Doi:10.1016/j.joen.2007.02.012


ARTICLE IN PRESS Basic Research—Technology
Comparative Evaluation of Antimicrobial Efficacy ofSodium Hypochlorite, MTAD, and Tetraclean AgainstEnterococcus faecalis Biofilm
Luciano Giardino, MD, DDS,* Emanule Ambu, MD, DDS,† Enrico Savoldi, MD, DDS,*Roberto Rimondini, PhD, MS,‡ Clara Cassanelli, MD,§ and Eugenio A. Debbia, MD§
AbstractThe aim of this study was to compare the antimicrobialefficacy of 5.25% NaOCl, BioPure MTAD (Dentsply
the major causative factors associated with endodontic treatment failures In
Tulsa Dental, Johnson City, TN), and Tetraclean (Ogna
infected and necrotic root canal systems, bacteria grow mostly in sessile biofilms,
Laboratori Farmaceutici, Milano, Italy) against Entero-
aggregates, and coaggregates in which they are embedded in an extracellular matrix
coccus faecalis biofilm generated on cellulose nitrate
material Biofilms are disrupted, and the microbial load is reduced by mechan-
membrane filters. After incubation, the membrane fil-
ical instrumentation, irrigation with tissue-lytic and microbicidal solutions, and anti-
ters were transferred into tubes containing 5 mL of the
microbial medicaments in the root canal. Irrigants are used during the endodontic
selected antimicrobial solution test agent or NaCl 0.9%
treatment to flush out loose debris, to lubricate the dentinal walls, to dissolve organic
(positive control) and incubated for 5, 30, and 60
matter in the canal, and to have antimicrobial effects Different concentrations of
minutes at 20°C. After each period of time, the test
sodium hypochlorite (NaOCl) were used as root canal irrigants in the past 7 decades
agents were vortexed for 60 seconds to resuspend the
because of its well-known antimicrobial action and its ability to dissolve tissue
microorganisms. Ten-fold serial dilutions were gener-
Previous studies have shown that instrumentation and antibacterial irrigation with
ated in reduced transport fluid. Each dilution was
sodium hypochlorite would eliminate bacteria in 50% to 75% of infected root canals at
plated onto a brain heart infusion plates. The plates
the end of the first treatment session, whereas the remaining root canals contain recov-
were then incubated for 48 hours in an aerobic atmo-
erable bacteria In their study Nair et al. showed that 88% of root canal–
sphere at 37°C and colony-forming units per membrane
treated mandibular molars revealed residual infection of mesial roots after instrumen-
was calculated. Statistical analysis showed that only
tation, irrigation with NaOCl, and obturation in a one-visit treatment. Because of these
5.25% NaOCl can disgregate and remove the biofilm at
limitations, a better root canal irrigant is still being searched for.
every time; however, treatment with Tetraclean caused
BioPure MTAD (Dentsply Tulsa Dental, Johnson City, TN) has been described as a
a high degree of biofilm disgregation in every consid-
universal irrigating solution Torabinejad et al. have shown that MTAD is able
ered time intervals as compared with MTAD (T5
to safely remove the smear layer and that it is effective against Enterococcus faecalis,
p ⬍ 0.05, T30 p ⬍ 0.01, and T60 p ⬍ 0.001). (J Endod
and it can also eliminate bacteria in human root canals that had been infected by whole
saliva A new irrigant, Tetraclean (Ogna Laboratori Farmaceutici, Milano, Italy),has been developed. It is a mixture of doxycycline hiclate at a lower concentration than
MTAD, an acid, and detergents. It is able to eliminate microorganisms and smear layer
Biofilm, Enterococcus faecalis, irrigants
in dentinal tubules of infected root canals with a final 4-minute rinse. E faecalis hasbeen often isolated from teeth with endodontic failed treatments Consequently,recent laboratory studies have focused on evaluating the effectiveness of root canalirrigants and medicaments against E faecalis. Many of these studies have grown the
From the *Department of Periodontology, Dental School,
bacterial strains as planktonic cultures (bacteria in suspension) However, plank-
University of Brescia, Brescia, Italy; †Department of Endodon-
tonic bacteria do not usually comply with the in vivo growth condition found in an
tics, Dental School, University of Modena e Reggio Emilia,Modena, Italy; ‡Department of Pharmacology, University of
infected tooth in which bacteria grow as a biofilm on the dentinal wall Bacterial
Bologna, Bologna, Italy; and §Department of Microbiology,
biofilm is made by bacteria in sessile form embedded in a polysaccharide matrix. This
Dental School, University of Genova, Genova, Italy.
