Cheongin.co.kr
1546
Current Pharmaceutical Design, 2009, 15, 1546-1558
The Role of the Gut Microbiota in Energy Metabolism and Metabolic
Disease
Patrice D. Cani* and Nathalie M. Delzenne*
Université catholique de Louvain, Louvain Drug Research Institute, Unit of Pharmacokinetics, Metabolism, Nutrition and Toxicology, Brussels, Belgium
Abstract: Obesity is now classically characterized by a cluster of several metabolic disorders, and by a low grade in-
flammation. The evidence that the gut microbiota composition can be different between healthy and or obese and type 2
diabetic patients has led to the study of this environmental factor as a key link between the pathophysiology of metabolic
diseases and the gut microbiota. Several mechanisms are proposed linking events occurring in the colon and the regulation
of energy metabolism, such as i.e. the energy harvest from the diet, the synthesis of gut peptides involved in energy ho-
meostasis (GLP-1, PYY…), and the regulation of fat storage. Moreover, the development of obesity and metabolic disor-
ders following a high-fat diet may be associated to the innate immune system. Indeed, high-fat diet feeding triggers the
development of obesity, inflammation, insulin resistance, type 2 diabetes and atherosclerosis by mechanisms dependent of
the LPS and/or the fatty acids activation of the CD14/TLR4 receptor complex. Importantly, fat feeding is also associated
with the development of metabolic endotoxemia in human subjects and participates in the low-grade inflammation, a
mechanism associated with the development of atherogenic markers. Finally, data obtained in experimental models and
human subjects are in favour of the fact that changing the gut microbiota (with prebiotics and/or probiotics) may partici-
pate in the control of the development of metabolic diseases associated with obesity. Thus, it would be useful to find spe-
cific strategies for modifying gut microbiota to impact on the occurrence of metabolic diseases.
Key Words: high fat diet, metabolic endotoxemia- obesity, prebiotics, gut peptides, bifidobacteria, gut bacteria, cardiovascular
diseases.
solely to changes in the human genome, nutritional habits, or
Obesity is now classically characterized by a cluster of
the reduction of physical activity in our daily lives [4]. Over
several metabolic disorders. Most of them are related to the
the past five years, studies have highlighted some key as-
glucose homeostasis and to the development of cardiovascu-
pects of the mammalian host-gut microbial relationship. Gut
lar diseases (Fig.
1) [1,2]. During the past decade, it became
microbiota could now be considered as a "microbial organ"
clear that a low-grade inflammation contributes to the devel-
placed within a host organism. In addition to the obvious
opment of the pathologies associated with obesity [3]. Une-
role of the intestine in the digestion and absorption of nutri-
quivocal experimental, clinical or epidemiological evidence
ents, the human gastrointestinal tract contains a diverse col-
have causally linked inflammation, or the inflammatory sig-
lection of microorganisms, residing mostly in the colon. So
nalling responses to the development of theses metabolic
far, the human gut microbiota has not been fully described,
disorders associated with obesity. The analysis of the nutri-
but it is clear that the human gut is home for a complex con-
tional disorders associated with obesity reveals that the ad-
sortium of around 1013 to 1014 bacterial cells. As a whole, the
verse health consequences of weight gain and obesity are
microorganisms that live inside humans are estimated to out-
especially prominent following prolonged periods of positive
number human cells by a factor of ten. The microbiome rep-
energy balance and is mostly associated with a high-fat diet
resents overall more than 100 times the human genome [5,6].
ingestion in our Western countries. However, it is more dif-
Therefore, the gut microbiota and its microbiome provide us
ficult to understand the mechanisms by which high-fat diet
with genetic and metabolic attributes, sparing us from the
feeding promotes low grade inflammation (Fig.
2). What is
need to evolve solely by our own. Accumulating evidence
the molecular link between high-fat or high-energy feeding
indicates that the gut microbiota is instrumental in the con-
and the development of this particular context? Why and by
trol of host energy metabolism. These findings open the way
which mechanisms such metabolic diseases are so com-
to better understand how the gut microbiota and the factors
monly linked to inflammatory processes? Those questions
that influence its distribution and constituent microorgan-
will constitute the core of this review paper (Fig.
2).
isms, are controlled and how they interact with the host or-
New evidence supports the idea that the increased preva-
lence of obesity and type 2 diabetes cannot be attributed
The present review will discuss the recent data in order to
propose how the gut microbiota may play an even more im-
*Address correspondence to these authors at the Unit PMNT-7369, Av. E. Mounier, 73/69, B-1200 Brussels, Belgium; Tel: +32 2 764 73 97; Fax: +32
portant role in the development of metabolic disorders asso-
2 764 73 59; E-mail:
[email protected] or
ciated with obesity.
1381-6128/09 $55.00+.00
2009 Bentham Science Publishers Ltd.
The Role of the Gut Microbiota in Energy Metabolism
Current Pharmaceutical Design, 2009, Vol. 15, No. 13 1547
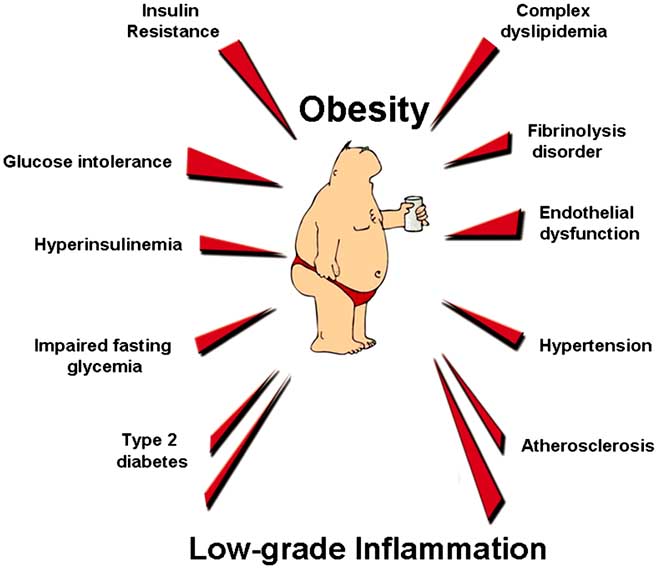
Fig. (1). Obesity and associated metabolic disorders.
Obesity is characterised by a cluster of metabolic disorders, related to the glucose homeostasis and to the development of cardiovascular diseases. Recently, the development of such pathologies has been associated with a low grade inflammatory tone.
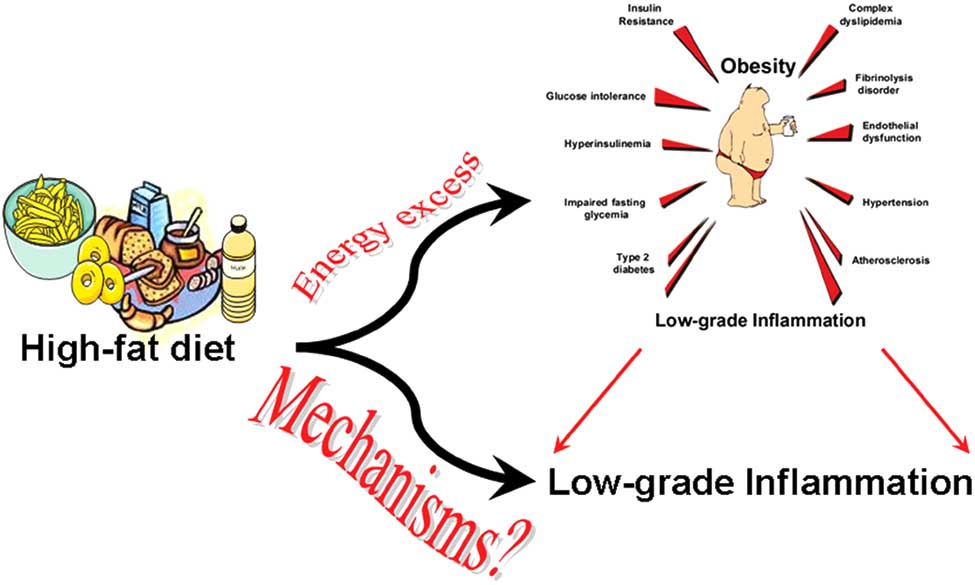
Fig. (2). Question: What are the mechanisms linking high-fat diet feeding to the development of a low grade inflammation?
