Bloodjournal.org
Tetracycline-controlled transgenic targeting from the
SCL locus directs conditionalexpression to erythrocytes, megakaryocytes, granulocytes, and c-kit–expressinglineage-negative hematopoietic cellsErnesto Bockamp, Cecilia Antunes, Marko Maringer, Rosario Heck, Katrin Presser, Sven Beilke, Svetlana Ohngemach, Rudiger Alt,Michael Cross, Rolf Sprengel, Udo Hartwig, Bernd Kaina, Steffen Schmitt, and Leonid Eshkind
The stem cell leukemia gene SCL, also
gene expression was restricted to erythro-
data therefore demonstrate that exog-
known as TAL-1, encodes a basic helix-loop-
cytes, megakaryocytes, granulocytes, and,
enously inducible and reversible expres-
helix transcription factor expressed in ery-
importantly, to the c-kit–expressing and lin-
sion of selected transgenes in myeloid,
throid, myeloid, megakaryocytic, and hema-
eage-negative cell fraction of the bone mar-
megakaryocytic, erythroid, and c-kit–ex-
topoietic stem cells. To be able to make use
row. In addition, conditional transgene acti-
pressing lineage-negative bone marrow cells
of the unique tissue-restricted and spatio-
vation also was detected in a very minor
can be directed through SCL regulatory
temporal expression pattern of the SCL
population of endothelial cells and in the
elements. The SCL knock-in mouse pre-
gene, we have generated a knock-in mouse
kidney. However, no activation of the re-
sented here represents a powerful tool for
line containing the tTA-2S tetracycline trans-
porter transgene was found in the brain of
studying normal and malignant hematopoi-
activator under the control of SCL regula-
adult mice. These findings suggested that
esis in vivo. (Blood. 2006;108:1533-1541)
tory elements. Analysis of this mouse using
the expression of tetracycline-responsive
reporter genes recapitulated the known en-
strains demonstrated that switchable trans-
dogenous expression pattern of SCL. Our
2006 by The American Society of Hematology
The basic helix-loop-helix transcription factor stem cell leukemia (
SCL)
endogenous
SCL expression pattern.18-24 Complementary studies
(also known as
TAL-1 or
TCL5) was originally identified by virtue of a
examining the expression of a
lacZ reporter knocked into exon III
chromosomal translocation associated with acute human lymphoblastic
of the
SCL gene locus provided evidence that
SCL regulatory
leukemia.1-3 In addition to its involvement in leukemia, loss-of-function
elements can direct expression of the
lacZ transgene to progenitors
studies in mice demonstrated an essential role of
SCL for the specifica-
of lymphoid, erythroid, and myeloid lineages.25 Analysis of
SCL
tion of mesoderm to primitive and definitive blood cell formation
lacZ knock-in embryos further revealed expression of the reporter
(reviewed in Begley and Green4 and Lecuyer and Hoang5). The absolute
gene in parts of the central nervous system, the vascular endothe-
requirement for
SCL expression during early embryonic development
lium, and in primitive and definitive blood cells.26 These findings,
has led to the view that
SCL acts as a master regulator of blood cell
together with the loss-of-function data, suggest that
SCL regulatory
formation.6 Furthermore, conditional gene targeting of
SCL in adult
elements are active in HSCs and blood progenitors and that this
mice not only has revealed a regulatory function of
SCL in both
activity is selectively maintained during ontogeny in myeloid,
erythropoiesis and megakaryopoiesis,7-9 but also has suggested that
SCL
erythroid, megakaryocytic, and HSCs/progenitors but extinguished
function is not required for self-renewal or long-term repopulation
in all other mature blood cell lineages.
capacity of hematopoietic stem cells (HSCs). Within blood cell lineages,
To be able to reversibly express transgenes in
SCL-positive
SCL expression has been reported in granulocytic, erythroid, megakaryo-
blood cells, we have made use of the tetracycline regulatory
cytic, and HSC/progenitor populations.4,5
system.27 Tetracycline-mediated control of transgenes has become
Human and murine
SCL genes are transcribed from 3 distinct
an excellent strategy for studying gene function in mice (Gossen
lineage-specific promoters leading to a complex pattern of differen-
and Bujard28 and Bockamp et al29). Since transgene expression in
tially spliced transcripts.10-16 DN
ase I hypersensitivity mapping,
these animals is exclusively dependent on the administration/
restriction endonuclease accessibility assays, and functional in
absence of tetracycline or tetracycline derivatives,30 the function of
vitro experiments revealed several enhancer and silencer elements
any gene product can be studied during selected developmental
within the
SCL genomic locus.17 In addition, reporter mice were
windows or at critical stages of disease. Furthermore, inducible
used to identify distinct regulatory elements of the
SCL locus
expression of toxic genes can be used to ablate selected cell
responsible for directing expression to specific subdomains of the
populations in vivo, allowing direct studies of the function of the
From the Institute of Toxicology/Mouse Genetics and the Department of
berg-Universita¨t Mainz (E.B.), and the Deutsche Krebshilfe (E.B.).
Hematology/Oncology, University Medical School, and the FACS and Array
E.B. and C.A. contributed equally to this work.
Core Facility, Johannes Gutenberg-Universita¨t Mainz; the Department ofHematology/Oncology, University of Leipzig; and the Max-Planck-Institute for
Reprints: Ernesto Bockamp, Institute of Toxicology/Mouse Genetics,
Medical Research, Heidelberg, Germany.
Johannes Gutenberg-Universita¨t Mainz, Obere Zahlbacher Str 67, 55131Mainz, Germany; e-mail:
[email protected].
Submitted December 12, 2005; accepted April 21, 2006. Prepublished online as
Blood First Edition Paper, May 4, 2006; DOI 10.1182/blood-2005-12-012104.
The publication costs of this article were defrayed in part by page chargepayment. Therefore, and solely to indicate this fact, this article is hereby
Supported by the European Union (E.B.), the Deutsche Forschungsge-
marked ‘‘advertisement'' in accordance with 18 U.S.C. section 1734.
meinschaft (E.B., L.E.), the Stiftung Rheinland-Pfalz fu¨r Innovation (E.B.),the Mainz-Forschungsfonds (MAIFOR) program from the Johannes Guten-
2006 by The American Society of Hematology
BLOOD, 1 SEPTEMBER 2006
䡠 VOLUME 108, NUMBER 5
BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
targeted cells and the creation of conditional disease models.31 The
blotting using an 800-bp fragment upstream of
SCL exon Ia as a 5⬘ outside
unique experimental potential of tet on/off mouse models for
probe and a 1025-bp polymerase chain reaction (PCR) fragment as an
approaching crucial questions about normal and malignant blood
inside probe to confirm correct integration. The 800-bp 5⬘ probe was
cell development is illustrated by numerous reports investigating
excised by
Hind III digestion of the ⫺2000
SCL Ia pGL-2 plasmid,13 andthe 3⬘ probe was generated by PCR using oligonucleotide 5⬘-CCTCA-
the in vivo function of conditionally expressed transgenes.32-41 In
GAAGCTGTCACTGTGTC-3⬘ as a forward and oligonucleotide 5⬘-
these reports, the combination of a tissue-specific effector with a
TTGCTCAGGGACTTTACTGTCAG-3⬘ as a reverse primer. For in vivo
responder mouse was used to express selected genes in a tetracy-
excision of the neomycin-resistant cassette, germ-line–transmitting
SCL-
TA-2S knock-in mice were crossed to the SYCP-Cre deleter line.49
For studying the etiology of hematologic malignancies and, in
Successful excision of the cassette was confirmed by using a 3-primer PCR
particular, leukemias, the ability to control gene function in vivo is
approach with the oligonucleotides 5⬘-TGGCCAAGTTACTCAATGACC-3⬘
a major advantage, since reversible induction can reveal whether
and 5⬘-GGAAGTATCAGCTCGACCAA-3⬘ as forward primers and the
transgene expression is needed for initiation, progression, mainte-
5⬘-GGATGGATCAACATGGACCT-3⬘ oligonucleotide as reverse primer.
