Pii: s0304-3940(00)01340-9
Neuroscience Letters 290 (2000) 137±140
Morphine and gabapentin decrease mechanical hyperalgesia and
escape/avoidance behavior in a rat model of neuropathic pain
Christopher J. LaBuda, Perry N Fuchs*
Department of Psychology, University of Texas at Arlington, PO Box 19528, Arlington, TX 76019, USA
Received 3 April 2000; received in revised form 30 June 2000; accepted 5 July 2000
A behavioral test paradigm that measures the aversive quality of stimulus-evoked pain in an animal model of neuro-
pathic pain (L5 ligation) was tested for sensitivity to (1) different forces (476 and 202 mN) and frequencies (once every 15
or 30 s) of mechanical stimulation to the hyperalgesic paw and (2) different doses of the common antinociceptive
compounds morphine (1 and 10 mg/kg) and gabapentin (30 and 90 mg/kg). Compared to non-ligated controls, the
greater force (476 mN) and frequency (every 15 s) of mechanical stimulation of the hyperalgesic paw was associated
with the greatest degree of escape/avoidance behavior. There was not a signi®cant degree of escape/avoidance behavior
at the lowest force (202 mN) and frequency (every 30 s) of mechanical stimulation. Compared to ligated vehicle treated
controls, morphine (1 mg/kg) and gabapentin (90 mg/kg) decreased mechanical hyperalgesia and also attenuated the
escape/avoidance behavior. The antinociceptive and antiaversive effects were found at doses that did not produce
evidence of decreased motor activity. It is concluded that the behavioral test paradigm used to measure the aversiveness
of stimulus-evoked nociceptive behavior is sensitive to different degrees of evoked pain and traditional analgesic
compounds. q 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords: Avoidance; Affect; Motivation; Mechanical hyperalgesia; Place preference
Traditional nociceptive tests typically measure the
nature of evoked pain in animal models of neuropathic and
response or a change in threshold to a noxious or non-
in¯ammatory conditions. However, it remains to be deter-
noxious mechanical or thermal stimulus, such as a change
mined if the behavioral paradigm is sensitive to differing
in mechanical threshold following nerve damage or a
forces and frequencies of mechanical stimulation and tradi-
response to radiant heat (tail-¯ick, paw withdrawal).
tional analgesic compounds. It is expected that if mechanical
These tests have been useful to elucidate spinal and suprasp-
stimulation of the hyperalgesic paw is aversive, then as the
inal mechanisms of nociception and screen compounds for
force and frequency of the stimulation decreases, there
analgesic ef®cacy. For instance, both morphine and gaba-
should be a decrease in the aversive nature of evoked pain
pentin have been extensively studied and demonstrated to
caused by the stimulus that should be re¯ected as a decrease
possess analgesic properties in many clinical pain states as
of escape/avoidance behavior. It is also expected that
well as animal models of in¯ammatory and neuropathic pain
compounds that decrease mechanical hyperalgesia should
be associated with an attenuation of the aversive nature of
We have recently developed a behavioral test paradigm
the noxious stimulus. Therefore, the purpose of the present
that measures the aversiveness of nociceptive stimuli as an
experiment was to (1) examine the effect of different forces
attempt to model the affective/motivational aspect of clinical
and frequencies of mechanical stimulation on the place
pain states [12]. The behavioral paradigm allows animals to
avoidance behavior; and (2) to determine, in a dose depen-
choose' an environment associated with the application of a
dent manner, if the behavioral test paradigm is sensitive to
mechanical stimulus to the hyperalgesic paw or to the non-
morphine and gabapentin.
operated contralateral paw. Our previous ®ndings indicate
One hundred and forty one male Sprague±Dawley rats
that the test paradigm is sensitive to measure the aversive
(UTA vivarium) were housed in pairs and allowed free
access to food and water throughout the study. Room
* Corresponding author. Tel.: 11-817-272-3427; fax: 11-817-
temperature and humidity were maintained at 218C and
E-mail address:
[email protected] (P.N. Fuchs).
