P209-17 ijmcm vol 4, no 4 dr. omrani [ararvtscp] 94-10-05
IJMCM
Original Article
Autumn 2015, Vol 4, No 4
Expression Pattern of Neuronal Markers in PB-MSCs Treated by
Growth Factors Noggin, bFGF and EGF
Zahra Fazeli1#, Sayyed Mohammad Hossein Ghaderian1,2#. Masoumeh Rajabibazl3, Siamak Salami3,
Nader Vazifeh Shiran4, Mir Davood Omrani1,2∗
1. Department of Medical Genetics, Faculty of Medicine, Shahid Beheshti University of Medical Sciences,
Tehran, Iran.
2. Urogenital Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Shahid Labbafi Nejad
Educational Hospital, Tehran, Iran.
3. Department of Clinical Biochemistry, Faculty of Medicine, Shahid Beheshti University of Medical Sciences,
Tehran, Iran.
4. Department of Hematology, School of Allied Medical Sciences, Shahid Beheshti University of Medical
Sciences, Tehran, Iran.
Submmited 15 September 2015; Accepted 1 December 2015; Published 26 December 2015
Mesenchymal stem cells (MSCs) have the ability to differentiate into neuronal like cells under appropriate
culture condition. In this study, we investigated whether MSCs derived from human peripheral blood (PB-
MSCs) can differentiate into neuronal like cells by synergic effect of the growth factors EGF, bFGF and Noggin.
For this purpose, the expression of five neuronal markers (Nestin, β III tubulin,
NFM,
MAP2 and
NSE) were
evaluated in treated PB-MSCs by SYBR Green Real time PCR. The expression analysis showed a higher
expression of β- tubulin and
NFM in treated BP-MSCs compared with untreated PB-MSCs as a control group.
The expression of Nestin was also diminished in PB-MSCs treated with Noggin. This study suggested that the
treatment of PB- MSCs with Noggin alongside with bFGF and EGF might differentiate these cells into neuronal
lineage cells. The obtained results could be further developed for useful applications in regenerative medicine.
Key words: Mesenchymal stem cells, differentiation, neuronal markers, Noggin
owadays, the regenerative medicine has
fetal umbilical cord have the ability to differentiate
Nprovided an alternative source of different
into cell types of different organs (3-4). In other
cell lines and organs through trans- differentiation
alternative methods, the induced pluripotent stem
process (1). During last decades, different strategies
cells (iPSCs) generated from somatic cells have
have been used to regenerate missing tissues (2).
opened a new window in regenerative medicine (5).
Some studies indicated that stem cells derived from
However, the use of these cells was accompanied
∗Corresponding author: Department of Medical Genetics, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Email:
[email protected] # These authors have equally contribution.
by some limitations. For example, lentivirus vectors
differentiation through blockage of Smad signalling
including transcription factors for transformation of
(20-21). Furthermore, it has been demonstrated that
somatic cells into iPSCs could cause the recipient
the inhibition of BMP signalling by Noggin along
cells to exhibit long-term genetic aberrations (6).
with activation of bFGF signalling could participate
The characterization and isolation of stem cells
into neural differentiation of MSCs (22).
from different organs and tissues has represented an
In this study, we attempted to differentiate
alternative source of cells in cell therapy or
peripheral blood derived MSCs (PB-MSCs) into
regenerative medicine. However
, it is not possible
neuronal cells by inhibition of BMP signalling upon
to isolate stem cells from some tissues including
treatment with growth factors such as Noggin,
central nervous system (CNS). Therefore, trans-
bFGF and EGF. The expression of neural markers
differentiation of stem cells derived from other
like nestin, β
III tubulin,
neurofilament
M (NFM),
tissues could provide a suitable supply for
microtubule- associated protein 2 (MAP2) and
regeneration of these tissues. Current studies have
neuron-specific enolase
(NSE)
in treated cells were
demonstrated that the mesenchymal stem cells
investigated to determine whether those growth
(MSCs) derived from different tissues have the
factors could influence the expression of these
ability to differentiate into different cell types
.