nance, or remission of the disease. In addition, for several
The LC-1, the enhanced green fluorescent protein (EGFP)–
lacZ, and the
leukemias, distinct oncogenes or leukemia-associated factors have
tetO-Cre tetracycline-responsive responder lines have been described.50-52
been reported to be already expressed in HSCs or blood cell
Genotyping of mice
progenitors.42,43 This observation, together with the obvious similar-ity between stem cells and cancer cells, has led to the emerging
For genotyping of the
SCL-tTA-2S knock-in mouse primers 5⬘-
concept of the leukemic stem cell.44,45 Research focusing on the
role of leukemic stem cells would therefore greatly benefit from
GCTCC-3⬘ were used. The LC-1 mouse was typed using primers
mouse models allowing the reversible induction of oncogenes
and/or leukemia-associated factors in HSCs or blood cell
GTTCTGCGGG-3⬘. The EGFP-
lacZ tetracycline-responsive respondermouse was typed using primers 5⬘-CTCAAGTTCATCTGCACCACC-3⬘
To be able to reversibly target the expression of transgenes to
SCL-positive cells, we have generated an
SCL tTA-2S knock-in
mouse. Detailed analysis of this mouse demonstrated that inhematopoietic tissues tetracycline-mediated transgene expression
Organs from adult mice were dissected, extracted, and assayed for
was completely restricted to myeloid, megakaryocytic, and ery-
luciferase activity as described.53 Luciferase activity was normalizedagainst the amount of 10 g protein. A linear relationship between light
throid cells, and, most importantly, to c-kit–expressing lineage-
units and volume was confirmed in all experiments. Luciferase values in the
negative cells of the bone marrow. In addition, conditional
presence and without doxycycline (DOX) were obtained in each case from
transgene expression also was found in a very minor fraction of
at least 3 different animals producing a similar pattern of activity.
platelet endothelial cell adhesion molecule 1 (PECAM-1)–expressing endothelial cells and in a subset of cells in the kidney.
However, no induction of transgenes was detected in histologicbrain sections. These findings suggest that the
SCL tTA-2S
Dissected tissues were digested at 37°C for 40 minutes in phosphatebuffered saline (PBS) (pH 7.4) containing 0.5 g/mL collagenase together
knock-in mouse recapitulates the known endogenous expression
with 50 units DN
ase I per mL (both Sigma, St Louis, MO) and subsequently
pattern of
SCL. The
SCL knock-in mouse presented here therefore
subjected to fluorescence activated cell sorting (FACS) analysis.
represents an excellent model for studying controlled gene expres-sion in
SCL-positive blood cells and, most importantly, to condition-
FACS analysis and cell sorting
ally direct expression of selected gene products to c-kit⫹/lin⫺hematopoietic cells of the bone marrow.
Lineage contribution of EGFP-marked blood cells was analyzed with a4-color–equipped FACSCalibur (Becton Dickinson [BD], San Jose, CA) byco-staining with phycoerythrin (PE)–conjugated antibodies against CD11b,CD19, Gr-1, TER119 (BD), CD3, CD11c, DX5 (Caltag, Burlingame, CA),
Materials and methods
CD23 (Southern Biotech, Birmingham, AL) or with purified antibodiesagainst CD41 (BD) detected with anti–rat-PE (Caltag). Collagenase-treated
Construction of the targeting vector
suspensions of peripheral organs were simultaneously incubated with an
The murine genomic
SCL locus was obtained by screening a 129/Sv lambda
endothelial-specific PECAM-1 rat monoclonal antibody (CD31, BD) and a
phage library. A 4.2-kb fragment upstream of
SCL exon V was used as the 5⬘
mix of TER119/CD45 antibodies (BD). Prior to staining, the samples (not
homology arm and an 8.1-kb fragment downstream of the unique XbaI site
the samples stained with secondary reagents) were blocked with PBS
in exon VI as the 3⬘ homology arm and cloned into pGem11 ZF⫹ (Promega,
supplemented with 5% rat serum for 10 minutes. Dead cells were excluded
Madison, WI). All ATG codons of exon IV and the first ATG codon in exon
from analysis via 7AAD staining (BD). Detection levels over background
V were changed to GGG codons, thus preventing translational initiation
were confirmed for the PECAM-1 antibody in parallel control experiments
from these sites. The unique Not I recognition site in exon V was used for
using a rat PE-conjugated IgG 2A isotype control antibody (BD). The stem
insertion of the tTA-2S transactivator,46 followed by the bovine growth
cell fraction was defined by lin⫺PE⫺ and c-kit⫹APC (CD117, BD) staining.
hormone polyA signal and a loxP-flanked neomycin-resistant cassette under
Data were analyzed using the CellQuest Pro software (BD). In all cases the
the control of the
Herpes simples virus TK promoter (Figure 1A). All
lineage contribution of EGFP-expressing cells was determined in 3
modified sequences were confirmed by sequence analysis.
independent experiments, analyzing each time a minimum of 5 ⫻ 105 cells.
Preparative FACS sorting of lin⫺ c-kit⫹ cells was performed using a
FACS Vantage SE Turbo (BD). Lin⫹ cells were first depleted from the
femoral mononuclear population using a magnetic affinity lineage depletion
The W9.5 embryonic stem (ES) cell line47 was electroporated with the
kit (MACS, Miltenyi Biotech, Auburn, CA). The lineage-depleted fraction
linearized targeting vector. G-418–resistant single clones containing the
was then stained with c-kit-APC antibody and the c-kit⫹ population sorted
correctly recombined locus were injected into blastocysts and transferred
simultaneously into EGFP⫹ and EGFP⫺ fractions. Because of the small
into pseudopregnant mothers following standard procedures.48 Successful
number of lin⫺ c-kit⫹ cells available, the EGFP sort gates were preset using
germ-line transmission and correct integration was confirmed by Southern
mononuclear cells from DOX-treated and untreated mice.
BLOOD, 1 SEPTEMBER 2006
INDUCIBLE EXPRESSION FROM THE SCL LOCUS
䡠 VOLUME 108, NUMBER 5
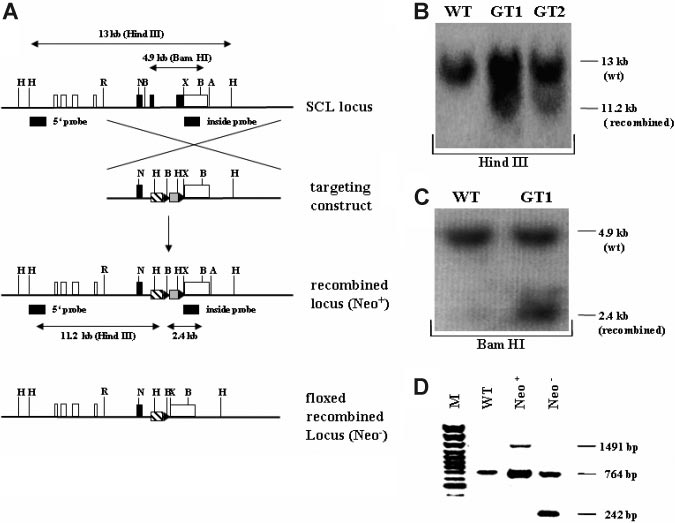
Figure 1. Targeting strategy and confirmation of the recombined SCL genomic locus. (A) Schematic overview of the targeting strategy. In the upper representation the
SCL wild-type genomic locus is shown. Coding exons (IV, V, and VI) are depicted as black, and noncoding exons (Ia, Ib, IIb, III, and part of VI) are depicted as white boxes. The
targeting construct is shown below the SCL genomic locus, consisting of 2 homology arms, the tTA-2S coding sequence (striped box), and the floxed neomycin-resistant
selection cassette (gray box). In the targeting construct all ATG codons in exon IV and the first ATG in exon V were changed to GGG codons. LoxP Cre-recombinase recognition
sites flanking the neomycin cassette are indicated as black triangles. Below the targeting construct the recombined mutant SCL locus is shown still containing the neomycin
cassette (Neo⫹). At the bottom of the representation the recombined SCL locus is depicted after excision of the neomycin cassette (Neo⫺). H indicates Hind III; R, EcoRI; N, Not
I; X, XbaI; A, ApaI, and B, BamHI. (B) 5⬘ confirmation of the recombined SCL locus by Southern blotting using a specific outside probe. Digestion with Hind III of wild-type (WT)
DNA gives rise to a 13-kb fragment, whereas the correctly recombined locus will result in a smaller 11.2-kb fragment (GT1 and GT2, germ-line–transmitting mouse founder line
1 and 2). (C) 3⬘ confirmation of the recombined SCL locus by Southern blotting. BamHI digestion of genomic DNA followed by hybridization with an inside probe produces a
4.9-kb fragment for the wild-type allele (WT) and a 2.4-kb fragment for the mutant knock-in allele (GT1). (D) In vivo excision of the neomycin-resistant cassette. PCR was used
to verify the excision of the neomycin-resistant cassette from the germ-line of the SCL tTA-2S knock-in mouse. The recombined SCL locus still containing the cassette will
produce a 1491-bp amplification product (Neo⫹). After excision of the neomycin cassette the same primers will amplify a 242-bp fragment (Neo⫺). The 764-bp amplification
product is specific for the SCL wild-type allele.