GUT MICROBIOTA AND ENERGY METABOLISM
development of the intestinal microvilli, the degradation of non digestible polysaccharides (fermentation of resistant
Gut Microbiota Regulates Fat Storage
starch, oligosaccharides, inulin). Hence, the gut microbiota
Gut microbiota is involved in several intestinal biological
harvests energy for the host from dietary compounds in-
functions such as defence against pathogens, immunity, the
gested but not digested by the host. In the majority of adults,
1548 Current Pharmaceutical Design, 2009, Vol. 15, No. 13
Cani and Delzenne
the qualitative and quantitative composition of food intake
significant lower food intake [9]. The mechanisms of the
varies considerably from meal to meal and from day to day,
apparent weight gain implied an increase in the intestinal
while adiposity and body weight are remarkably constant
glucose absorption, energy extraction from non-digestible
despite huge short-term variations in energy balance. When
food component and concomitant higher glycemia and insu-
recording food intake and activity within a period including
linemia, two key metabolic factors regulating lipogenesis.
several meals, most individuals are able to compensate their
Moreover, glucose and insulin are also known to promote
cumulative energy intake with their energy expenditure with
hepatic de novo lipogenesis through the expression of several
great precision [7]. Such an active process - energy homeo-
key enzymes such as aceyl-CoA carboxylase (ACC) and
stasis - stabilizes the amount of body energy stored as fat.
fatty acid synthase (FAS). Strikingly, a two weeks conven-
However, an excess of energy intake by less than 1% com-
tionalization of germ free mice is accompanied by a two-fold
pared to the daily energy expenditure, can lead to a detrimen-
increase in hepatic triglyceride content. Both ACC and FAS
tal increase of body weight and metabolic complications in
are controlled by ChREBP (Carbohydrate Responsive Ele-
the long term (several years) [8]. Consequently, all the mech-
ment Binding Protein) and SREBP-1 (Sterol Responsive
anisms influencing calorie ingestion and subsequent harvest-
element Binding Protein) [10]. Accordingly, the convention-
ing should contribute to the balance of the body weight. Sev-
alized mice exhibited an increased hepatic ChREBP and
eral recent studies from the group of J. Gordon (USA) high-
SREBP-1 mRNA levels (Fig. 3) [9]. In addition to a modula-
lighted that gut microbiota composition is involved in the
tion of de novo lipogenesis, the authors found that germ free
regulation of energy homeostasis. Backhed, et al. found that
mice had a lower monosaccharide uptake from the intestine
the mice raised in the absence of microorganisms (germ free)
to the portal blood. This last phenomenon could be partly
had about 40% less total body fat than mice with a normal
explained by the lower capillary density of the small intes-
gut microbiota, eventhough the latter ate 30% less diet than
tine of germ free mice as compared to their conventionalized
did the germ free mice. To get more insight to those find-
counterparts. Finally, all these data provide evidence that the
ings, the authors performed a key experiment: they conven-
digestion of polysaccharides by microbial enzymes and the
tionalized germ free mice with a normal gut microbiota har-
increased saccharides delivery to the liver, participate in
vested from the cecum of a "normal" mouse, and found that
higher lipogenesis (Fig. 3). However, in the adipose tissue,
this conventionalization produced a 60% increase in body fat
the adipocytes hypertrophy observed in the mice harbouring
content and insulin resistance within two weeks, despite a
gut microbiota was not explained by the modulation of the
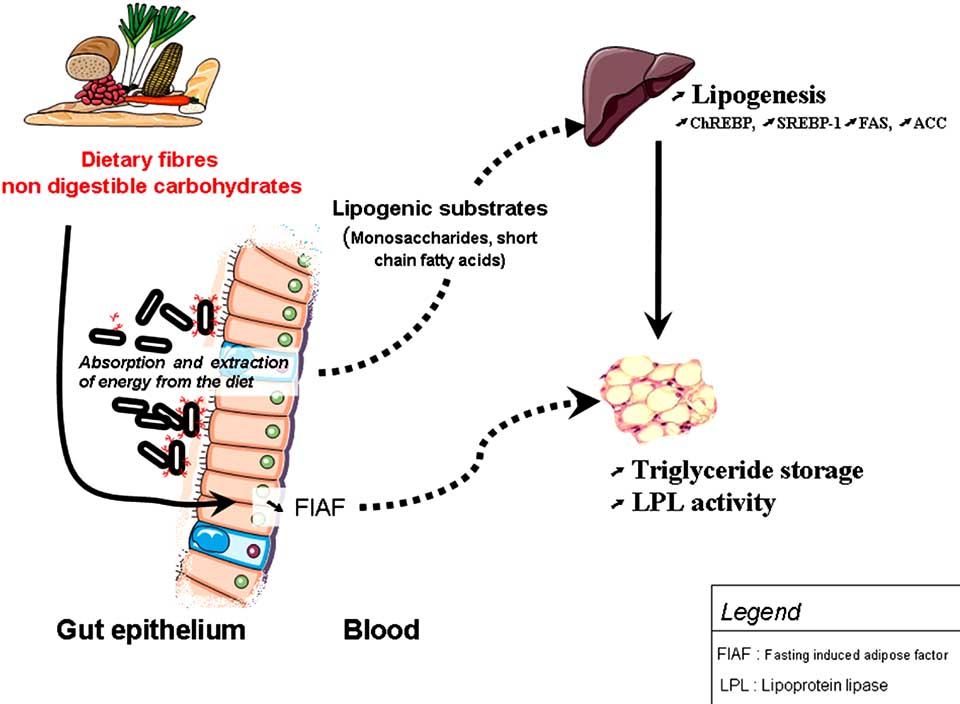
Fig. (3). Gut microbiota helps harvesting energy from the diet and increases lipogenesis. Environmental factor such as gut microbiota may
regulate energy storage: 1) by providing lipogenic substrates (short chain fatty acids, monosaccharides) to the liver, 2) by increasing the en-
zyme lipoprotein lipase (LPL) activity (as a consequence of suppressing the Fasting-Induced Adipose Factor (FIAF) in the gut).
Both phenomenon, contribute to the release of fatty acids and triacylglycerol from circulating lipoproteins in muscle, and adipose tissue.
The Role of the Gut Microbiota in Energy Metabolism
Current Pharmaceutical Design, 2009, Vol. 15, No. 13 1549
adipogenesis or the lipogenesis. Interestingly, the conven-
tained germ free mice or conventionalized mice on a high-
tionalization also brought about a general increase in the
fat/high-carbohydrates diet (western diet). They found that
activity of the enzyme lipoprotein lipase (LPL), catalyzing
conventionalized mice fed a high-fat diet gained signifi-
the release of fatty acids and triacylglycerol from circulating
cantly more weight and fat mass than the germ free mice. In
lipoproteins in muscle, and adipose tissue. The authors pro-
addition, the germ free mice were also protected against the
posed that such an increase was the consequence of suppres-
high-fat diet induced glucose intolerance and insulin resis-
sion of the Fasting-Induced Adipose Factor (FIAF) in the
tance. Strikingly, and opposite to the results previously ob-
gut. FIAF inhibits the LPL activity. The blunted FIAF ex-
served in germ free mice fed a normal chow diet, germ free
pression in conventionalized germ free mice could thus par-
mice consumed similar amounts of high-fat diet than the
ticipate to the accumulation of triacylglycerol in the adipose
conventionalized mice and had a similar energy content in
tissue. This set of experiments demonstrated for the first time
their feces. These last observations are not completely in
that an environmental factor such as gut microbiota may
favour of a better energy harvest from the high-fat diet in the
regulate energy storage Fig. (3) [9].
conventionalized mice, as previously suggested in normal chow fed mice. The authors have proposed a mechanism
Obesity and Gut Microbiota
dependent of the activation of a cellular energy-dependent protein kinase activated in response to metabolic stresses,
Ley, et al. demonstrated, in a rodent model, that obesity
namely AMP-activated protein kinase (AMPK) [14]. Com-
can be associated with an altered gut microbiota [11]. After
parisons of germ free mice and colonized mice fed a high-fat
the characterisation of more than five thousands bacterial
diet indicate that the gut microbiota can be involved in the
16S RNA gene sequences from gut microbiota of genetically
regulation of AMPK activity and fatty acids oxidation. The
obese ob/ob mice and their lean counterparts, they pointed
resistance to diet induced obesity observed in germ free mice
out that ob/ob mice had a 50% reduction in the abundance of
can be also explained by the following metabolic sequence :
Bacteroidetes and a proportional increase in Firmicutes. The
in the absence of gut microbiota, AMPK activity is constitu-
observed alterations in community may represent an unher-
tively higher in muscle, leading to a higher phosphorylation
alded contributing factor to the pattern of fuel partitioning
of its specific target acetylCoA carboxylase (ACC), reducing
between lean and obese subjects. Accordingly, these authors
thereby malonyl CoA production. This drop in malonyCoA
have also compared the distal gut microbiota of obese and
increases carnitine palmitoyl transferase-1 (CPT-1), and ther-
lean human subjects [12]. To investigate the relation be-
fore promotes mitochondrial fatty acid oxidation.
tween gut microbial ecology and body fat mass in humans, they studied 12 obese subjects assigned to a fat restricted or
Thus, these last experiments strongly suggest that a bac-
a carbohydrate restricted low calorie diet. They found that
terially related factor/mechanism other than energy harvest-
before the dietary intervention, obese people had lower Bac-
ing may be responsible for the development of diet-induced
teroidetes and more Firmicutes than did lean control subjects
obesity and diabetes.