multipotent cells that can
Materials and methods
adipocytes, myocytes, endothelial cells, and
PB-MSCs isolation
neurons (7-10). Although the primary sources of
The peripheral blood (almost 6 ml) was
MSCs are bone marrow, umbilical-cord blood,
obtained from three healthy individuals. The blood
olfactory bulb, amniotic fluid (AF), and Wharton's
was collected in EDTA-treated tubes and layered
jelly, these cells have also been found in peripheral
over ficoll in a 2:1 ratio. The peripheral blood
blood (11-12). Studies have demonstrated that
mononuclear cells (PBMCs) were separated by
MSCs have the ability to spontaneously express the
density gradient centrifugation, plated in DMEM/F-
neural markers including nestin, NeuN, gilal
12 medium containing 10% fetal bovine serum
fibrillary acidic protein (GFAP) and βIII tubulin
(FBS), 2 mM L- Glutamate and 100 units/ml
(13). These observations could support the
Penicillin/ Streptomycin (medium A) and then,
predisposition of MSCs to differentiate toward
incubated at 37 °C in a 5% CO2 humidified
atmosphere. After 48 hours, media and unattached
oligodendrocytes
cells were removed by washing with
phosphate-
protocols have been published for differentiation of
buffered saline (PBS). The adherent cells were
MSCs into neuronal lineage cells (9, 14-16). There
maintained in a fresh medium until approximately
is increasing evidence about neural induction of
80% confluence was reached on day 6 of culture.
MSCs by numerous growth factors (9, 17). These
Flow cytometry analysis
growth factors are able to regulate neuronal
The adherent cells were confirmed to be
differentiation through different mechanisms (18).
MSCs by flow cytometry. On day 6, the cells were
It has been known that BMP2 is one of the most
harvested with trypsin. After centrifuging at 450 g
important
bone morphogenetic proteins (BMPs) in
for 5 min, the cells were suspended in DPBS and
regulating the osteogenic differentiation (19).
Previous studies indicated that the inhibition of
(Fluorescein isothiocyanate) conjugated CD45 (BD
BMP2 by Noggin prevented from osteogenic
Biosciences, Cat# 347463, RRID: AB_400306) as
Int J Mol Cell Med Autumn 2015; Vol 4 No 4 210
Treatment of PB-MSCs and Neuronal Gene Expression
leukocyte marker, PE (Phycoerythrin) conjugated
Total RNA was extracted from untreated and
CD14 (BD Biosciences, Cat# 347497, RRID:
growth factor-treated PBMSCs (day 14) using the
total RNA purification kit (Jena Bioscience,
conjugated CD44 (BD Biosciences, Cat# 347943,
RRID: AB_400360), PE conjugated CD105 (BD
instructions. DNase I treatment of RNA was
Biosciences, Cat# 560839, RRID: AB_2033932)
performed in a final volume of 50
µl containing 40
and PE conjugated CD73 (BD Biosciences, Cat #
µl RNA, 5
µl RNase-free DNase I and 5
µl 10x
550257, RRID: AB_393561) for 30 min in the dark.
reaction buffer (Fermentas, Thermo Scientific,
The CD73, CD105 and CD44 served as surface
Waltham, MA, USA). The mixture was incubated
markers of MSCs. Negative control staining was
for 30 min at 37 °C. Then, the enzyme was
performed by using IgG1- FITC and IgG1-PE
inactivated at 65 °C for 10 min. The complete
isotype controls. Then, the cells were analyzed on
removal of DNA was confirmed by electrophoresis
Partec
CyFlow Space cytometer
using FloMax
on 1% agarose gel. Finally, the cDNA template was
software (http://flomax.software.informer. com/ 2.2 /).
synthesized from extracted RNA using random
Neuronal differentiation
hexamer primers and dART reverse transcriptase
On day 6, the medium A was removed and the
(EURx Ltd, Gdansk, Poland).
cells were plated in medium A supplemented
Real time quantitative PCR
with 0.1 mM NEAA, 2% B27 supplement, 1%
The expression levels of neuronal marker
N2 supplement, 50 ng/ml Noggin, 20 ng/ml EGF
genes were evaluated by quantitative PCR (qPCR)
and 10 ng/ml recombinant human bFGF (medium
after 14 days of culture. The SYBR Green based
B). The medium A was used as the control medium.