0304-3940/00/$ - see front matter q 2000 Elsevier Science Ireland Ltd. All rights reserved.
C.J. LaBuda, P.N. Fuchs / Neuroscience Letters 290 (2000) 137±140
70%, respectively. All procedures were approved by the
For experiment 1, the analysis of time spent within the
UTA Institutional Animal Use and Care Committee.
light side of the chamber for the different force/frequency
Nerve injury was produced by tightly ligating the L5 spinal
combinations revealed a signi®cant group £ time interaction
nerve (n 94) [11,12]. Forty-seven additional animals
for the 476 mN force applied at 15 s intervals (P , 0:01),
served as sham surgery control without ligation of the L5
with the L5 ligated group spending signi®cantly more time
spinal nerve. Behavioral testing was performed on the second
within the light side of the chamber at 20 min compared to the
day following the surgical procedure which involved
sham surgery group (Fig. 1). Analysis of the other force/
measures of mechanical paw withdrawal thresholds
frequency combinations revealed no signi®cant group differ-
(MPWT) using the up/down technique [6,12] immediately
ences or group £ time interactions. However, visual inspec-
followed by further behavioral testing described for experi-
tion of Fig. 1 indicates a strong trend towards a group £ time
ment 1 (force/frequency) or experiment 2 (morphine/gaba-
interaction for the 202 mN force applied at 15 s intervals
(P , 0:15), with the L5 ligated group spending signi®cantly
Experiment 1 examined different forces and frequencies
more time in the light side of the chamber at 20 min compared
of mechanical stimulation on escape/avoidance behavior.
to the sham surgery group. All L5 ligated groups demon-
Sham surgery and L5 ligated animals were randomized to
strated a signi®cant decrease of MPWT that did not differ
one of four groups (476 mN/15 s, 476 mN/30 s, 202 mN/15
among the groups (data not shown).
s, 202 mN/30 s) and tested for escape/avoidance behavior
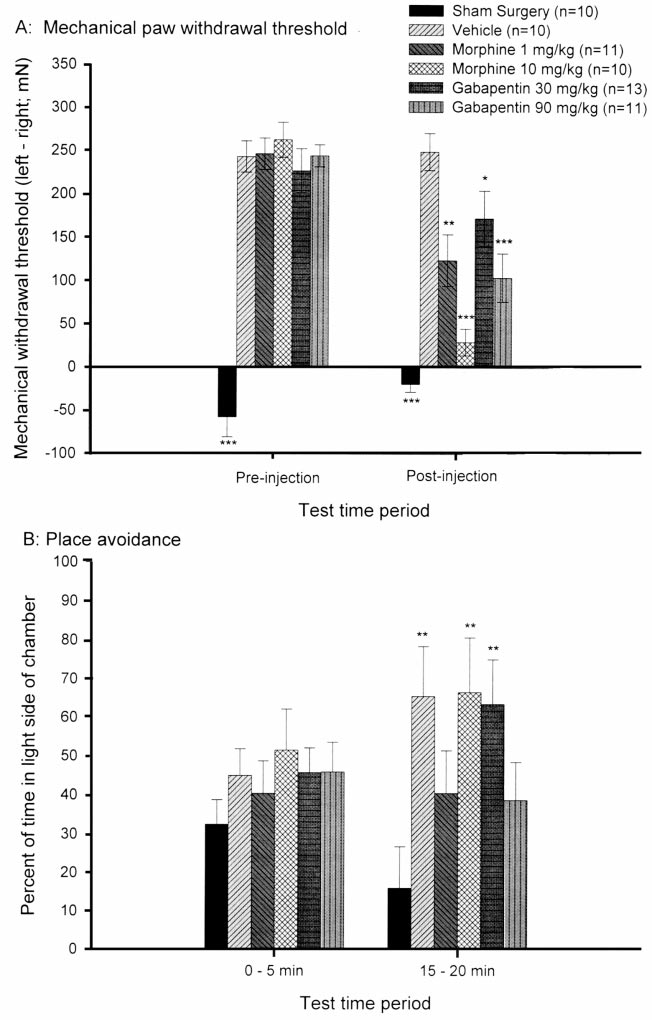
For experiment 2, the analysis of MPWT for animals that
using methods previously reported [12]. Immediately
received L5 ligation or sham surgery revealed a signi®cant
following MPWT testing, animals were placed within a
main effect for group at the pre-injection time point
30 £ 30 £ 30-cm Plexiglas chamber (half painted black
(P , 0:001), with all ®ve L5 ligated groups demonstrating
and the other half painted white) and allowed unrestricted
a signi®cant decrease of left hindpaw MPWT compared to
movement for the duration of a 20-min test period. The
sham surgery controls (Fig. 2A) with no signi®cant pre-
mechanical stimulus (either 202 or 476 mN) was applied
injection MPWT differences among the ®ve L5 spinal
to the plantar surface of the hindpaws at a constant interval
nerve ligated groups. The analysis of MPWT following
of time (either 15 or 30 s). When the animal was within the
the escape/avoidance test revealed a signi®cant main effect
dark side of the chamber, the mechanical stimulus was
for group (P , 0:001), with the sham surgery group, both
