Faculty.olin.edu
NIH Public Access
Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
NIH-PA Author Manuscript
Published in final edited form as:
Prog Polym Sci. 2007 ; 32(8-9): 991–1007. doi:10.1016/j.progpolymsci.2007.05.013.
Silk as a Biomaterial
Charu Vepari and
David L. Kaplan
Departments of Chemical & Biological Engineering and Biomedical Engineering, Tufts University,
4 Colby St, Room 153, Medford, MA 02155, Tel: 617-627-3251, Fax: 617-627-3231, Email:
[email protected]
Silks are fibrous proteins with remarkable mechanical properties produced in fiber form by silkwormsand spiders. Silk fibers in the form of sutures have been used for centuries. Recently regenerated silksolutions have been used to form a variety of biomaterials, such as gels, sponges and films, for medicalapplications. Silks can be chemically modified through amino acid side chains to alter surface
NIH-PA Author Manuscript
properties or to immobilize cellular growth factors. Molecular engineering of silk sequences has beenused to modify silks with specific features, such as cell recognition or mineralization. Thedegradability of silk biomaterials can be related to the mode of processing and the correspondingcontent of beta sheet crystallinity. Several primary cells and cell lines have been successfully grownon different silk biomaterials to demonstrate a range of biological outcomes. Silk biomaterials arebiocompatible when studied
in vitro and
in vivo. Silk scaffolds have been successfully used in woundhealing and in tissue engineering of bone, cartilage, tendon and ligament tissues.
Silk; Fibroin; Spidroin; Scaffold; Tissue Engineering; Biomaterial
Silk, popularly known in the textile industry for its luster and mechanical properties, isproduced by cultured silkworms. Silks are produced by members of the class Arachnida (over30,000 species of spiders) and by several worms of the order Lepidoptera, which includes
NIH-PA Author Manuscript
mites, butterflies and moths. Silk are fibrous proteins synthesized in specialized epithelial cellsthat line glands in these organisms [1].
Silk fibroin polymers consist of repetitive protein sequences and provide structural roles incocoon formation, nest building, traps, web formation, safety lines and egg protection [1,2].
Silks are generally composted of β-sheet structures due to the dominance of hydrophobicdomains consisting of short side chain amino acids in the primary sequence. These structurespermit tight packing of stacked sheets of hydrogen bonded anti-parallel chains of the protein.
Large hydrophobic domains interspaced with smaller hydrophilic domains foster the assemblyof silk and the strength and resiliency of silk fibers [3].
Correspondence to: David L. Kaplan.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers
we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting
proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could
affect the content, and all legal disclaimers that apply to the journal pertain.
Vepari and Kaplan
Silks from silkworms (e.g.,
Bombyx mori) and orb-weaving spiders (e.g.,
Nephila clavipes)have been explored to understand the processing mechanisms and to exploit the properties of
NIH-PA Author Manuscript
these proteins for use as biomaterials. Silks from silkworms and orb-weaving spiders haveimpressive mechanical properties (Table 1), in addition to environmental stability,biocompatibility, controlled proteolytic biodegradability, morphologic flexibility, and theability for amino acid side change modification to immobilize growth factors [2,4–15].
Biomaterial design is an important element of tissue engineering, incorporating physical,chemical, and biological cues to guide cells into functional tissues via cell migration, adhesion,and differentiation. Many biomaterials need to degrade at a rate commensurate with new tissueformation to allow cells to deposit new extracellular matrix (ECM) and regenerate functionaltissue. In addition, biomaterials may need to include provisions for mechanical supportappropriate to the level of functional tissue development. In general, biomaterials must bebiocompatible and elicit little to no host immune response.
Thus, silks have been investigated as biomaterials due to the successful use of silk fibers from
B. mori as suture material for centuries [16]. Functional differences among silks of differentspecies and within a species are a result of structural differences due to differences in primaryamino acid sequence, processing, and the impact of environmental factors [17]. Silks representa unique family of structural proteins that are biocompatible, degradable, mechanicallysuperior, offer a wide range of properties, are amenable to aqueous or organic solvent
NIH-PA Author Manuscript
processing, and can be chemically modified to suit a wide range of biomedical applications.
2. Silkworm (Bombyx mori) Silk
The domesticated silkworm (
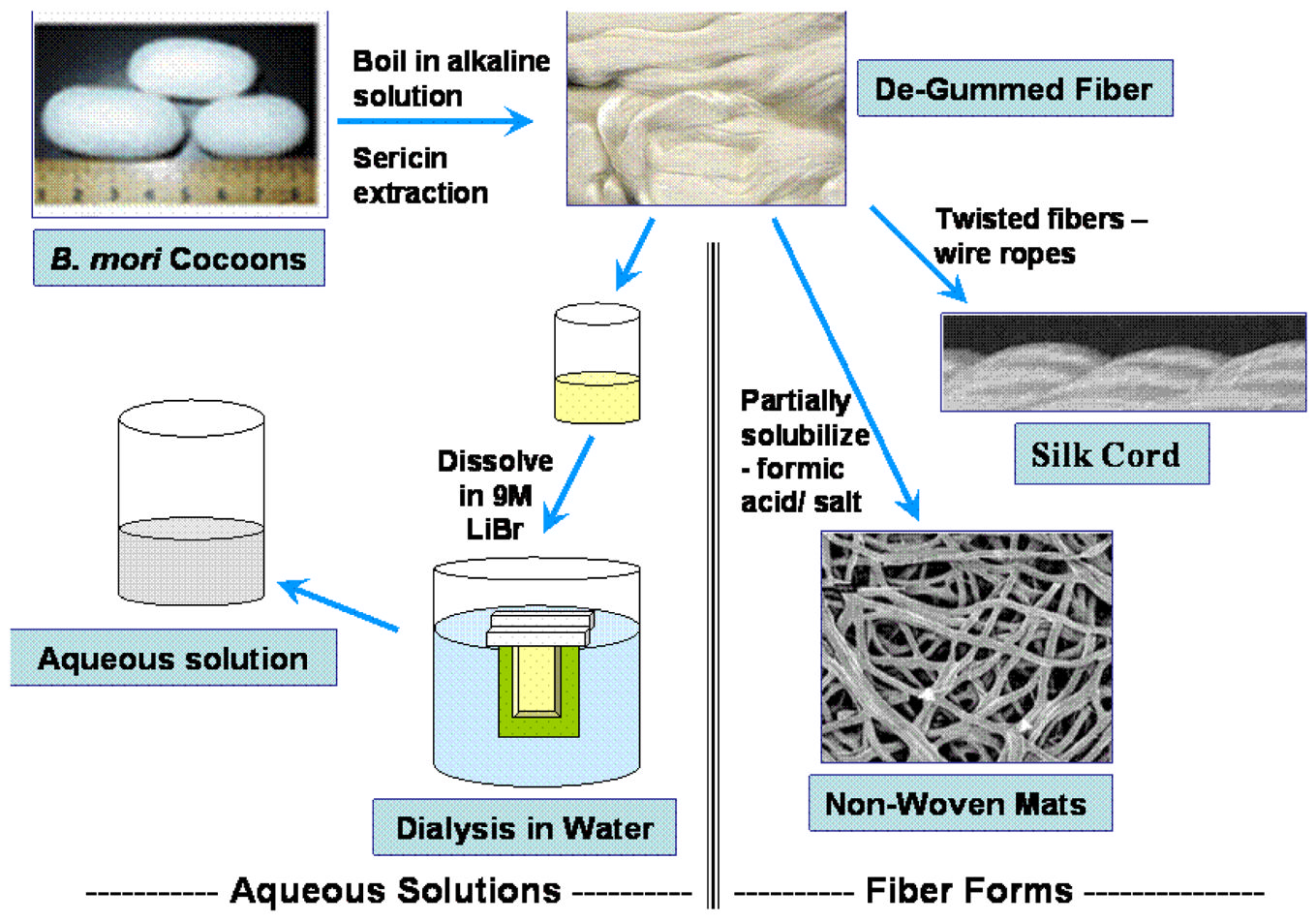
B. mori) silk fibroin fibers are about 10–.25 μm in diameter andconsist of two proteins: a light chain ( 26 kDa) and heavy chain ( 390 kDa) which are presentin a 1:1 ratio and linked by a single disulfide bond [18]. These proteins are coated with a familyof hydrophilic proteins called sericins (20–310 kDa) [1,18–20]. The disulfide linkage betweenthe Cys-c20 (twentieth residue from the carboxyl terminus) of the heavy chain and Cys-172of the light chain holds the fibroin together and a 25 kDa glycoprotein, named P25, is non-covalently linked to these proteins [21]. Silk fibroin is purified from sericins by boiling silkcocoons in an alkaline solution (Figure 1A). Twenty-five to thirty percent of the silk cocoonmass is sericin, which is removed during the de-gumming process.
2.1 B. mori silk fibroin structure
The amino acid composition of silk fibroin from
B. mori consists primarily of glycine (Gly)(43%), alanine (Ala) (30%) and serine (Ser) (12%) [1]. The heavy chain consists of 12 domains
NIH-PA Author Manuscript
that form the crystalline regions in silk fibers, which are interspersed with primary sequencethat is non-repetitive and thus forms fewer organized domains in the fibers. The crystallinedomains in the fibers consist of Gly-X repeats, with X being Ala, Ser, Threonine (Thr) andValine (Val) [22]. The crystalline forming domains consist of an average of 381 residues (596in size in the seventh domain to 36 in the twelfth domain). Each domain consists of sub-domainhexapeptides including: GAGAGS, GAGAGY, GAGAGA or GAGYGA where G is Glycine,A is Alanine, S is Serine and Y is Tyrosine. These sub domains end with tetrapeptides such asGAAS or GAGS [18,22,23]. The less crystalline forming regions of the fibroin heavy chain,also known as linkers, are between 42–44 amino acid residues in length. All the linkers havean identical 25 amino acid residue (non-repetitive sequence) which is composed of chargedamino acids not found in the crystalline regions [22]. The primary sequence results in ahydrophobic protein with a natural co-block polymer design. Efficient secretion of fibroin isbelieved to be due in part to the formation of a disulfide bond between the heavy and lightfibroin chains. A naked pupa mutation in
B. mori has been mapped to the same locus as of thelight chain on the 14th chromosome. The resulting fibroin light chain does not have a disulfide
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
bond with the fibroin heavy chain and the cocoon has less than 0.3% fibroin protein content[24].