qPCR was carried out on Rotor-Gene 6000 Real
The growth factors Noggin, EGF and recombinant
time PCR system. The qPCR reaction was prepared
human bFGF were added to the medium every
in a total volume of 25 µl containing 12.5 µl of 2X
day. After three days culture in the medium B,
SYBR Green master mix (Eurex, Poland), 5 µl of
the EGF and recombinant human bFGF were
the cDNA template, 0.2 µl of each primer (10
removed from the medium (medium C) and the
pmol/µl) and 7.1 µl of deionized water. A negative
cells were cultured for an additional six days
control was used by replacing the cDNA template
in medium C. The medium was changed every
with deionized water. Primer sequences used in this
study and their annealing temperature are shown in
RNA extraction and cDNA synthesis
Table 1. Characteristics of qPCR primers pairs used in this study
Accession number
Primer sequences
temperature (ºC) ence
GGAAGTGCACCATGGAGAGGA
(Reference gene)
GCGAATCTTGTCCAAGGCATCAG
CTCAGGGGCCTTTGGACATC
CAGGCAGTCGCAGTTTTCAC
ATCGCTCAGGTCCTGGAA
AAGCTGAGGGAAGTCTTGGA
CATGGGTCACAGGGCACCTATTC
GGAGAACAGTGAAGCCTTGG
GGTCAAATGGGTCCTCAATG
211 Int J Mol Cell Med Autumn 2015; Vol 4 No 4
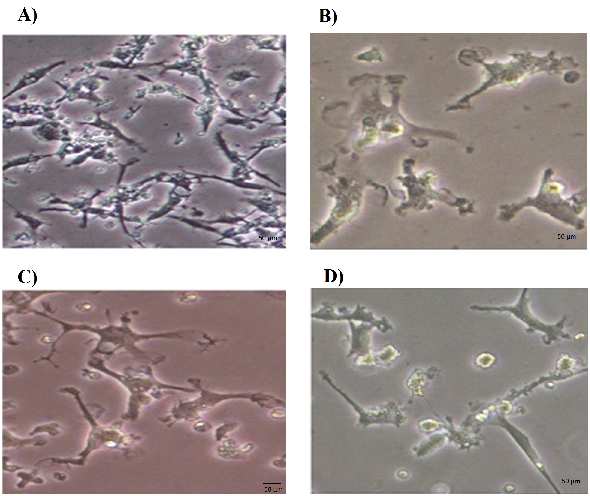
The PCR amplification consisted of an initial
Morphological changes of the growth factor-
denaturation at 95 ºC for 10 min, followed by 40
treated PBMSCs
cycles of denaturation at 95 ºC for 30 s, annealing
The morphological features of untreated and
at 60 ºC for 30 s and extension at 72 ºC for 30 s.
growth factor-treated PBMSCs were observed
The specificity of PCR products was verified by
under inverted microscope. After being cultured for
melting curves and electrophoresis through 3%
6 days, the PBMSCs adhered to the culture surfaces
reached 70-80% confluence (Figure 1A). The
The expression level of each gene was
untreated PBMSCs showed mainly spindle- shaped
calculated as fold change relative to the expression
morphology. These cells had a tendency to become
of reference gene (HSP90AB1) using pfaffl method
flatter and wider over time. The neurosphere like
(28). The statistical analysis was performed using
cells were suspended 2-3 days after culture in the
Social Science Statistics website (http: //www.
medium induction containing growth factors
socscistatistics.com /tests/studentttest/ Default2.
Noggin, bFGF and EGF. Within 4-5 days after
aspx). The ∆Ct value of treated versus untreated
addition of growth factors, some cells began to look
PBMSCs was compared by t-test. Data were
like oligodendrocytes or astrocytes. After 8 days
represented as fold change relative to the cell
treatment with growth factors, the PBMSCs
identifier using GraphPad Prism software (http:
displayed multipolar shapes and bright cell bodies
oligodendrocytes
Fig. 1. Morphological features of PBMSCs treated with Noggin. A) Prior to treatment, PBMSCs showed fibroblast like shape on day 6. B)
PBMSCs after 4 days treatment with Noggin (Day 10). C) PBMSCs after 8 days treatment with Noggin (Day 14). The cells showed the
multipolar processes. D) PBMSCs after 8 days treatment with Noggin (Day 14). The cells in this figure displayed synaptic structure.