structure makes it more difficult for drugs to reach bacteria. Therefore, all studies about
Address requests for reprints to Dr. Luciano Giardino,
the "clinical" action of endodontic irrigants should be conducted with bacteria in
Via Marinella 12, 88900 Crotone, Italy. E-mail address:
"biofilm form." Up to now, however, very few studies about the action of antimicrobic
[email protected].
0099-2399/$0 - see front matter
irrigants against biofilm have been published. As a result, recent laboratory studies have
Copyright 2007 by the American Association of
attempted to evaluate the efficacy of antimicrobial agents used in root canal treatment
against E faecalis grown as a biofilm The aim of this study was to assess the
antimicrobial efficiency of endodontic irrigants against E faecalis biofilms.
Materials and Methods
The methodology used was adapted from Spratt et al. with some modifica-
tions. Biofilms of E faecalis strain ATCC 29212 were generated on cellulose nitratemembrane filters. An overnight culture of E faecalis grown in brain heart infusion(BHI) broth (Difco Co; Becton Dickinson, Sparks, MD), adjusted to 0.5 Mc Farland
JOE — Volume xx, Number x, Month 2007
Enterococcus faecalis Biofilm


ARTICLE IN PRESS
variances revealed that data were normally distributed (Cochran, Bart-lett test; T5 p ⫽ 0.4, T30 p ⫽ 0.67, and T60 p ⫽ 0.35). Statistical
significance was set at p ⬍ 0.05. The overall analysis of variance re-vealed a highly significant either for treatment effect (F[3,8,rsqb] ⫽177.87, p ⬍ 0.00) or for interaction effects (treatment ⫻ time)(F[6,16] ⫽ 5.86, p ⬍ 0.002).
Bonferroni post hoc analysis showed that treatment with Tetra-
clean induced an increase in biofilm disgregation in every consideredtime intervals compared with the positive control NaCl 0.9% (T5 p ⬍0.01, T30 p ⬍ 0.001, and T60 p ⬍ 0.001), MTAD (T5 p ⬍ 0.05, T30p ⬍ 0.01, and T60 p ⬍ 0.001), and NAClO 5% (T5 p ⬍ 0.0001, T30p ⬍ 0.00001, and T60 p ⬍ 0.00001).
In any natural environment, bacteria show the tendency to aggre-
gate in adherent microbic communities. The biofilm formation ispresent on any surface that comes in contact with natural liquids. Theformation of biofilms follows the same stages starting with theformation of a conditioning film; the adhesion of planktonic bacteria
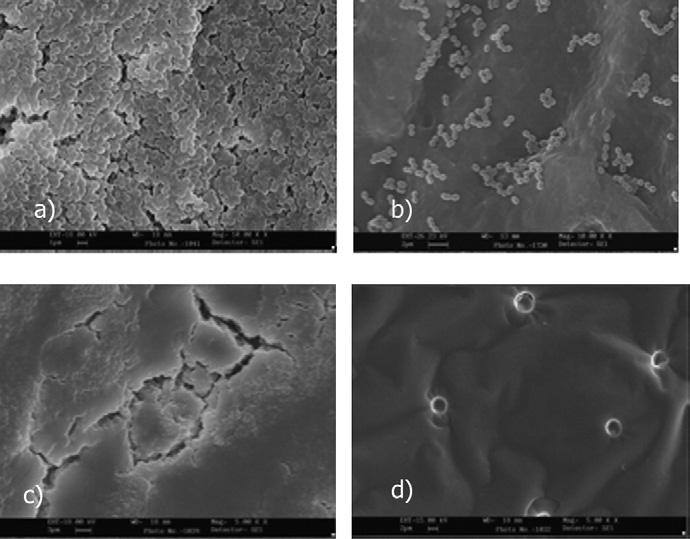
Figure 1. (a) E faecalis biofilm on cellulose nitrate membrane filter after 48
takes place on this surface, and this attachment may be strengthened
hours of incubation (original magnification ⫻10,000). (b) E faecalis biofilm
through polymer production and the unfolding of cell-surface struc-
after 30 minutes of contact with Tetraclean (original magnification ⫻5,000).