NIH-PA Author Manuscript
A number of silk polymorphs have been reported, including the glandular state prior tocrystallization (silk I), the spun silk state which consists of the β-sheet secondary structure (silkII), and an air/water assembled interfacial silk (silk III, with a helical structure) [1,25,26]. Thesilk I structure is the water-soluble state and upon exposure to heat or physical spinning easilyconverts to a silk II structure. The silk I structure is observed in vitro in aqueous conditionsand converts to a β-sheet structure when exposed to methanol or potassium chloride [27]. Theβ-sheet structures are asymmetrical with one side occupied with hydrogen side chains fromglycine and the other occupied with the methyl side chains from the alanines that populate thehydrophobic domains. The β-sheets are arranged so that the methyl groups and hydrogengroups of opposing sheets interact to form the inter-sheet stacking in the crystals. Stronghydrogen bonds and van der Waals forces generate a structure that is thermodynamically stable[1]. The inter- and intra-chain hydrogen bonds form between amino acids perpendicular to theaxis of the chains and the fiber [1]. The silk II structure excludes water and is insoluble inseveral solvents including mild acid and alkaline conditions, and several chaotropes.
2.2. Control of morphology of silk biomaterials
Due to the well-established sericulture process, 400,000 tons of dry silkworm cocoons are
NIH-PA Author Manuscript
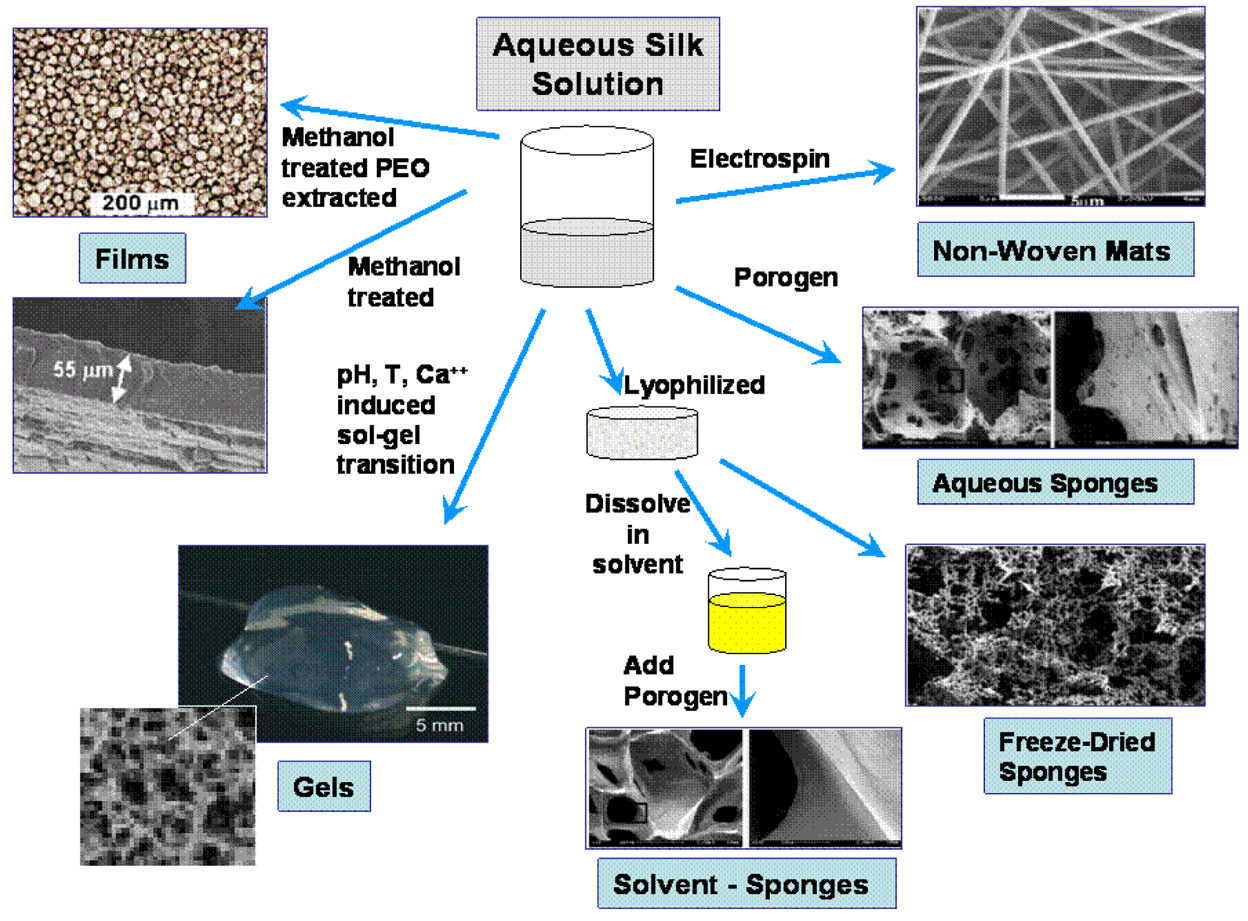
available worldwide per annum for the textile industry [28] and thus for biomaterialsapplications. Several different material morphologies can be formed from aqueous or solventformulations of the natural fiber form of silk for utilization in biomaterials for biomedicalapplications (Figure 1B and Table 2). The fibers must first be dissolved in aqueous systems,followed by reprocessing into desired material formats.
2.2.1 Silk fibers—Silk fibers can be obtained by reeling from cocoons [1]. Sutures braided
from silk fibers have been used for centuries in gummed (virgin) and de-gummed (black braided
silk) forms as sutures for surgical options [4]. A thorough review of the utilization of virgin
and black braided silk (coated with silicone or wax to prevent fraying) and associated immune
responses has been previously described [4]. Silk sutures have been used for tendon tissue
engineering [29]. Sutures modified with immobilized Arg-Gly-Asp (RGD) peptide to increase
cell attachment, were cultured with human tenocytes and supported increased adhesion after
3 days when compared with unmodified silk fiber and tissue cultured plastic [29]. An increase
in collagen type I and decorin transcript levels was observed on the RGD-modified sutures
compared with unmodified silk and tissue culture plastic at six weeks [29]. Textile engineering
techniques with silk fibers were used to generate biomaterial replacements for ligaments. A
wire rope design was studied to generate silk protein devices with mechanical strength
NIH-PA Author Manuscript
equivalent to the human anterior cruciate ligament (ACL). Human bone marrow stromal cells(hMSCs) and fibroblasts obtained from ligaments seeded on these silk fibroin wire ropesattached and proliferated [30]. Cells cultured with mechanical stimulation expressed collagentypes I and III and tenascin C characteristic of human ligaments [4,31].
2.2.1 Non-woven silk fibroin mats—Non-woven mats are of interest as biomaterials due
to the increased surface area and rougher topography for cell attachment. Silk fibroin has been
used to generate non-woven silk mats from reprocessed native silk fibers or by electrospinning.
Non-woven silk fibroin mats were prepared by partial solubilization of native silk fibers,
usually in formic acid and small amounts of calcium chloride. The mats were washed and
planted subcutaneously in rats, where they demonstrated good biocompatibility. Histology,
mRNA transcript levels, and immunohistochemistry all suggest that the silk fibroin non-woven
mat guided the formation of vascularized reticular connective tissue [32].
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Silk fibers can also be treated by homogenization to achieve similar outcomes [33]. Silk fibroinmats 10–30 μm in diameter and pores of about 300 μm in diameter? were obtained. These non-
NIH-PA Author Manuscript
woven mats were studied with a variety of cells, including keratinocytes, fibroblasts,osteoblasts, and cell lines from epithelial lung, colon, and cervical carcinomas for up to 7 weeks.
No degradation of the silk fibers was observed during culture [33], possibly due to lowinfiltration of the cells within the matrix. Endothelial cells (both primary and transformed),when cultured on the silk fibroin mats, attached and proliferated. This outcome improved witha coating of collagen type I or fibronectin, most likely due to the presence of RGD sequencesavailable for cell binding. Endothelial cells cultured for a week formed microvessel-likestructures on the nonwoven mats [34].
Electrospun fibers can be produced in a wide range of diameters, ranging from a fewnanometers to a few microns depending on mode of processing [35]. Electrospinning fromaqueous silk fibroin solution mixed with poly(ethylene oxide) (PEO) was established and fibermorphology based on scanning electron microscopy (SEM) analysis showed uniform fibersless than 0.8 μm in diameter [36]. hMSC's cultured on these mats showed attachment andspreading [37]. Atomic force microscopy nano-indentation was used to analyze the mechanicalproperties of the fibers. Electrospun silk fibers prepared from silk fibroin and poly(ethyleneoxide) (PEO) had a lateral modulus of 8 GPa, compared with 13.6 GPa for native silk fibers[38]. Silk fibroin mats prepared from formic acid with fiber diameters averaging 100 nm
NIH-PA Author Manuscript
showed a Young's modulus of 515 MPa, ultimate tensile strength (UTS) of 7.25 MPa and strainof 3% [39].
Electrospun non-woven meshes can be prepared as predominately random coil structure fromwhich β-sheet structures can be formed via methanol treatment [40]. The porosity changedfrom 76 to 68% due to dehydration after methanol treatment of these non-woven meshes[40]. Silk fibroin non-woven mats prepared using formic acid contained? 30–120 nm diameterfibers. Fibroblasts were cultured on the non-woven meshes and attached on silk and silk fiberscoated with collagen type I, fibronectin, and laminin [40].
