Nswhealth.moodle.com.au

National Inpatient Medication Chart
Guidelines for use of the National Inpatient Medication Chart
All nursing, medical and pharmacy staff and administrative and allied
Audience:
health staff who are authorised to access and use patient medication
Exceptions:
The National Inpatient Medication Chart is intended to be used as a record
of orders and administration of general medicines. Where they exist for more specialised purposes (such as intravenous fluids, anticoagulants,
management of diabetes, palliative care and acute pain) separate, specific charts should be used
ITEMS COVERED IN THIS PROCEDURE
1. Purpose. 2
2. General instructions. 3
3. Front page of medication chart . 4
3.1 Identification of the patient. 4
3.2 Numbering of medication chart . 4 3.3 Additional charts . 4 3.4 Adverse Drug Reaction Alerts. 5
3.5 Once only, pre-medication, telephone orders and nurse initiated medicines . 6 3.6 Medicines taken prior to admission . 7
4. Second & third page of medication chart . 8
4.1 Variable dose medicines . 8
4.2 Warfarin ordering section . 8 4.3 Warfarin education record . 9
4.4 Regular medicines . 9 4.5 Limited duration and ceased medicines. 13
4.6 Administration record . 13 4.7 Reason for not administering . 14 4.8 Patient weight and height . 14
4.9 Clinical pharmacist review . 14 4.10 Discharge supply . 15
5. Back page of medication chart . 16
5.1 As required (‘prn') medication orders . 16
Appendix A: Dangerous abbreviations . 17
Appendix B: Guidelines for withholding medicines. 18
Acknowledgements:
These Guidelines were adapted by the Department of Human Services Victoria from material
provided by the Australian Council for Safety and Quality in Health Care and the Council's National Inpatient Medication Chart Working Group.
Department of Human Services Draft copy for reference only
1. Purpose
Consistent documentation allows accurate interpretation of orders
The National Inpatient Medication Chart (NIMC) is an initiative of the Australian Council for
Safety and Quality in Health Care (the Council). Research shows that many adverse events reported in Australian hospitals are associated with medications. Research also demonstrates that improvements to medication chart design can
improve the safety of medication processes in hospitals. The Council has developed the NIMC collaboratively with a group of health care professionals (including nursing, medical, pharmacy
and the private sector) from states and territories across Australia who have been involved in similar medication chart standardising projects within their own organisations.
Australian Health Ministers have endorsed the recommendation made by the Council that a standard inpatient medication chart be in use in all public hospitals by June 2006 to assist in
standardisation and consistent documentation of medications. Council's vision is that this chart will be used in health care facilities nationally, and that it will be a valuable precursor to
the electronic health environment. The chart is intended to reflect best practice and assist clinicians in improving all steps of the medication management cycle for safer prescribing, dispensing and administration of medicines in order to minimise the risk of adverse medication events.
The following are general requirements regarding use of the medication chart:
• All medical officers must order medicines for inpatients in accordance with legislative
requirements as required by state/territory Health (Drugs and Poisons) Regulations. • The medication chart is to be completed for all admitted patients and placed at the foot of
the bed unless ward/unit procedures state otherwise.
• All medications should be reviewed regularly to identify potential drug interactions and to
discontinue medicines that are no longer required.
• Specific charts are required for specialised medication orders such as insulin, intravenous
fluids, anticoagulants, parenteral cytotoxic and immunosuppressive agents, epidural and regional infusion and patient controlled analgesia.

2. General instructions
No matter how accurate or complete an order is, it may be misinterpreted if it cannot be read. The following are general requirements regarding the use of the NIMC: • All orders are to be written legibly in ink. Water-soluble ink (for example fountain pen)
should not be used. Black ink is preferred.
• A medication order is valid only if the medical officer enters all the required items (refer
Section 4.4).
• All information, including drug names, should be printed.
• Only accepted abbreviations may be used. Dangerous abbreviations must be avoided
(refer Appendix A). • A separate order is required for each medicine.
