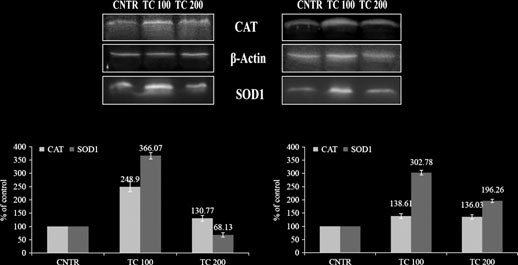
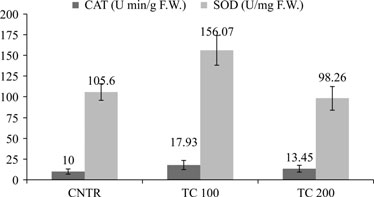
Untitled
IEEE TRANSACTIONS ON INFORMATION THEORY, VOL. 56, NO. 9, SEPTEMBER 2010 Thinning, Entropy, and the Law of Thin Numbers Peter Harremoës, Member, IEEE, Oliver Johnson, and Ioannis Kontoyiannis, Senior Member, IEEE Abstract—Rényi's thinning operation on a discrete random vari- where the random variables are independent and identi-