Blends of silk fibroin-PEO have been electrospun into nanoscale diameter fibers for thedelivery of cell morphogens like bone morphogenetic protein-2 (BMP-2) [11]. BMP-2 is amorphogen which induces osteogenesis from mesenchymal stem cells. Nanoparticles ofhydroxyapaptite were added with or without BMP-2 and the mats were seeded with hMSCsand grown under osteogenic conditions. Differentiation was evaluated by calcium depositionand transcript levels of osteogenic markers. The silk fibroin mats supported the differentiationof the hMSCs to bone-like cells, and the mats formed with hydroxyapaptite and BMP-2 showedthe most calcium deposition and upregulation of osteogenic markers. This difference was
NIH-PA Author Manuscript
attributed to the availability and release of BMP-2. Electrospun mats with hydroxyapatite alsoinduced significant osteogenesis and the upregulation of BMP-2 transcript [11]. Silk fibroinnon-woven mats electrospun from a 98% silk formic acid solution were implanted in calvarialdefects of rabbits for bone regeneration and resulted in complete healing with new bone at 12weeks [41].
Aside from biologically generated bone as above, options to control hydroxyapatitemineralization on silk biomaterial matrices have also been reported [42]. In nature, the organic/inorganic interface gives rise to unique material properties. Silk fibroin, when blended withadded poly(L-aspartate), was successfully used as a template for the growth of apatite crystals[42].
2.2.2 Silk fibroin films—Silk fibroin films have been cast from aqueous or organic solvent
systems, as well as after blending with other polymers. Silk films prepared from aqueous silk
fibroin solution had oxygen and water vapor permeability dependent on the content of silk I
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
and silk II structures [43,44]. Alteration of silk structure was induced by treatment with 50%methanol for varying times. Changes in silk structure resulted in differing mechanical and
NIH-PA Author Manuscript
degradability properties of the films [44]. Nanoscale silk fibroin films can also be formed fromaqueous solution using a layer-by-layer technique (Wang et al., 2005). These ultrathin filmswere stable due to hydrophobic interactions and predictable film thickness could be obtainedbased on control of solution conditions. The films supported hMSC adhesion and proliferation[45].
Microstructures in films, which are advantageous for increasing surface roughness for cellattachment, were formed via blending of silk with poly(ethylene oxide) (PEO) [46]. The roughsurfaces were exposed by extracting the PEO with water, after locking in the beta sheetcrystallinity with methanol [46]. The roughness was directly related to the content of PEO usedin the process.
Fibroblast attachment to silk films has been shown to be as high as for collagen films [12,47]. Other mammalian and insect cells also showed good attachment on silk fibroin films whencompared with collagen films [48]. Silk films, employed for healing full thickness skin woundsin rats, healed in seven days faster with a lower inflammatory response than traditional porcinebased wound dressings [49]. Silk films have also been used for improved cell attachment andbone formation, particularly when chemically modified with RGD cell binding domains [50].
Silk fibroin films coupled with BMP-2 showed increased bone formation compared with the
NIH-PA Author Manuscript
same silk fibroin films without the BMP-2 [9].
Transparent films cast from a blend of silk and cellulose showed increased mechanical strengthcompared with silk films alone [51]. Films cast from blends of silk fibroin and recombinanthuman-like collagen were seeded with hepatocytes and showed higher cell viability than silkfibroin films alone [8]. Silk fibroin solution, when coated on polyurethane and poly(carbonate)urethane films and scaffolds, increased the adhesion and proliferation of human fibroblasts[52,53]. Films cast from silk fibroin and S-carboxymethyl kerateine (SCMK) showeddecreased blood coagulation compared with silk fibroin or SCMK films alone [54].
2.2.4 Silk fibroin hydrogels—Hydrogels are three-dimensional polymer networks which
are physically durable to swelling in aqueous solutions but do not dissolve in these solutions.
Hydrogel biomaterials provide important options for the delivery of cells and cytokines. Silk
fibroin hydrogels have been prepared from aqueous silk fibroin solution and are formed from?
β-sheet structures [55,56]. The pH of the silk fibroin solution impacted the rate of solution
gelation. Gelation of a 3% solution was obtained in two days at pH 3–4, compared with eight
days as required from a solution with pH 5–12 [56]. Other factors important in gelation included
NIH-PA Author Manuscript
silk polymer concentration and Ca++ [55]. An increase in silk fibroin concentration, increasein temperature, decrease in pH, and an increase in Ca++ concentration decreased the time ofsilk fibroin gelation. Hydrogel pore size was controllable based on silk fibroin concentrationand temperature [55].
Supplementation of silk solutions with poloxamer 407 (a nonionic surfactant) induced gelation;however, additional poloxamer reversed the sol-gel transition [57]. Semi-interpenetratingpolymer networks (SIPNs) formed by mixing poloxamer 407 and silk fibroin solution increasedthe mechanical properties of the hydrogel [58]. A hydrogel blend of silk fibroin and gelatinshowed a temperature dependent helix coil transition of the gelatin that impacted therheological and mechanical properties of the gel. Composition and temperature dependentproperties of gelatin-silk fibroin hydrogels were examined for drug delivery purposes [59,60]. The release of benfotiamide for oral delivery was dependent upon the concentration offibroin in silk fibroin-glycerol hydrogels [61]. The hydrolysis of trichlormethiazide in silkfibroin hydrogels prepared in various monosaccharides (ribose, fructose, glucose, and
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
mannose) was dependent upon the number of hydroxyl groups on the various monosaccharidemolecules [62].
NIH-PA Author Manuscript
Osteoblasts-like cells that attached when cultured on 2% (w/v) silk fibroin hydrogels showedadherence and biocompatibility [13]. Addition of 30% glycerol to the hydrogel increased theproliferation of the cells [13]. Silk fibroin hydrogels injected in critical-sized femur defects inrabbits resulted in greater trabecular bone volume and thickness, significantly higher mineraland rate of bone formation when compared to poly(D,L lactide-glycolide) [63].
Hydrogels combining the properties of silk and elastin were formed to generate biomaterialscalled silk-elastin-like protein polymers (SELPs). The water content in SELP hydrogels couldbe managed by time of gelation and concentration of polymer, while the properties were notaffected by pH, ionic strength, or temperature [64,65]. SELP hydrogels have been employedfor the release of small molecules like theophylline, vitamin B12, and cytochrome c [65]. SELPhydrogels were also used for the controlled release of DNA. Size, conformation, andconcentration of DNA determined release rates of DNA from SELP hydrogels. The transfectionefficiency was 1–3 orders higher than DNA delivered without hydrogel [66].
2.2.5 Silk fibroin porous sponges—Porous sponge scaffolds are important for tissue
engineering applications for cell attachment, proliferation, and migration, as well as for nutrient
NIH-PA Author Manuscript
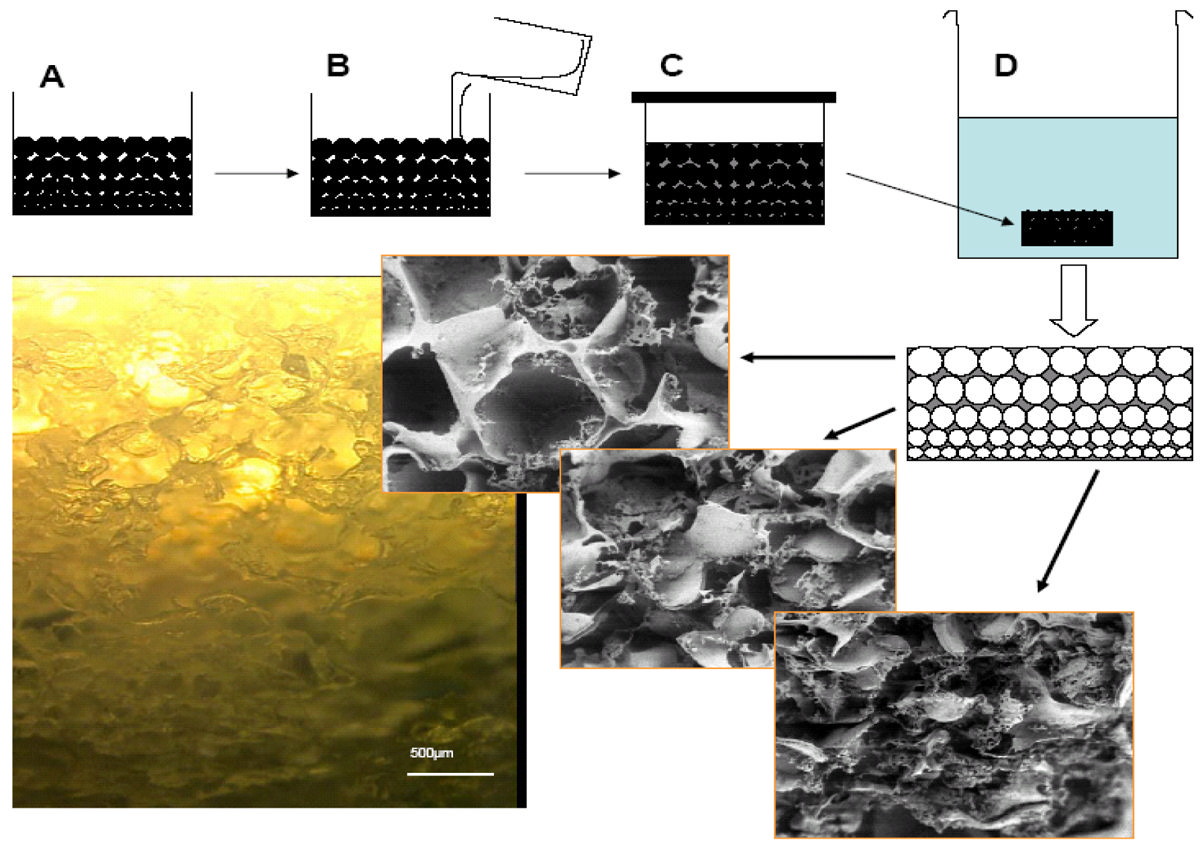
and waste transport. Regenerated silk fibroin solutions, both aqueous and solvent, have beenutilized in the preparation of porous sponges. Sponges have been formed using porogens, gasfoaming, and lyophilization [67]. Solvent-based sponges were prepared using salt (e.g., sodiumchloride) or sugar as porogen. Solvents such as 1,1 3,3 hexafluropropanol (HFIP) do notsolubilize salt or sugar; therefore, pores sizes in the sponges reflect the size of the porogen usedin the process [67]. A gradient of pore sizes can be generated by stacking porogens of differentsizes (Figure 2). Sponges with varying porosity can be controlled by stacking variations of salt/HFIP-silk solutions. Solvent-based porous sponges can also be prepared by addition of a smallamount of solvent (ethanol, methanol, DMSO) into aqueous silk fibroin solution before pouringinto a mold and freezing [68].