• No erasers or ‘whiteout' can be used. Orders must be rewritten if any changes are made,
especially changes to dose and/or frequency.
• The patient's current location should be clearly marked on the medication chart, in the
section illustrated below. This section is located on the left hand side of the front page of the

3. Front page of medication chart (including top section of page three)
3.1 Identification of the patient
A watermark has been placed on the ‘patient identification section' as a reminder that a
prescription is not valid unless the patient's identifiers are present, that is:
• the current patient identification label
• or, as a minimum, the patient name, UR number, date of birth and gender written
in legible print.
The first prescriber must print the patient's name. This will reduce the risk of wrong
identification label being placed on the chart.
Medication orders cannot be administered if the prescriber does not document the patient
identification.
3.2 Numbering of the medication chart
If more than one general medication chart is in use, this must be indicated by circling the
appropriate numbers provided. For example, Medication chart 1 of 2
If additional charts are written, this information will need to be updated.
3.3 Additional (specialised) charts
When additional (specialised) charts are written, this should be indicated by placing a tick or
cross in the space provided.



3.4 Adverse drug reaction (ADR) alerts
Doctors, nurses and pharmacists are obliged to complete ‘Allergies and Adverse Drug Reactions (ADR)' details for all patients. (Patients may be more familiar with the term allergy,
than ADR, so this may be a better prompt). Once the information has been documented, the person documenting the information must sign, print their name and date the entry.
If any information is added to this section after the initial interview, the person adding the information must initial the designated area.
If the patient is not aware of any previous ADRs, then the Nil known box should be ticked
and the person documenting the information must sign, print their name and date the entry.
If a previous ADR exists, the following steps must be completed:
a) Document the following information in the space provided on the medication chart and in
the patient's medical notes: -
name of drug/substance
reaction details (for example rash)
date the reaction occurred (or approximate timeframe for example ‘20 years ago')
Note this is the minimum information that should be documented. It is preferable to also
document how the reaction was managed (for example ‘withdraw and avoid offending agent') and the source of the information (for example patient self report, previous documentation in
medical notes)
b) Affix ADR alert sticker to the front and back page of the medication chart in space
provided
c) Affix large, ADR alert sticker to front of patient's medical record and complete the
relevant information

d) Attach red ADR alert bracelet to patient's wrist. Details of the ADR should not be written
on the bracelet. The bracelet is only to be used as an alert. For allergy details refer to the
medication chart The bracelet may be annotated with the patient name, UR number and date of birth, in legible print using a permanent marker, if this is required by local policy/procedure.
3.5 Once only, pre-medication, telephone orders and nurse initiated medicines
Once only and pre-medication orders:
The following must be documented for once only and pre-medication orders:
-
generic name of medicine
route of administration (accepted abbreviations may be used, refer Appendix A)
dose to be administered
date and time medicine is to be administered
prescriber's signature and printed name
initials of person that administers the medicine
time medicine administered
pharmacy confirmation that medicine requires supply (S) or is on imprest (I).
Nurse initiated medicines
The following must be documented for nurse initiated medicines
-
generic name of medicine
route of administration (accepted abbreviations may be used, refer Appendix A)
dose to be administered
date and time that medicine nurse initiated is to be administered
nurse initiator to sign and print name
initials of person that administers the medicine.
Local hospital policy/guidelines will outline when nurses can initiate medicines and will
specify a limitation on nurse initiated medicines such as ‘for one dose only' or ‘for a
maximum of 24 hours only'.
Generally the capacity applies to a limited list of medicines only. Typically this includes:
simple analgesics, aperients, antacids, cough suppressants, sublingual nitrates, inhaled bronchodilators, artificial tears, sodium chloride 0.9% flush or IV infusion to keep IV line(s)
patent as per local policy.
Telephone orders:
The following must be documented for telephone orders:
-
generic name of medicine
route of administration (accepted abbreviations may be used, refer Appendix A)
dose to be administered
date and time medicine is to be administered
name of doctor giving verbal order
initials of two nurses to confirm that verbal order heard and checked (see example below)
time of administration.
