Cadsur.sr
Epidemiological Alert
Neurological syndrome, congenital malformations, and
Zika virus infection. Implications for public health
in the Americas
1 December 2015
Given the increase of congenital anomalies, Guil ain-Barré syndrome, and other neurological and autoimmune syndromes in areas where Zika virus is circulating and their possible relation to the virus, the Pan American Health Organization / World Health Organization (PAHO/WHO) recommends its Member States establish and maintain the capacity to detect and confirm Zika virus cases, prepare healthcare facilities for the possible increase in demand at al healthcare levels and specialized care for neurological syndromes, and to strengthen antenatal care. In addition, Member States should continue efforts to reduce the presence of mosquito vectors through an effective vector control strategy and public communication.
Situation summary
Autochthonous transmission of Zika virus
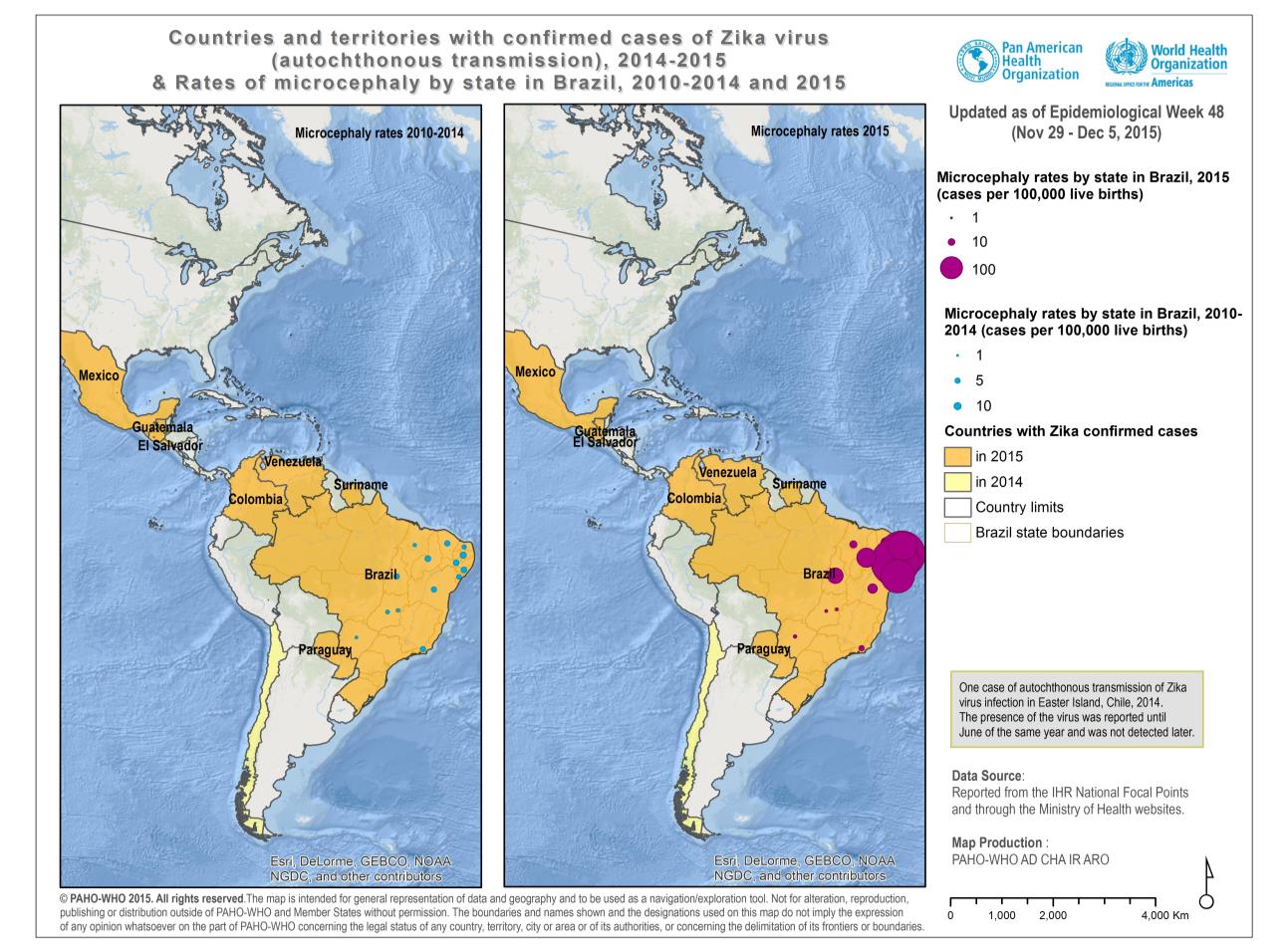
As of 1 December 2015, nine Member States in the Americas have confirmed autochthonous
circulation of Zika virus: Brazil, Chile (on Easter Island), Colombia, El Salvador, Guatemala, Mexico,
Paraguay, Suriname, and Venezuela.1
Autochthonous circulation of Zika virus (ZIKV) in the Americas was first confirmed in February of 2014
on Easter Island, Chile, and cases were reported there up to June of 2014.