Aqueous based porous silk sponges can be prepared using variable size salt crystals as porogen,with control of pore sizes from 490 to 940 μm, by manipulating the percent silk solution andsize of salt crystals. Pore sizes are 80–90% smaller than the size of salt crystals due to thelimited solubilization of the surface of the crystals during supersaturation of the silk solutionprior to solidification [10]. Aqueous-based sponges have rougher surface morphology, basedon SEM, than solvent-based sponges due to this partial solubilization. Aqueous silk fibroinsponges demonstrated improved cell attachment than the solvent-based porous sponges, likely
NIH-PA Author Manuscript
due to these rougher surfaces. Sponges with high porosity and better mechanical strength wereobtained with aqueous-based processing. Stiffness, compressive strength, and modulus wereelevated with an increase in percent silk fibroin solution utilized in the process (Table 3)[10]. Enzymatic degradation of aqueous based sponges was more rapid than the solvent-basedsponges [10].
A phase diagram for the processing of aqueous- and solvent-based porous sponges has beendeveloped. The concentration of silk fibroin solution and size of sodium chloride crystals(porogen) can be related to stable sponge formation with predictable morphological andstructural features [69].
Porous three-dimensional silk sponges have been utilized in a number of studies with cells togenerate various connective tissues. RGD coupled silk sponges seeded with hMSCs that havebeen cultured in osteogenic media resulted in differentiation of the cells, deposition ofhydroxyapaptite, and upregulation of bone markers
in vitro [70]. Tissue engineered silk
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
sponges were useful for healing critical size femur defects in rats [71]. Aqueous porous spongesponges with large pore sizes (900 μm) were used for bone tissue engineering. Structures
NIH-PA Author Manuscript
similar to trabecular bone were observed after 28 days of hMSC differentiation in osteogenicmedia [72]. Solvent-based silk sponges were cultured with hMSCs in chondrogenic media andcollagen type II and glycosaminglycan transcripts were upregulated to a higher degree thansponges composed of collagen or cross-linked collagen [73]. The structural integrity of silksponges compared with rapidly degrading collagen-based sponges was in part responsible forthese differences [73]. Aqueous-based silk fibroin sponges seeded with chondrocytes alsosupported cartilage tissue engineering [74]. Chondrocytes originating from New Zealand whiterabbits were cultured in silk fibroin sponges and proliferated faster in the silk fibroin spongesand generated a higher content of glycosaminoglycan compared with collagen sponges [75].
Porous silk fibroin scaffold sponges seeded with rabbit chondrocytes [76], cultured inchondrogenic media, yielded a frictional coefficient similar to that of native cartilage after 28days of culture [77]. Sponges formed from a blend of poly(vinyl alcohol) (PVA), chitosan, andsilk fibroin showed the best healing of the epidermis and dermis of rats when compared to thepaired and single polymers [78].
2.3 Surface modification
Surface modification can be used to alter cell attachment and impact cell proliferation. Surfacemodification with the integrin recognition sequence RGDS can increase cell attachment [79].
NIH-PA Author Manuscript
Modification of silk fibroin with poly(ethylene glycol) (PEG) showed decreased attachmentof fibroblasts [80]. Silk fibroin surfaces coupled with PEG also showed contact angle, proteinadsorption, and hMSC attachment related to the amount of PEG on the surface of the fibroin[81]. Surface modification includes physical adsorption or chemical immobilization of aprotein or ligand. Silk surfaces are hydrophobic, and attract and repel proteins depending uponthe pI and hydrophobicity of the protein and pH of the solution. Differential adsorption ofhorseradish peroxidase (HRP) to silk fibroin porous sponges depended on the pH of thesolution; HRP precipitated on the surface when the pI and solution pH were similar [14].
Chemical immobilization of HRP increased the amount of HRP available on the silk fibroinsurface [14].
Silk fibroin can be functionalized using the amino acid side chain chemistry. The limitation tousing silk fibroin for chemical modification is the limited total content of modifiable aminoacid side chain groups: 3.3% of the amino acids contain carboxyl side groups [1] comparedwith 9.5% found in bovine collagen [82]. Carbodiimide chemistry, which uses amine orcarboxyl groups on silk for modification, has been used to modify the surface of silk fibroinbiomaterials or to react in solution followed by biomaterials formation. Glucose-oxidase wasimmobilized on silk fibroin films for use as a glucose sensor [83]. Arginine residues of silk
NIH-PA Author Manuscript
fibroin were modified by 1,2-cyclohexanedione, altering cell attachment and proliferation[84]. Silk fibers modified with RGD (covalently coupled) resulted in improved cell attachmentand proliferation and subsequently increased collagen type I production by hMSCs andligament fibroblast cells [30]. Covalent attachment of RGD to silk fibroin films increased thenumber of mineral modules formed by Saos-2 cells in osteogenic media [50]. Interestingly,some wild type silkworms like
Antheraea pernyi have RGD, an integrin recognition sequence,in their silk fibroin sequence, which improves cell adhesion [47]. BMP-2 immobilized vs.
adsorbed on silk fibroin films showed increased inducement of bone markers from hMSCs,including alkaline phosphatase, calcium deposition, and transcripts for collagen type I, bonesialoprotein, osteopontin, and BMP-2 [9].
Tissue development occurs in response to gradients of morphogens during embryogenesis andis recapitulated during adult tissue regeneration [85]. These signaling cues required by cells todevelop into tissues would be useful to incorporate into biomaterial designs. Silk fibroin
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
sponges were used to form immobilized gradients of morphogens [14]. Covalently coupledand adsorbed BMP-2 gradients within three-dimensional silk fibroin sponges were studied with
NIH-PA Author Manuscript
hMSCs and a gradient response of calcium deposition was observed (Vepari et al., unpublishedresults). Immobilized gradients provide new options for biomaterial scaffolds for thegeneration of more complex cell and tissue outcomes
in vitro and
in vivo.
Organic-inorganic composites can also be generated through covalent linkage. Nanoscaleparticles of hydroxyapatite, less than 200 nm in diameter, were chemically bonded to silkfibroin [86]. Vinyl groups were introduced on silk fibroin by treatment with 2-methacryloxyethyl isocyanate. Subsequently, the modified silk fibroin surface was grafted withpoly γ-methacryloxypropyl trimethoxysilane (polyMPTS). Hydroxyapatite particles werereacted with alkoxysilyl group of MPTS to form a siloxane bond. The crystallinity of silkfibroin remained constant during the coupling procedure and resulted in a silk-hydroxyapatitecomposite with properties of both components [86].
The degradation of biomaterials is important in terms of restoring full tissue structure andfunction
in vivo. Control over the rate of degradation is an important feature of functional tissuedesign, such that the rate of scaffold degradation matches the rate of tissue growth [87]. Silkfibroin fibers retain more than 50% of their mechanical properties after two months of
NIH-PA Author Manuscript
implantation
in vivo; thus, they are defined as a non-degradable biomaterial by the United StatesPharmacopeia [7]. Other polymers like poly (lactic acid) (PLA), poly (glycolic acid) (PGA),and PLGA have degradation rates related to hydrolysis based on the polyester composition,purity, and processing conditions. The degradation of these polymers is usually controlled byvarying ratios of polymers with different degradation rates or by altering molecular weight ofthe polymer [88]. These polymers only exhibit limited degradation by enzymes, such asmetalloproteinases (MMPs). Furthermore, the degradation products of synthetic polymers likePLA decrease local pH and result in inflammation [89].
Natural polymers like collagen and silks degrade via the action of proteases. Typically, the rateof collagen degradation is altered by cross-linking in order to reduce enzymatic degradation.
Cross-linking of collagen may also reduce immunogenicity [90]. The degradation byproductsof collagen are peptides and amino acids. The rate of silk fibroin degradation depends uponthe structure, morphology, and mechanical and biological conditions at the location ofimplantation. Degradation of silk fibroin films and fibers has been explored using several typesof proteases, including α-chymotrypsin and collagenases [5,7]. Interestingly, a 6 kDa trypsininhibitor isolated from the water extract from silkworm cocoons, termed cocoon shellassociated trypsin inhibitor (CSTI), protected the light chain of silk fibroin against tryptic
NIH-PA Author Manuscript
degradation [91]. This inhibitor may be a useful tool in biomaterials design for spatialrestriction of the degradation process.
A correlation between
in vitro and
in vivo rates of degradation of silk fibroin fibers has alsobeen established (Horan et al. unpublished). Arai
et al. [5] compared degradation of silk fiberswith silk films when exposed to different amounts and types of enzymes. Exposure to similarenzymes resulted in faster film degradation than the fibers based on weight loss. The weightloss was accompanied by a change in average molecular weight of silk from 120 kDa for controlsilk films to 53 kDa for silk films degraded with α-chymotrypsin for 17 days. An increase inthe crystallinity of the silk was observed with degradation. Tensile properties of silk fibersdecreased without a large change in molecular weight of the silk fibers [5].
Silk fibroin porous sponges from regenerated
B. mori fibers degraded differently with differentprocessing conditions [10]. Aqueous processed 3D sponges, with similar pore sizes, degradedmore slowly upon exposure to proteases with an increased percent silk present during the
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
processing. Solvent-processed sponges degraded more slowly than the aqueous-processedsystems with similar pore sizes: 65% mass remained after 21 days as compared to aqueous-
NIH-PA Author Manuscript
based sponges, which degraded completely in 4 days. The difference in degradation was dueto increased surface roughness or differences in content or distribution of crystallinity [10].