Int J Mol Cell Med Autumn 2015; Vol 4 No 4 212
Treatment of PB-MSCs and Neuronal Gene Expression
Fig. 2. Flow cytometry analysis of adherent cells derived from peripheral blood. The cultured cells were CD45 and CD14 negative. In
contrast, presence of MSCs markers (CD73, CD105 and CD44) confirmed that the majority of these cells are MSCs.
Fig. 3. Analysis of neuronal markers expression of the PBMSCs treated with growth factor Noggin. Graphs were generated using
GraphPad Prism 6. The results were obtained from three independent experiments.
213 Int J Mol Cell Med Autumn 2015; Vol 4 No 4
Expression pattern of surface markers on
used to establish neuronal stem cells (NSCs) and
neuronal lineage cells. The safety problem is an
The adherent cells derived from peripheral
important issue to use of these cells as a therapeutic
blood were analyzed by flow cytometry. As
method (32). MSCs are an alternative source of
expected, these cells were negative for leukocyte
cells for use in treating patients with neurological
marker CD45 as well as monocytic marker CD14.
disease. It has been demonstrated that these cells
The majority of these cells showed positive signal
have the ability to differentiate into neuron like
for mesenchymal cell makers CD105, CD44 and
cells (9). Numerous reports have described different
CD73 (Figure 2).
protocols for differentiation of stem cells derived
Expression levels of neural markers in growth
from peripheral blood. The previous studies
factor treated-PBMSCs
revealed that PBMCs have ability to differentiate
Quantitative analysis with qPCR revealed that
into neural like cells in the presence of different
the expression of NFM and βIII tubulin increased
combinations of growth factors (33-34). In this
significantly in the growth factor-treated group.
study, a new combination of growth factors
Almost three fold increase of βIII tubulin
including Noggin, bFGF and EGF was used to
expression was observed upon treatment in the two
induce neural differentiation of MSCs derived from
cultures of PBMSCs. The nestin expression level
peripheral blood (PBMSCs) by inhibition of BMP
was markedly reduced in the PBMSCs treated with
signaling. To confirm the differentiation of
Noggin. Treatment with Noggin, bFGF and EGF
PBMSCs, the expression level of neural cell
caused an increased expression of MAP2 and
specific markers were assessed with qPCR.
diminished expression of NSE in one of the treated-
PB-MSCs showed changes in morphology and
PBMSCs. In contrast, the other culture displayed a
expression of neural markers upon treatment with
reduction of MAP2 expression and augmentation of
growth factors Noggin, bFGF and EGF. The cells in
NSE expression after treatment with Noggin. The
the present study had morphology different from
third culture showed reduced expression of MAP2
the neural like cells described in the previous
as well as NSE in growth factor-treated PBMSCs.
studies. These observations suggested that these
The graphs derived from these data are presented in
cells belonged to different cell types of neural
figure 3. The results obtained from statistical
analysis indicated that there was no statistically
Nestin is a marker of NSCs (35) that its
significant difference between treated and untreated
expression has also been observed in MSCs (36). It
PBMSCs. This is probably due to the low number
was revealed that the expression of nestin is
inversely correlated with cellular differentiation
(35). The expression of nestin decreased upon
Discussion
treatment with growth factor Noggin, consistent
Stem cell therapy is a new approach for the
with differentiation of MSCs. Although the
previous studies showed that the expression of
neurological diseases (29). Different studies have
nestin needs at least 10 passages of the cultured
demonstrated that the embryonic stem cells can
MSCs in serum free medium (37), but we observed
give rise to neuronal cells (30-31). However, the
nestin expression in MSCs following culture in
ethical problems are major concerns in the use of
medium supplemented with fetal bovine serum for
these cells in cell therapy. In some studies, the
14 days. These results were consistent with the
induced pluripotent stem cells (iPSCs) have been
finding obtained by Foudah et al. (38).