In May 2015, the first autochthonous cases of Zika virus in Brazil were confirmed. As of 1 December
2015, a total of 18 states in Brazil have confirmed autochthonous circulation: North region
(Amazonas, Pará, Rondônia, Roraima, and Tocantins), Northeast region (Alagoas, Bahía, Ceará,
Maranhão, Paraíba, Pernambuco, Piauí, and Rio Grande do Norte), Southeast region (Espírito
Santo, Rio de Janeiro, and São Paulo), Central-West region (Mato Grosso), and South region
(Paraná)(1).
In October 2015, the Colombia health authorities notified the detection of the first autochthonous
case of Zika virus infection in the state of Bolívar.2 To date, 26 out of 36 territorial entities in Colombia
have reported autochthonous circulation of the virus (2).
Additional y, in November 2015, El Salvador, Guatemala, Mexico, Paraguay, Suriname, and
Venezuela each confirmed autochthonous circulation of Zika virus.
1 Notification received through the International Health Regulations (IHR) National Focal Points (NFPs) of Brazil, Chile, Colombia, El Salvador, Guatemala, Mexico, Suriname, and Venezuela. 2 Complete information is available at
Increase in congenital anomalies
In October 2015, the Brazil International Health Regulations (IHR) National Focal Point (NFP) notified
the detection of an unusual increase in microcephaly3 cases in public and private healthcare
facilities in Pernambuco state, Northeast Brazil. Seefrom 17 November 2015.4
As of 30 November 2015, 1,248 cases (99.7/100,000 live births) of microcephaly, including 7 deaths,
have been reported in 14 states of Brazil, which are under investigation (3). In 2000, the prevalence
of microcephaly in newborns in Brazil was 5.5 cases/ 100,000 live births and in 2010 it was 5.7 cases /
100,000 live births. This data demonstrates a twentyfold increase in comparison to the rate
observed in previous years. The data was obtained from the Live Births Information System (Sinac,
per its acronym in Portuguese) which captures epidemiological data related to pregnancy, births
and congenital malformation, in addition to the socio-demography of the mothers.5 The
distribution of microcephaly cases is shown in Figure 1.
Figure 1. Countries and territories with confirmed cases of Zika virus (autochthonous transmission), 2014- 2015
and rates of microcephaly by state in Brazil, 2010-2014 and 2015
3 Microcephaly is a neurological disorder in which the occipitofrontal circumference is smal er than that of other children of the same age, race, and sex. 4 PAHO/WHO 17 November 2015, Epidemiological Alert available at: 5 Sinac is a universal system, which capturesinformation on births in the entire national territory of Brazil. Available at:
On 17 November 2015, the Flavivirus Laboratory at the Osvaldo Cruz Institute indicated that Zika
virus genome was detected through RT-PCR in amniotic fluid samples of two pregnant women
from Paraíba, whose fetuses have been diagnosed with microcephaly by ultrasound exams (4).
On 24 November 2015, the French Polynesia health authorities reported an unusual increase of
central nervous system malformation in fetuses and newborns registered during 2014-2015,
coinciding with the Zika virus outbreaks on the islands. Out of 17 malformations registered, 12 were
fetal cerebral malformations or polymalformative syndromes, including brain lesions, and five
infants were reported as having brainstem dysfunction and absence of swal owing. None of the
pregnant women described clinical signs of Zika virus, but the four women that were tested were
found positive by IgG serology assays for flavivirus, suggesting a possible asymptomatic Zika virus
infection. Further serological investigations are ongoing. Based on the temporal correlation of these
cases with the Zika epidemic, the French Polynesia health authorities hypothesize that Zika virus
infection may be associated with these abnormalities if mothers are infected with the virus during
the first or second trimester of pregnancy (5).