tures. In the third stage, the biofilm is already formed, and, in its growth,
(c) Undisgregated biofilm after 30 minutes of contact with MTAD (originalmagnification ⫻5,000). (d) Biofim completely removed after 30 minutes of
a continuous detachment of bacteria to the planktonic phase is present.
contact with 5.25% NaOCl (original magnification ⫻5,000).
These detached cells serve as a steady source for chronic infection This phenomenon starts after initial adhesion, and the number of bac-teria released is related to the number of bacteria forming the biofilm.
scale (1 ⫻ 108 CFU/mL), was used. An aliquot of 20 L of E faecalis
Moreover, the stage of biofilm is a defense of the microbic community
was seeded onto 13.0-mm diameter cellulose nitrate membrane filters
against host defenses and against antimicrobic agents Central to
(0.22-m pore diameter; Millipore Corporation, Bedford, MA), which
the theme of biofilm control is the use of surfactants, antimicrobial
were placed on the surfaces of BHI agar plates. Nine membranes were
agents, and preservatives. The microbic communities grown in biofilm
used for each plate. Plates containing membranes were then incubated
are remarkably difficult to eradicate with antimicrobial agents; bacteria
for 48 hours at 37°C in an aerobic atmosphere. The efficiency of the
in mature biofilm can resist the action of antibacterial irrigants, but the
method for biofilm generation was observed in a pilot study visually and
reasons for this resistance are not completely understood. Bacteria in
by scanning electron microscopy (SEM) After incubation,
biofilm are 2- to 1,000-fold more resistant than the corresponding
membrane filters were removed aseptically from the agar plate and
bacteria in planktonic form Biofilm bacteria may also show a
transferred carefully to avoid any disruption of the biofilm into tubes
different phenotype that could increase an enhanced resistance, and
containing 5 mL of the selected antimicrobial solution test agent, NaCl
this could be because of different metabolic pathways. Biofilm bacteria
0.9% (positive control), and 5.25% NaOCl (negative control) and in-
might not express the drug target; they have been found to be more
cubated for 5, 30, and 60 minutes at 20°C. After each period of time, the
resistant to amoxicillin, doxycycline, and metronidazole.
membrane filters were then carefully transferred aseptically into tubes
Furthermore, the "biofilm model"seems to be more realistic than
containing 5 mL of neutralizing broth (D/E Neutralizing Broth, Difco
the "direct contact method" to test antimicrobic agents. Spratt et al.
Co) for 5 minutes to stop the antimicrobial action of the test agents and
used a simple model to evaluate the activity of several irrigants
vortexed for 60 seconds to resuspend the microorganisms. Ten-fold
against 5 different root canal bacterial isolates. This study reported that
serial dilutions were generated in reduced transport fluid. Each dilution
sodium hypochlorite is the most effective agent tested.
was plated onto BHI plates. The plates were then incubated for 48 hours
In a study published by Sena et al. the capability of different
in an aerobic atmosphere at 37°C and colony-forming units (CFU) per
irrigants with andwithout agitation against 5 strains of bacteria was
membrane were calculated. Controls were exposed to sterile saline forthe same periods. Three replicates were performed for each antimicro-bial agent and control.