CAFC assay
(Perkin Elmer Life Sciences, Shelton, CT). Images were captured using acolor view digital camera running on an Olympus BX50 WI microscope
The cobblestone area-forming cell (CAFC) assay was performed essentially
(Olympus, Hamburg, Germany) and a 20⫻/0.50 numeric aperture objec-
as described.54,55 Briefly, the lin⫺ c-kit⫹ EGFP⫹, lin⫺ c-kit⫹ EGFP⫺, and
tive. Images were captured using a Color View 12 digital charge-coupled
the whole mononuclear cell populations were counted, then titrated through
device (CCD) camera (Olympus). Images were digitalized using the
serial dilutions onto established OP-9 stromal feeder layers, each cell
analySIS software package 3.1 (Soft Image Systems, Mu¨nster, Germany)
concentration being represented by 20 independent wells. Cultures were fed
and imported into Adobe Photoshop 4.0 (Adobe Systems, San Jose, CA). In
by refreshing half of the medium weekly. All wells were scored for the
all cases, electronic adjustments were applied to the whole image.
presence of cobblestone areas (groups of 5 or more hematopoietic cells
-Galactosidase expression and Cre expression in the brains of mice
growing underneath the stromal layer) at day 14 and day 35 of culture, and
were analyzed as described.51
the frequency of CAFCs calculated using Poisson statistics.
Controlled expression of transgenes
To exogenously switch the expression of luciferase, EGFP, and -galactosi-
dase in tTA-2S-SCL/LC-1 or tTA-2S-SCL/EGFP-lacZ tetracycline-responsive mice, animals were either provided with normal drinking water
Generation of the SCL tTA-2S knock-in mouse
(reporter gene expression on) or fed a solution of 7.5 mg DOX (Sigma)/mL
To conditionally express transgenes under the control of SCL
water containing 1% sucrose (reporter gene expression off).
regulatory elements, gene targeting was used to insert the coding
Immunofluorescence and X-gal staining
sequence for the tTA-2S transactivator46 into exon V of the SCLgene locus. We selected insertion of tTA-2S into exon V to ensure
Mice were killed by cervical neck dislocation and organs snap frozen in
that all known SCL regulatory elements were present in the
isopenthane. Cryostat sections (5-12 m) were fixed in 100% acetone at
recombined locus.12-23,56,57 Figure 1A shows a schematic represen-
4°C for 1 hour, air dried, and stained for -galactosidase by washing twice
tation of the targeting strategy. Correct homologous recombination
in PBS (pH 7.4), followed by overnight incubation at 37°C in X-gal
in ES cells and germ-line transmission was confirmed by Southern
solution (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 1 mg/mLX-gal in PBS). To visualize endothelial cells, sections were incubated with a
blotting (Figure 1B,C). Consistent with the introduction of 2 novel
purified rat anti–mouse CD31 monoclonal antibody against PECAM-1
Hind III sites in the recombined locus, an 11.2-kb band was
(BD), followed by a second biotin-conjugated goat anti–rat Ig–specific
detected in addition to the 13-kb wild-type band after digestion of
polyclonal antibody (BD) using the Renaissance TSA fluorescence system
genomic DNA from the germ-line–transmitting founder animals
BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
and hybridization with the 5⬘ outside probe (Figure 1B). Similarly,
will bind to the tetO sequence upstream of the cytomegalovirus
correct 3⬘ recombination was confirmed by BamHI digestion of
(CMV) minimal promoter, resulting in transcriptional activation of
genomic DNA, followed by Southern hybridization with an inside
the luciferase transgene.
probe. As shown in Figure 1C in the germ-line–transmitting
SCL expression in the adult is mainly restricted to hematopoi-
founder GT1, the expected 2.4 kb was detected in addition to the
etic tissues.4,5 In addition, the presence of a small number of
4.9-kb wild-type specific band (see also the schematic representa-
SCL-positive cells also has been reported for the adult kidney.58 To
tion of the expected fragments in Figure 1A). Correct recombina-
evaluate if the SCL-tTA-2S effector mouse also will direct condi-
tion was further confirmed for the overlap between the 3⬘ targeting
tional expression of transgenes to these cells, SCL-tTA-2S knock-in
arm and the adjacent genomic SCL locus using 2 additional probes
effector mice were crossed to the LC-1 reporter mouse line.50 In
(data not shown). Taken together, Southern blot analysis of the
this mouse the luciferase gene is under the control of a tetracycline-
germ-line–transmitting founder GT1 demonstrated correct homologous
responsive promoter element. As expected, extracts prepared from
recombination into the SCL locus.
different organs of bitransgenic SCL-tTA-2S/LC-1 mice, kept in the
To completely exclude unwanted transcriptional interference
presence of DOX, did not show luciferase activity (bottom bar
effects from the TK promoter governing the expression of the
graph ⫹DOX in Figure 2B, luciferase off). By contrast, high levels
neomycin-resistant cassette, this cassette was removed from the
of luciferase activity were detected in bone marrow and spleen of
recombined SCL locus by in vivo excision using the SYCP-Cre-
bitransgenic littermates that were never exposed to DOX (upper bar
deleter mouse line.49 Successful excision of the floxed neomycin-
graph ⫺DOX in Figure 2B, luciferase on). In addition, lower
resistant cassette was confirmed by PCR. As shown in Figure 1D,
luciferase activity was found in the thymus of induced animals.
removal of the floxed cassette resulted in a 242-bp PCR product
Interestingly, extracts prepared from brain, heart, kidney, liver,
(lane Neo⫺). By contrast, the recombined locus still containing the
lung, tongue, esophagus, and pancreas also exhibited luciferase
neomycin-resistant cassette produced a 1491-bp PCR product (lane
activity over background, suggesting the presence of tTA-2S–
Neo⫹). A 764-bp product specific for the wild-type SCL locus was
expressing cells in these tissues. No substantial luciferase activity
detected both in wild-type (lane WT) and rearranged mice (lanes
was detectable in the salivary gland, the stomach, the small and
Neo⫹ and Neo⫺), indicating the presence of at least one SCL
large intestine, the muscle, or the lymph nodes. These results
wild-type allele. For all subsequent experiments heterozygous
demonstrated that the SCL-tTA-2S effector mouse induced reporter
SCL-tTA-2S mice lacking the neomycin-resistant cassette were
gene activity in adult hematopoietic tissues and that this expression
used (homozygous SCL-tTA-2S knock-in mice were embryonic
was strictly dependent on DOX (compare luciferase activity
lethal, data not shown).
between bitransgenic mice in the presence and absence of DOX inFigure 2B). The observed high levels of luciferase activity in bone
Tissue-specific expression of transgenes with the SCL tTA-2S
marrow and spleen were expected, as SCL is known to be expressed
knock-in mouse is completely dependent on DOX
in these tissues. The low luciferase activity in the thymus is
The schematic representation in Figure 2A illustrates the DOX-
probably explained by the presence of a minor population of
dependent regulatory strategy used here. As shown in Figure 2A, in
CD8/CD4 double-negative and/or positive thymocytes or other
the presence of DOX the tTA-2S transactivator does not bind to the
cells of hematopoietic origin. Whether the somewhat unexpected
tetO binding sequence and, thus, transgene expression is not
luciferase activity in brain, heart, liver, lung, tongue, esophagus,
initiated. Conversely, in the absence of DOX tTA-2S homodimers
and pancreas represented organ-specific activation of the reporter
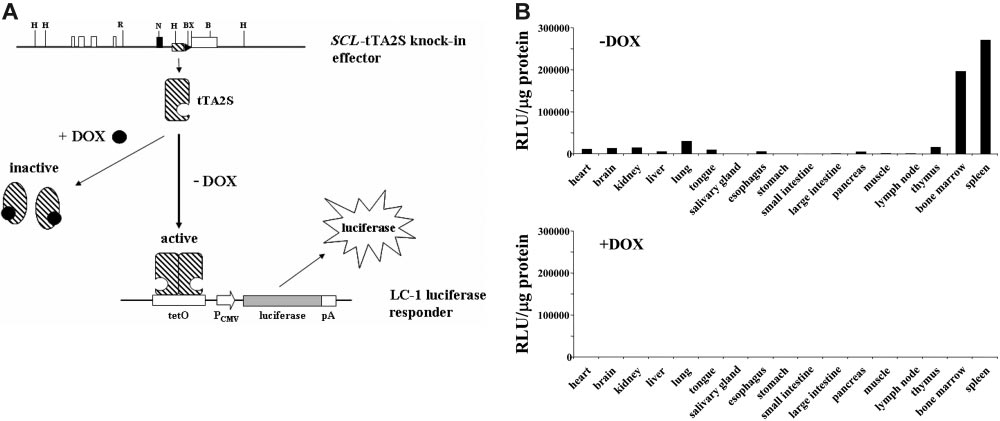
Figure 2. Tissue-specific induction of the luciferase transgene is completely DOX-dependent. (A) Schematic representation of the tetracycline regulatory system.