[12]. Whereas the ratio of Bacteroidetes to Firmicutes ap-
Although all these elegant studies revealed that the gut
proached a lean type profile after 52 weeks of diet-induced
microbiota exerts a crucial role in the development of adi-
posity and the regulation of homeostasis, it remains to be
Together, the results obtained in rodents and in humans,
demonstrated how the gut microbiota can be involved in the
suggest that obesity alters the nature of the gut microbiota,
development of a low-grade inflammation classically associ-
but they did not prove that the relative difference of bacterial
ated with the metabolic disorders related to high-fat diet in-
proportions leads to different body weights.
duced obesity [15,16].
To determine if the gut microbial community from ob/ob
Gut Microbiota-Related Factor Responsible for Low-
mice can increase capacity for energy harvest from the diet,
Grade Inflammation
Turnbaugh, et al. transplanted caecal microbiota from lean and ob/ob mice to germ free wild-type recipients. They
Experimental Data
found that after only two weeks, mice harbouring the micro-
Recently, a new hypothesis linking gut microbiota to the
biota from obese mice had a modest fat gain, and extracted
metabolic homeostasis has been proposed. High-fat diet-
more calories from their food compared to the lean mice
induced obesity and metabolic disorders are associated with
having received the gut microbiota from lean mouse donors
an increased expression of several inflammatory related fac-
[13]. Together, these data suggest that the characteristics of
tors IL-1, TNF-�, MCP-1, and IL-6 in muscle, liver and adi-
gut microbiota of obese mice participate per se to the accre-
pose tissue [17-19]. These markers are involved in the de-
tion of fat and body weight gain.
velopment of impaired insulin action and induce insulin re-
GUT MICROBIOTA AND METABOLIC DISORDERS
sistance. For instance, TNF-� phosphorylates serine residue substrate (IRS-1) from the insulin receptor, leading to its
Gut Microbiota Controls the Occurrence of High-Fat
inactivation [20].
Diet Metabolic Disorders
Since type 2 diabetes and obesity are closely associated
The contribution of energy harvesting for the host due to
to a low-grade inflammatory state when feeding a high-fat
bacterial colonization is not the sole and crucial metabolic
diet, we have been seeking a bacterially related factor able to
exchange between the host and the intestinal bacteria. A re-
trigger the development of high-fat diet-induced obesity,
cent study performed in germ free mice, has analyzed their
diabetes and inflammation. The eligible candidate should
resistance to diet-induced obesity [14]. The authors main-
be an inflammatory compound of bacterial origin, continu-
1550 Current Pharmaceutical Design, 2009, Vol. 15, No. 13
Cani and Delzenne
ously produced within the gut and its absorption/action
shown to reduce intestinal endotoxin levels in rodents and
should be associated with high-fat diet feeding. We hypothe-
improve mucosal barrier function Fig. (4) [29-31]. In order
sized that the bacterial lipopolysaccharide (LPS) could be the
to determine the role of metabolic endotoxemia as a trigger-
eligible candidate, for the following reasons : 1) LPS is a
ing factor in the development of metabolic disorders associ-
constituent of Gram negative bacteria present in the gut mi-
ated with obesity, we mimicked the metabolic endotoxemia
crobiota, 2) LPS triggers the secretion of proinflammatory
by developing a mouse model chronically infused with a
cytokines when it binds to the complex of CD14 and the toll-
very low dose of LPS to reach the same plasma LPS levels
like receptor 4 (TLR4) at the surface of innate immune cells
as the one measured in the high-fat diet fed mice [27]. The
[21] , 3) LPS is continuously produced within the gut by the
four weeks chronic low dose LPS infusion mimicked the
death of Gram negative bacteria and is physiologically car-
high-fat diet fed mice phenotype namely, fasting hypergly-
ried into intestinal capillaries through a TLR4 dependent
cemia, obesity, steatosis, adipose tissue macrophages infil-
mechanism [22], 4) LPS is transported from the intestine
tration, hepatic insulin resistance and hyperinsulinemia Fig.
towards target tissues by a mechanism facilitated by lipopro-
(4). Finally, in order to demonstrate the causative link be-
teins, notably chylomicrons freshly synthesized from epithe-
tween LPS and the development of metabolic diseases, we
lial intestinal cells in response to fat feeding [23-26]. We
challenged LPS receptor knock out mice (CD14 knock out
have recently demonstrated that mice fed a high-fat diet for
mice-CD14KO) with a high-fat diet and/or a chronic low
as short a term as 2 to 4 weeks, exhibited a significant in-
dose LPS infusion. CD14 is a key molecule involved in the
crease in plasma LPS (Fig. 4) [27]. This can be considered as
innate immune system [32]. CD14 is a multifunctional re-
a "metabolic endotoxemia", since, the LPS plasma concen-
ceptor constituted by a phosphatidyl inositol phosphate-
trations were very much lower than those obtained during a
anchored glycoprotein of 55kDa expressed on the surface of
septic shock [28]. We have demonstrated that high-fat diet
monocytes, macrophages and neutrophils [33-36]. We have
feeding changed gut microbiota profile. Indeed, the popula-
shown that CD14KO mice were completely resistant to the
tion levels of Bifidobacterium spp. and E. rectale/Cl. coccoi-
development of the inflammation induced by both, high-fat
des group were significantly reduced in high fat fed animals
feeding or following the chronic low dose LPS administra-
versus mice receiving the standard high carbohydrate diet
tion in the visceral and subcutaneous adipose depots, the
(Fig. 4) [27]. Importantly, Bifidobacterium spp. have been
liver and the muscle. Moreover, CD14KO mice are hyper-
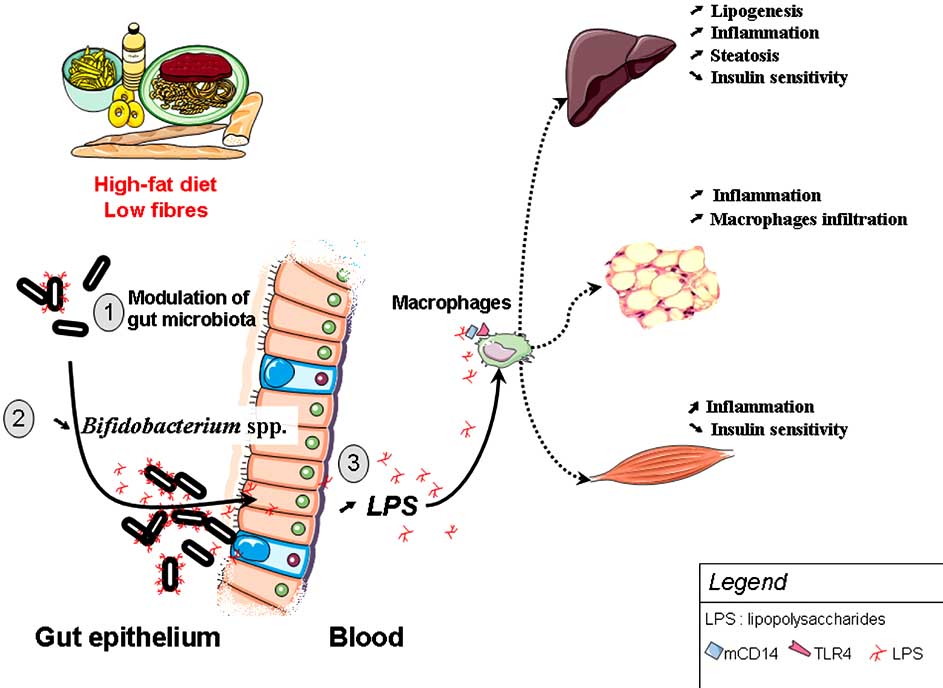
Fig. (4). High-fat diet feeding changes gut microbiota, promotes metabolic endotoxemia and triggers the development of metabolic disorders,
via a CD14/TLR4 dependent mechanism.
(1) High-fat diet feeding changes gut microbiota in a complex way and (2) specifically decreases Bifidobacterium spp. (3) This phenomenon is associated with a higher plasma LPS content (metabolic endotoxaemia), a LPS-dependent secretion of proinflammatory cytokines. High-fat feeding and LPS promotes low-grade inflammation-induced metabolic disorders (insulin resistance, diabetes, obesity, steatosis, adipose tissue macrophages infiltration).