Silk fibroin degradation could be regulated by changing crystallinity [44], pore size, porosity,and molecular weight distribution (MWD) of the silk fibroin. A change in MWD can beachieved by treating silk fibroin under alkaline conditions and heat. A decrease in MWD maydisrupt ordered structures and reduce cross-links, potentially resulting in faster degradation. Itwill be useful to understand the mechanism and correlation of silk fibroin degradation withmechanical properties. Poly(D,L-lactic acid) surfaces coated with silk fibroin of differentMWD resulted in differential attachment of osteoblasts. The adhesion of osteoblasts could havebeen a result of differences in surface hydrophilicity [92].
2.5 Immunological responses
Immunological reactions to biomaterials are an important consideration. Sutures made fromvirgin silk compared with sutures from de-gummed silk showed differences in hypersensitivity[4]. The inflammatory response of de-gummed silk fibroin
in vitro compared with polystyreneand poly(2-hydroxyethyl methacrylate) showed less adhesion of immuno-competent cells[93]. Silk films implanted
in vivo induced a lower inflammatory response than collagen films
NIH-PA Author Manuscript
and PLA films [94]. Silk fibroin non-woven mats implanted subcutaneously in rats induced aweak foreign body response and no occurrence of fibrosis. There was little upregulation ofinflammatory pathways at the implantation site and no invasion by lymphocytes after sixmonths
in vivo [32].
Sericins, which have been known to cause hypersensitivity [4], have been used to increase cellproliferation and attachment. Sericin M, a 400 kDa protein, supported cell attachment of skinfibroblasts to collagen [95]. Sericin-S (5–100 kDa) have been used to increase proliferation ofmammalian cells like T-lymphocytes and hybridomas [96]. Fibroblast cells cultured on sericinmatrices showed non-elongated morphology, unlike the normal spindle shaped morphologyobserved on silk fibroin and collagen matrices [12]. Biomaterials like tricalcium phosphatehave also been coated with sericin to improve biocompatibility [97].
An important feature of silk as a biomaterial, compared with other fibrous proteins such ascollagen, is the versatility of options for sterilization [49]. Sterilization of silk fibroin scaffoldsby autoclaving does not change morphology [73] or β-sheet structure when heated to 120°C[86]. Comparatively, collagen denatures at these temperatures [98]. Silk fibroin scaffolds can
NIH-PA Author Manuscript
also be sterilized using ethylene oxide [4], γ-radiation, or 70% ethanol [9,11].
3. Spider (Nephilia clavipes) silk
Spider silk from
N. clavipes has been studied extensively and is characterized by its remarkablemechanical strength and thermal stability in fiber form [2]. The different types of silks formedby spiders serve various functions. The mechanical properties of the different silks are due tostructural differences derived from different amino acid compositions and sequences. Draglinesilk for safety and web construction is one the strongest natural materials and is composed oftwo proteins: major ampullate spidroins protein 1 and 2 (MaSp1 and (MaSp2). A putativemolecular weight of 275 kDa based on gel electrophoresis of MaSp1 [1] and 740 kDa basedon the major ampullate gland silk by size exclusion chromatography [99] this sentence soundsa little strange, but I'm not sure why, so I left it alone. The molecular weight of minor ampullategland silk was determined to be 290 kDa, as measured by size exclusion chromatography
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
[99]. Unlike silkworms, spider silks characterized to date do not indicate the presence of sericinproteins. The dragline silks from different species of spiders have different mechanical
NIH-PA Author Manuscript
properties [100] (Table 4).
The superior mechanical properties of dragline spider silks can be used as a template fordeveloping specific structures for various biomaterial needs. Spider silks have not beencommercialized in a similar fashion as has been done with silkworm silk due to the lack ofdomestication and lower productivity of spiders. However, genetic engineering provides theopportunity to produce spider silks, both native and hybrids, for biomaterial applications.
3.1 Structure of Dragline silk
The amino acid composition of dragline silk, MaSp1 from
N. clavipes, consists mainly of theamino acids glycine and alanine, like silkworm silk, while glutamic acid, proline, and arginineare also significant in content [1]. This silk consists of repetitive blocks of peptides which giverise to the unique structural properties. The crystalline domains, which contribute to the tensilestrength, contain repeats of alanine or glycine-alanine in MaSp1 and MaSp2. Another motifconsisting of GPGXX (where X is most likely Glutamine and P is Proline) found only in MaSp2is responsible for β-turn spiral and results in the elasticity of silk. Flagelliform silk from
N.
clavipes is rich in this motif and is highly elastic to serve its function in prey capture. Anothermotif, GGX, a glycine helix found in MaSp1, is responsible for the less crystalline regions of
NIH-PA Author Manuscript
the silk structure. These domains also give rise to elasticity of dragline silk. At the carboxy-and amino- termini of the protein, non-repetitive sequences are found which have beenproposed to have a role in assembly of the protein [101].
3.2 Large scale production of spider silk
MaSp1 from
N. clavipes was expressed in
E. coli; however, gene stability and complicationsin processing long repetitive sequences were encountered [102]. Therefore, alternative hostsystems have been explored for the cloning and expression of MASp1- and MaSp2-likeproteins, such as transgenic tobacco [103]. Spider dragline-like protein with yields of 1 g/Lwere expressed in the yeast,
Pichia pastoris [104]. MaSp1 and MaSp2/ADF-3 were clonedand expressed in baby hamster kidney cells and bovine mammary epithelial alveolar cells[105]. Proteins of 60–140 kDa were produced and spun into fibers with reasonable mechanicalproperties [105]. Genes encoding spider silk have also been expressed in mouse milk [106].
Despite the above efforts, no substantial effort at commercialization of spider silk via geneticengineering has been successful to date, a required step to realize biomaterials applicationsfrom spider silks.
NIH-PA Author Manuscript
3.3 Morphology of recombinant spider silk
Environmental factors such as pH, water content, concentration of protein, salt, and chargeimpact the processing and assembly of spider silk
in vivo and
in vitro. For example, interchaininteractions increased with enhanced calcium concentration and low water content [107]. Filmscast from dragline silk proteins of
Araneus diadematus in HFIP exhibited α-helical structurewhich dissolved in aqueous solution. Insoluble films were prepared by treatment with methanoland resulted in β-sheet structures. These films could be chemically coupled with fluoresceinand β-galactosidase, which showed higher activity in chemically coupled than adsorbed forms[27]. A higher percentage of aspartic acid and glutamic acid found in MaSp1 (11.7%) thanfibroin (3.3) [1] provides enhanced options for the chemical modification or coupling of factorsto MaSp silk when compared to silk fibroin from silkworm. Hydrogels have also been preparedfrom recombinant spider silk and were found to be stable for several weeks and demonstratedhigh elastic modulus [108].
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Silks are a unique group of fibrous proteins with unusually high mechanical strength in fiber
NIH-PA Author Manuscript
form. The clinical success of silk sutures and availability of silkworm silk have encouraged arecent expansion of new biomaterials generated from the original suture-based proteinharvested and processed from silkworms. Native silk fibers can be processed into wire ropesand non-woven silk mats. The same fibers can be solubilized and regenerated in aqueoussolution, then further processed into sponges, films, hydrogels, and nanoscale electrospun non-woven mats. Surface modification of silk fibroin biomaterials can be used to alter cellresponses. Cell culture on silk-based biomaterials has resulted in the formation of a variety oftissues including bone, cartilage, and ligament, both
in vitro and
in vivo. The degradability ofsilk fibroin can be altered by processing conditions. Molecular biology has been used togenerate spider silks, although reasonable quantities are still not available. These geneticallyengineered spider silk proteins which have been formed into films can be chemically modified[27]. The unique structure of silk, versatility in processing, biocompatibility, availability ofdifferent biomaterial morphologies, options for genetic engineering of variations of silks, theease of sterilization, thermal stability, surface chemistry for facile chemical modifications, andcontrollable degradation features make silks promising biomaterials for many clinicalfunctions. Since the exploration of biomaterial applications for silks, aside from sutures, isonly a relatively recent advance, the future for this family of structural proteins to impact
NIH-PA Author Manuscript
clinical needs appears promising.
We thank our many colleagues inside and outside of Tufts University for their contributions to the studies on silk overthe years. We also thank various funding agencies for support of different aspects of our studies on silks, includingthe NIH, the NSF, and the DoD.
1. Kaplan, DL.; Mello, SM.; Arcidiacono, S.; Fossey, S.; Senecal, KWM. Protein based materials.
McGrath, KKD., editor. Boston: Birkhauser; 1998. p. 103-131.
2. Wong Po Foo C, Kaplan DL. Genetic engineering of fibrous proteins: spider dragline silk and collagen.
Adv Drug Deliv Rev 2002;54(8):1131–1143. [PubMed: 12384311]
3. Bini E, Knight DP, Kaplan DL. Mapping domain structures in silks from insects and spiders related
to protein assembly. J Mol Biol 2004;335(1):27–40. [PubMed: 14659737]
4. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-
based biomaterials. Biomaterials 2003;24(3):401–416. [PubMed: 12423595]
5. Arai T, Freddi G, Innocenti R, Tsukada M. Biodegradation of Bombyx mori Silk Fibroin Fibers and
NIH-PA Author Manuscript
Films. J Appl Polym Sci 2004;91:2383–2390.
6. Fuchs S, Motta A, Migliaresi C, Kirkpatrick CJ. Outgrowth endothelial cells isolated and expanded
from human peripheral blood progenitor cells as a potential source of autologous cells forendothelialization of silk fibroin biomaterials. Biomaterials 2006;27(31):5399–5408. [PubMed:16837042]
7. Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH.
In vitro degradation of silk fibroin. Biomaterials 2005;26(17):3385–3393. [PubMed: 15621227]
8. Hu K, Lv Q, Cui F, Feng Q, Kong X, Wang H, Huang L, Li T. Biocompatible fibroin blended films
with recombinant human-like collagen for hepatic tissue engineering. J Bioact Compat Polym 2006;21(1):23–37.
9. Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic
protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromalcells. J Biomed Mater Res A 2004;71(3):528–537. [PubMed: 15478212]
10. Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial
scaffolds from silk fibroin. Biomaterials 2005;26(15):2775–2785. [PubMed: 15585282]
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
11. Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue
engineering. Biomaterials 2006;27(16):3115–3124. [PubMed: 16458961]
NIH-PA Author Manuscript
12. Minoura N, Aiba S, Gotoh Y, Tsukada M, Imai Y. Attachment and growth of cultured fibroblast cells
on silk protein matrices. J Biomed Mater Res 1995;29(10):1215–1221. [PubMed: 8557723]
13. Motta A, Migliaresi C, Faccioni F, Torricelli P, Fini M, Giardino R. Fibroin hydrogels for biomedical
applications: preparation, characterization and in vitro cell culture studies. J Biomater Sci Polym Ed2004;15(7):851–864. [PubMed: 15318796]
14. Vepari CP, Kaplan DL. Covalently immobilized enzyme gradients within three-dimensional porous
scaffolds. Biotechnol Bioeng 2006;93(6):1130–1137. [PubMed: 16444737]
15. Arai T, Freddi G, Colonna GM, Scotti E, Boschi A, Murakami R, Tsukada M. Absorption of Metal
Cations by Modified B. mori Silk and Preparation of Fabrics with Antimicrobial Activity. J ApplPolym Sci 2001:80297–303.
16. Moy RL, Lee A, Zalka A. Commonly used suture materials in skin surgery. Am Fam Physician
1991;44(6):2123–2128. [PubMed: 1746393]
17. Vollrath F, Knight DP. Liquid crystalline spinning of spider silk. Nature 2001;410(6828):541–548.
[PubMed: 11279484]
18. Zhou CZ, Confalonieri F, Medina N, Zivanovic Y, Esnault C, Yang T, Jacquet M, Janin J, Duguet
M, Perasso R, Li ZG. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic AcidsRes 2000;28(12):2413–2419. [PubMed: 10871375]
19. Yamaguchi K, Kikuchi Y, Takagi T, Kikuchi A, Oyama F, Shimura K, Mizuno S. Primary structure
of the silk fibroin light chain determined by cDNA sequencing and peptide analysis. J Mol Biol
NIH-PA Author Manuscript
1989;210(1):127–139. [PubMed: 2585514]
20. Inoue S, Tanaka K, Arisaka F, Kimura S, Ohtomo K, Mizuno S. Silk fibroin of Bombyx mori is
secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25,with a 6:6:1 molar ratio. J Biol Chem 2000;275(51):40517–40528. [PubMed: 10986287]
21. Tanaka K, Inoue S, Mizuno S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide
chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem Mol Biol1999;29(3):269–276. [PubMed: 10319440]
22. Zhou CZ, Confalonieri F, Jacquet M, Perasso R, Li ZG, Janin J. Silk fibroin: structural implications
of a remarkable amino acid sequence. Proteins 2001;44(2):119–122. [PubMed: 11391774]
23. Gage LP, Manning RF. Internal structure of the silk fibroin gene of Bombyx mori. I The fibroin gene
consists of a homogeneous alternating array of repetitious crystalline and amorphous codingsequences. J Biol Chem 1980;255(19):9444–9450. [PubMed: 6157693]
24. Mori K, Tanaka K, Kikuchi Y, Waga M, Waga S, Mizuno S. Production of a chimeric fibroin light-
chain polypeptide in a fibroin secretion-deficient naked pupa mutant of the silkworm Bombyx mori.
J Mol Biol 1995;251(2):217–228. [PubMed: 7643398]
25. Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature 2003;424(6952):
1057–1061. [PubMed: 12944968]
26. Motta A, Fambri L, Migliaresi C. Regenerated silk fibroin films: Thermal and dynamic mechanical
NIH-PA Author Manuscript
analysis. Macromolecular Chemistry and Physics 2002;203(10–11):1658–1665.
27. Huemmerich D, Slotta U, Scheibel T. Processing and modification of films made from recombinant
spider silk proteins. Appl Phys A 2006;82:219–222.
28. Zhang YQ. Applications of natural silk protein sericin in biomaterials. Biotechnol Adv 2002;20(2):
91–100. [PubMed: 14538058]
29. Kardestuncer T, McCarthy MB, Karageorgiou V, Kaplan D, Gronowicz G. RGD-tethered silk
substrate stimulates the differentiation of human tendon cells. Clin Orthop Relat Res 2006:448234–239.
30. Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. Human
bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J BiomedMater Res A 2003;67(2):559–570. [PubMed: 14566798]
31. Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue
engineered anterior cruciate ligaments. Biomaterials 2002;23(20):4131–4141. [PubMed: 12182315]
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
32. Dal Pra I, Freddi G, Minic J, Chiarini A, Armato U. De novo engineering of reticular connective
tissue in vivo by silk fibroin nonwoven materials. Biomaterials 2005;26(14):1987–1999. [PubMed:15576173]
NIH-PA Author Manuscript
33. Unger RE, Wolf M, Peters K, Motta A, Migliaresi C, James Kirkpatrick C. Growth of human cells
on a non-woven silk fibroin net: a potential for use in tissue engineering. Biomaterials 2004;25(6):1069–1075. [PubMed: 14615172]
34. Unger RE, Peters K, Wolf M, Motta A, Migliaresi C, Kirkpatrick CJ. Endothelialization of a non-
woven silk fibroin net for use in tissue engineering: growth and gene regulation of human endothelialcells. Biomaterials 2004;25(21):5137–5146. [PubMed: 15109837]
35. Reneker D, Chun I. Nanometer diameter fibers of polymer, produced by electrospinning.
36. Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene
oxide). Biomacromolecules 2002;3(6):1233–1239. [PubMed: 12425660]
37. Jin HJ, Chen J, Karageorgiou V, Altman GH, Kaplan DL. Human bone marrow stromal cell responses
on electrospun silk fibroin mats. Biomaterials 2004;25(6):1039–1047. [PubMed: 14615169]
38. Wang M, Jin HJ, Kaplan DL, Rutledge GC. Mechanical properties of electrospun silk fibers.
39. Ayutsede J, Gandhi M, Sukigara S, Micklus M, Chen HE, Ko F. Regeneration of Bombyx mori silk
by electrospinning, Part 3: characterization of electrospun nonwoven mat. Polymer 2005;46(5):1625–1634.
40. Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and
NIH-PA Author Manuscript
its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro.
Biomaterials 2004;25(7–8):1289–1297. [PubMed: 14643603]
41. Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, Kim TI, Park YJ, Seol YJ, Lee YM, Ku Y,
Rhyu IC, Han SB, Chung CP. Biological efficacy of silk fibroin nanofiber membranes for guidedbone regeneration. J Biotechnol 2005;120(3):327–339. [PubMed: 16150508]
42. Li C, Jin H, Botsaris G, Kaplan D. Silk apatite composites from electrospun fibers. Journal of Materials
43. Minoura N, Tsukada M, Nagura M. Fine structure and oxygen permeability of silk fibroin membrane
treated with methanol. Polymer 1990;31(2):265–269.
44. Minoura N, Tsukada M, Nagura M. Physico-chemical properties of silk fibroin membrane as a
biomaterial. Biomaterials 1990;11(6):430–434. [PubMed: 2207234]
45. Wang X, Kim HJ, Xu P, Matsumoto A, Kaplan DL. Biomaterial coatings by stepwise deposition of
silk fibroin. Langmuir 2005;21(24):11335–11341. [PubMed: 16285808]
46. Jin HJ, Park J, Valluzzi R, Cebe P, Kaplan DL. Biomaterial films of Bombyx mori silk fibroin with
poly(ethylene oxide). Biomacromolecules 2004;5(3):711–717. [PubMed: 15132651]
47. Minoura N, Aiba S, Higuchi M, Gotoh Y, Tsukada M, Imai Y. Attachment and growth of fibroblast
cells on silk fibroin. Biochem Biophys Res Commun 1995;208(2):511–516. [PubMed: 7695601]
48. Inouye K, Kurokawa M, Nishikawa S, Tsukada M. Use of Bombyx mori silk fibroin as a substratum
NIH-PA Author Manuscript
for cultivation of animal cells. J Biochem Biophys Methods 1998;37(3):159–164. [PubMed:9870190]
49. Sugihara A, Sugiura K, Morita H, Ninagawa T, Tubouchi K, Tobe R, Izumiya M, Horio T, Abraham
NG, Ikehara S. Promotive effects of a silk film on epidermal recovery from full-thickness skinwounds. Proc Soc Exp Biol Med 2000;225(1):58–64. [PubMed: 10998199]
50. Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone
formation. J Biomed Mater Res 2001;54(1):139–148. [PubMed: 11077413]
51. Freddi G, Romano M, Massafra M, Tsukada M. Silk fibroin/cellulose blend films—preparation,
structure, and physical-properties. J Appl Polym Science 1995;56(12):1537–1545.
52. Petrini P, Parolari C, Tanzi MC. Silk fibroin-polyurethane scaffolds for tissue engineering. J Mater
Sci Mater Med 2001;12(10–12):849–853. [PubMed: 15348328]
53. Chiarini A, Petrini P, Bozzini S, Pra I, Armato U. Silk fibroin/poly(carbonate)-urethane as a substrate
for cell growth: in vitro interactions with human cells. Biomaterials 2003;24(5):789–799. [PubMed:12485797]
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
54. Lee K, Kong S, Park W, Ha W, Kwon I. Effect of surface properties on the antithrombogenicity of
silk fibroin/S-carboxymethyl kerateine blend films. J Biomater Sci—Polym Ed 1998;9(9):905–914.
[PubMed: 9747984]
NIH-PA Author Manuscript
55. Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels.
Biomacromolecules 2004;5(3):786–792. [PubMed: 15132662]
56. Ayub Z, Arai M, Hirabayashi K. Mechanism of the gelation of fibroin solution. Biosci Biotechnol
57. Kang G-D, Nahm J-H, Park J-S, Moon J-Y, Cho C-S, Yeo J-H. Effects of Poloxamer on the gelation
of silk fibroin. Macromolecular Rapid Communications 2000;21(11):788–791.