Restriction endonuclease recognition sites are as in Figure 1. DOX indicates doxycycline; tTA-2S, tetracycline-dependent transactivator; tetO, DNA-binding consensus for
tTA-2S homodimers; pCMV, human cytomegalovirus minimal promoter; pA, polyA signal. (B) Luciferase activity expressed as relative light units (RLU) per microgram of protein
extract was determined for different organs as indicated. The top bar graph shows luciferase activities of double heterozygous SCL-tTA-2S/LC-1 mice in the absence of DOX
(⫺DOX, luciferase on). The bottom bar graph represents luciferase values obtained from double-transgenic SCL-tTA-2S/LC-1 mice that were kept from conception onwards in
the presence of DOX (⫹DOX, luciferase off). The luciferase values in each graph are shown for a single bitransgenic mouse. A similar pattern of activity was obtained also in 2
additional independent experiments using different mice.
BLOOD, 1 SEPTEMBER 2006
INDUCIBLE EXPRESSION FROM THE SCL LOCUS
䡠 VOLUME 108, NUMBER 5
gene or was the result of tTA-2S expressing circulating blood
and that each individual cell can be simultaneously analyzed for the
and/or endothelial cells could not be addressed at this point.
presence of several different tissue-specific markers. First, we
Finally, the detected luciferase activity in the kidney was in line
wanted to determine the overall percentage of transgene-expressing
with the published expression of SCL in this organ.58
cells in lung, heart, kidney, tongue, and esophagus. The result ofthis analysis is shown in Figure 4 and demonstrated that lung, heart,
Histologic and flow-cytometric analysis of transgene induction
kidney, tongue, and esophagus of noninduced bi-transgenic ani-
in peripheral organs
mals did not contain any EGFP⫹ cells (data not shown). Consistent
In the adult, SCL is restricted to hematopoietic cells and the
with the previously detected luciferase activity, a small fraction of
kidney.4,5,58 In addition, expression of endogenous SCL in endothe-
EGFP-expressing cells was present in lung (1.8%), heart (1.71%),
lial cells has been described for the early embryo, the vasculature of
kidney (1.29%), tongue (0.67%), and esophagus (1.09%) of
tumors, and the lining of newly arising blood vessels but is absent
induced animals (Figure 4, ⫺DOX).
in quiescent adult vasculature.59-63 Intriguingly, lysates obtained
To distinguish whether conditionally induced EGFP-expressing
from SCL-tTA-2S/LC-1 mice exhibited luciferase activity in heart,
cells were organ specific or represented migrating blood cells
liver, lung, tongue, esophagus, and pancreas (Figure 2B). To
and/or rare tTA-2S–expressing endothelial cells, EGFP⫹ cells were
clarify, if transgene induction in the SCL-tTA-2S knock-in mouse
tested for co-expression of the endothelial marker PECAM-1
was due to endogenous organ-specific expression or reflected the
together with CD45 and TER119 pan-hematopoietic markers. The
presence of circulating blood cells and/or resident endothelial cells,
result of these experiments is shown in the central panel of Figure 4
SCL tTA-2S knock-in mice were mated to EGFP-lacZ tetracycline-
and indicates that in lung, heart, esophagus, and tongue the
responsive reporter mice.51 The resulting bitransgenic SCL tTA-2S/
majority of EGFP⫹ cells were of hematopoietic origin (CD45⫹/
EGFP-lacZ mice were either kept from conception onwards in the
TER119⫹ cells contained in the 2 upper quadrants of each organ
presence of DOX (reporter gene off) or kept on normal drinking
plot). In the boxes on the right of Figure 4 the percentage of
water (reporter gene on). At the age of 6 to 8 weeks organs from
EGFP-expressing cells falling either into the category blood
these mice were subjected to histologic analysis. As shown in the
(CD45⫹/TER119⫹, large upper box) or endothelium (exclusively
left panel of Figure 3, heart, liver, and kidney of bitransgenic SCL
PECAM-1 expressing, bottom right box) and other cell types
tTA-2S/EGFP-lacZ mice harbored blue -galactosidase–express-
(CD45⫺/TER119⫺ and PECAM-1⫺, bottom left box) is indicated
ing cells, consistent with the previously detected luciferase activity
for each organ. Even though a significant proportion of EGFP⫹
in these organs. No -galactosidase activity was detected in
cells of the kidney expressed hematopoietic markers (52.4%), a
bitransgenic animals permanently kept in the presence of DOX
major population of kidney cells lacked expression of both the
(data not shown) or in muscle (Figure 3G). Immunofluorescence
endothelial PECAM-1 marker and the pan-hematopoietic combina-
analysis for the endothelial-specific PECAM-1 marker further
tion of CD45/TER119 surface antigens (47.1%). The presence of a
revealed that -galactosidase–expressing cells typically did not
significant population of EGPF-expressing cells lacking blood and
colocalize with PECAM-1–positive endothelial populations (Fig-
endothelial markers suggests that in renal tissues tTA-2S is
ure 3, right panel). These results indicated that in the analyzed
expressed in a kidney-specific fashion. This observation is in line
organs transgene expression was in general not directed to endothe-
with the preciously described presence of SCL-expressing cells in
the kidney.58 Finally, in all analyzed peripheral organs very few
To analyze transgene-expressing cells of different organs more
EGFP⫹ cells exclusively expressed the PECAM-1 endothelial
precisely, dissected tissues from induced and noninduced SCL-tTA-
marker (bottom right quadrant of each plot). This suggested that
2S/EGFP-lacZ bitransgenic mice were treated with collagenase and
conditional transgene expression also was directed to very rare
the resulting cell suspensions examined by FACS. Major advan-
endothelial cells. This finding was further supported by control
tages of this strategy are that large numbers of cells can be tested
experiments using an isotype antibody instead of PECAM-1. In
Figure 3. DOX-induced expression of -galactosidase in peripheral organs of SCL-tTA-2S/EGFP-lacZ double-transgenic mice does not generally colocalize to
vascular endothelium. Representative sections from (A) heart, (C) liver, (E) kidney, and (G) muscle of double-transgenic mice were analyzed for the presence of
-galactosidase–expressing cells (left panel). Vascular endothelium was identified by immunofluorescence using a monoclonal antibody against murine PECAM-1 (B, D, F,
and H, right panel). The location of -galactosidase–expressing cells is indicated by arrows.

BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
lymph nodes (0.13%). These results indicated that induction of theEGFP reporter gene in these mice was strictly dependent on DOXand that expression of EGFP occurred only in a subset of cells.
To investigate more precisely whether conditional induction of
EGFP was tissue restricted to certain blood cell types or whether allhematopoietic lineages contained EGFP-expressing cells, distincthematopoietic cell types were analyzed for the presence of EGFP.
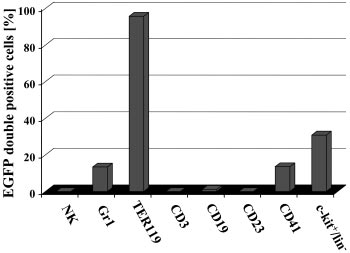
As shown in Figure 6, no EGFP-positive DX5⫹ NK-cells, CD3⫹T-lymphoid cells, a very minor fraction of CD19⫹ cells, no CD23⫹mature B cells, activated macrophages, eosinophils, and folliculardendritic cells were detected in hematopoietic organs of inducedbitransgenic mice. Indeed, as no EGFP⫹ cells expressed CD23, thevery minor fraction of CD19-expressing EGFP⫹ cells mightrepresent early myelomonocytic cells and/or immature B cells. Bycontrast, in the same animals EGFP⫹ cells were detected in Gr1⫹granulocytes, TER119⫹ erythrocytes, CD41⫹ megakaryocytes, andthe c-kit/lin⫺ fraction.
To further evaluate the presence of HSCs/progenitor cells
within the EGFP-expressing c-kit⫹/lin⫺ population, limiting dilu-tion cobblestone area-forming cell (CAFC) assays were performed.