On 28 November 2015, the Brazil Ministry of Health established the relationship between the
increase in occurrence of microcephaly and Zika virus infection through the detection of Zika virus
genome in the blood and tissue samples of a baby from the state of Pará. The newborn presented
microcephaly and other congenital anomalies and died within five minutes of being born. The
confirmation of the presence of the viral genome was provided by the Evandro Chagas Institute,
national reference laboratory for arboviruses in Belém, Pará.
According to the preliminary analysis of the investigation conducted by the Brazil health
authorities, the greatest risk of microcephaly or congenital anomalies in newborns is associated
with Zika virus infection in the first trimester of pregnancy.
Zika virus related deaths
As of 28 November 2015, the Brazil Ministry of Health has notified three deaths associated with Zika
virus infection. The fatal cases are two adults and one newborn.
The first fatal case is a male adult with no neurological disorders, with history of lupus
erythematosus, chronic use of corticosteroid drugs, rheumatoid arthritis, and alcoholism. He was
admitted as a suspected dengue case, however, dengue was discarded and the final laboratory
diagnosis by RTp-PCR technique was Zika virus infection. Zika virus genome was detected by RT-
PCR in the blood and organ samples (brain, liver, spleen, kidney, lung, and heart). Additional y, Zika
virus was identified through partial sequencing of the virus.
The second fatal case is a 16 year old female from the Benevides municipality in the state of Pará.
She had no neurological disorder, and was admitted to the hospital as a suspected dengue case.
The onset of her symptoms (headache, nausea, and petechiae) was on 29 September 2015 and
she died in late October. Zika virus infection was confirmed by RTp-PCR.
The third fatal case is the newborn described above.
Increase in neurological syndromes
In July 2015, the Brazil IHR NFP reported the detection of patients with neurological syndromes with
recent history of Zika virus infection, especial y in the state of Bahía. Up to 13 July, 76 neurological
syndrome patients had been identified, of which 55% (42/76) were confirmed as Guil ain-Barré syndrome (GBS), 5 out of the 76 were confirmed for other neurological syndromes, 4 out of the 76 were discarded, and 25 of the 76 remain under investigation. Among patients with GBS and based on clinical history, 62% (26/42) had symptoms consistent with Zika virus (6). In addition, on 25 November of 2015, the Aggeu Magalhães Research Center of the Oswaldo Cruz
Foundation Institute reported that of the 224 suspected dengue patients whose samples were analyzed for Zika virus infection, ten were confirmed positive. Seven of the ten samples confirmed with Zika virus infection correspond to patients with neurological syndrome (7). On the other hand, during the Zika virus outbreak in French Polynesia in which 8,750 suspected cases were detected6, 74 patients presented neurological syndromes or autoimmune syndromes fol owing an il ness with symptoms compatible with Zika virus infection in previous days. Of these, 42 were confirmed as Guil ain-Barré syndrome, 37 of which had presented with a previous viral syndrome (5, 8). Although neither event establishes a causal relation with Zika virus, the hypothesis cannot be discarded.
Recommendations for public health authorities
Considering the expansion of the Zika virus transmission in the Region of the Americas, and taking
into account the recent findings related to Zika virus, the Pan American Health Organization/ World
Health Organization (PAHO/WHO) has updated the recommendations on surveil ance of Zika virus.
This includes the surveil ance for neurological syndromes and congenital malformations, and also
provides recommendations for the fol ow up of pregnant women and newborns in areas where
Zika virus is circulating.
PAHO/WHO reinforces earlier recommendations issued on the same vector-borne diseases, and
urge Member States where the Aedes mosquito is circulating to continue their efforts to implement
an effective vector control strategy with emphasis on communication with communities to reduce
the density of the vector and protect the population.
The fol owing recommendations expand on the surveil ance recommendations, including
surveil ance for neurological syndromes and congenital malformations (updated), clinical
management (updated) including fol ow up for pregnant women and newborns, and prevention
control measures. These recommendations wil be reviewed and updated as new evidence of the
disease becomes available.