The telephone order MUST be signed, or otherwise confirmed in writing, within 24
hours
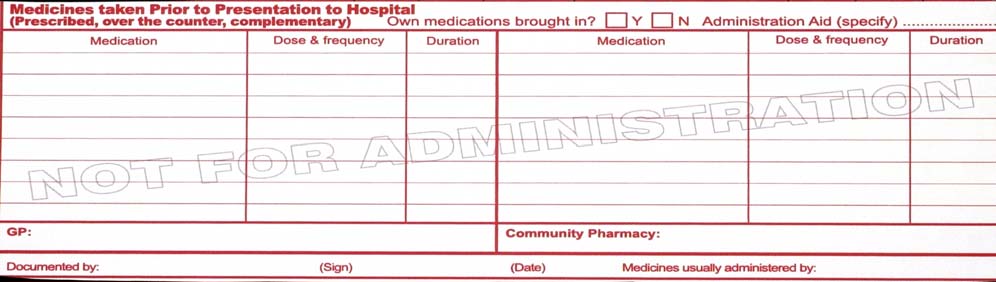
3.6 Medicines taken prior to admission
The admitting medical officer, a pharmacist or other clinician trained in medication history documentation may complete this section. The following must be documented: -
a complete list of all medicines taken normally at home (prescription and non-prescription) including drug identification details (generic name, strength and form), dose
and frequency, and duration of therapy/when therapy started
whether the patient has their own medicines with them
whether the patient uses a dose administration aid (for example Webster Pack or other blister pack)
contact details for patient's community health providers (GP and community pharmacist)
whether the patient usually receives assistance to administer/manage their medicines.
Any discrepancies noted by the person documenting the medication history must be brought
to the attention of the attending medical officer.
Note: The medication chart provides space for the minimum information that should be
documented. It is helpful to also document the indication for use and to use a checklist as a prompt to ensure a comprehensive history is obtained. For more information about medication
history documentation refer to local health service policy.
Note: This section is included in the medication chart to facilitate quick and effective
documentation of, and access to, medication history information. At local levels, facilities may choose to implement a more comprehensive approach to documentation.
4. Second and third page of medication chart
4.1 Variable dose medicines
This section has been formatted to facilitate ordering of medicines that require variable dosing
based on laboratory test results or as a reducing protocol, for example gentamicin and steroids. If these agents are ordered in the regular ordering section, there is no designated
area to record drug levels. If they are ordered in the ‘once-only' ordering section, the risk of omission errors is increased.
For each day of therapy, the following information should be documented:
-
drug level results
time drug level taken.
For each dose, the following information must be documented:
-
doctor's initials
actual time of administration (this may be different from the dose time)
initials of nurse that administers the dose.
If a patient requires a second variable dose medication, or twice daily dosing, prescribe in the
regular section using the above format.
4.2 Warfarin ordering section
The warfarin ordering section is printed in red as an extra alert to indicate that it is an anticoagulant and a high risk medicine. It is recommended that a laminated copy of th is available to assist the doctor/pharmacist/nurse when a patient is commenced on warfarin.
The Guidelines offer information about target INR, duration of therapy, dosing, management of excessive bleeding and drug interactions.
A standard dose time of 1600 hours (4pm) is recommended as this allows the medical team
caring for the patient to order the next dose based on INR results, rather than the after-hours team.
The indication and target INR (based on Guidelines for Anticoagulation using Warfarin) should be included when warfarin is initially ordered.
For each day of therapy, the following information should be documented:
-
doctor's initials
initials of nurse that administers the dose and the checking nurse.
4.3 Warfarin education record
Because of the well-documented risks associated with use of
warfarin, all patients should receive counselling about the use of warfarin and given a warfarin book or list. This
section is included as a record that these risk mitigation activities have been completed.