CAFC assays are providing a generally accepted in vitro readout ofboth primitive and progenitor HSCs in mice.54,55,64 Cobblestone
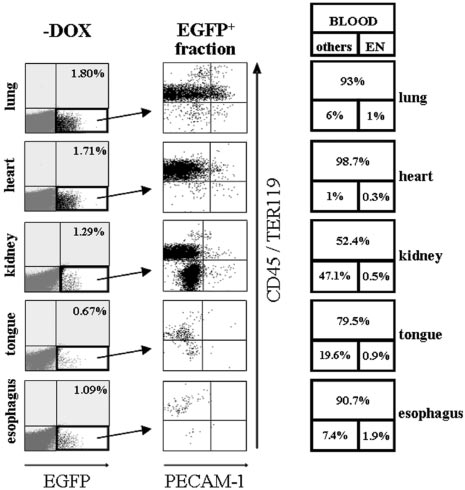
Figure 4. Induction of EGFP in peripheral organs of SCL-tTA-2S/EGFP-lacZ
areas apparent after 14 days accurately measure spleen colony-
double-transgenic mice is primarily restricted to hematopoietic cells and a
subset of organ-specific cells in the kidney. Representative FACS profiles of
forming units (CFU-S) day 12, and those present after 35 days of
collagenase-digested tissues from lung, heart, kidney, tongue, and esophagus are
culture contain long-term HSC repopulating activity.54,55,64 To
shown. Left panel (⫺DOX): Induced organs of bitransgenic mice do contain a small
investigate if the EGFP-expressing population of bone marrow
fraction of EGFP⫹ cells (bottom right quadrant). Percentages of EGFP-expressingcells are shown in the top right quadrant. Central panel: The EGFP⫹ fraction of cells
cells did contain CAFC activity, lin⫺/c-kit⫹ EGFP⫹, lin⫺/c-kit⫹
from the left panel of organ plots (indicated by an arrow) was used for plotting
EGFP⫺, and, as a negative control, mononuclear bone marrow cells
CD45/TER119 pan-hematopoietic markers (y-axis) against the PECAM-1 endothelial
of induced SCL-tTA-2S/EGFP-lacZ mice were preparatively sorted
marker (x-axis). Right panel: Percentages of EGFP⫹ hematopoietic cells are shownin the large top box and percentages of endothelial cells in the bottom right box. The
and tested for their CAFC activities. As expected the mononuclear
percentage of EGFP-expressing cells lacking blood and endothelial markers is
fraction of bone marrow cells essentially contained no CAFCs
indicated in bottom box on the left. Note the substantial increase of EGFP-expressing
(Table 1, MNC). In contrast, day 14 and day 35 CAFCs were
double-negative CD45⫺/TER119⫺and PECAM-1⫺ cells in the kidney.
several control experiments the absolute percentage of PECAM-1single-positive cells was in all cases higher than the percentagesdetected with the matched isotype antibody (Figure S2, available atthe Blood website; see the Supplemental Figures link at the top ofthe online article). For this reason we conclude that a very minorpopulation of all PECAM-1⫹ cells did express the tTA-2S transac-tivator. It is most likely that these cells represented newly formingor regenerating vasculature known to express SCL.59-63
In conclusion, our data suggest that in lung, heart, tongue, and
esophagus, expression of tTA-2S was almost completely restrictedto hematopoietic cells. In the kidney the majority of EGFP-expressing cells were either hematopoietic or organ specific.
SCL regulatory elements target induction of EGFP to red blood
cells, megakaryocytes, granulocytes, and the c-kitⴙ/linⴚ
population of the bone marrow
Next, we wanted to determine in which hematopoietic lineages theSCL-tTA-2S effector mouse could induce expression of conditionaltransgenes. For this purpose reporter mice carrying the EGFPcoding region under the control of a tetracycline-inducible pro-moter51 were mated to the SCL-tTA-2S effector mouse line. In theresulting bitransgenic animals hematopoietic organs were analyzedfor the presence of EGFP⫹ cells by FACS. As shown in Figure 5,hematopoietic organs from bitransgenic effector/reporter mice
Figure 5. Expression of EGFP in hematopoietic organs is dependent on DOX.
permanently kept in the presence of DOX did not contain any
FACS analysis of adult spleen, bone marrow, thymus, and lymph nodes from
EGFP⫹ cells (right panel ⫹DOX, EGFP off). By contrast, bi-
double-transgenic effector/responder mice, demonstrating that the induction ofEGFP was strictly dependent on DOX. Note the lack of EGFP⫹ cells in the FACS plots
transgenic mice without DOX contained a fraction of EGFP⫹ cells
on the right where EGFP expression was inhibited by DOX. The percentage of
in spleen (1.3%), bone marrow (1.72%), thymus (0.03%), and
EGFP-positive cells in each organ is indicated in the top right quadrant.
BLOOD, 1 SEPTEMBER 2006
INDUCIBLE EXPRESSION FROM THE SCL LOCUS
䡠 VOLUME 108, NUMBER 5
generate a mouse line allowing reversible targeting of transgeneexpression to HSCs and blood progenitors. Such a conditional SCLeffector mouse would be an invaluable experimental tool forapproaching fundamental issues concerning normal and malignanthematopoiesis.
The basic helix-loop-helix transcription factor SCL is one of the
very few genes known to be expressed both in embryonic and adultHSCs.4,5 This unique expression pattern suggests that SCL regula-tory elements could be used to direct conditional expression toHSCs and blood cell progenitors. Radomska and colleagues36 hadpreviously used the human CD34 locus to direct tetracycline-controlled expression of heterologous transgenes to HSCs and
Figure 6. Induction of EGFP expression in SCL-tTA-2S/EGFP-lacZ double-
early progenitors. In this mouse inducible transgene expression was
transgenic mice is restricted to granulocytes, red blood cells, megakaryocytes,
and c-kitⴙ/linⴚ cells of the bone marrow. The presence of EGFP⫹ cells in DX5⫹ NK
reported for endothelial and early blood cell progenitors. In a
cells, Gr1⫹ myeloid cells, TER119⫹ red blood cells, CD3⫹ T-lymphoid cells, CD19⫹
similar fashion elements from the 3⬘ SCL enhancer were used to
cells, CD41⫹ megakaryocytes, CD23 mature B cells, activated macrophages,
direct DOX-inducible expression of transgenes to hematopoietic
eosinophils, follicular dendritic cells, and the bone marrow lin⫺/c-kit⫹ population wasdetermined by FACS.
tissues and HSCs.41 However, in this study, only lung, intestine,and hematopoietic organs were analyzed for DOX-dependenttransgene induction. For this reason it is not clear to what extent
generated from the lin⫺/c-kit⫹ EGFP-expressing fraction, indicat-
conditional expression was exclusively restricted to hematopoietic
ing the presence of progenitors/HSCs proficient to generate early
tissues and the lung but was absent from other organs. Interestingly,
and late CAFCs (Table1). Furthermore, the lin⫺/c-kit⫹ EGFP-
when this effector mouse was used to express the BCR-ABL
negative fraction also contained CAFC activity. The presence of
oncogene, a chronic myeloid leukemia (CML)–like disease was
CAFC activity in both the lin⫺/c-kit⫹ EGFP-expressing and
induced.41 However, since overexpression of SCL under the control
EGFP-negative fraction is not surprising, since SCL is not homoge-
of the 3⬘ SCL enhancer led only to a partial rescue of the lethal SCL
neously expressed in hematopoietic progenitors/HSCs.65,66 How-
knock-out phenotype, it is to be assumed that the 3⬘ enhancer is not
ever, the generation of day 14 and day 35 CAFC with the
sufficient for recapitulating the endogenous SCL expression pat-
EGFP-expressing lin⫺/c-kit⫹ fraction suggests that the SCL-
tern.23 Here, we report the generation of a tTA-2S knock-in mouse
tTA-2S knock-in mouse line directs expression of EGFP to a subset
line that mirrors the known expression pattern of SCL in the adult.