The results of this study are shown in 5.25% NaOCl was
the only irrigant capable of removing biofilm after only 5 minutes,whereas the same effect was reached by Tetraclean after 60 minutes.
Biopure MTAD was unable to reach this goal at every considered time.
Furthermore, the use of Tetraclean was able to reduce 90% bactericload after 5 minutes and ⬎99.9% after 30 minutes of application. Thebacterial load reduction using Biopure MTAD has not been significantafter 5 minutes, whereas it has been much lower after 30 minutes than
Figure 2. Bonferroni post hoc analysis showed that treatment with Tetraclean
using Tetraclean or 5.25% NaOCl.
induced an increase in biofilm disgregation in every considered time intervals
Statistical analysis was performed by using two-way analysis of
compared with the negative control NaCl 0.9% (T5 p ⬍ 0.01, T30 p ⬍ 0.001, and
variance followed by Bonferroni post hoc test. A test of homogeneity of
T60 p ⬍ 0.001) and MTAD (T5 p ⬍ 0.05, T30 p ⬍ 0.01, and T60 p ⬍ 0.001).
Giardino et al.
JOE — Volume xx, Number x, Month 2007


ARTICLE IN PRESS Basic Research—Technology
investigated. In this study, all strains were eliminated by 5.25% sodium
different components of the mixture rather than the concentration of
hypochlorite after 30 seconds, both with or without agitation, whereas
antibiotics. In any case, only 5.25% NaOCl seems to be able to remove
2.5% sodium hypochlorite showed the same action against E faecalis
completely the biofilm organized on the surface of the membrane,
after 5 minutes only for E faecalis the "agitation group." E faecalis, a
whereas "new" irrigants fail in this action; Tetraclean, compared with
saprophytic component of the enteric flora, is the bacteria most com-
MTAD BioPure, shows a better action, but the goal of the total disap-
monly isolated in endodontic retreatment of apical periodontitis
pearance of the biofilm is reached only after 30 to 60 minutes of irri-
where it is often isolated either as a monoinfection or mixed with one or
gation. This is too long for a clinical use of this irrigant; however,
more species. According to Molander et al. E faecalis can survive
Tetraclean seems to cause a valid reduction in bacteria after only 5
in a quiescent phase with low metabolic activity for a period of time.
Distel et al. have shown that in both short-term and long-term
According to this work, further studies should be performed to
incubation periods, E faecalis colonized medicated root canals with
understand the correct action and the correct sequence of different
possible biofilm formation in the long-term experiments. This is the
irrigants against bacteria both in the plaktonic phase and organized in
reason why E faecalis is often used in studies regarding the efficiency of
biofilm on the surface of the root canal wall or inside the dentinal
the endodontic irrigants in cleaning the root canal system. Two new
irrigants, MTAD BioPure and Tetraclean, have recently been proposedas the final rinse in endodontic treatment. Both these irrigants are amixture of doxycycline, citric acid, and a surfactant. Using the "direct
method," a study made by measuring zones of inhibition on agar plates
The authors want to thank Mrs Roberta Penna and Mrs Angelica
has shown that MTAD was as effective as 5.25% NaOCl in eradicating
Mercurio Ciampi for their help in the draft of this article.
A final rinse of MTAD, used in combination with 1.3% NaOCl as theroot canal irrigant, seems to be significantly more effective than 5.25%
NaOCl in disinfecting root canal systems contaminated with whole salivaor with E faecalis Furthermore, when the "biofilm method"
1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental
is used, BioPure MTAD efficacy in root canal disinfection seems to be
pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol1965;20:34 –5.
much lower. Dunavant et al. have shown that only NaOCl is able to
2. Nair PNR. Light and electron microscopic studies of root canal flora and periapical
kill the whole bacteria population organized in biofilm and that its
lesions. J Endod 1987;13:29 –39.
activity is strictly correlated to its concentration. The percentage of
3. Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C. The application of biofilm
bacteria killed by BioPure MTAD was only 16.08%.
science to the study and control of chronic bacterial infections. J Clin Invest2003;112:1466 –77.