4.4 Regular medicines
A medication order is valid only if the prescribing medical officer enters all listed
items.
a) Date: The date that the medication order was started during this hospital admission
should be entered. It is not the date that the chart was written or rewritten.
b) Generic drug name: Generic drug name: Because there may be several brands of one
agent available, the generic name should be used if possible unless combination preparations are being ordered (for example Timentin, Panadeine etc). Generally the
pharmacy department will stock and supply only one brand of each generic drug. The red ‘Tick if Slow Release' box is included as a prompt to prescribers to consider
whether or not the standard release form of the drug is required. This box must be ticked to indicate a sustained or modified release form of an oral drug (for example, verapamil
SR, diltiazem CD). If not ticked, it is assumed that the standard release form is to be administered. Further explanation, as below, is in the margin of the medication chart.
c) Route: Only commonly used and understood abbreviations should be used to indicate the
route of administration. Acceptable abbreviations are listed as follows:
COMMONLY USED AND UNDERSTOOD ABBREVIATIONS
Abbreviation
per oral / by mouth
nasogastric
SUBLINGUAL
sublingual
intravenous
injection
injection
intrathecal
ointment
nebulised
nebuliser
DANGEROUS ABBREVIATIONS
NOT TO BE USED
Abbreviation to
Intended
Reason for avoiding
Acceptable
alternative
S/C subcutaneous
Mistaken
write subcut or
S/L sublingual
Mistaken for S/C and
write subling or
under tongue
Misinterpreted as the
write ear or eye
d)Dose: must be written using metric and Arabic (1,2,3…) systems. Never use
Roman numerals (i, ii, iii, iv…). Acceptable abbreviations are listed below.
Always use zero ( 0. ) before a decimal point (for example, 0.5g) otherwise the decimal point
may be missed. However if possible it is preferable to state the dose in whole numbers, not
decimals (for example, write 500mg instead of 0.5g or write 125mcg instead of 0.125mg).
Never use a terminal zero ( .0 ) as it may be misread if the decimal point is missed (for
example, 1.0 misread as 10)
Do not use U or IU for Units because it may be misread as zero. Always write units in full.
Note: In the case of liquid medicines, the strength and the dose in milligrams or
micrograms (not millilitres) must always be specified, for example morphine mixture
(10mg/mL), Give 10mg every 8 hours.
Note: The ward/clinical pharmacist will clarify when the strength supplied is different from
that ordered, for example, for 10mg, the pharmacist may write 2 x 5mg tablets or for 25mg, the pharmacist may write ½ x 50mg.
COMMONLY USED AND UNDERSTOOD ABBREVIATIONS
Abbreviation:
Meaning:
Millilitre
Milligram
Mcg (safer to write
Microgram
microgram in full)
DANGEROUS ABBREVIATIONS
NOT TO BE USED
Abbreviation to
Intended
Reason for avoiding
Acceptable
alternative
ug or μg microgram
mistaken
milligram
write mcg clearly
when handwritten
write microgram
unit or units
mistaken for 0
write unit(s)
international unit mistaken as iv
write unit(s)
(for example 3 IU)
or as 31u (thirty-one
No zero before
decimal point
Misread as 5mg
Write 0.5mg or write
(for example
.5mg)
Zero after
Do not use decimal
decimal point
Misread as 50mg
points after whole
(for example
5.0mg)
e) Frequency and administration times. The medical officer writing the order must
enter the frequency and administration time(s) when writing the medication order.
This will prevent errors where a nurse may misinterpret the frequency and write
down the wrong times. If these details are not entered, the dose may not be
administered by nursing staff.
Acceptable abbreviations are listed below.
Times should be entered using the 24-hour clock (this nomenclature is the global standard).
Unless drugs must be given at specific times, (for example, antibiotics with or before food),
they should be administered according to the recommended administration times.
The ward/clinical pharmacist or nurse will clarify (and annotate the chart) to record the administration time and to optimise drug administration, for example, in relation to food.