Surveil ance
Zika infection surveil ance should be set up based on the existing surveil ance systems for dengue and chikungunya, while taking into account the differences in clinical presentation. As appropriate per a country's epidemiological situation, surveil ance should be focused to:
(i) determine if the Zika virus has been introduced to an area,
(i ) monitor the spread of Zika virus infection once autochthonous transmission is confirmed, and
6 32,000 people are estimated to have been infected.
(i i) monitor for neurological and autoimmune complications.
In countries without autochthonous transmission of Zika virus infection, they are recommended to:
Strengthen event-based surveil ance to detect the first cases. Based on the experiences of
Brazil and Colombia, health authorities must be on alert for the emergence of clusters of rash febrile syndrome of unknown etiology (in which dengue, chikungunya, measles, rubella, and parvovirus B19 have been ruled out), and laboratory tests for Zika virus detection.
Potential cross reactivity with dengue in serological samples must be considered, especial y if there
has been prior dengue infection. Early detection wil al ow the identification of circulating viral
strains, the proper characterization of the outbreak and implementation of a proportionate
response.
In countries with autochthonous transmission of Zika virus infection, they are recommended to:
Monitor the trend and geographical spread of the virus to detect the introduction into new
areas of a country,
Monitor potential neurological and autoimmune complications, as wel as the potential
impact on public health,
Identify risk factors associated with Zika virus infection, and
Identify circulating Zika virus lineages, when the capacity exists.
Once the introduction of the virus is documented, ongoing surveil ance should be maintained in order to monitor epidemiological and entomological changes that may affect the transmission of Zika virus. Any changes detected by the surveil ance system should be promptly communicated to the national authorities in order to ensure timely decisions for actions as warranted.
Below is the provisional7 case definition for Zika virus infection.
Suspected case: Patient with rash or elevation of body temperature (> 37.2 °C) with one or more of
the fol owing symptoms (not explained by other medical conditions):
Arthralgia or myalgia
Non-purulent conjuntivitis or conjunctival hyperemia
Headache or malaise
Confirmed case: A suspected case with laboratory positive result for the specific detection of Zika
virus (see algorithm for laboratory diagnosis).8
Surveillance of neurological and autoimmune complications
7 This case definition is based on the definition used during the outbreak in French Polynesia, 2013-2014 (Direction de la Santé BdVs,
Polynesie Francaise. Surveil ance de la dengue et du zika en Polynésie Française, 2013-2014. Available in: The case definition has been adapted according to the clinical description available after the introduction of the Zika virus in the Region of the Americas and may be subject to further modifications as new knowledge and information on the disease and the etiological agent is available. 8 The algorithm for laboratory diagnosis is available at:
Given that neurological and autoimmune complications have been reported during some Zika
virus and dengue outbreaks, Member States are recommended to, particularly in situations of
possible Zika virus circulation, establish or strengthen surveil ance of neurological syndromes for al
age groups.
This surveil ance of neurological syndromes aimed to increase the awareness of health care
professional to provide adequate clinical management to those cases presenting neurological
complications and contribute to establishing the possible relationship between neurological
complications, Zika virus infection, and previous infection with other agents.
Surveillance of congenital anomalies
PAHO/WHO recommends analyzing live birth databases, specifical y in relation to malformations/
neurological disorders, in order to detect any unusual increase in occurrence.
Surveil ance of the neurological anomalies must be integrated into the monitoring of congenital
malformations. This surveil ance should be continuous to better determine the magnitude and
burden due to the disorders.
Event-based surveil ance is a useful tool in this situation. For this reason, healthcare professionals
involved in antenatal care, as well as child care, should be encouraged to notify all unusual
events.
Microcephaly is defined as a head circumference of 2 standard deviations (SD) below the mean
for age and sex or about less than the second percentile. There are no absolute values to define
microcephaly given that it varies by race, sex, and gestational age. For this reason, the WHO child
growth standards tables on head circumference-for-age, with percentiles, and expanded tables
for
Any increase of microcephaly or other neurological congenital disorder must be assessed, investigated, and reported to competent public health authorities.