Int J Mol Cell Med Autumn 2015; Vol 4 No 4 214
Treatment of PB-MSCs and Neuronal Gene Expression
β III tubulin and NFM are known to be the
expression level was increased in one of treated cell
early and late neuronal markers, respectively. These
cultures with Noggin, consistent with their
differentiation into neuron like cells. The previous
undifferentiated MSCs (39). As observed in Figure
studies indicated that the expression level of NSE
3, Noggin treatment of PBMSCs resulted in
increased during the oligodendrocyte differentiation
increased β III tubulin and NFM expression. The
and NSE expression was repressed in mature
results obtained from previous studies suggested
oligodendrocytes (44). Furthermore, it has been
that the in vitro culture could induce the
found that low levels of NSE expression are present
spontaneous expression of neural markers in MSCs.
in astrocytes. Therefore, NSE expression data in our
However, it has remained to be demonstrated (40).
study suggested that treatment with Noggin was
Different isoforms of MAP2 are expressed in
accompanied by differentiation of PBMSCs into
the neural lineage cells (41). The primer pair used
different types of neurons, astrocytes and
in the present study detects MAP2a, MAP2b and
oligodendrocytes.
MAP2c isoforms. These isoforms of MAP2 were
In general, our results showed that PBMSCs
could express some neural markers including
differentiation. MAP2c is an early neuronal marker
Nestin, βIII tubulin, NFM, MAP2 and NSE.
and its expression decreased in the mature neurons.
Accordingly, PBMSCs are a potential source of
MAP2b was expressed in terminally differentiated
cells that can be used to generate neuronal cells.
neurons as well as during differentiation. MAP2a
Although different induction protocols were
expression was detectable in mature neurons (39).
published about differentiation of MSCs into
We observed a two-fold increase in the expression
neuron like cells, the introduction of new protocols
of MAP2 in one of treated PBMSCs as compared
could improve our understanding from the
with non-treated PBMSCs. This data along with
characteristics of MSCs and the neuron like cells
expression pattern of other markers suggested that
derived from MSCs. Furthermore, the results
PBMSCs differentiated into neuron like cells
obtained from this study provide evidence of
following treatment with Noggin. In contrast, qPCR
neuronal differentiation of MSCs upon treatment
analysis showed a decrease of MAP2 expression in
with Noggin. However, neural marker expression
the other two PBMSCs treated with noggin,
analysis cannot be used as the only proof to
proposing the differentiation of these treated cells
demonstrate the neuronal differentiation of MSCs
into oligodendrocytes. This fact was confirmed by
following treatment with Noggin and the complete
morphology assessment of treated PBMSCs. A
understanding of these cells needs additional
previous study on differentiating oligodendrocytes
studies from the molecular, biological and
has demonstrated that MAP2 expression transiently
physiological aspects.
increased in preoligodendrocytes. Its expression
Conflict of interest
The authors declared no conflict of interests.
differentiation of oligodendrocytes (42).
Enolase is a key enzyme in the glycolytic
References
pathway which plays an important role in energy
production for cells. It has been revealed that γ-
transdifferentiation shift the landscape of regenerative medicine.
enolase (Eno2) was only found in cells of neuronal
DNA and cell biology 2013;32:565-72.
lineage (43). Our results showed that NSE
2. Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation,
215 Int J Mol Cell Med Autumn 2015; Vol 4 No 4
transdifferentiation and reprogramming: three routes to
of biomedicine & biotechnology 2012;2012:820821.
regeneration. Nature reviews Molecular cell biology 2011;12:79-
14. Bae KS, Park JB, Kim HS, et al. Neuron-like differentiation
of bone marrow-derived mesenchymal stem cells. Yonsei
3. Fu YS, Shih YT, Cheng YC, et al. Transformation of human
medical journal 2011;52:401-12.
umbilical mesenchymal cells into neurons in vitro. Journal of
15. Guan M, Xu Y, Wang W, et al. Differentiation into neurons
biomedical science 2004;11:652-60.
of rat bone marrow-derived mesenchymal stem cells. European
4. Fu YS, Cheng YC, Lin MY, et al. Conversion of human
cytokine network 2014;25:58-63.
umbilical cord mesenchymal stem cells in Wharton's jelly to
16. Hosseini SM, Talaei-Khozani T, Sani M, et al.
dopaminergic neurons in vitro: potential therapeutic application
Differentiation of human breast-milk stem cells to neural stem
for Parkinsonism. Stem cells 2006;24:115-24.
cells and neurons. Neurol Res Int 2014;2014:1-8.