Transcriptional regulation of the murine SCL gene has been
Taken together, our results show that the SCL-tTA-2S knock-in
extensively studied in vitro and in vivo.12-23,56 Based on this
line exclusively targeted EGFP expression to a subset of hematopoi-
information, we reasoned that inserting the tTA-2S coding se-
etic lineages, namely, erythrocytes, megakaryocytes, granulocytes,
quence into exon V of the SCL locus would ensure the conservation
and also to c-kit⫹/lin⫺ bone marrow cells. These findings sug-
of critical regulatory elements and result in a faithful recapitulation
gest that conditional targeting of the EGFP transgene recapitu-
of the endogenous SCL expression pattern by tTA-2S. The capacity
lated the reported lineage-restricted expression pattern of SCL in
and tissue specificity of the SCL-tTA-2S effector mouse line was
adult blood.
tested using luciferase, lacZ, and EGFP tetracycline-dependentreporter mice. In a first series of experiments the LC-1 luciferase
Analysis of transgene induction in the brain
responder line50 was used to determine in which organs the
Expression of SCL has been reported in V2b interneurons of the
expression of the luciferase transgene was induced. Since lucif-
developing embryo.67-69 In addition, in a recent report it was shown
erase is known to be a sensitive reporter, low levels of transgene
that SCL plays a critical role for the initial specification of primitive
induction should be detectable. These experiments demonstrated
neural precursors to astrocytes.69 However, SCL mRNA is not
high and strictly DOX-dependent transgene induction in bone
expressed in the brain of postnatal mice.70 Using the EGFP-lacZ
marrow and spleen and intermediate levels in brain, heart, kidney,
and the tetO-Cre responder mouse lines,51,52 functional tTA-2S
liver, lung, tongue, esophagus, pancreas, and thymus (Figure 2B).
activity could not be detected in coronal sections through the entire
The intermediate induction of luciferase activity in these organs
brain of induced SCL-tTA-2S mice. The lack of Cre-recombinase
was somewhat unexpected, as SCL expression in the adult had been
expression in SCL-tTA-2S/tetO-Cre mice (data not shown) and theabsence of detectable -galactosidase activity in induced SCL-tTA-
Table 1. EGFP-expressing c-kitⴙ/linⴚ cells from the bone marrow
2S/lacZ-EGFP mice (compare induced and noninduced sections in
of induced SCL-tTA-2S/EGFP-lacZ mice contain early and late
Figure S1) indicated that tTA-2S expression in the brain was either
absent or too low to drive the expression of the indicator
Experiment 1, CAFCs
Experiment 2, CAFCs
transgenes. We conclude, therefore, that the SCL-tTA-2S effector
per 104 cells
per 104 cells
Tested cell
mouse is not suitable for robust expression of transgenes in the
adult brain.
lin⫺ c-kit⫹ EGFP⫹
lin⫺ c-kit⫹ EGFP⫺
Bone marrow cells were isolated and cultured on OP-9 cells for limiting dilution
analysis of CAFC activity as described in "Materials and methods." Mean CAFC
The aim of this study was to generate a conditional mouse model
frequencies scored at day 14 and day 35 are shown for 2 independent experimentsusing, in total, 4 different mice. Numbers in parentheses indicate the range of the 95%
that recapitulates the unique spatio-temporal and lineage-restricted
confidence limit.
expression pattern of the SCL gene. In particular, we wished to
MNCs indicates mononuclear cells.
BLOOD, 1 SEPTEMBER 2006 䡠 VOLUME 108, NUMBER 5
reported only for hematopoietic tissues and the kidney.4,5,58 How-
cells was determined. In a previously published report of mice
ever, given that the analyzed organs were not perfused prior to
harboring a lacZ reporter gene in exon III of the SCL locus, lacZ
dissection, we could not exclude the possibility that the measured
expression was confined to HSCs, blood cell progenitors, and red
luciferase activities were due to tTA-2S–expressing, circulating
blood cells.25 These findings contrast with the endogenous SCL
blood, and/or resident endothelial cells. To address this question
expression pattern and also with the induced transgene expression
and to visualize transgene expressing cells in situ, sections from
pattern observed here, which also included megakaryocytes and
kidney, muscle, liver, and heart of induced bitransgenic SCL-tTA-
granulocytes. However, the differences between these 2 SCL
2S/EGFP-lacZ mice were stained for -galactosidase activity.
knock-in lines are most likely explained by differences in the
Inspection of these sections revealed the presence of lacZ-
design of the targeting strategy (lack of the third SCL promoter and
expressing blue cells in kidney, liver, and heart, but not in the
actively transcribing neomycin gene in the case of the lacZ
muscle (Figure 3). Subsequent staining of these sections with the
knock-in line). Most notably, the SCL-tTA-2S knock-in mouse
PECAM-1 endothelial-specific marker further revealed no obvious
directed expression of inducible transgenes to c-kit⫹/lin⫺ bone
general co-localization of tTA-2S and PECAM-1–expressing cells
marrow cells known to contain blood progenitors/HSCs. Further-
(Figure 3, right panel). Therefore, the histologic analysis suggested
more, measurement of CAFC frequencies from tTA-2S–targeted
that tTA-2S was not expressed in the majority of endothelial cells
EGFP-expressing c-kit⫹/lin⫺ bone marrow cells demonstrated the
of these organs. To further clarify the origin of tTA-2S–expressing
presence of day 14 CAFCs and day 35 CAFCs, which are an
cells in peripheral organs and to permit analysis of large numbers of
accepted in vitro correlate for CFU-S and bone marrow repopulat-
individual cells, we used FACS. As the EGFP-lacZ responder mice
ing stem cell activity (Table1). The ability of EGFP⫹/c-kit⫹/lin⫺
will simultaneously express EGFP and lacZ upon induction,51
cells from the bone marrow to generate day 35 CAFCs thus
kidney, heart, lung, esophagus, and tongue tissues were subjected
strongly suggests that the SCL-tTA-2S knock-in mouse is suitable
to collagenase digestion followed by FACS analysis. These experi-
for conditional expression of transgenes in adult HSCs/progenitors.
ments showed that all analyzed tissues contained a fraction of cells
Taken together, our data show that the SCL-tTA-2S knock-in
expressing EGFP, thus confirming the previously measured lucif-
mouse model will direct conditional DOX-dependent expression of
erase activities in these organs (Figure 4). In addition, examination
transgenes within blood to erythrocytes, megakaryocytes, granulo-
of EGFP-expressing cells using blood- and endothelial-specific
cytes, and, most importantly, to c-kit⫹/lin⫺ cells of the bone
markers revealed that the majority of the analyzed cells were of
marrow. This expression profile therefore represents a recapitula-
hematopoietic origin and that only a minor subset represented
tion of the known endogenous SCL expression pattern. It is to be
endothelial or other cell types that were not analyzed further.
expected that the mouse presented here will be a valuable tool for
Moreover, the kidney contained a significant proportion of EGFP-
raising fundamental questions about normal and malignant blood
expressing cells lacking both blood and endothelial markers,
cell development.
directly suggesting that these cells were organ-specific (Figure 4).
This finding is in line with a recent report showing the expressionof SCL in the kidney.58 To determine if adult brain tissues were
targeted by the SCL-tTA-2S knock-in mouse, -galactosidaseinduction of neuronal tissues also was determined in SCL-tTA-2S/
We thank H. Bujard for the LC-1 reporter mouse line and the
EGFP-lacZ mice. No difference between induced and noninduced
tTA-2S transactivator cDNA. In addition, we are grateful to J.
brain tissues was seen in these mice, demonstrating that SCL
Mann, who gave us the W9.5 ES cell line. We also would like to
regulatory elements did not direct transgene induction to the brain.
thank the animal technicians of the Mainz animal house for
Taken together, histologic and flow cytometric analyses suggested
excellent assistance and mouse care, and the Interdisciplinary
that the observed induction of transgenes closely mirrored the
Center for Clinical Research (IZKF) Core Unit of Fluorescence
known expression pattern of SCL.
Technology in Leipzig for preparative cell sorting. Finally, we would
Finally, the specificity of tTA-2S–mediated transgene expres-
like to acknowledge Annette Herold for her excellent technical assis-
sion in mature blood cells and c-kit–expressing lineage-negative
tance in the preparation and analysis of brain sections.
1. Begley CG, Aplan PD, Davey MP, et al. Chromo-
7. Hall MA, Slater NJ, Begley CG, et al. Functional
13. Bockamp EO, McLaughlin F, Murrell AM, et al.
somal translocation in a human leukemic stem-
but abnormal adult erythropoiesis in the absence
Lineage-restricted regulation of the murine SCL/
cell line disrupts the T-cell antigen receptor delta-
of the stem cell leukemia gene. Mol Cell Biol.
TAL-1 promoter. Blood. 1995;86:1502-1514.
chain diversity region and results in a previously
14. Bockamp EO, McLaughlin F, Gottgens B, Murrell
unreported fusion transcript. Proc Natl Acad Sci
8. Curtis DJ, Hall MA, Van Stekelenburg LJ, Robb L,
AM, Elefanty AG, Green AR. Distinct mechanisms
U S A. 1989;86:2031-2035.
Jane SM, Begley CG. SCL is required for normal
direct SCL/tal-1 expression in erythroid cells and
2. Chen Q, Cheng JT, Tasi LH, et al. The tal gene
function of short-term repopulating hematopoietic
CD34 positive primitive myeloid cells. J Biol
undergoes chromosome translocation in T cell
stem cells. Blood. 2004;103:3342-3348.
leukemia and potentially encodes a helix-
9. Mikkola HK, Klintman J, Yang H, et al. Haematopoi-
loop-helix protein. EMBO J. 1990;9:415-424.