The Role of the Gut Microbiota in Energy Metabolism
Current Pharmaceutical Design, 2009, Vol. 15, No. 13 1551
sensitive to insulin, even when they are fed a normal diet,
biotic treatment dramatically changed the gut microbiota;
suggesting that CD14 could be a modulator of insulin sensi-
reduced the Lactobacillus spp., Bifidobacterium spp.; and
tivity in physiological conditions [27]. As a matter of fact,
Bacteroides-Prevotella spp. All these features were associ-
CD14KO mice were completely resistant to the insulin resis-
ated with a strong decrease of metabolic endotoxemia. Fur-
tance induced by the high-fat diet and chronic LPS treat-
thermore, these parameters were associated with a signifi-
ment. In these sets of experiments, we showed that high-fat
cantly lower inflammatory tone in ob/ob antibiotic-treated
feeding induced a low-grade inflammation which originates
mice [44]. Macrophages infiltration, inflammatory markers
from the intestinal absorption of the LPS. Thus taken to-
and oxidative stress were reduced in the visceral adipose
gether our data support the key idea that the gut microbiota
depots and to a lesser extent in the subcutaneous fat. This
can contribute to the pathophysiology of obesity and type 2
experiment demonstrates that the gut microbiota is an impor-
diabetes (Fig. 4).
tant factor involved in the development of the metabolic dis-orders in ob/ob mice. Finally, we wanted to demonstrate that
Importantly, the real mechanism by which in the absence
the metabolic endotoxemia per se was the triggering factor
of the complex CD14/TLR4 receptor the mice are resistant
of the inflammatory tone characterizing these obese and dia-
to the high-fat diet induced metabolic disorders remains a
betic leptino-deficient mice. Therefore, we used two differ-
matter of debate. Several studies have demonstrated that the
ent approaches to block the endogenous LPS action, the first
TLR4 receptor could also be activated by specific saturated
one consisted of a pharmacological administration of a LPS-
fatty acids [37-39]. Hence, TLR4KO mice fed a high-fat diet
quencher molecule inactivating the circulating LPS, and the
are resistant to the development of a high-fat diet induced
second one consists of a genetic model of obese mice lacking
obesity and related disorders (obesity, inflammation, insulin
the LPS receptor CD14, the double knock out mice ob/ob-
resistance,…) [37,40-43]. However, none of these studies
CD14-/-. In both models, impairing the endogenous LPS ac-
have investigated the putative modulation by the dietary in-
tion, recapitulated the phenotype observed during the modu-
tervention of either the gut microbiota nor the putative meta-
lation of gut microbiota by antibiotics [44]. In addition to the
bolic endotoxemia. Therefore, it is difficult to conclude
improved inflammatory status, all the models were also
whether the protective effect linked to the invalidation of the
characterized by a significant improvement of glucose toler-
TLR4 receptor is a mechanism dependent of the high-fat diet
ance and insulin resistance [44]. These last results have been
induced endotoxemia and/or a direct effect of the fatty acid
confirmed in a study using a similar approach [47]. Alto-
pattern of the diet. Previous experiments performed in germ
gether, this set of data confirms that the gut microbiota and
free mice fed a high-fat diet helped to answer this question.
the consequent increased bacteria-related factor LPS exert a
The fact that the germ free mice resist the deleterious effects
key role in the development of adipose depots and inflamma-
of a high-fat diet supports the idea that the phenomenon is
tion in ob/ob mice.
not exclusively mediated through a fatty acids/TLR4 de-pendent mechanism. To ascertain this hypothesis and to as-
Several studies have shown that bifidobacteria, seen as
sess the contribution of gut microbiota to the development of
beneficial members of the gut microbiota, lower intestinal
high-fat diet-induced metabolic disorders, we used intestinal-
endotoxin levels and improve mucosal barrier function [29-
focused antibiotic treatment in high-fat fed mice. The antibi-
31]. Conversely, we reported that high-fat feeding alters the
otic treatment completely abolished the high-fat diet-induced
intestinal microbiota composition where Bifidobacterium
metabolic disorders, namely metabolic endotoxemia, the
spp. were reduced. Therefore, we addressed the following
development of visceral adipose tissue inflammation, macro-
phages infiltration, oxidative stress and metabolic disorders [44]. These last experiments clearly demonstrate the contri-
Could the Selective Increase of Bifidobacteria in Gut Mi-
bution of the gut microbiota to the metabolic endotoxemia.
crobiota Improve High-Fat Diet-Induced Diabetes in
Mice?
Together, these findings strongly suggest that the gut
microbiota contributes to the metabolic endotoxemia related
To answer this question, we used prebiotic dietary fibres
to high-fat diet feeding. In the same line of our results, recent
(oligofructose, OFS) [48] to specifically increase the gut
studies report that plasma LPS is increased in ob/ob and
bifidobacteria content in high-fat fed mice. We confirmed
db/db mice [45]. Furthermore, polymyxin B treatment,
that mice fed a high-fat diet exhibit a higher endotoxemia, a
which specifically eliminates Gram-negative bacteria and
phenomenon completely abolished through dietary supple-
further quenches LPS, diminishes hepatic steatosis [46].
mentation with the prebiotic dietary fibres (Fig. 5). In prebi-
However, these studies did not demonstrate that the gut bac-
otic treated-mice, Bifidobacterium-spp. significantly and
teria determine the threshold at which metabolic endotoxe-
positively correlated with improved glucose-tolerance, glu-
mia occurs and that the modulation of gut microbiota in
cose-induced insulin-secretion, and normalized low-grade
obese and diabetic ob/ob mice controls the occurrence of
inflammation (decreased endotoxemia, plasma and adipose
metabolic and inflammatory disorders.
tissue proinflammatory cytokines) (Fig. 5) [49]. We also
Therefore, we asked the following question.
found that metabolic endotoxemia correlated negatively with Bifidobacterium spp [49].
What is the Contribution of Gut Microbiota to the Devel-
opment of Metabolic Endotoxemia, Inflammation, Oxida-
Thus, it would be useful to develop specific strategies for
tive Stress and Metabolic Disorders in ob/ob Mice?
modifying gut microbiota to favour specific gut microbiota (i.e. bifidobacteria) to prevent the deleterious effect of high-
To test this hypothesis, we changed the gut microbiota of
ob/ob mice using antibiotic treatment for four weeks. Anti-
fat or obesity-induced metabolic diseases.
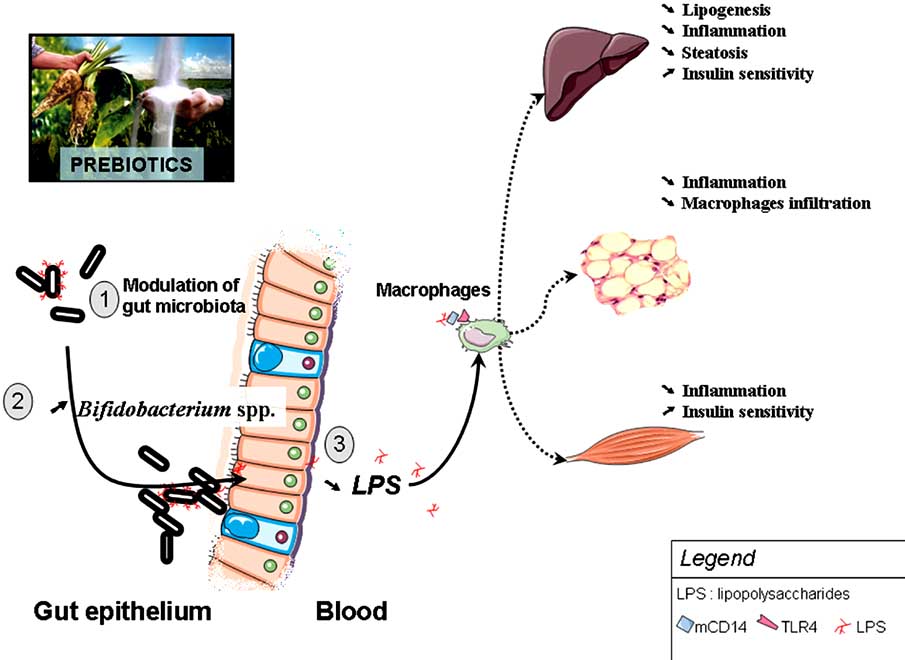
1552 Current Pharmaceutical Design, 2009, Vol. 15, No. 13
Cani and Delzenne
Fig. (5). Changing gut microbiota by the mean of prebiotics protects against high-fat diet induced metabolic endotoxemia and the develop-
ment of metabolic disorders. Prebiotic treatment increases Bifidobacterium-spp., decreases plasma LPS levels and improved insulin sensitiv-
ity, steatosis, and normalized low-grade inflammation (decreased endotoxemia, plasma and adipose tissue proinflammatory cytokines).