applied to the hyperalgesic paw; when the animal was
groups that received morphine (1 and 10 mg/kg) and both
within the light side of the chamber, the mechanical stimu-
groups that received gabapentin (30 and 90 mg/kg) demon-
lus was applied to the non-ligated control paw. The amount
strating signi®cantly less mechanical hyperalgesia than the
of time that each animal stayed within the light side of the
chamber was recorded.
The second experiment examined the effect of morphine
and gabapentin on MPWT and escape/avoidance behavior.
Immediately following pre-drug administration baseline
MPWT measurement, L5 ligated animals were randomized
to receive one of ®ve (two doses of morphine and gabapen-
tin and a vehicle control) coded drug solutions. Morphine
and gabapentin (Sigma Chemical Co., St. Louis, Mo) were
prepared on the day prior to the injection. Morphine was
dissolved in a 0.9% saline solution to form either a 1 or 10
mg/ml solution and was delivered s.c. (10 ml/kg). Gabapen-
tin was dissolved in a 0.9% saline solution to form either a
30 or 90 mg/ml solution and was delivered s.c. (10 ml/kg).
The vehicle solution consisted of the 0.9% saline solution
delivered in the same manner. A sham surgery group that
did not receive injection served as an additional control
group. At 20-min post-injection, animals were tested in
Fig. 1. Mean ( SEM) percent of time spent within the light side of
the escape/avoidance test using the same methods as
the chamber for animals that had L5 spinal nerve ligation or
described for experiment 1. Based on the results from
received a sham surgery without ligation of the L5 spinal
nerve. Groups of animals were tested for 20 min in the escape/
experiment 1, testing was performed for 20 min using the
avoidance test while being administered a von Frey force of
476 mN force applied at 15 s intervals. Quanti®cation of
either 476 or 202 mN at a frequency of once every 15 or every
motor behavior consisted of counting the number of center-
30 s. The duration of time spent within the light side of the
line crossings, as de®ned as all four paws crossing the line,
chamber for each force/frequency combination was analyzed
during the 20-min test period. Following the escape/avoid-
separately for group differences using repeated measures
ANOVA followed by post-hoc comparison (LSD) of group differ-
ance test, animals were tested for MPWT. The experimenter
ences at each test time period. *P , 0:05 compared to vehicle
was blind to the content of each solution.
C.J. LaBuda, P.N. Fuchs / Neuroscience Letters 290 (2000) 137±140
The overall analysis (one-way ANOVA followed by post-
hoc comparison (LSD) of total crosses from the light to dark
side of the chamber revealed a signi®cant main effect for
group (P , 0:01). Animals treated with 10 mg/kg morphine
made signi®cantly fewer line crosses than all other ligated
groups (data not shown). In addition, there was no signi®-
cant difference in line crosses between the 10 mg/kg
morphine and sham surgery groups (P . 0:05).