Guidelines for international reporting
Given the recent introduction of Zika virus in the Americas and to contribute to integrated arbovirus surveil ance, national public health authorities are encouraged to inform PAHO/WHO through the established IHR channels, of any laboratory-confirmed cases of Zika virus infection that are registered in the countries and territories of the Region of the Americas. Additional y, in order to contribute to the knowledge of the progression of this virus, PAHO/WHO urges Member States to notify any increase of neurological and autoimmune syndromes (in adults and children), or congenital malformations in newborns that cannot be explained by other known causes.
Laboratory detection of Zika virus
During the first 5 days after the onset of clinical picture, (acute phase, viremic period) viral ribonucleic acid (RNA) can be detected in serum by molecular techniques (conventional or real-
time RT-PCR). The reverse transcription-polymerase chain reaction (RT-PCR) for dengue as the main differential diagnosis should be negative. In addition, a generic assay against flavivirus fol owed by genetic sequencing can be used to establish the specific etiology.
For a case clinical y suggestive of infection and where dengue has been discarded, further tests for other flaviviruses, including Zika virus, should be performed.
The serological tests (ELISA or immunofluorescence) to detect specific IgM or IgG against Zika virus can be positive after 5 to 6 days fol owing the onset of symptoms. The tests must detect an increased antibody titer in paired samples, with an interval of about two weeks. However, confirmation of positive results with plaque reduction neutralization test (PRNT) showing at least a four-fold increase in the titer of neutralizing antibodies to Zika virus is recommended. There can be cross-reactivity with other flaviviruses, especial y dengue and yellow fever or, less frequently, with West Nile virus. Accordingly, a four-fold rise or more of the neutralizing antibody titer against dengue in a patient infected with Zika virus, particularly if the patient previously had dengue, could be detected. Given this cross-reactivity between flavivirus serology, results should be interpreted with caution.
Case management
There is no specific antiviral treatment for Zika virus. Symptomatic treatment after excluding more
severe conditions such as malaria, dengue, and bacterial infection is recommended.
It is important to differentiate Zika virus infection from dengue due to severe clinical outcomes in
some dengue cases. In addition, cases of co-infection, Zika and dengue, could occur. Compared
with dengue, Zika virus infection has a mild to moderate clinical picture, the onset of fever is more
acute and shorter in duration. To date, severe cases have been observed sporadical y and further
investigation to better understand the risk factors linked to severe presentations is needed.
Because Zika virus outbreaks could cause additional burdens on al levels of the health care
system, it is necessary to develop and implement (or adapt based on what already exists)
protocols and wel established plans for the patient screening and treatment.
Treatment for Zika virus
There is no vaccine or specific treatment for Zika virus infection. For this reason, treatment is
geared toward relieving symptoms.
Symptomatic and supportive treatment includes rest and the use of acetaminophen or
paracetamol to relieve fever. The use of antihistamines to control pruritus usually associated with the maculo-papular rash could be recommended.
Using aspirin is not advised due to the risk of bleeding and developing Reye's syndrome in
children younger than 12 years of age. The use of other nonsteroidal anti-inflammatory drugs is not advised either, since the cause of the clinical symptoms could be dengue or chikungunya, pathologies in which the use of Non-steroidal Anti-inflammatory Drugs (NSAIDs) is contraindicated.
Patients should be advised to drink plenty of fluids to replenish fluid lost from sweating,
vomiting and other insensible losses.
Patient isolation
To prevent infection of other persons, a Zika virus-infected patient should avoid being bitten by
mosquitoes during the first week of il ness (viremic phase). The patient is recommended to stay
under a bed net (treated or without insecticide), or stay in a place with intact window/door
screens. In addition, physicians or health care workers who attend to Zika virus-infected patients
should protect against mosquito bites by using insect repel ent (IR3535 or Icaridin) and wearing
long sleeves and pants.
Pregnant women and newborns
It is important to ensure antenatal care for pregnant women, including basic clinical assessment and paraclinical tests according to established national protocols.
In areas where Zika virus is circulating, it is recommended to emphasize the need for pregnant women to take personal precautions to avoid contact with the vector (see recommendations under personal prevention measures on page 9-10 of this document).
Newborns with congenital malformations must be monitored to determine any neurodevelopmental outcomes. The evaluation of newborns with congenital anomalies requires the participation of multidisciplinary teams including neuropediatricians, geneticist, rehabilitators, psychologists, and social service among others.
Parents or guardians should be properly informed about conditions of the fetus or newborn, especial y if it increases the risk of unfavorable outcomes. They should be made aware of the importance of fol ow-up appointments and fol owing recommendations for health and disease prevention measures.