Human Evidence
a population based-study, we found a link between energy (food) intake and metabolic endotoxemia [55]. Furthermore,
Even if from a mechanistic point of view, the results ob-
a similar metabolic endotoxemia has been shown to increase
tained in rodent models are very encouraging, it remains to
adipose TNF-� and IL-6 concentrations and insulin resis-
be demonstrated that such a mechanism is also observed in
tance in healthy volunteers [56]. By linking energy intake
and endotoxemia in a large sample of healthy men, the study
Is a High-Fat Meal Associated with Metabolic Endotoxe-
adds important information to this body of evidence. This
mia in Humans?
study shows for the first time that the confounding factor of the relation between fat intake and metabolic endotoxemia is
Interesting data suggest that high-fat feeding is associated
likely to be energy intake. Taken together, both human stud-
with a higher endotoxemia in humans. Erridge, et al. have
ies suggest that diet-induced changes in endotoxemia may
highlighted the putative role of a high-fat meal and devel-
bridge the gap between food intake behaviour and metabolic
opment of metabolic endotxemia. The study is the first to
diseases in humans.
examine the kinetics of baseline endotoxemia concentrations in healthy human subjects. Even if, in humans plasma endo-
What is the Contribution of Gut Microbiota to the Devel-
toxin levels are classically associated with sepsis, many stud-
opment of Metabolic Disorders in Humans?
ies have also reported that in healthy subjects plasma endo-
et al. recently reinforced the hypothesis that
toxin concentrations range from 1 to 200 pg/ml [50-53]. In
metabolic endotoxemia might act as a gut microbiota related
this study, the authors found that a high-fat meal induces a
factor involved in the development of type 2 diabetes and
metabolic endotoxemia which fluctuates rapidly in healthy
obesity in humans [57]. The authors found that endotoxemia
subjects, from a very low concentration at baseline (between
was 2-fold higher in the BMI-, sex-, and age-matched type 2
1 to 9 pg/mL) to concentrations that may be sufficient to in-
diabetes patients group than in the non diabetic subjects.
duce some degree of cellular activation in in vitro experi-
Furthermore, they found that fasting insulin significantly
ments [54]. They found that the metabolic endotoxemia ob-
correlated with metabolic endotoxemia in the whole non
served following a high-fat meal is sufficient to activate cul-
diabetic population, and this correlation persisted when con-
tured human aortic endothelial cells, and that this endothelial
trolled for sex, age, and BMI [57]. The quest for the gut–
cell activation is likely to be due to the release of soluble
dependent source of endotoxemia in these patients remains
inflammatory mediators, such as TNF- �, from monocytes.
Along the same line, in a large sample of men (n=211) from
The Role of the Gut Microbiota in Energy Metabolism
Current Pharmaceutical Design, 2009, Vol. 15, No. 13 1553
Specific Modulation of Gut Microbiota by Prebiotic Nutri-
[77]. Two years later, Kok, et al. observed that feeding rats
ents : A Rationale to Support Nutritional Advices in the
with a prebiotic fibre oligofructose (OFS) lead to an increase
Context of Obesity?
in total caecal GLP-1 and jejunum GIP concentrations [78]. Several data show that prebiotics containing short chain oli-
Current recommendations for the management of obesity
gosaccharides reduce food intake, body weight gain and fat
and diabetes propose an increase in dietary fibre which may
mass development. All these features are associated with a
contribute to the control of several metabolic disorders (i.e.
significant 2 fold increase of the portal plasma levels of two
lower fasting glycemia, an improved glucose tolerance,
gut peptide GLP-1 and PYY (anorexigenic) and a decrease in
lower body weight gain, decreased food intake,…) [58-60].
Ghrelin (orexigenic) (Fig. 6) [79,80]. Prebiotic feeding pro-
Among the dietary fibres which seem to be effective in this
motes GLP-1 synthesis (mRNA and peptide content) in the
context, prebiotics dietary fibres are now well described in
proximal colon by a mechanism linked to the differentiation
the literature [48,61,62]. Prebiotics can be used as a tool to
of precursor cells into enteroendocrine cells [81]. Moreover,
modulate the gut microbiota. A prebiotic is "a selectively
in another set of experiments performed in high-fat diet-
fermented ingredient that allows specific changes, both in the
induced obesity and type 2 diabetes, the modulation of gut
composition and/or activity in the gastrointestinal microflora
microbiota using prebiotics protects against body weight
that confers benefits upon host well-being and health" [63]
gain, fat mass development (visceral, epidydimal and subcu-
and probiotic are live bacteria given in oral quantities that
taneous), glucose intolerance, and hepatic insulin resistance
allow for colonization of the colon [64]. Inulin-type fructans,
[82-84]. Accordingly, prebiotics like fructans added in the
namely inulin and fructooligosaccharides are prebiotic die-
diet are able to counteract diabetes when given in streptozo-
tary fibres well studied and clearly effective in humans to
tocin-treated diabetic rats [85]. Studies showing similar ef-
stimulate growth of health-promoting species belonging to
fects to those observed in fructans studies, for example, with
the genera Bifidobacterium spp. and Lactobacillus spp. The
lactitol or resistant starch (both fermentable carbohydrates)
daily amount taken in the diet necessary to exert a prebiotic
added into the diet of rats, lowers food intake, body weight
effect is relatively small (5–20 g/day) [61,65].
gain and increases plasma GLP-1 and PYY (Fig. 6) [86-88].
Besides their effect on metabolic endotoxemia previously
Nevertheless, the putative role of a specific gut microbiota
described, prebiotic dietary fibers may also modulate other
profile has not been studied.
targets prone to influence metabolic disorders associated
What is the Relevance of Prebiotics-Dependent Gut Micro-
with obesity, such as gut peptides.
biota Modulation and Energy Metabolism in Humans?
Are Gut Peptides Involved in the Effect Of Prebiotics Die-
To date, only a few studies have reported the effects of
tary Fibres on Energy Metabolism and Metabolic Disor-
prebiotics on energy homeostasis and metabolism in humans.
ders Associated with Obesity?
Interestingly, one study reported that oligofructose feeding
The modulation of gut peptides involved in the control of
(20g/d) significantly increased plasma GLP-1 after a mixed
energy and glucose homeostasis could be one of the mecha-
meal [89]. Moreover, in healthy humans, feeding 16g/d OFS
nisms by which the modulation of gut microbiota via spe-
promotes satiety following breakfast and diner, and reduces
cific dietary fibres is associated with an improvement of
hunger and prospective food consumption after the diner.
metabolic disorders. Endocrine cells present in the intestinal
This was accompanied by a significant 10% lower total en-
mucosa secrete peptides involved in the regulation of energy
ergy intake [90]. Along the same lines, Archer, et al. have
homeostasis, and/or pancreatic functions - the later being
demonstrated that of fructans, added in food as fat-replacer,
called incretins (GLP-1 and GIP) [66-69]. Among those pep-
were able to lower energy intake during a test day [91]. The
tides, GLP-1, PYY, Ghrelin and oxyntomodulin have re-
role of fermentable dietary fibres in the management of ap-
cently been proposed as important modulators of food intake
petite in healthy human has been recently confirmed [92].
and energy expenditure (Fig. 6) [70-74]. Several experimen-
Finally, in the quest for the role of prebiotic in the control of
tal data suggest that those peptides could constitute a link
body weight and fat mass development, a recent study dem-
between the outcome of gut microbiota fermentation in the
onstrated that supplementation with a prebiotic, in addition
lower part of the gut and systemic consequences.
to its benefit to bone mineralization, had a significant benefit in the maintenance of an appropriate BMI, and fat mass in
The putative link between gut microbiota fermentation of
primarily nonobese young adolescents [93]. Altogether,
non digestible carbohydrate and the modulation of gut pep-
these human studies provide evidence that the modulation of
tides secretion was proposed in 1987 by Goodlad, et al.,
gut microbiota by using prebiotics impacts on energy ho-
demonstrating that inert bulk fibre cannot stimulate colonic
meostasis and body weight gain.
epithelial cell proliferation, but that fermentable fibres were capable of stimulating proliferation in the colon, linking
What is the Contribution of Bifidobacterium spp. in the
these effects to the increased enteroglucagon plasma levels
Prebiotic-Improved Metabolic Status?
[75,76]. And along the 20 years, other reports suggesting new mechanisms of such a dietary compound have appeared
A recent study has shown for the first time in human that
in the literature. In 1996, the first study demonstrating a role
differences in the gut microbiota may precede overweight
of the fermentation occurring in the lower part of the gut was
development [94]. The authors found that Bifidobacterium
associated with an increase of GLP-1 synthesis, secretion
spp., affecting both the quantity and quality of the microbiota
and insulin metabolism. The study demonstrated that rats fed
during the first year of life, was higher in number in children
a high fiber diet (300 g/kg of diet) had a higher plasma GLP-
who exhibited a normal weight at 7 years than in children
1, insulin and c-peptide 30 min after an oral glucose load
developing overweight. More importantly and according to the results obtained in experimental models, they found that
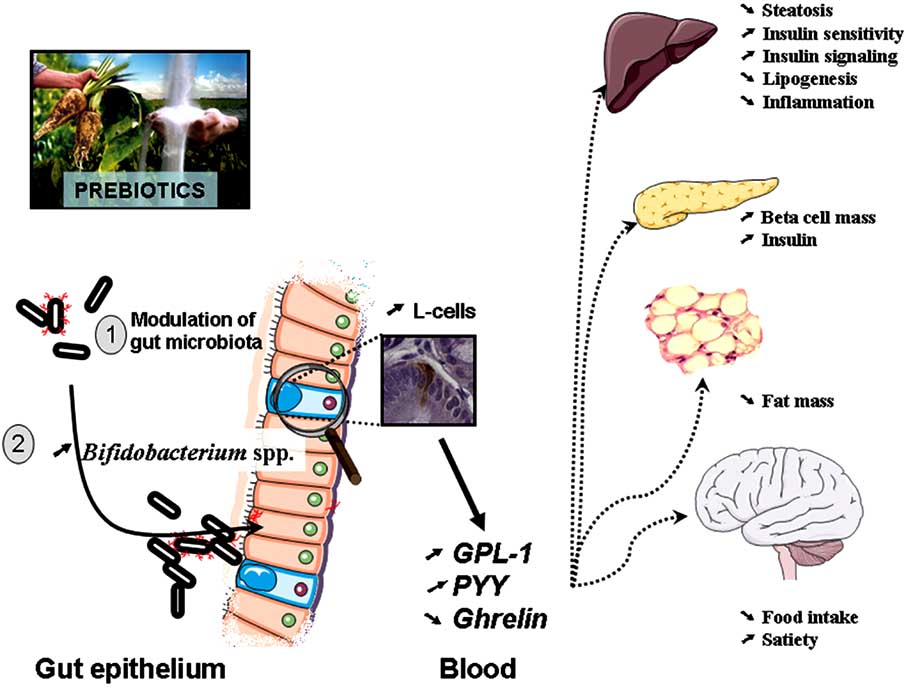
1554 Current Pharmaceutical Design, 2009, Vol. 15, No. 13
Cani and Delzenne
Fig. (6). The modulation of gut microbiota by prebiotics treatment modulates the endogenous production of gut peptides associated with
energy homeostasis.