The rationale for developing the present behavioral
escape/avoidance test paradigm is based on the need for a
method to quantify the negative affective dimension of pain
in various animal models. The present results con®rm our
previous report indicating that animals quickly begin to
spend less time in the naturally preferred environmental
area (i.e. dark) that is associated with mechanical stimula-
tion of the hyperalgesic paw [12]. In the present experiment,
both the L5 ligated vehicle treated and sham surgery groups
started out with an equal preference for the light side of the
chamber (Fig. 2B, 0±5 min). However, by 15±20 min the
amount of time spent within the light side of the chamber
was approximately 15% for the sham surgery group versus
70% for the L5 ligated vehicle treated group. This ®nding is
a clear indication that animals ®nd mechanical stimulation
of the L5 spinal nerve ligated paw aversive, and when given
a choice, will perform purposeful behavior to minimize
stimulation of the hyperalgesic part. In addition, our predic-
tion that as the force and frequency of mechanical stimula-
tion decreased, there would be an associated decrease in the
shift from the dark area of the chamber to the light side of
the chamber was con®rmed (Fig. 1).
A second purpose of the present experiment was to exam-
ine if the behavioral test paradigm to measure the aversive
Fig. 2. (A) Mean ( SEM) mechanical paw withdrawal threshold
nature of mechanical stimulation following nerve ligation
(left paw±right paw) for animals that did not receive L5 ligation
was sensitive to known analgesic compounds. First, we
(Sham Surgery) or received different doses of different
compounds following L5 ligation. Mechanical paw withdrawal
ensured that morphine and gabapentin reversed mechanical
thresholds for the pre-injection and post-injection time periods
hyperalgesia following L5 nerve ligation. Our results
were analyzed using one way ANOVA on the right±left paw differ-
con®rm that mechanical hyperalgesia produced by L5 spinal
ence score for each animal at each time point followed by post-
nerve ligation can be attenuated with gabapentin at doses
hoc comparison (LSD) for group differences. *P , 0:05,
previously found to be effective in neuropathic conditions
**P , 0:01, ***P , 0:001 compared to vehicle control. (B) Mean
( SEM) percent of time spent within the light side of the chamber
[1,17]. It should be noted that the dose of gabapentin that
from 0±5 and 15±20 min for animals that did not receive L5 ligation
was observed to decrease mechanical hyperalgesia did not
(Sham Surgery) or received different doses of different
produce any obvious effect on motor activity. In addition,
compounds following L5 ligation. The duration of time spent
both doses of morphine (1 and 10 mg/kg) were found to
within the light side of the chamber was analyzed for group differ-
possess anti-allodynic properties. Although there is contro-
ence using one-way ANOVA for the 0±5 min and the 15±20 min
time periods followed by post-hoc comparison (LSD) for group
versy as to the clinical utility of morphine for the treatment of
differences. **P , 0:01 compared to sham surgery control.
neuropathic pain [5], our ®nding was not entirely surprising
considering reports that morphine can be an effective treat-
L5 ligated vehicle treated group. The overall analysis of
ment for neuropathic pain [4,7,18]. The attenuation of hyper-
percent time spent within the light area of the test chamber
algesia at a dose of 1 mg/kg morphine occurred in
during the ®rst 5 min of the test period revealed no signi®-
the absence of signi®cant sedative effects as revealed by
cant difference among the groups (P . 0:05). However, at
normal motor behavior re¯ected by total number of line
15±20 min, there was a signi®cant main effect for group
crossings, while the dose of 10 mg/kg morphine signi®cantly
(P , 0:05), with no signi®cant difference in the amount of
decreased mechanical hyperalgesia and motor activity.