Vector control and prevention measures
Among prevention and control measures, those aimed at the reduction of vector density are critical, as their effective application can prevent transmission of this virus and other mosquito-borne viruses.
The Integrated Management Strategy for the Prevention and Control of Dengue (IMS –Dengue) provides the basis for preparedness for Zika virus. In the current situation, PAHO/WHO recommends the intensification of comprehensive prevention and control of IMS-dengue. In particular, these recommendations include:
Intersectoral participation and col aboration at all levels of government and health,
education, environment, social development and tourism sectors, among other sectors.
Participation of non-governmental organizations (NGOs) and private organizations, while at
the same time maintaining risk communication and mobilization for the whole community.
Mosquito control is the only measure that can interrupt the transmission of vector borne viruses such as dengue, chikungunya, and Zika. The key elements of the vector control program that should guide the response are listed below.
Integrated Vector Management (IVM)
An effective and operational dengue and chikungunya vector control program provides the basis for adequate preparation against Zika virus, because these viruses are transmitted by the same mosquito, Ae. Aegypti. Therefore, it is recommended to apply and intensify the surveil ance and
vector control measures developed for dengue and chikungunya as part of the Integrated Vector Management (IVM). Given the broad distribution of Ae. aegypti and Ae. albopictus in the Americas, prevention and control measures should be aimed at reducing vector density, and obtaining the acceptance and col aboration of communities to adopt such measures.
Prevention and control measures by national authorities should include:
Strengthening environmental management and eliminating vector breeding sites in
household and common areas (e.g., parks, schools, cemeteries, etc.) to prevent or minimize vector propagation and human contact with the vector-mosquito
Organizing mass sanitation campaigns for the elimination of breeding sites, specifical y in
areas where routine garbage col ection has been interrupted
Implementing breeding site control measures through physical, biological, and chemical
methods while actively involving families and communities
Identifying areas of high risk of transmission (risk stratification), and prioritizing places where
people gather (e.g., schools, transport terminals, hospitals, health centers, etc.). Mosquitoes should be removed with a radius of at least 400 meter around these places,
In areas where autochthonous or imported cases of dengue, chikungunya, and/or Zika virus
are detected, it is suggested to use adulticide treatment (primarily through spraying), to remove infected adult mosquitoes and interrupt transmission. It is important to take into account that this action is exceptional and is only effective when executed by adequately trained personnel fol owing international y accepted technical guidelines. This is to be carried out together with other proposed actions, as described above. Spraying is the primary manner to intensively interrupt transmission and obtain time to consolidate the removal of vector larval breeding sites
Selecting appropriate insecticide (in accordance with PAHO/WHO recommendations),
verifying the product label and formula, and considering the susceptibility of mosquito populations to that insecticide
Maintaining and using spraying equipment in an appropriate manner and maintaining a
stockpile of insecticides
Ensuring intensified monitoring (e.g., quality control) of fieldwork operators both during larval
control and adult insecticide treatment (fumigation)
Integrated (simultaneous or coordinated) actions for vector control (e.g., adulticide and larval control by trained personnel, coupled with sanitation and the promotion of community actions) are essential to achieve the greatest impact on vector populations in the shortest amount of time. It is crucial that the personnel involved in the chemical control of vector control use, without exception, the appropriate personal protective equipment. It is the responsibility of vector control programs to supply this equipment to its staff, to monitor its use, and to have enough stockpile stored under appropriate conditions.
Personal prevention measures
It is important for patients infected with dengue, chikungunya, or Zika virus to minimize contact with the vector. This measure helps prevent the spread of the virus and therefore the disease. Patients,
their household members, and the community must be educated about the risk of transmission to others and the ways to minimize this risk by reducing vector population and human-vector contact.
The fol owing actions are recommended to minimize vector-patient contact:
Patients should rest under mosquito nets (bed nets), treated with or without insecticide.
Patients and other members of the household should wear clothes that cover the
Repel ents containing DEET (N, N-diethyl-3-methylbenzamide), IR3535 (3- [N-butyl-N-acetyl]
aminopropionic acid ethyl-ester), or Icaridin (piperidinecarboxílico acid-1, 2- (2-
hydroxyethyl) - 1-metilpropilester), Icaridin (DEET or IR 3535) can be applied to exposed skin
or clothing; its use must be strictly in accordance with the instructions indicated on the
product label. There is no evidence of any restriction of the use of these repellents by
pregnant women if they are used in accordance with the instructions on the product label
(9, 10, 11).