5. Toyoda T, Mae S, Tanaka H, et al. Cell aggregation optimizes
17. Nandy SB, Mohanty S, Singh M, et al. Fibroblast Growth
the differentiation of human ESCs and iPSCs into pancreatic
Factor-2 alone as an efficient inducer for differentiation of
bud-like progenitor cells. Stem cell research 2015;14:185-97.
human bone marrow mesenchymal stem cells into dopaminergic
6. Gore A, Li Z, Fung HL, et al. Somatic coding mutations in
neurons. Journal of biomedical science 2014;21:83.
human induced pluripotent stem cells. Nature 2011;471:63-7.
18. Gaulden J, Reiter JF. Neur-ons and neur-offs: regulators of
7. Barry F, Boynton RE, Liu B, et al. Chondrogenic
neural induction in vertebrate embryos and embryonic stem
differentiation of mesenchymal stem cells from bone marrow:
cells. Human molecular genetics 2008;17:R60-6.
differentiation-dependent gene expression of matrix components.
19. Bais MV, Wigner N, Young M, et al. BMP2 is essential for
Experimental cell research 2001;268:189-200.
post natal osteogenesis but not for recruitment of osteogenic
8. Jaiswal RK, Jaiswal N, Bruder SP, et al. Adult human
stem cells. Bone 2009;45:254-66.
mesenchymal stem cell differentiation to the osteogenic or
20. Edgar CM, Chakravarthy V, Barnes G, et al. Autogenous
adipogenic lineage is regulated by mitogen-activated protein
regulation of a network of bone morphogenetic proteins (BMPs)
kinase. The Journal of biological chemistry 2000;275:9645-52.
mediates the osteogenic differentiation in murine marrow
9. Kim EY, Lee KB, Yu J, et al. Neuronal cell differentiation of
stromal cells. Bone 2007;40:1389-98.
mesenchymal stem cells originating from canine amniotic fluid.
21. Wang Y, Hong S, Li M, et al. Noggin resistance contributes
Human cell 2014;27:51-8.
to the potent osteogenic capability of BMP9 in mesenchymal
10. Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal
stem cells. Journal of orthopaedic research : official publication
stem cells can be differentiated into endothelial cells in vitro.
of the Orthopaedic Research Society 2013;31:1796-803.
Stem cells 2004;22:377-84.
22. Delaune E, Lemaire P, Kodjabachian L. Neural induction in
11. Chong PP, Selvaratnam L, Abbas AA, et al. Human
Xenopus requires early FGF signalling in addition to BMP
peripheral blood derived mesenchymal stem cells demonstrate
inhibition. Development 2005;132:299-310.
similar characteristics and chondrogenic differentiation potential
23. Jouhilahti EM, Peltonen S, Peltonen J. Class III beta-tubulin
to bone marrow derived mesenchymal stem cells. Journal of
is a component of the mitotic spindle in multiple cell types. The
orthopaedic research : official publication of the Orthopaedic
journal of histochemistry and cytochemistry : official journal of
Research Society 2012;30:634-42.
the Histochemistry Society 2008;56:1113-9.
12. Montesinos JJ, Flores-Figueroa E, Castillo-Medina S, et al.
24. Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression
Human mesenchymal stromal cells from adult and neonatal
and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Molecular cancer 2006;5:67.
immunophenotype, differentiation patterns and neural protein
25. Mareschi K, Novara M, Rustichelli D, et al. Neural
expression. Cytotherapy 2009;11:163-76.
differentiation of human mesenchymal stem cells: Evidence for
13. Foudah D, Redondo J, Caldara C, et al. Expression of neural
expression of neural markers and eag K+ channel types.
markers by undifferentiated rat mesenchymal stem cells. Journal
Experimental hematology 2006;34:1563-72.