58. Yoo MK, Kweon HY, Lee KG, Lee HC, Cho CS. Preparation of semi-interpenetrating polymer
networks composed of silk fibroin and poloxamer macromer. Int J Biol Macromol 2004;34(4):263–270. [PubMed: 15374683]
59. Gil ES, Spontak RJ, Hudson SM. Effect of beta-sheet crystals on the thermal and rheological behavior
of protein-based hydrogels derived from gelatin and silk fibroin. Macromol Biosci 2005;5(8):702–709. [PubMed: 16080165]
60. Gil ES, Frankowski DJ, Spontak RJ, Hudson SM. Swelling behavior and morphological evolution of
mixed gelatin/silk fibroin hydrogels. Biomacromolecules 2005;6(6):3079–3087. [PubMed:16283730]
61. Hanawa T, Watanabe A, Tsuchiya T, Ikoma R, Hidaka M, Sugihara M. New oral dosage form for
elderly patients, II: release behavior of benfotiamine from silk fibroin gel. Chem Pharm Bull 1995;43(5):872–876. [PubMed: 7553973]
NIH-PA Author Manuscript
62. Hanawa T, Maeda R, Muramatsu E, Suzuki M, Sugihara M, Nakajima S. New oral dosage form for
elderly patients. III. Stability of trichlormethiazide in silk fibroin gel and various sugar solutions.
Drug Dev Ind Pharm 2000;26(10):1091–1097. [PubMed: 11028224]
63. Fini M, Motta A, Torricelli P, Giavaresi G, Nicoli Aldini N, Tschon M, Giardino R, Migliaresi C.
The healing of confined critical size cancellous defects in the presence of silk fibroin hydrogel.
Biomaterials 2005;26(17):3527–3536. [PubMed: 15621243]
64. Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered
silk-elastinlike protein polymer hydrogel. Biomaterials 2002;23(21):4203–4210. [PubMed:12194523]
65. Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Solute diffusion in genetically engineered silk-
elastinlike protein polymer hydrogels. J Control Release 2002;82(2–3):277–287. [PubMed:12175743]
66. Megeed Z, Haider M, Li D, O'Malley BW Jr, Cappello J, Ghandehari H. In vitro and in vivo evaluation
of recombinant silk-elastinlike hydrogels for cancer gene therapy. J Control Release 2004;94(2–3):433–445. [PubMed: 14744493]
67. Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin.
Biomacromolecules 2004;5(3):718–726. [PubMed: 15132652]
68. Tamada Y. New process to form a silk fibroin porous 3-D structure. Biomacromolecules 2005;6(6):
NIH-PA Author Manuscript
3100–3106. [PubMed: 16283733]
69. Kim H, Kim H, Matsumoto A, Chin I, Jin H, Kaplan D. Processing windows for forming silk fibroin
biomaterials into a 3D porous matrix. Aust J Chem 2005:58716–720.
70. Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R,
Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects ofscaffold material and medium flow. Ann Biomed Eng 2004;32(1):112–122. [PubMed: 14964727]
71. Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D. Silk
implants for the healing of critical size bone defects. Bone 2005;37(5):688–698. [PubMed:16140599]
72. Kim HJ, Kim UJ, Vunjak-Novakovic G, Min BH, Kaplan DL. Influence of macroporous protein
scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 2005;26(21):4442–4452. [PubMed: 15701373]
73. Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, Vunjak-Novakovic G.
Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds.
Biotechnol Bioeng 2004;88(3):379–391. [PubMed: 15486944]
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
74. Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds
and human articular chondrocytes. Biomaterials 2006;27(25):4434–4442. [PubMed: 16677707]
NIH-PA Author Manuscript
75. Aoki H, Tomita N, Morita Y, Hattori K, Harada Y, Sonobe M, Wakitani S, Tamada Y. Culture of
chondrocytes in fibroin-hydrogel sponge. Biomed Mater Eng 2003;13(4):309–316. [PubMed:14646046]
76. Morita Y, Tomita N, Aoki H, Wakitani S, Tamada Y, Suguro T, Ikeuchi K. Visco-elastic properties
of cartilage tissue regenerated with fibroin sponge. Biomed Mater Eng 2002;12(3):291–298.
[PubMed: 12446944]
77. Morita Y, Tomita N, Aoki H, Sonobe M, Wakitani S, Tamada Y, Suguro T, Ikeuchi K. Frictional
properties of regenerated cartilage in vitro. J Biomech 2006;39(1):103–109. [PubMed: 16271593]
78. Yeo JH, Lee KG, Kim HC, Oh HYL, Kim AJ, Kim SY. The effects of Pva/chitosan/fibroin (PCF)-
blended spongy sheets on wound healing in rats. Biol Pharm Bull 2000;23(10):1220–1223. [PubMed:11041255]
79. Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small
synthetic fragments of the molecule. Nature 1984;309(5963):30–33. [PubMed: 6325925]
80. Gotoh Y, Tsukada M, Minoura N, Imai Y. Synthesis of poly(ethylene glycol)-silk fibroin conjugates
and surface interaction between L-929 cells and the conjugates. Biomaterials 1997;18(3):267–271.
[PubMed: 9031729]
81. Vepari C, Matheson D, Naik R, Kaplan DL. Regulation of mesenchymal stem cell behavior on silk
fibroin surfaces modified with polyethylene glycol - in preparation.
82. Bowes JH, Elliott RG, Moss JA. The composition of collagen and acid-soluble collagen of bovine
NIH-PA Author Manuscript
skin. Biochem J 1955;61(1):143–150. [PubMed: 13260189]
83. Demura M, Asakura T. Porous membrane of Bombyx mori silk fibroin—structure characterization,
physical-properties and application to glucose-oxidase immobilization. J Membr Sci 1991;59(1):39–52.
84. Gotoh Y, Tsukada M, Minoura N. Effect of the chemical modification of the arginyl residue in
Bombyx mori silk fibroin on the attachment and growth of fibroblast cells. J Biomed Mater Res1998;39(3):351–357. [PubMed: 9468042]
85. Teixeira J, Urist M. Bone morphogenetic protein induced repair of compartmentalized segmental
diaphyseal defects. Arch Orthop Trauma Surg 1998;117(1–2):27–34. [PubMed: 9457332]
86. Furuzono T, Kishida A, Tanaka J. Nano-scaled hydroxyapatite/polymer composite I. Coating of
sintered hydroxyapatite particles on poly(gamma-methacryloxypropyl trimethoxysilane)grafted silkfibroin fibers through chemical bonding. J Mater Sci Mater Med 2004;15(1):19–23. [PubMed:15338587]
87. Lanza, R.; Langer, R.; Vacanti, J. Principles of Tissue Engineering. San Diego, CA: Academic Press;
88. Oh JE, Nam YS, Lee KH, Park TG. Conjugation of drug to poly(D,L-lactic-co-glycolic acid) for
controlled release from biodegradable microspheres. J Control Release 1999;57(3):269–280.
[PubMed: 9895414]
NIH-PA Author Manuscript
89. Solheim E, Sudmann B, Bang G, Sudmann E. Biocompatibility and effect on osteogenesis of poly
(ortho ester) compared to poly(DL-lactic acid). J Biomed Mater Res 2000;49(2):257–263. [PubMed:10571914]
90. Basu S, Cunningham LP, Pins GD, Bush KA, Taboada R, Howell AR, Wang J, Campagnola PJ.
Multiphoton excited fabrication of collagen matrixes cross-linked by a modified benzophenonedimer: bioactivity and enzymatic degradation. Biomacromolecules 2005;6(3):1465–1474. [PubMed:15877366]
91. Kurioka A, Yamazaki M, Hirano H. Primary structure and possible functions of a trypsin inhibitor
of Bombyx mori. Eur J Biochem 1999;259(1–2):120–126. [PubMed: 9914483]
92. Cai K, Yao K, Lin S, Yang Z, Li X, Xie H, Qing T, Gao L. Poly(D,L-lactic acid) surfaces modified
by silk fibroin: effects on the culture of osteoblast in vitro. Biomaterials 2002;23(4):1153–1160.
[PubMed: 11791919]
93. Santin M, Motta A, Freddi G, Cannas M. In vitro evaluation of the inflammatory potential of the silk
fibroin. J Biomed Mater Res 1999;46(3):382–389. [PubMed: 10397996]
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
94. Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer
R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo.
Biomaterials 2005;26(2):147–155. [PubMed: 15207461]
NIH-PA Author Manuscript
95. Tsubouchi K, Igarashi Y, Takasu Y, Yamada H. Sericin enhances attachment of cultured human skin
fibroblasts. Biosci Biotechnol Biochem 2005;69(2):403–405. [PubMed: 15725668]
96. Terada S, Sasaki M, Yanagihara K, Yamada H. Preparation of silk protein sericin as mitogenic factor
for better mammalian cell culture. J Biosci Bioeng 2005:6667–671.
97. Takeuchi A, Ohtsuki C, Kamitakahara M, Ogata S, Tanihara M, Miyazaki T, Yamazaki M, Furutani
Y, Kinoshita H. Biodegradation of porous alpha-tricalcium phosphate coated with silk sericin. KeyEngineering Materials 2005:284–286. 329–332.
98. McClain PE, Wiley ER. Differential scanning calorimeter studies of the thermal transitions of
collagen. Implications on structure and stability. J Biol Chem 1972;247(3):692–697. [PubMed:5058222]
99. Jackson C, O'Brien J. Molecular weight distribution of Nephila clavipes dragline silk.
100. Swanson BO, Blackledge TA, Beltran J, Hayashi CY. Variation in the material properties of spider
dragline silk across species. Appl Phys 2006:A 82213–218.