These findings could be correlated with the bacteriostatic activity
4. Siqueira JF, Rôças IN, Santos SRLD, Lima KC, Magalhães FAC, de Uzeda M. Efficacy of
of doxycycline; therefore, no destructive activity of this mixture may be
Instrumentation techniques and irrigation regimens in reducing the bacterial pop-
present. Kho and Baumgartner compared the action of 1.3%
ulation within root canals. J Endod 2002;28:181– 4.
NaOCl/BioPure MTAD versus 5.25% NaOCl/17% EDTA in the apical 5
5. Walker A. Definitive and dependable therapy for pulpless teeth. J Am Dent Assoc
mm of teeth infected with E faecalis. After irrigation, the root canal
1936;23:1418 –25.
6. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium
apexes were resected and pulverized to expose E faecalis in dentinal
hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol 1983;55:
tubules. In this study, there were no differences in antimicrobial efficacy
for irrigation with 5.25% NaOCl/17% EDTA versus 1.3% NaOCl/BioPure
7. Peters LB, van Winkelhoff AJ, Buijs JF, Wesselink PR. Effects of instrumentation,
MTAD in 5-mm apical dentinal tubules infected with E faecalis. In a
irrigation and dressing with calcium hydroxide on infection in pulpless teeth withperiapical bone lesions. Int Endod J 2002;35:13–21.
recent study, Clegg et al. reported that only 6% and 3% NaOCl were
8. Nair PNR, Henry S, Cano V, Vera J. Microbial status of apical root canal system of
capable of disrupting and removing the biofilm, but only the 6% con-
human mandibular first molars with primary apical periodontitis after "one-visit⬙
centration was capable of both rendering bacteria nonviable and phys-
endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;
ically removing the biofilm; 1.6% NaOCl/BioPure MTAD was capable of
disrupting but not eliminating bacteria. Tetraclean has shown a good
9. Torabinejad M, Khademi AA, Babagoli J, et al. A new solution for the removal of the
smear layer. J Endod 2003;29:170 –5.
antimicrobial activity when used against bacteria in the planktonic
10. Torabinejad M, Shabahang S, Aprecio R, Kettering JD. The antimicrobial effect of
phase but it seems to also have an excellent antimicrobial activity
MTAD: An in vitro investigation. J Endod 2003;29:400 –3.
against E faecalis in an "ex vivo" model Tetraclean has shown the
11. Shabahang S, Pouresmail M, Torabinejad M. In Vitro antimicrobial efficacy of MTAD
lowest value of surface tension, and this could increase the adaptation of
and sodium hypochlorite. J Endod 2003;29:450 –2.
12. Chavez De Paz LE, Dahlen G, Molander A, Möller A, Bergenholtz G. Bacteria recovered
the mixture to dentinal walls and to biofilm As seen in the results
from teeth with apical periodontitis after antimicrobial endodontic treatment. Int
of other studies, even in the present study, 5.25% NaOCl was the only
Endod J 2003;36:500 – 8.
irrigant capable of eliminating biofilm at every tested time, whereas
13. Abdullah M, Ng YL, Gulabivala K, Moles DR, Spratt DA. Susceptibilities of two Entero-
MTAD BioPure seems to be unable to remove biofilm at any tested time.
coccus faecalis phenotypes to root canal medications. J Endod 2005;31:30 – 6.
Tetraclean was able to eliminate biofilm after 60 minutes of con-
14. Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial
efficacy of irrigants on biofilms of root canal isolates. Int Endod J 2001;34:300 –7.
tact, but its action of disrupting the biofilm was statistically better in
15. Svensäter G, Bergenholtz G. Biofilms in endodontic infections. Endod Top
every considered time interval compared with the NaCl 0.9% (positive
control) and the MTAD one. Moreover, it must be emphasized that
16. Costerton JW. Cystic fibrosis pathogenesis and the role of biofilms in persistent
Tetraclean removes 90% of the bacteria present in biofilm after 5 min-
infection. Trends Microbiol 2001;9:50 –2.
