.
17. Mueller RJ, Stussi G, Puga Yung G, et al. Persistence of
10. Henry S, Oriol R, Samuelsson B. Lewis histo-blood group
recipient-type endothelium after allogeneic hematopoietic
system and associated secretory phenotypes. Vox Sang
stem cell transplantation. Haematologica 2011;96:
11. Grubb R. Correlation between Lewis blood group and
18. Stussi G, Huggel K, Schanz U, et al. Levels of anti-A/B anti-
secretor character in man. Nature 1948;162:933.
bodies after ABO-incompatible hematopoietic stem cell
12. Kelly RJ, Rouquier S, Giorgi D, et al. Sequence and expres-
transplantation. Transplant Proc 2005;37:1385-7.
sion of a candidate for the human secretor blood group
19. Martinez MT, Bucher C, Stussi G, et al. Transplant-
alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity
associated microangiopathy (TAM) in recipients of alloge-
for an enzyme-inactivating nonsense mutation commonly
neic hematopoietic stem cell transplants. Bone Marrow
correlates with the non-secretor phenotype. J Biol Chem
20. Shanwell A, Andersson TM, Rostgaard K, et al.
13. Kormoczi GF, Wagner T, Jungbauer C, et al. Genetic diver-
Post-transfusion mortality among recipients of
sity of KELnull and KELel: a nationwide Austrian survey.
ABO-compatible but non-identical plasma. Vox Sang
Source: http://51083757.de.strato-hosting.eu/author/resources/2014-holbro-secretor-in-mino-abo-incompatible-hscttransfusion.pdf
Creation of a Bacterial Cell Controlled by a Chemically SynthesizedGenome Daniel G. GibsonScience 329 This copy is for your personal, non-commercial use only. , you can order high-quality copies for your If you wish to distribute this article to otherscolleagues, clients, or customers by can be obtained by
International Journal of Risk & Safety in Medicine 27 (2015) 85–91 DOI 10.3233/JRS-150645IOS Press Suicidal risk from TADS study was higherthan it first appeared G¨oran H¨ogberga,b,∗, David O. Antonuccioc and David Healyda Department of Women's and Children's Health, Child and Adolescent Psychiatric Unit,Karolinska Institutet, Astrid Lindgren Children's Hospital, Stockholm, SwedenbStockholm Child and Adolescent Psychiatry, BUP Huddinge, Stockholm, SwedencDepartment of Psychiatry and Behavioral Sciences, University of Nevada School of Medicine,Reno, NV, USAdBangor University, Wales, UK