15. Bockamp EO, Fordham JL, Gottgens B, Murrell
etic stem cells retain long-term repopulating activity
AM, Sanchez MJ, Green AR. Transcriptional
3. Finger LR, Kagan J, Christopher G, et al. Involve-
and multipotency in the absence of stem-cell leukae-
regulation of the stem cell leukemia gene by PU.1
ment of the TCL5 gene on human chromosome 1
mia SCL/tal-1 gene. Nature. 2003;421:547-551.
and Elf-1. J Biol Chem. 1998;273:29032-29042.
in T-cell leukemia and melanoma. Proc Natl Acad
10. Aplan PD, Begley CG, Bertness V, et al. The SCL
Sci U S A. 1989;86:5039-5043.
gene is formed from a transcriptionally complex
16. Bernard O, Azogui O, Lecointe N, et al. A third
4. Begley CG, Green AR. The SCL gene: from case
locus. Mol Cell Biol. 1990;10:6426-6435.
tal-1 promoter is specifically used in human T cell
report to critical hematopoietic regulator. Blood.
11. Begley CG, Robb L, Rockman S, et al. Structure
leukemias. J Exp Med. 1992;176:919-925.
of the gene encoding the murine SCL protein.
17. Gottgens B, McLaughlin F, Bockamp EO, et al. Tran-
5. Lecuyer E, Hoang T. SCL: from the origin of he-
scription of the SCL gene in erythroid and CD34
matopoiesis to stem cells and leukemia. Exp He-
12. Lecointe N, Bernard O, Naert K, et al. GATA-and
positive primitive myeloid cells is controlled by a
SP1-binding sites are required for the full activity
complex network of lineage-restricted chromatin-
6. Shivdasani RA, Orkin SH. The transcriptional control
of the tissue-specific promoter of the tal-1 gene.
dependent and chromatin-independent regulatory
of hematopoiesis. Blood. 1996;87:4025-4039.
elements. Oncogene. 1997;15:2419-2428.
BLOOD, 1 SEPTEMBER 2006
INDUCIBLE EXPRESSION FROM THE SCL LOCUS
䡠 VOLUME 108, NUMBER 5
18. Gottgens B, Nastos A, Kinston S, et al. Establish-
Winkler TH. Induction of pre-B cell proliferation
the small intestinal and colonic epithelium. J Biol
ing the transcriptional programme for blood: the
after de novo synthesis of the pre-B cell receptor.
SCL stem cell enhancer is regulated by a multi-
Proc Natl Acad Sci U S A. 2001;98:1745-1750.
53. Kistner A, Gossen M, Zimmermann F, et al. Doxy-
protein complex containing Ets and GATA factors.
36. Radomska HS, Gonzalez DA, Okuno Y, et al. Trans-
cycline-mediated quantitative and tissue-specific
EMBO J. 2002;21:3039-3050.
genic targeting with regulatory elements of the hu-
control of gene expression in transgenic mice.
19. Delabesse E, Ogilvy S, Chapman MA, Piltz SG,
man CD34 gene. Blood. 2002;100:4410-4419.
Proc Natl Acad Sci U S A. 1996;93:10933-10938.
Gottgens B, Green AR. Transcriptional regulation
37. Huettner CS, Koschmieder S, Iwasaki H, et al.
54. Klug A, Jordan T. The cobblestone-area-forming
of the SCL locus: identification of an enhancer
Inducible expression of BCR/ABL using human
cell assay. Methods in molecular medicine: he-
that targets the primitive erythroid lineage in vivo.
CD34 regulatory elements results in a mega-
matopoietic stem cell protocols. Totowa, NJ: Hu-
Mol Cell Biol. 2005;25:5215-5225.
karyocytic myeloproliferative syndrome. Blood.
mana Press; 2002:63.
20. Gottgens B, Broccardo C, Sanchez MJ, et al. The
55. Ploemacher RE, van der Sluijs JP, van Beurden
scl ⫹18/19 stem cell enhancer is not required for
CA, Baert MR, Chan PL. Use of limiting-dilution
38. Manfra DJ, Chen SC, Jensen KK, Fine JS,
hematopoiesis: identification of a 5⬘ bifunctional
type long-term marrow cultures in frequency anal-
Wiekowski MT, Lira SA. Conditional expression of
hematopoietic-endothelial enhancer bound by
ysis of marrow-repopulating and spleen colony-
murine Flt3 ligand leads to expansion of multiple
Fli-1 and Elf-1. Mol Cell Biol. 2004;24:1870-1883.
forming hematopoietic stem cells in the mouse.
dendritic cell subsets in peripheral blood and tis-
21. Sanchez M, Gottgens B, Sinclair AM, et al. An SCL
sues of transgenic mice. J Immunol. 2003;170:
3⬘ enhancer targets developing endothelium together
56. Courtes C, Lecointe N, Le Cam L, Baudoin F,
with embryonic and adult haematopoietic progeni-
Sardet C, Mathieu-Mahul D. Erythroid-specific
39. Obst R, van Santen HM, Mathis D, Benoist C.
tors. Development. 1999;126:3891-3904.
inhibition of the tal-1 intragenic promoter is due to
Antigen persistence is required throughout the
binding of a repressor to a novel silencer. J Biol
22. Sinclair AM, Gottgens B, Barton LM, et al. Distinct
expansion phase of a CD4(⫹) T cell response.
5⬘ SCL enhancers direct transcription to develop-
J Exp Med. 2005;201:1555-1565.
ing brain, spinal cord, and endothelium: neural
57. Gottgens B, Gilbert JG, Barton LM, et al. Long-
40. Nguyen HG, Yu G, Makitalo M, et al. Conditional
expression is mediated by GATA factor binding
range comparison of human and mouse SCL loci:
overexpression of transgenes in megakaryocytes
sites. Dev Biol. 1999;209:128-142.
localized regions of sensitivity to restriction endo-
and platelets in vivo. Blood. 2005;106:1559-1564.
nucleases correspond precisely with peaks of
23. Sanchez MJ, Bockamp EO, Miller J, Gambardella
conserved noncoding sequences. Genome Res.
L, Green AR. Selective rescue of early haemato-
41. Koschmieder S, Gottgens B, Zhang P, et al. In-
poietic progenitors in Scl(⫺/⫺) mice by express-
ducible chronic phase of myeloid leukemia with
58. Dekel B, Hochman E, Sanchez MJ, et al. Kidney,
ing Scl under the control of a stem cell enhancer.
expansion of hematopoietic stem cells in a trans-
blood, and endothelium: developmental expres-
genic model of BCR-ABL leukemogenesis. Blood.
2005;105:324-334.
sion of stem cell leukemia during nephrogenesis.
24. Silberstein L, Sanchez MJ, Socolovsky M, et al.
Kidney Int. 2004;65:1162-1169.
Transgenic analysis of the stem cell leukemia
42. Cozzio A, Passegue E, Ayton PM, Karsunky H,
59. Chetty R, Dada MA, Boshoff CH, et al. TAL-1 pro-
⫹19 stem cell enhancer in adult and embryonic
Cleary ML, Weissman IL. Similar MLL-associated
tein expression in vascular lesions. J Pathol.
hematopoietic and endothelial cells. Stem Cell.
leukemias arising from self-renewing stem cells
and short-lived myeloid progenitors. Genes Dev.
2003;17:3029-3035.
60. Kallianpur AR, Jordan JE, Brandt SJ. The SCL/
25. Elefanty AG, Begley CG, Metcalf D, Barnett L,
TAL-1 gene is expressed in progenitors of both
Kontgen F, Robb L. Characterization of hemato-
43. Jamieson CH, Ailles LE, Dylla SJ, et al. Granulo-
the hematopoietic and vascular systems during
poietic progenitor cells that express the transcrip-
cyte-macrophage progenitors as candidate leuke-
embryogenesis. Blood. 1994;83:1200-1208.
tion factor SCL, using a lacZ "knock-in" strategy.
mic stem cells in blast-crisis CML. N Engl J Med.
61. Pulford K, Lecointe N, Leroy-Viard K, Jones M,
Proc Natl Acad Sci U S A. 1998;95:11897-11902.
Mathieu-Mahul D, Mason DY. Expression of
26. Elefanty AG, Begley CG, Hartley L, Papaevange-
44. Passegue E, Jamieson CH, Ailles LE, Weissman
TAL-1 proteins in human tissues. Blood. 1995;85:
liou B, Robb L. SCL expression in the mouse em-
IL. Normal and leukemic hematopoiesis: are leu-
bryo detected with a targeted lacZ reporter gene
kemias a stem cell disorder or a reacquisition of
62. Drake CJ, Brandt SJ, Trusk TC, Little CD. TAL1/
demonstrates its localization to hematopoietic,
stem cell characteristics? Proc Natl Acad Sci
SCL is expressed in endothelial progenitor cells/
vascular, and neural tissues. Blood. 1999;94:
U S A. 2003;100(suppl 1):11842-11849.
angioblasts and defines a dorsal-to-ventral gradi-
45. Huntly BJ, Gilliland DG. Leukaemia stem cells
ent of vasculogenesis. Dev Biol. 1997;192:17-30.