Prebiotics change gut microbiota, increase portal plasma levels of two gut peptide GLP-1 and PYY (anorexigenic) and decrease Ghrelin (orexigenic). Prebiotics feeding promotes GLP-1 synthesis in the proximal colon by a mechanism linked to the differentiation of precursor cells into enteroendocrine L-cells. All these features are associated with a reduced food intake, body weight gain and fat mass development, a restored beta cell mass and glucose-induced insulin secretion.
the fecal numbers of Staphylococcus aureus were lower in
tion with HDL cholesterol [101]. Whether the metabolic en-
children remaining normal weight than in children who be-
dotoxemia measured in these studies derives from periodon-
came overweight several years later. These results unequivo-
tal pathogens alone or not remains to be demonstrated.
cally imply that the gut microbiota profile in favour of a
What is the Contribution of the Gut Microbiota to the De-
higher bifidobacteria and a lower number of S. aureus in
velopment of Atherosclerosis?
infancy may provide protection against overweight and obe-sity development. The authors proposed that S. aureus may
Experimental Evidence
act as a trigger of low-grade inflammation [95], contributing to the development of obesity [27].
Several authors have demonstrated that the link between
metabolic endotoxemia and the development of systemic low
GUT MICROBIOTA AND CARDIOVASCULAR RISK
grade inflammation and cardiovascular diseases is mediated through a LPS receptor dependent mechanism [102-105]. In
The link between periodontal diseases and cardiovascular
accordance with the recent evidence suggesting that inflam-
diseases is now well established [96-99]. The accumulated
matory process induced by high-fat diet feeding causes insu-
evidence supports that periodontal infections and atheroscle-
lin resistance via a mechanism involving CD14 and TLR4,
rosis are causally linked by the metabolic endotoxemia. Sev-
two recent studies have proposed that inflammation can be
eral marker related to the metabolic endotoxemia (LPS bind-
activated in the vasculature of mice fed a high-fat diet [43,
ing protein, soluble CD14, and antibodies to LPS of perio-
106]. Mice fed a high-fat diet for 8 weeks developed vascu-
dontal pathogens) have been reported to be also elevated in
lar inflammation (higher thoracic aorta I�B�-phosphoryla-
the plasma of affected patients. Porphyromonas gingivalis
tion, ICAM, IL-6) and vascular insulin resistance (lower
systemic exposure appears to predispose to incident stroke
thoracic aorta insulin dependent AKT-phosphorylation and
eNOS-phosphorylation). All these features were completely
Metabolic endotoxemia is positively correlated with total
absent in mice lacking the TLR4 receptor. Furthermore, de-
cholesterol, diastolic blood pressure, waist to hip ratio, BMI,
ficiency of either TLR4 or Myd88 attenuates the high-fat
and antibody levels to P gingivalis, and a negative correla-
diet induced atherosclerosis, chemokine secretion and mac- rophage infiltration in apolipoprotein E deficient mice
The Role of the Gut Microbiota in Energy Metabolism
Current Pharmaceutical Design, 2009, Vol. 15, No. 13 1555
(ApoE-/-) [107-111]. These studies support the idea that
CONCLUSIONS
TLR4 and Myd88 likely contribute to atherosclerosis pro-
The evidence that the gut microbiota composition can be
gression via a fatty acid dependent mechanism. In addition,
different between healthy and/or obese and type 2 diabetic
it has been proposed that modulation of gut microbiota in
patients has led to the study of this environmental factor as
ApoE-/- also contribute to the reduction of inflammation and
an important contributor to the pathophysiology of metabolic
atherosclerosis development [112]. The authors found that
diseases. Different and complementary mechanisms have
changing the gut microbiota of atherosclerotic prone ApoE-/-
been recently proposed. The gut microbiota may participate
mice by feeding mice with prebiotics for 16 weeks, signifi-
to the regulation of energy metabolism by several mecha-
cantly reduce the development of atherosclerotic lesions by
nisms, i.e. energy harvest from the diet, regulation of fat
about 35% as compared to the mice fed a control diet [112].
storage (FIAF expression), regulation of lipogenesis (ACC,
However, the authors did not propose any putative mecha-
FAS, chREBP and SREBP-1 expression), or regulation of
nisms related to the modulation of gut microbiota, inflamma-
fatty acid oxidation (AMPK activity). Moreover, the devel-
tion or metabolic endotoxemia.
opment of obesity and metabolic disorders following a high-
Human Evidence
fat diet may be associated to the innate immune system. In-deed, high-fat diet feeding triggers the development of obe-
The notion that gut microbiota may participate in the
sity, inflammation, insulin resistance, type 2 diabetes and
prevention of coronary artery disease has been already inves-
atherosclerosis by mechanisms dependent of the LPS and/or
tigated and proposed several years ago. Based on previous
the fatty acids activation of the CD14/TLR4 receptor com-
animal studies demonstrating that probiotic feeding partici-
plex. Importantly, fat feeding is also associated with the de-
pate in the improvement of atherogenic markers (LDL-
velopment of metabolic endotoxemia in human subjects and
cholesterol, fibrinogen), Bukowska, et al. decided to test this
participates in the low-grade inflammation, a mechanism
interesting possibility in human subjects. The authors inves-
associated with the development of atherogenic markers.
tigated in a double blind cross over study with 30 male sub-
Among the mechanisms linking the gut microbiota to the
jects the role of both a fermentable carbohydrate (ferment-
control of body weight, insulin secretion and appetite, the
able oat fraction) and a probiotic (Lactobacillus plantarum)
modulation of gut peptides (i.e. GLP-1, PPY, …) by prebiot-
supplementation on two key atherogenic parameters, namely
ics seems to be of interest. Several data obtained in experi-
LDL-cholesterol and fibrinogen. After 6 weeks of treatment,
mental models and human subjects are in favour of the fact
levels of LDL-cholesterol and fibrinogen were significantly
that changing the gut microbiota by the means of prebiotics
reduced [113]. This study showed for the first time that the
and/or probiotics may participate in the control of several
modulation of gut microbiota may participate to the modula-
parameters involved in the development of metabolic dis-
tion of two key risk factors.
eases associated with obesity. Nevertheless, progress in un-
Along the same line, the same group documented the
derstanding the mechanisms by which the gut microbiota
influence of L. plantarum in a controlled double-blind study
interact with the host will, provide new basis for putative
with placebo on 36 smokers [114]. The authors found that a
pharmacological or dietary intervention. Moreover, the tre-
6 weeks treatment reduces several proatherogenic markers.
mendous lack of data limits our current knowledge of the
Plasma fibrinogen concentrations decreased by 21%, plasma
complexity of gut microbiota-host interactions and proposal
IL-6 concentrations decreased by 41% and F2-isoprostanes
of exact mechanisms linking dietary habits, gut microbiota
(markers of lipid oxidant stress) decreased by 31%. Moreo-
and metabolic disorders. Multidisciplinary research in this
ver, the authors found that L. plantarum administration in
field will be helpful to provide evidence-based data, which
smokers markedly decreased the adherence of monocytes to
will be taken into account to consider the gut microbiota as a
resting (40%) and tumor necrosis factor–activated (36%)
putative target to prevent metabolic disorders.
endothelial cells [114]. These studies demonstrate that sup-plementation of the diet with L. plantarum may contribute to
the prevention and treatment of metabolic disorders in smok-
PDC is postdoctoral researcher from the FRS-FNRS
ers. The authors proposed that this positive effect may be
(Fonds de la Recherche Scientifique, Belgique). NMD and
directly associated with the production of propionic acid
PDC are recipients of subsides from fonds spéciaux de re-
through the bacterial fermentation of fiber [114]. In accor-
cherche, UCL (Université catholique de Louvain) and FNRS.
dance with these studies, Kullissar, et al. demonstrated that changing gut microbiota by means of probiotic lactobacilli
REFERENCES
fermented goat milk feeding impacted on several atherogenic markers. The authors found that a 3 weeks treatment signifi-
Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new
cantly improved the oxidative status (lower conjugated diene
worldwide definition
Matarese G, Mantzoros C, La CA. Leptin and adipocytokines:
level in plasma lipoprotein fraction, diminished the level of
bridging the gap between immunity and atherosclerosis. Curr
oxidized LDL and suppressed production of 8-isoprostanes)
Pharm Des 2007; 13: 3676-80.