COMMONLY USED AND UNDERSTOOD ABBREVIATIONS
Abbreviation
Three times a day
Four times a day
DANGEROUS ABBREVIATIONS
NOT TO BE USED
Abbreviation to
Intended
Reason for avoiding
Acceptable
alternative
OD, od or d
Once a day
mistaken for twice a
write mane, nocte
or specific time
d is easily missed
Every day
Mistaken as qid
write mane, nocte
(four times a day)
or specific time
Mistaken for n (night)
Write mane
Mistaken for m
Write nocte
(morning)
Every six hours
Mistaken for six times
Write q6h or 6
For one day
Mistaken for one week Write for one day
For 3 days
Mistaken as for three
Write for 3 days in
g) Pharmacy: This section is for use by the ward/clinical pharmacist.
Annotations include:
• I for medicines available on imprest
• S for non-imprest items that will be supplied and labelled for individual use from the
• Pts own for medicines checked by the pharmacist and confirmed to be acceptable for use
during the patient's admission
• CD to indicate a Schedule 8 medicine (stored in CD cupboard)
Fridge to indicate a medicine that is stored in the fridge.
h) Indication. This section is for the doctor to document the indication for use or
pharmacist to add or clarify any specific details (for example, may be used to specify
administration methods or rates).
i) Prescriber Signature and Print Name. The signature of the doctor must be written to
complete each medication order. For each signature (doctor), the name must be printed at
least once on the medication chart.
4.5 Limited duration and ceased medicines
When a medicine is ordered for a limited duration, or only on certain days, it must be
clearly indicated using crosses (X) to block out day/times when the drug is NOT to be given.
When stopping a medicine, the original order must not be obliterated. The doctor must
draw a clear line through the order in both the prescription and the administration record
sections, taking care that the line does not impinge on other orders. The doctor must write the reason for changing the order (for example, cease, written in error, increased dose) at an appropriate place in the administration record section.
Note the acronym ‘D/C' should not be used for ceased orders since this can be confused with
‘DISCHARGE'. Always use ‘CEASE'.
When a medication order needs to be changed, the doctor must not over write the
order. The original order must be ceased and a new order written.
4.6 Administration record
The medication administration record provides
space to record up to eleven days of therapy. At the
end of eleven days, a new chart should be written.
The last column (which is partially blocked out) is present
only as a safety net if the order has not been rewritten. If the medication chart is full, then the medication orders
written in it should not be considered valid/current prescriptions.
The shading of alternate columns is intended to reduce
the risk of administering a drug on the wrong day.
4.7 Reasons for not administering
When it is not possible to administer the prescribed
medicine, the reason for not administering must be
recorded by entering the appropriate code (refer below)
and circling. By circling the code it will not accidentally
be misread as someone's initials.
If a patient refuses medicine(s), then the doctor must be notified.
If medicine(s) are withheld, the reason must be
documented in the patient's medical notes. If the medicine is not available on the ward, it is the
nurse's responsibility to notify the pharmacy and/or obtain supply or to contact the doctor to advise that the
medicine ordered is not available.
(Refer to Appendix B - Guidelines for withholding medicines)
4.8 Patient weight and height
This information should be documented in the space provided, as it is important clinical information for
calculating doses of certain medicines).
4.9 Clinical pharmacist review
The clinical pharmacist will sign this section as a record that they have reviewed the medication chart (on that day) to ensure that all orders are clear, safe and appropriate for that individual patient to minimise the risk of an adverse drug event.
4.10 Discharge Supply
The discharge supply section on the NIMC should be used to minimise the potential for
transcription errors, except in sites not using the PBS system to supply discharge medications,
For each drug prescribed while an inpatient, the following information must be documented
in the discharge supply section:
• discharge supply required yes/no
• duration / quantity.
For each page the following information is only required to be documented once
• prescriber's signature
• prescriber to print name
• date discharge required
• pharmacist signature
• date discharge information completed.