Int J Mol Cell Med Autumn 2015; Vol 4 No 4 216
Treatment of PB-MSCs and Neuronal Gene Expression
26. Constantinescu R, Constantinescu AT, Reichmann H, et al.
express a new class of intermediate filament protein. Cell
Neuronal differentiation and long-term culture of the human
neuroblastoma line SH-SY5Y. Journal of neural transmission
36. Wong A, Ghassemi E, Yellowley CE. Nestin expression in
Supplementum 2007:17-28.
mesenchymal stromal cells: regulation by hypoxia and
27. Hafizi M, Atashi A, Bakhshandeh B, et al. MicroRNAs as
osteogenesis. BMC veterinary research 2014;10:173.
markers for neurally committed CD133+/CD34+ stem cells
37. Wislet-Gendebien S, Hans G, Leprince P, et al. Plasticity of
derived from human umbilical cord blood. Biochemical genetics
cultured mesenchymal stem cells: switch from nestin-positive to
excitable neuron-like phenotype. Stem cells 2005;23:392-402.
28. Pfaffl MW. A new mathematical model for relative
38. Foudah D, Redondo J, Caldara C, et al. Human mesenchymal
quantification in real-time RT-PCR. Nucleic acids research
stem cells express neuronal markers after osteogenic and
adipogenic differentiation. Cellular & molecular biology letters
29. Lescaudron L, Naveilhan P, Neveu I. The use of stem cells in
regenerative medicine for Parkinson's and Huntington's
39. Chung WJ, Kindler S, Seidenbecher C, et al. MAP2a, an
Diseases. Current medicinal chemistry 2012;19:6018-35.
alternatively spliced variant of microtubule-associated protein 2.
30. Morizane A, Takahashi J, Shinoyama M, et al. Generation of
Journal of neurochemistry 1996;66:1273-81.
graftable dopaminergic neuron progenitors from mouse ES cells
40. Croft AP, Przyborski SA. Formation of neurons by non-
by a combination of coculture and neurosphere methods. Journal
neural adult stem cells: potential mechanism implicates an
of neuroscience research 2006;83:1015-27.
artifact of growth in culture. Stem cells 2006;24:1841-51.
31. Park CH, Minn YK, Lee JY, et al. In vitro and in vivo
41. Shafit-Zagardo B, Kalcheva N. Making sense of the multiple
analyses of human embryonic stem cell-derived dopamine
MAP-2 transcripts and their role in the neuron. Molecular
neurons. Journal of neurochemistry 2005;92:1265-76.
neurobiology 1998;16:149-62.
32. Velasco I, Salazar P, Giorgetti A, et al. Concise review:
42. Vouyiouklis DA, Brophy PJ. Microtubule-associated
Generation of neurons from somatic cells of healthy individuals
proteins in developing oligodendrocytes: transient expression of
and neurological patients through induced pluripotency or direct
a MAP2c isoform in oligodendrocyte precursors. Journal of
conversion. Stem cells 2014;32:2811-7.
neuroscience research 1995;42:803-17.
33. Horschitz S, Meyer-Lindenberg A, Schloss P. Generation of
43. Marangos PJ, Parma AM, Goodwin FK. Functional
neuronal cells from human peripheral blood mononuclear cells.
properties of neuronal and glial isoenzymes of brain enolase.
Neuroreport 2010;21:185-90.
Journal of neurochemistry 1978;31:727-32.
34. Liu Q, Guan L, Huang B, et al. Adult peripheral blood
44. Sensenbrenner M, Lucas M, Deloulme JC. Expression of two
mononuclear cells transdifferentiate in vitro and integrate into
neuronal markers, growth-associated protein 43 and neuron-
the retina in vivo. Cell biology international 2011;35:631-8.
specific enolase, in rat glial cells. Journal of molecular medicine
35. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells
217 Int J Mol Cell Med Autumn 2015; Vol 4 No 4
Source: http://www.ijmcmed.org/article-1-384-en.pdf
iQ Global Managed Portfolio SEGREGATED MANAGED SHARE PORTFOLIO iQ Global Managed SP iQ Global Managed Share Portfolio Company Descriptions Fees and reporting iQ Global Managed Share Portfolio March 2016 iQ Global Managed SP PURPOSE STATEMENT To provide clients with market leading investment solutions seeking to maximise risk-adjusted returns
Your Guide to Coumadin®/Warfarin Therapy This booklet is based on a product developed by Carla Huber, A.R.N.P., M.S., Cedar Rapids Community Anticoagulation Clinic, Cedar Rapids, Iowa, under Agency for Healthcare Research and Quality (AHRQ) Grant No. 1 U18 HSO15830-01 to Kirkwood Community College. This document is in the public domain and may be used and