[115]. Altogether, these data suggest that the modulation of
Heilbronn LK, Campbell LV. Adipose tissue macrophages, low
gut microbiota may positively impact on several atherogenic
grade inflammation and insulin resistance in human obesity. Curr
markers. However, systemic investigations are needed to
Pharm Des 2008; 14: 1225-30.
Kahn SE, HulRFl RL, Utzschneider KM. Mechanisms linking
clarify the molecular mechanisms linking the modulation of
obesity to insulin resistance and type 2 diabetes.
gut microbiota by pre/probiotics and the positive effect ob-
Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, et al. Evolution of symbiotic bacteria in the distal human intes-tine. PLoS Biol 2007; 5: e156.
1556 Current Pharmaceutical Design, 2009, Vol. 15, No. 13
Cani and Delzenne
Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proc Natl
Wang ZT, Yao YM, Xiao GX, Sheng ZY. Risk factors of devel-
opment of gut-derived bacterial translocation in thermally injured
Edholm OG. Energy balance in man studies carried out by the
Division of Human Physiology, National Institute for Medical Re-
Kitchens RL, Thompson PA. Modulatory effects of sCD14 and
search. J Hum Nutr 1977; 31: 413-31.
LBP on LPS-host cell interactions.
Hill JO. Understanding and addressing the epidemic of obesity: an
energy balance perspective.
Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, et
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al.
al. Resistance to endotoxin shock and reduced dissemination of
The gut microbiota as an environmental factor that regulates fat
gram-negative bacteria in CD14-deficient mice.
Denechaud PD, Dentin R, Girard J, Postic C. Role of ChREBP in
Haziot A, Ferrero E, Lin XY, Stewart CL, Goyert SM. CD14-
hepatic steatosis and insulin resistance.
deficient mice are exquisitely insensitive to the effects of LPS.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD,
Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, Le
Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad
Beau MM. The CD14 monocyte differentiation antigen maps to a
region encoding growth factors and receptors.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology:
human gut microbes associated with obesity
Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The
monocyte differentiation antigen, CD14, is anchored to the cell
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER,
membrane by a phosphatidylinositol linkage.
Gordon JI. An obesity-associated gut microbiome with increased
capacity for energy harvest
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4
Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mecha-
links innate immunity and fatty acid-induced insulin resistance. J
nisms underlying the resistance to diet-induced obesity in germ-
Clin Invest 2006; 116: 3015-25.
Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J
Attenuation of obesity-induced adipose tissue inflammation in
Clin Invest 2005; 115: 1111-9.
C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem
Hotamisligil GS. Inflammation and metabolic disorders.
Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al.
receptor 4 is associated with insulin resistance in adipocytes. Bio-
Local and systemic insulin resistance resulting from hepatic activa-
tion of IKK-beta and NF-kappaB. Nat Med 2005; 11: 183-90.
Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO,
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression
Hirabara SM, Schenka AA, et al. Loss-of-function mutation in
of tumor necrosis factor-alpha: direct role in obesity-linked insulin
Toll-like receptor 4 prevents diet-induced obesity and insulin resis-
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL,
Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C,
Ferrante AW, Jr. Obesity is associated with macrophage accumula-
et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are
tion in adipose tissue.
protected against the development of insulin resistance in white
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF,
adipose tissue in response to a high-fat diet
Spiegelman BM. IRS-1-mediated inhibition of insulin receptor ty-
rosine kinase activity in TNF-alpha- and obesity-induced insulin
Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 defi-
ciency selectively protects against obesity induced by diets high in
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC.
saturated fat. Obesity (Silver Spring) 2008; 16: 1248-55.
CD14, a receptor for complexes of lipopolysaccharide (LPS) and
Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et
LPS binding protein.
al. Toll-like receptor-4 mediates vascular inflammation and insulin
Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, et
resistance in diet-induced obesity
al. Enterocyte TLR4 mediates phagocytosis and translocation of
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne
bacteria across the intestinal barrier
NM, et al. Changes in gut microbiota control metabolic endotoxe-
Tomita M, Ohkubo R, Hayashi M. Lipopolysaccharide transport
mia-induced inflammation in high-fat diet-induced obesity and dia-
system across colonic epithelial cells in normal and infective rat.
betes in mice.
Drug Metab Pharmacokinet 2004; 19: 33-40.
Brun P, Castagliuolo I, Leo VD, Buda A, Pinzani M, Palu G, et al.
Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM,
Increased intestinal permeability in obese mice: new evidence in
Abernathy CM, et al. Gut bacterial translocation via the portal vein:
the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gas-
a clinical perspective with major torso trauma.
trointest Liver Physiol 2007; 292: G518-25.
Pappo I, Becovier H, Berry EM, Freund HR. Polymyxin B reduces
Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van ', V,
cecal flora, TNF production and hepatic steatosis during total par-
Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates
enteral nutrition in the ra
LPS detoxification by chylomicrons
Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin
RG, et al. Gut microbiota modulation with norfloxacin and am-
Black DD, Tso P, Weidman S, Sabesin SM. Intestinal lipoproteins
picillin enhances glucose tolerance in mice
in the rat with D-(+)-galactosamine hepatitis
Tuohy KM, Rouzaud GC, Bruck WM, Gibson GR. Modulation of
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et
the human gut microflora towards improved health using prebiot-
al. Metabolic endotoxemia initiates obesity and insulin resistance.
ics--assessment of effi
Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy
Mitaka C. Clinical laboratory differentiation of infectious versus
KM, et al. Selective increases of bifidobacteria in gut microflora
non-infectious systemic inflammatory response syndrome. Clin
improve high-fat-diet-induced diabetes in mice through a mecha-
Chim Acta 2005; 351: 17-29.
nism associated with endotoxaemia.
Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leav-
ens A, et al. In vivo effects of bifidobacteria and lactoferrin on gut
Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger
endotoxin concentration and mucosal immunity in Balb/c mice.
G, Oberhollenzer F, et al. Association of endotoxemia with carotid
atherosclerosis and cardiovascular disease: prospective results from
Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifi-
the Bruneck Study. J Am Coll Cardiol 1999; 34: 1975-81.
dobacteria in gut barrier function after thermal injury in rats. J
Bolke E, Jehle PM, Storck M, Nothnagel B, Stanescu A, Orth K.
Trauma 2006; 61: 650-7.
Endotoxin release and endotoxin neutralizing capacity during colonoscopy.
The Role of the Gut Microbiota in Energy Metabolism
Current Pharmaceutical Design, 2009, Vol. 15, No. 13 1557
Goto T, Eden S, Nordenstam G, Sundh V, Svanborg-Eden C, Mat-
lium: relationship to gastrin, enteroglucagon and PYY. Gut 1987;
tsby-Baltzer I. Endotoxin levels in sera of elderly individuals. Clin
28(Suppl): 221-6.
Diagn Lab Immunol 1994; 1: 684-8.
Reimer RA, McBurney MI. Dietary fiber modulates intestinal
Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial
proglucagon messenger ribonucleic acid and postprandial secretion
endotoxin is an active component of cigarette smoke.
of glucagon-like peptide-1 and insulin in rats
Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal in-
Kok NN, Morgan LM, Williams CM, Roberfroid MB, Thissen JP,
duces low-grade endotoxemia: evidence of a novel mechanism of
Delzenne NM. Insulin, glucagon-like peptide 1, glucose-dependent
insulinotropic polypeptide and insulin-like growth factor I as puta-
Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC,
tive mediators of the hypolipidemic effect of oligofructose in rats. J
et al. Energy intake is associated with endotoxemia in apparently
Nutr 1998; 128: 1099-103.
Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate
Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L,
gastrointestinal peptides involved in appetite regulation (glucagon-
Comiskey LL, et al. Innate immunity modulates adipokines in hu-
like peptide-1 and ghrelin) in rats.
Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of
Creely SJ, McTernan PG, Kusminski CM, Fisher M, da Silva NF,
inulin and oligofructose on gastrointestinal peptides. Br J Nutr
Khanolkar M, et al. Lipopolysaccharide activates an innate immune
2005; 93(Suppl 1): S157-61.
system response in human adipose tissue in obesity and type 2 dia-
Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible
betes. Am J Physiol Endocrinol Metab 2007; 292: E740-7.
carbohydrates promote L-cell differentiation in the proximal colon
Vinik AI, Jenkins DJ. Dietary fiber in management of diabetes.
Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose
American Diabetes Association. Nutrition recommendations and
promotes satiety in rats fed a high-fat diet: involvement of gluca-
principles for people with diabetes mellitus. Diabetes Care 2000;
gon-like Peptide-1. Obes Res 2005; 13: 1000-7.
23(Suppl 1): S43-6.
Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Bur-
Delzenne NM, Cani PD. A place for dietary fibre in the manage-
celin R. Improvement of glucose tolerance and hepatic insulin sen-
ment of the metabolic syndrome.
sitivity by oligofructose requires a functional glucagon-like peptide
Macfarlane S, Macfarlane GT, Cummings JH. Review article:
Delmee E, Cani PD, Gual G, Knauf C, Burcelin R, Maton N, et al.
prebiotics in the gastrointestinal trac
Relation between colonic proglucagon expression and metabolic
response to oligofructose in high fat diet-fed mice
Tuohy KM, Probert HM, Smejkal CW, Gibson GR. Using probiot-
ics and prebiotics to improve gut health.
Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Del-
zenne NM. Involvement of endogenous glucagon-like peptide-1(7-
Gibson GR, Roberfroid MB. Dietary modulation of the human
36) amide on glycaemia-lowering effect of oligofructose in strepto-
colonic microbiota: introducing the concept of prebiotics. J Nutr
1995; 125: 1401-12.
Gee JM, Johnson IT. Dietary lactitol fermentation increases circu-
Hord NG. Eukaryotic-microbiota crosstalk: Potential mechanisms
lating peptide YY and glucagon-like peptide-1 in rats and humans.
for health benefits of prebiotics and probiotics.
Nutrition 2005; 21: 1036-43.
Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG,
Flamm G, Glinsmann W, Kritchevsky D, Prosky L, Roberfroid M.
Todd E, et al. Effects of resistant starch, a non-digestible ferment-
Inulin and oligofructose as dietary fiber: A review of the evidence.
able fiber, on reducing body fat. Obesity (Silver Spring) 2006; 14:
Burcelin R, Cani PD, Knauf C. Glucagon-like peptide-1 and energy
Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, Xi X, Raggio
homeostasis. J Nutr 2007; 137: 2534S-8S.
AM, et al. Peptide YY and proglucagon mRNA expression patterns
Cani PD, Holst JJ, Drucker DJ, Delzenne NM, Thorens B, Burcelin
and regulation in the gut. Obesity (Silver Spring) 2006; 14: 683-9.
R, et al. GLUT2 and the incretin receptors are involved in glucose-
Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC,
induced incretin secretion.
Galmiche JP. Colonic fermentation influences lower esophageal
Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C,
sphincter function in gastroesophageal reflux disease. Gastroen-
et al. Brain glucagon-like peptide 1 signaling controls the onset of
terology 2003; 124: 894-902.
high-fat diet-induced insulin resistance and reduces energy expen-
Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose pro-
motes satiety in healthy human: a pilot study.
Knauf C, Cani PD, Kim DH, Iglesias MA, Chabo C, Waget A, et
al. Role of central nervous system glucagon-like Peptide-1 recep-
Archer BJ, Johnson SK, Devereux HM, Baxter AL. Effect of fat
tors in enteric glucose sensing.
replacement by inulin or lupin-kernel fibre on sausage patty ac-
Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal
ceptability, post-meal perceptions of satiety and food intake in
satiety signals.
Druce MR, Small CJ, Bloom SR. Minireview: Gut peptides regu-
Whelan K, Efthymiou L, Judd PA, Preedy VR, Taylor MA. Appe-
lating satiety.
tite during consumption of enteral formula as a sole source of nutri-
Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J
tion: the effect of supplementing pea-fibre and fructo-
Endocrinol 2005; 184: 291-318.
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove
Abrams SA, Griffin IJ, Hawthorne KM, Ellis KJ. Effect of prebi-
KL, et al. The distribution and mechanism of action of ghrelin in
otic supplementation and calcium intake on body mass index. J Pe-
the CNS demonstrates a novel hypothalamic circuit regulating en-
diatr 2007; 151: 293-8.
ergy homeostasis. Neuron 2003; 37: 649-61.
Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differ-
Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, et
ences in fecal microbiota composition in children may predict
al. Brain glucagon-like peptide-1 increases insulin secretion and
muscle insulin resistance to favor hepatic glycogen storage. J Clin
Lundell AC, Adlerberth I, Lindberg E, Karlsson H, Ekberg S,
Invest 2005; 115: 3554-63.
Aberg N, et al. Increased levels of circulating soluble CD14 but not
Goodlad RA, Lenton W, Ghatei MA, Adrian TE, Bloom SR,
CD83 in infants are associated with early intestinal colonization
Wright NA. Effects of an elemental diet, inert bulk and different
with Staphylococcus aureus.
types of dietary fibre on the response of the intestinal epithelium to
Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN,
refeeding in the rat and relationship to plasma gastrin, enterogluca-
Pischon T. Obesity, inflammation, and periodontal disease. J Dent
gon, and PYY concentrations
Res 2007; 86: 400-9.
Goodlad RA, Lenton W, Ghatei MA, Adrian TE, Bloom SR,
Saito T, Hayashida H, Furugen R. Comment on: Cani, et al. (2007)
Wright NA. Proliferative effects of 'fibre' on the intestinal epithe-
Metabolic endotoxemia initiates obesity and insulin resistance: Diabetes 56: 1761-1772. Diabetes 2007; 56: e20.
1558 Current Pharmaceutical Design, 2009, Vol. 15, No. 13
Cani and Delzenne
Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al.
Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty
The severity of periodontal disease is associated with the develop-
TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation
ment of glucose intolerance in non-diabetics: the Hisayama study. J
factor 88 reduces atherosclerosis and alters plaque phenotype in
Dent Res 2004; 83: 485-90.
mice deficient in
Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N
[109] Bjorkbacka H. Multiple roles of Toll-like receptor signaling in
Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J. Sys-
atherosclerosis.
temic exposure to Porphyromonas gingivalis predicts incident
Bjorkbacka H, Fitzgerald KA, Huet F, Li X, Gregory JA, Lee MA,
et al. The induction of macrophage gene expression by LPS pre-
Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J,
dominantly utilizes Myd88-independent signaling cascades. Physiol
Salomaa V. Endotoxemia, immune response to periodontal patho-
Genomics 2004; 19: 319-30.
gens, and systemic inflammation associate with incident cardiovas-
Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM,
cular disease events
Lee MA, et al. Reduced atherosclerosis in MyD88-null mice links
elevated serum cholesterol levels to activation of innate immunity
Smith BJ, Lightfoot SA, Lerner MR, Denson KD, Morgan DL,
signaling pathways.
Hanas JS, et al. Induction of cardiovascular pathology in a novel
Rault-Nania MH, Gueux E, Demougeot C, Demigne C, Rock E,
model of low-grade chronic inflammation.
Mazur A. Inulin attenuates atherosclerosis in apolipoprotein E-
deficient mice.
Smith BJ, Lerner MR, Bu SY, Lucas EA, Hanas JS, Lightfoot SA,
Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Na-
et al. Systemic bone loss and induction of coronary vessel disease
ruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels
in a rat model of chronic inflammati
upon supplementation of diet with Lactobacillus plantarum in sub-
Joyee AG, Yang X. Role of toll-like receptors in immune responses
jects with moderately elevated cholesterol.
to chlamydial infections.
Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling
Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska
and vascular inflammation: potential therapeutic targets in cardio-
H. Effect of Lactobacillus plantarum 299v on cardiovascular dis-
ease risk factors in smokers.
Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG,
Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T,
Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left
Zilmer M. Antioxidative probiotic fermented goats' milk decreases
ventricular remodeling and impairs cardiac function after myocar-
oxidative stress-mediated atherogenicity in human subjects. Br J
dial infarction.
Nutr 2003; 90: 449-56.
Michelsen KS, Arditi M. Toll-like receptor signaling and athero-
Source: http://www.cheongin.co.kr/shop/board/download.php?id=thesis&no=11&div=1
Wholesale Product Catalogue Professional Division Table of Contents About NaturTech . 3 Formulation Abbreviations . 4 Referral Program . 4 Dosage Information . 5 Understanding Dosage Ratios Children's Dosage Information Herbal Blends . 10 Herbal Drops . 13 Topical Herbal Blends . 14 Terms and Conditions . 15 Common - Latin Names . 16 Table of Contents NaturTech is a line of Canadian made remedies exclusively sold to health care professionals. We
PROTOCOLE/parcours de soins HYPERTENSION ARTERIELLE GRAVIDIQUE Références: Prise en charge multidisciplinaire des formes graves de Prééclampsie ; recommandations formalisées d'experts communes SFAR/CNGOF/SFMP/SFNN. HAS-Grossesses à risque : orientation des femmes enceintes entre les maternités en vue de RPC RCIU CNGOF 2013