5. Back page of medication chart
5.1 As required (‘prn') medicines
Prescribing:
The medical officer must write:
• dose and hourly frequency. ‘PRN' (pre-printed) alone is not sufficient
• indication and maximum daily dose (that is, maximum dose in 24 hours), for example
paracetamol 4g/24 hrs.
Administration:
• The actual dose given must be recorded.
• The person administering each dose is responsible for checking that the maximum daily
dosage will not be exceeded.
APPENDIX A – DANGEROUS ABBREVIATIONS
Avoid these
Intended
What should I use?
OD can be mistaken as Preferably write the time
d can easily be missed
administration
for example mane,
midday, or nocte
Mistaken as three
Write out in full and
specify which days
Mistaken for sublingual Use subcut or
Mistaken as Q.I.D or
Specify time of day for
four times a day
example mane, nocte etc
Use units
for example 3 IU
(intravenous) or
misread as 31 U (i.e. 31 units)
Misread as u when
microgram Mistaken
milligram Write out in full
when handwritten
Mistaken as three
Use for three days
Opposite of intended
Use greater than or less
Zero after a decimal
Misread as 50mg if
Do not use decimal points
decimal point not seen
after whole numbers
for example 5.0 No decimal point
Always use a zero before a
before fractional
decimal when dose is less
dose for example
.5mg Chemical symbols
May not be understood Write out in full
for example MgSO4
misunderstood for example morphine
Mistaken as evening
Write all drug names out in
primrose oil
full – generic name for
single active ingredient,
and trade name for combination drugs
Mistaken as six times a Use q6h or 6 hourly
day
Mistaken for one week
Write for one day
ear or eye
Misinterpreted as the
Write ear or eye
Mistaken for S/C -
Write subling or
sublingual or under
Misinterpreted as the
Write out discontinue or
discharge
APPENDIX B – GUIDELINES FOR WITHHOLDING MEDICINES
The medication chart is a legal document and therefore must be written in a clear, legible and
unambiguous form.
Every nurse has a responsibility to ensure they can clearly read and understand the order
before administering any medicines. For all incomplete or unclear orders, the doctor should be
contacted to clarify.
Never make any assumptions about the prescriber's intent.
Every medication chart must have the patient's identification details completed.
Every medication order must be complete and include:
• route
• generic drug name
• dose ordered in metric units & arabic numerals
• frequency (using only accepted abbreviations)
• times (must be entered by the doctor)
• doctor's signature
It is appropriate to withhold the medicine if there is a known adverse drug reaction (ADR) to
the prescribed medicine. If the medication chart is full (that is, there is no appropriate space to sign for administration) then the medication order is not valid. The chart must be re-written as soon as possible.
Generally medicines should not be withheld if the patient is pre-operative or nil by mouth
(NBM)/fasting unless specified by the doctor.
Remember the five Rs:
• right drug
• right dose
• right route
• right time
• right patient
Source: http://www.nswhealth.moodle.com.au/DOH/Child_Prescribing/nimcAdult.pdf
January - April 2012 Dulsco Employee Newsletter Volume 9 Issue 1 January - April 2012 Dulsco Day Celebrated On UAE National Day Dulsco News Page 9-11 Dulsco Human Resources Page 12-13 Inauguration by Prakash Mahadalkar, Managing Director, Dulsco.
scientific report Mps1 promotes rapid centromere accumulationof Aurora BMaike S. van der Waal1*, Adrian T. Saurin1,2*, Martijn J.M. Vromans1, Mathijs Vleugel1,2,Claudia Wurzenberger3, Daniel W. Gerlich3w, Rene´ H. Medema4, Geert J.P.L. Kops1,2 & Susanne M.A. Lens1+1Department of Medical Oncology, 2Department of Molecular Cancer Research, University Medical Center Utrecht, Utrecht,The Netherlands, 3Institute of Biochemistry, Department of Biology, Swiss Federal Institute of Technology, Zu¨rich, Switzerland,and 4Division of Cell Biology, Netherlands Cancer Institute, Amsterdam, The Netherlands