Use wire-mesh screens on doors and windows.
These personal prevention measures are also effective in preventing transmission of the virus to healthy people.
Travelers
Prior to departure Health authorities should advise travelers heading to any country with documented circulation of dengue, chikungunya, and/or Zika virus to take the necessary measures to protect themselves from mosquito bites, such as using repel ents, wearing appropriate clothing that minimize skin exposure, and using insecticides or nets. It is also important to inform travelers of the symptoms of dengue, chikungunya, and Zika virus, in order for them identify it promptly during their trip. This advice could be relayed through travel medicine services, clinics, and travel health web pages of the Ministry of Health or other relevant government web pages.
While visiting places with dengue, chikungunya and/or Zika virus transmission
Travelers are advised to:
Take appropriate measures to protect themselves from mosquito bites by using repel ents or
wearing appropriate clothes that minimize skin exposure
Avoid mosquito-infested areas
Use nets and/or insecticide
Recognize symptoms of dengue, chikungunya, and Zika virus, and seek professional health
care if any of these symptoms occur
Upon returning from places with dengue, chikungunya, and/or Zika virus transmission
Travelers must be advised to contact their healthcare provider if they suspect they have dengue, chikungunya, or Zika virus upon returning home.
References
1. The public health Emergency Operations Center report on microcephaly. Epidemiological
Week 46 of 2015. Brazil Ministry of Health. Available at:
2. Epidemiological Bul etin. Colombia National Institute of Health. Epidemiological Week 46 of
2015. Available at
3. The public health Emergency Operations Center report on microcephaly. Epidemiological
Week 47 of 2015. Brazil Ministry of Health. Available at:
4. Brazil Ministry of Health. Microcephaly – Ministry of Health releases epidemiological bul etin
[Internet]. Available at:
5. European Centre for Disease Prevention and Control. Rapid risk assessment: Microcephaly in
Brazil potential y linked to the Zika virus epidemic – 24 November 2015. Stockholm: ECDC; 2015. Available at
6. Information provided by the Brazil International Health Regulations (IHR) National Focal Point
(NFP). July 2015.
7. Fiocruz Pernambuco answers questions about Zika virus. Agencia Fiocruz de Noticias. Available
8. Bul etin hebdomadaire international du 5 au 11 mars 2014. N°442. Available at:
9. Dengue. Guidelines for diagnosis, treatment, prevention and control. World Health
Organization, 2009. WHO/HTM/NTD/DEN/2009.1
10. Koren G, Matsui D and Bailey B. DEET-based insect repel ants: safety implications for children
and pregnant and lactating women. Canadian Medical Association Journal 2003;169(3):209-12
11. United States Center for Disease Control and Prevention (CDC). Insect Repel ent Use & Safety.
Available at(accessed 29 November 2015)
Source: http://www.cadsur.sr/EPI%20alert%20ZIKA%201%20dec.pdf
Evaluation of a Newly Developed Lateral FlowImmunoassay for the Diagnosis of Cryptococcosis Mark D. Lindsley,1 Nanthawan Mekha,2 Henry C. Baggett,3 Yupha Surinthong,2 Rinrapas Autthateinchai,2Pongpun Sawatwong,3 Julie R. Harris,1 Benjamin J. Park,1 Tom Chiller,1 S. Arunmozhi Balajee,1 andNatteewan Poonwan2 1Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia; 2National Institute of Health, Department of Medical Sciences,Ministry of Public Health, Nonthaburi, Thailand; and 3International Emerging Infections Program, Thailand Ministry of Public Health–Centers forDisease Control and Prevention Collaboration, Nonthaburi, Thailand
Time to Register for Religious Educa- tion Classes for 2008/09 - R.E. Classes forGrades 1 - 6 will meet on Tuesday, Wednesday, and July 26 - August 3 Thursday afternoons from 3:45 - 4:45 PM. Grades 7& 8 will meet on Thursdays from 6 - 7 PM with 7th Seventeenth Sunday of Ordinary Time and 8th Grade Youth Group to follow from 7- 8:30