27. Gossen M, Bujard H. Tight control of gene ex-
and the evolution of cancer-stem-cell research.
63. Drake CJ, Fleming PA. Vasculogenesis in the day 6.5
pression in mammalian cells by tetracycline-re-
Nat Rev Cancer. 2005;5:311-321.
to 9.5 mouse embryo. Blood. 2000;95:1671-1679.
sponsive promoters. Proc Natl Acad Sci U S A.
46. Urlinger S, Baron U, Thellmann M, Hasan MT,
64. Neben S, Anklesaria P, Greenberger J, Mauch P.
Bujard H, Hillen W. Exploring the sequence
Quantitation of murine hematopoietic stem cells
28. Gossen M, Bujard H. Studying gene function in
space for tetracycline-dependent transcriptional
in vitro by limiting dilution analysis of cobblestone
eukaryotes by conditional gene inactivation. Annu
activators: novel mutations yield expanded range
area formation on a clonal stromal cell line. Exp
Rev Genet. 2002;36:153-173.
and sensitivity. Proc Natl Acad Sci U S A. 2000;
29. Bockamp E, Maringer M, Spangenberg C, et al.
65. Brady G, Billia F, Knox J, et al. Analysis of gene
Of mice and models: improved animal models for
47. Szabo P, Mann JR. Expression and methylation
expression in a complex differentiation hierarchy
biomedical research. Physiol Genomics. 2002;11:
of imprinted genes during in vitro differentiation of
by global amplification of cDNA from single cells.
mouse parthenogenetic and androgenetic embry-
Curr Biol. 1995;5:909-922.
30. Eger K, Hermes M, Uhlemann K, et al. 4-Epidoxycy-
onic stem cell lines. Development. 1994;120:
66. Zinovyeva MV, Zijlmans JM, Fibbe WE, Visser
cline: an alternative to doxycycline to control gene
JW, Belyavsky AV. Analysis of gene expression in
expression in conditional mouse models. Biochem
48. Hogan B, Beddington RS, Constantini F, Lacey E.
subpopulations of murine hematopoietic stem
Biophys Res Commun. 2004;323:979-986.
Manipulating the mouse embryo—a laboratory
and progenitor cells. Exp Hematol. 2000;28:318-334.
31. Lee P, Morley G, Huang Q, et al. Conditional lin-
manual. 2nd ed. Cold Spring Harbor, NY: Cold
eage ablation to model human diseases. Proc
Spring Harbor Press; 1994.
67. Zhou Y, Yamamoto M, Engel JD. GATA2 is re-
Natl Acad Sci U S A. 1998;95:11371-11376.
quired for the generation of V2 interneurons.
49. Vidal F, Sage J, Cuzin F, Rassoulzadegan M. Cre
32. Huettner CS, Zhang P, Van Etten RA, Tenen DG.
expression in primary spermatocytes: a tool for
68. Smith E, Hargrave M, Yamada T, Begley CG,
Reversibility of acute B-cell leukaemia induced by
genetic engineering of the germ line. Mol Reprod
Little MH. Coexpression of SCL and GATA3 in the
BCR-ABL1. Nat Genet. 2000;24:57-60.
V2 interneurons of the developing mouse spinal
33. Rhoades KL, Hetherington CJ, Harakawa N, et
50. Schonig K, Schwenk F, Rajewsky K, Bujard H. Strin-
cord. Dev Dyn. 2002;224:231-237.
al. Analysis of the role of AML1-ETO in leukemo-
gent doxycycline dependent control of CRE recombi-
69. Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH.
genesis, using an inducible transgenic mouse
nase in vivo. Nucleic Acids Res. 2002;30:e134.
Specification of astrocytes by bHLH protein SCL
model. Blood. 2000;96:2108-2115.
51. Krestel HE, Mayford M, Seeburg PH, Sprengel R.
in a restricted region of the neural tube. Nature.
34. Hess J, Nielsen PJ, Fischer KD, Bujard H, Wirth
A GFP-equipped bidirectional expression module
T. The B lymphocyte-specific coactivator BOB.1/
well suited for monitoring tetracycline-regulated
70. Gray PA, Fu H, Luo P, et al Mouse brain organiza-
OBF.1 is required at multiple stages of B-cell de-
gene expression in mouse. Nucleic Acids Res.
tion revealed through direct genome-scale TF
velopment. Mol Cell Biol. 2001;21:1531-1539.
expression analysis. Science. 2004;306:
35. Hess J, Werner A, Wirth T, Melchers F, Jack HM,
52. Saam JR, Gordon JI. Inducible gene knockouts in
2006 108: 1533-1541
originally published
doi:10.1182/blood-2005-12-012104online May 4, 2006
locus directs
Tetracycline-controlled transgenic targeting from the
conditional expression to erythrocytes, megakaryocytes, granulocytes,
and c-kit-expressing lineage-negative hematopoietic cells
Ernesto Bockamp, Cecilia Antunes, Marko Maringer, Rosario Heck, Katrin Presser, Sven Beilke,
Svetlana Ohngemach, Rudiger Alt, Michael Cross, Rolf Sprengel, Udo Hartwig, Bernd Kaina, Steffen
Schmitt and Leonid Eshkind
Updated information and services can be found at:
Articles on similar topics can be found in the following Blood collections
Information about reproducing this article in parts or in its entirety may be found online at:
Information about ordering reprints may be found online at:
Information about subscriptions and ASH membership may be found online at:
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Societyof Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Source: http://www.bloodjournal.org/content/108/5/1533.full.pdf
Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance of job duties. Post exposure prophylaxis (PEP) refers to comprehensive medical management to minimise the risk of infection among Health Care Personnel (HCP) following potential exposure to blood-borne pathogens (HIV, HBV, HCV). This includes counselling, risk assessment, relevant laboratory investigations based on informed consent of the source and exposed person, first aid and depending on the risk assessment, the provision of short term (four weeks) of antiretroviral drugs, with follow up and support. Who is at risk? All Health Care Personnel, including emergency care providers, laboratory personnel, autopsy personnel, hospital employees, interns and medical students, nursing staff and students, physicians, surgeons, dentists, labour and delivery room personnel, laboratory technicians, health facility sanitary staff and clinical waste handlers and health care professionals at all levels. Also at risk are public safety workers, including law enforcement personnel, prison staff, fire-fighters, workers in needle exchange programme and workers in HIV programmes. What is the risk? Health Care Personnel are at risk of blood-borne infection transmission through exposure of a percutaneous injury (e.g. needle-stick or cut with a sharp instrument), contact with the mucous membranes of the eye or mouth of an infected person, contact with non-intact skin (particularly when the exposed skin is chapped, abraded, or afflicted with dermatitis or contact with blood or other potentially infectious body fluids. potentially infectious body fluids Any direct contact (i.e., contact without barrier protection) with concentrated virus in a research laboratory or production facility requires clinical evaluation. Transmission of HIV infection from human bites is rarely reported. The average risk of acquiring HIV infection from different types of occupational exposure is low compared to risk of infection with HBV or HCV. In terms of occupational exposure the important routes are needle stick exposure (0.3% risk for HIV, 9–30% for HBV and 1–10% for HCV) and mucous membrane exposure (0.09% for HIV).e What is infectious and what is not? Exposure to blood, semen, vaginal secretions, cerebrospinal fluid, synovial, pleural, peritoneal, pericardial fluid, amniotic fluid and other body fluids contaminated with visible blood can lead to infection. Exposure to tears, sweat, saliva, urine and faeces is non-infectious unless these secretions contain visible blood. Step 1: First aid in management of exposure For skin — if the skin is broken after a needle-stick or sharp instrument:
Εργαστήριο Σπουδών Φύλου και Ισότητας Λ. Συγγρού 134, 1ος όροφος, 17671 Αθήνα, τηλ. 210 9210177-8, fax 210 9210178 http://www.genderpanteion.gr, e-mail: [email protected] ΕΙΣΗΓΗΣΗ 22 Μαΐου 2007 Elizabeth Dermody Leonard, καθηγήτρια κοινωνιολογίας στο Πανεπιστήµιο Vanguard της Νότιας Καλιφόρνιας, Η.Π.Α