time spent within the light side of the chamber for animals
It was hypothesized that if animals were less hyperalgesic,
treated with 1 mg/kg morphine and 90 mg/kg gabapentin
then mechanical stimulation during the escape/avoidance
compared to sham surgery treated animals (Fig. 2B).
test should be less aversive which should be re¯ected as an
C.J. LaBuda, P.N. Fuchs / Neuroscience Letters 290 (2000) 137±140
attenuation in the amount of time that animals spent in the
Research (Technology) Program. We wish to thank Vivian
light side of the chamber. Indeed, morphine and gabapentin,
Rivera for help during a portion of data collection.
two drugs that are able to attenuate mechanical hyperalgesia
(Fig. 2A) also attenuate the aversive nature of the mechanical
[1] Abdi, S., Lee, D.H. and Chung, J.M., The anti-allodynic ef ects
stimulus (Fig. 2B). The lack of a signi®cant effect of the 1 mg/
of amitriptyline, gabapentin, and lidocaine in a rat model of
kg morphine and 90 mg/kg gabapentin on total number of
neuropathic pain, Anesth. Analg., 87 (1998) 1360±1366.
line crossings rules out the possibility that sedative properties
[2] Ageel, A.M., Acute effects of morphine and chlorpromazine
account for the lack of shift from the dark side to the light side
on the acquisition of shuttle-box avoidance response,
of the chamber. Rather, it seems more likely that mechanical
Psychopharmacology, 46 (1976) 311±315.
[3] Attal, N., Brasseur, L., Parker, F., Chauvin, M. and Bouhas-
stimulation of the L5 ligated paw is less aversive in animals
sira, D., Effects of gabapentin on the different components
treated with morphine and gabapentin. The failure of the 10
of peripheral and central neuropathic pain syndromes: a
mg/kg morphine group to show attenuation in the shift from
pilot study, Eur. Neurol., 40 (1998) 191±200.
the light side to the dark side of the chamber most likely
[4] Backonja, M.-M., Miletic, G. and Miletic, V., The effect of
re¯ects impaired motor activity.
continuous morphine analgesia on chronic thermal hyper-
algesia due to sciatic constriction injury in rats, Neurosci.
In the present paradigm, animals must acquire an associate
Lett., 196 (1995) 61±64.
between the applications of the mechanical stimulus to the
[5] Brena, S.F. and Sanders, S.H., Opioids in nonmalignant pain:
hyperalgesic paw with some external cue (i.e. dark vs. light
questions in search of answers, Clin. J. Pain, 7 (1991) 342±345.
area of the test chamber). It is possible that the attenuation of
[6] Dixon, W.J., Ef®cient analysis of experimental observa-
time spent within the light side of the chamber seen with
tions, Annu. Rev. Pharmacol. Toxicol., 20 (1980) 441±462.
[7] Field, M.J., Bramwell, S., Hughes, J. and Singh, L., Detection
morphine and gabapentin is caused by an interference with
of static and dynamic components of mechanical allodynia
acquisition and retention of this relationship rather than a
in rat models of neuropathic pain: are they signaled by
change in the negative hedonic value of the mechanical
distinct primary sensory neurons? Pain, 83 (1999) 303±311.
stimulus. Indeed, morphine has been found to impair perfor-
[8] Fuchs, P.N. and Gamsa, A., Chronic use of opioids for
mance on the Morris water maze [14] and the radial arm maze
nonmalignant pain: a prospective study, Pain Res. Manage.,
2 (1997) 101±107.
[19], which is a test of spatial memory [16]. However, it
[9] Galizio, M., Robinson, E.G. and Ordronneau, C., Opioid
should be noted that the effect of morphine on the radial
drugs and timeout from avoidance, Behav. Pharmacol., 5
arm maze requires chronic high dose administration (up to
(1994) 125±130.