101. Scheibel T. Spider silks: recombinant synthesis, assembly, spinning, and engineering of synthetic
proteins. Microb Cell Fact 2004;3(1):14. [PubMed: 15546497]
102. Arcidiacono S, Mello C, Kaplan D, Cheley S, Bayley H. Purification and characterization of
recombinant spider silk expressed in Escherichia coli. Appl Microbiol Biotechnol 1998;49(1):31–
NIH-PA Author Manuscript
38. [PubMed: 9487707]
103. Menassa R, Zhu H, Karatzas C, Lazaris A, Richman A, Brandle J. Spider dragline silk proteins in
transgenic tobacco leaves: accumulation and field production. Plant Biotechnology Journal 2004;2(5):431–438. [PubMed: 17168889]
104. Fahnestock SR, Bedzyk LA. Production of synthetic spider dragline silk protein in Pichia pastoris.
Appl Microbiol Biotechnol 1997;47(1):33–39. [PubMed: 9035408]
105. Lazaris A, Arcidiacono S, Huang Y, Zhou JF, Duguay F, Chretien N, Welsh EA, Soares JW, Karatzas
CN. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science2002;295(5554):472–476. [PubMed: 11799236]
106. Karatzas CN, Chretien N, Duguay F, Bellemare A, Zhou JF, Rodenhiser A, Islam SA, Turcotte C,
Huang Y, Lazaris A. High-toughness spider silk fibers spun from soluble recombinant silk producedin mammalian cells. Biopolymers 2003:897–117.
107. Wong Po Foo C, Bini E, hensman J, Knight DP, Lewis RV, Kaplan DL. Role of pH and charge on
silk protein assembly in insects and spiders. Appl Phys A 2006:82223–233.
108. Rammensee S, Huemmerich D, Hermanson KD, Scheibel T, Bausch AR. Rheological
characterization of hydrogels formed by recombinantly produced spider silk. Appl Phys A2006:82261–264.
109. Wang X, Kim HJ, Wong C, Vepari C, Matsumoto A, Kaplan DL. Fibrous Proteins - Role in
NIH-PA Author Manuscript
Biomaterials and Tissue Engineering. Materials Today. In press
110. Perez-Rigueiro J, Viney C, Llorca J, Elices M. Mechanical properties of single-brin silkworm silk.
J Appl Polym Sci 2000:751270–1277.
111. Cunniff P, Fossey S, Auerbach M, Song J, Kaplan D, Adams WW, Eby R, Mahoney D, Vezie D.
Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polymers forAdvanced Technologies 1994;5(8):401–410.
112. Pins G, Christianse D, Patel R, Silver F. Self-assembly of collagen fibers: influence of fibrillar
alignment and decorin on mechanical properties. Biophys J 1997:732164–2172.
113. Engelberg I, Kohn J. Physicomechanical properties of degradable polymers used in medical
applications: a comparative study. Biomaterials 1991:12292–304.
114. Meinel L, Betz O, Fajardo R, Hofmann S, Nazarian A, Cory E, Hilbe M, McCool J, Langer R,
Vunjak-Novakovic G, Merkle HP, Rechenberg B, Kaplan DL, Kirker-Head C. Silk basedbiomaterials to heal critical sized femur defects. Bone. 2006
115. Meinel L, Hofmann S, Betz O, Fajardo R, Merkle HP, Langer R, Evans CH, Vunjak-Novakovic G,
Kaplan DL. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials:
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials2006;27(28):4993–5002. [PubMed: 16765437]
NIH-PA Author Manuscript
116. Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, Chen J, Vunjak-Novakovic
G, Kaplan DL. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 invitro and in vivo. J Biomed Mater Res A 2006;78(2):324–334. [PubMed: 16637042]
117. Marolt D, Augst A, Freed LE, Vepari C, Fajardo R, Patel N, Gray M, Farley M, Kaplan D, Vunjak-
Novakovic G. Bone and cartilage tissue constructs grown using human bone marrow stromal cells,silk scaffolds and rotating bioreactors. Biomaterials. 2006
118. Kino R, Ikoma T, Monkawa A, Yunoki S, Munekata M, Tanaka J, Asakura T. Deposition of bone-
like apatite on modified silk fibroin films from simulated body fluid. Journal of Applied PolymerScience 2006;99(5):2822–2830.
119. Moreau JE, Chen J, Horan RL, Kaplan DL, Altman GH. Sequential growth factor application in
bone marrow stromal cell ligament engineering. Tissue Eng 2005;11(11–12):1887–1897. [PubMed:16411835]
120. Kim S, Cho Y, Kang E, Kwon I, Lee E, Kim J, Chung H, Jeong S. Three-dimensional porous
collagen/chitosan complex sponge for tissue engineering. Fibers and Polymers 2001;2(2):64–70.
121. Lv Q, Feng Q, Hu K, Cui F. Three-dimensional fibroin/collagen scaffolds derived from aqueous
solution and the use for HepG2 culture. Polymer 2005;46(26):12662–12669.
122. Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a
scaffold for articular cartilage repair. Ann Biomed Eng 2004;32(3):391–397. [PubMed: 15095813]
123. Hou Q, Grijpma DW, Feijen J. Preparation of interconnected highly porous polymeric structures by
NIH-PA Author Manuscript
a replication and freeze-drying process. J Biomed Mater Res B Appl Biomater 2003;67(2):732–740. [PubMed: 14598400]
124. Nam YS, Yoon JJ, Park TG. A novel fabrication method of macroporous biodegradable polymer
scaffolds using gas foaming salt as a porogen additive. J Biomed Mater Res 2000;53(1):1–7.
[PubMed: 10634946]
125. Moran JM, Pazzano D, Bonassar LJ. Characterization of polylactic acid-polyglycolic acid
composites for cartilage tissue engineering. Tissue Eng 2003;9(1):63–70. [PubMed: 12625955]
126. Heijkants R, Van Tienen T, De Groot J, Pennings A, Buma P, Veth R, Schouten A. Preparation of
a polyurethane scaffold for tissue engineering made by a combination of salt leaching and freeze-drying of dioxane. Journal of Materials Science 2006;41(8):2423–2428.
127. Meretoja VV, Helminen AO, Korventausta JJ, Haapa-aho V, Seppala JV, Narhi TO. Crosslinked
poly(epsilon-caprolactone/D,L-lactide)/bioactive glass composite scaffolds for bone tissueengineering. J Biomed Mater Res A 2006;77(2):261–268. [PubMed: 16392138]
128. Kang Y, Yang J, Khan S, Anissian L, Ameer GA. A new biodegradable polyester elastomer for
cartilage tissue engineering. J Biomed Mater Res A 2006;77(2):331–339. [PubMed: 16404714]
129. Fisher JP, Holland TA, Dean D, Mikos AG. Photoinitiated cross-linking of the biodegradable
polyester poly(propylene fumarate). Part II. In vitro degradation. Biomacromolecules 2003;4(5):1335–1342. [PubMed: 12959603]
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Fig. 1.
Fig. 1A: Silk fibroin is purified from sericins via boiling in an alkaline solution. The de-
gummed or purified silk fibers can be processed into silk cords by twisting [4]; non-woven silk
NIH-PA Author Manuscript
mats by partial solubilization [32]; or dissolved in lithium bromide, dialyzed and formed into
aqueous silk fibroin solution [67] for preparation of other material morphologies (See figure
1B).
Fig. 1B: Processing of silk morphologies from aqueous silk fibroin solution into non-woven
silk fibers [11]; aqueous and solvent based porous sponges [67,69]; hydrogels [108]; and films
[46].
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Fig. 2.
Porous gradient silk sponges prepared by stacking a water soluble porogen from smallest to
largest (A). Solvent based silk solution is added and allowed to diffuse through the salt crystals
(B & C). The porogen is dissolved in water leaving the sponge with pore gradient (D) [109].
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Fig. 3.
Covalent coupling versus adsorption of proteins on silk surfaces. A: Modifiable amino acid
side chains; presence of amine, carboxyl and hydroxyl groups. B: Carboxyl side groups
activated by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) for 10–15 minutes. A
protein like BMP-2 can be introduced via amine groups reacting with the activated silk to form
amide bonds. Cyanuric activated polyethylene glycol (PEG) reacts with amine and hydroxyl
groups on silk fibroin surface. C: Coupling of PEG on silk fibroin films generates a more
hydrophilic surface and reduced attachment of human mesenchymal stem cells (hMSCs)
[81]. D: Coupling BMP-2 to silk fibroin films via carbodiimide coupling results in increased
calcium deposition (increased calcein labeling) by differentiated hMSCs [9].
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Mechanical properties of biodegradable polymeric materials (modified from [4]).
NIH-PA Author Manuscript
Source of Biomaterial
Strain (%) at break
B. mori silk
B. mori silk (without sericin)
B. mori silk
N. clavipes silk
Crosslinked collagen
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Cell and tissue applications of silk fibroin scaffolds.
NIH-PA Author Manuscript
Bone tissue engineering
Cartilage Tissue engineering
Ligament tissue engineering
Tendon tissue engineering
Hepatic tissue engineering
Connective tissue
Endothelial and blood vessel
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Comparison of compressive strength and modulus of degradable polymeric porous sponge scaffolds (modified from[10,67].
NIH-PA Author Manuscript
Porous sponge material
Silk fibroin (aqueous)
Silk fibroin (solvent)
crosslinked Hyaluronan
Poly(lactic acid)
Poly(lactic acid)/poly(glycolic acid)
NIH-PA Author Manuscript
Poly(propylene fumarate)
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Vepari and Kaplan
Mechanical properties of dragline silk from different species (abridged from [100].
NIH-PA Author Manuscript
Nephila clavipes
Leucauge verusta
NIH-PA Author Manuscript
NIH-PA Author Manuscript
Prog Polym Sci. Author manuscript; available in PMC 2009 June 19.
Source: http://faculty.olin.edu/~asieminski/topics/papers/Silk%20Lab%20Field%20Trip/Vepari%20et%20al%202007%20silk%20biomaterial.pdf
Vol 23 Number 1 2013 Fiji Medical Journal Instructions to Authors Foreword byFiji National University Vice Chancellor Dr Ganesh Chand Foreword by Fiji Medical Association President Dr. James Fong 9 Evaluation & Assessment of Diabetes Knowledge Among Trainee Teachers Alka Sewram 10-13 Original ResearchSome common barriers to self-care amongst Type 2 diabetic patients in Sigatoka medical area Dr. Sravaniya Dasi
An essential guide on the importance of iron The Salus guide ‘Iron and Pregnancy' informs pregnant women as well as health care professionals about the importance of iron for the human body, particularly during pregnancy; how to identify the first signs of an iron deficiency; and what you can do to maintain healthy iron levels before, during and after pregnancy.