17. Mah TFC, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents.
utes of contact and ⬎99% after 30 minutes. According to Torabinejad
Trends Microbiol 2001;9:34 –9.
et al. the action against bacteria of these irrigants should be
18. Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobial. Adv Dent Res
caused by the doxycycline present in the mixture, but this is a bacteri-
1997;11:160 –7.
ostatic antibiotic and it cannot kill bacteria. Moreover, BioPure MTAD
19. Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet
contains a quantity of doxycyclin three times greater than Tetraclean,
2001;358:135– 8.
20. Sena NT, Gomes BPFA, Vianna E, et al. In vitro antimicrobial activity of sodium
but it shows a lower effect in removing biofilm; the better action of the
hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J
latter in disrupting biofilm should be caused by the synergic effect of
2006;39:878 – 85.
JOE — Volume xx, Number x, Month 2007
Enterococcus faecalis Biofilm

ARTICLE IN PRESS
21. Haapasalo M, Endal U, Zandi H, Coil JM. Eradication of endodontic infection by
27. Clegg MS, Vertucci FJ, Walker C, Belanger M, Britto LR. The effect of exposure to
instrumentation and irrigant solutions. Endod Top 2005;10:77–102.
irrigant solution on apical dentin biofilms in vitro. J Endod 2006;32:434 –7.
22. Molander A, Reit AC, Dahlen G, Kvist T. Microbiological status of root-filled teeth with
28. Giardino L, Ambu E, Generali L, Savoldi E. Effetto antimicrobico di due nuovi irriganti
apical periodontitis. Int Endod J 1998;31:1–7.
nei confronti dell'Enterococcus faecalis: studio comparativo in vitro. G Ital Endod
23. Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J
2006;20:91– 4.
Endod 2002;28:689 –93.
29. Rimoldi C, Ardizzoni A, Neglia R, Blasi E, Generali L, Giardino L. In vitro and ex vivo
24. Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis— contaminated
studies on the antibacterial efficacy of sodium hypochlorite and two new-generation
root canals of extracted human teeth. J Endod 2003;29:576 –9.
endodontic irrigants, Tetraclean and MTAD, in comparison with sodium hypochlo-
25. Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of
rite. G Ital Endod 2006;20:37– 8.
endodontic irrigants against Enterococcus faecalis biofilms. J Endod 2006;32:527–31.
30. Giardino L, Ambu E, Becce C, Rimondini L, Morra M. Surface tension comparison of
26. Kho P, Baumgartner JC. A comparison of antimicrobial efficacy of NaOCl/Biopure MTAD
four common root canal irrigants and two new irrigants containing antibiotic. J
versus NaOCl/EDTA against Enterococcus faecalis. J Endod 2006;32:652–5.
Giardino et al.
JOE — Volume xx, Number x, Month 2007
Source: http://www.endodonzia.it/wp-content/uploads/allegatiforum/luciano/2007527202814_biofilm.pdf
Second, updated edition Maija Saxelin, Ph.D. Published byValio Ltd, R&DP.O. Box 30, FIN-00039Helsinki, FinlandTel. +358 10 381 121Fax +358 10 381 3019http://www.valio.com Lay-out by Imageneering Printed in Finland by Hämeen Kirjapaino Oy2002 © Valio Ltd 2002 Table of contents
Time to Register for Religious Educa- tion Classes for 2008/09 - R.E. Classes forGrades 1 - 6 will meet on Tuesday, Wednesday, and July 26 - August 3 Thursday afternoons from 3:45 - 4:45 PM. Grades 7& 8 will meet on Thursdays from 6 - 7 PM with 7th Seventeenth Sunday of Ordinary Time and 8th Grade Youth Group to follow from 7- 8:30