40 mg/kg) and most likely is related to impaired acquisition
[10] Jones, D.L. and Sorkin, L.S., Systemic gabapentin and S(1)-
of procedures necessary to perform the task rather than with
3-isobutyl-g-aminobutyric acid block secondary hyperalge-
sia, Brain Res., 810 (1998) 93±99.
interference of working memory [19]. Other investigators
[11] Kim, S.H. and Chung, J.M., An experimental model for
report biphasic results in rats such that lower doses of
peripheral neuropathy produced by segmental spinal
morphine enhance while higher doses impair memory
nerve ligation in the rat, Pain, 50 (1992) 355±363.
[2,9]. Avoidance responding has been reported to be unal-
[12] LaBuda, C.J. and Fuchs, P.N., A behavioral test paradigm to
tered following morphine administration at doses that inhibit
measure the aversive quality of in¯ammatory and neuro-
pathic pain in rats, Exp. Neurol., 163 (2000) 490±494.
re¯exive withdrawal responding in non-human primates
[13] Lu, Y. and Westlund, K.N., Gabapentin attenuates nocicep-
[20]. The effect of gabapentin on the acquisition and reten-
tive behaviors in an acute arthritis model in rats, J. Pharma-
tion of spatial memory tasks in animals remains unknown.
col. Exp. Ther., 290 (1999) 214±219.
Taken together, the most parsimonious interpretation of the
[14] McNamara, R.K. and Skelton, R.W., Pharmacological disso-
present results is that a decrease of mechanical hyperalgesia
ciation between the spatial learning de®cits produced by
morphine and diazepam, Psychopharmacology, 108 (1992)
is associated with a decrease in the aversiveness of a mechan-
ical stimulus applied to the hyperalgesic body region.
[15] Mellick, G.A. and Mellicy, L.B., Gabapentin in the manage-
In conclusion, the present experiment provides additional
ment of re¯ex sympathetic dystrophy, J. Pain Symptom
support that a behavioral paradigm based on a shift in the
Manage., 10 (1995) 265±266.
amount of time that animals spend in an environmental
[16] Olton, D.S., The radial arm maze as a tool in behavioral
pharmacology, Physiol. Behav., 40 (1987) 793±797.
location associated with mechanical stimulation of the
[17] Pan, H.-L., Eisenach, J.C. and Chen, S.-R., Gabapentin
hyperalgesic paw can be used to measure the affective
suppresses ectopic nerve discharge and reverses allodynia
dimension of pain in rats. In addition, it is concluded that
in neuropathic rats, J. Pharmacol. Exp. Ther., 288 (1999)
two commonly prescribed analgesic compounds are directly
affective against stimulus-evoked nociceptive responses
[18] Portenoy, R.K., Chronic opioid therapy in nonmalignant
pain, J. Pain Symptom Manage., 5 (1990) S46±S62.
(mechanical paw withdrawal threshold) as well as the aver-
[19] Spain, J.W. and Newsom, G.C., Chronic opioids impair
sive nature of neuropathic pain. Future studies will examine
acquisition of both radial maze and Y-maze choice escape,
additional compounds and also explore supraspinal struc-
Psychopharmacology, 105 (1991) 101±106.
tures related to the limbic system to dissociate sensory
[20] Yeomans, D.C., Cooper, B.Y. and Vierck Jr., C.J., Compari-
from affective nociceptive processing.
sons of dose-dependent effects of systemic morphine on
of awake non-human primates, Brain Res., 670 (1995) 297±
This research was supported by the Texas Advanced
Source: http://www.ndineuroscience.com/userfiles/LaBuda%20and%20Fuchs,%20morphine,%20GP.pdf
This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or
Development Bank December 9 – 10, 2004 "Una Aproximación al Enfoque de Derechos en las Estrategias y Políticas de Desarrollo de América Latina" Víctor Abramovich CELS (Centro de Estudios Legales y Sociales, Argentina) Documento preparado para: "Derechos y Desarrollo en América Latina: Una Reunión de Trabajo" Santiago, Chile Diciembre 9 y 10 del 2004








