Pnas201201089 3808.3813

Aerobic kinetoplastid flagellate Phytomonas does not
require heme for viabilityLud ˇek Ko ˇrenýa,b, Roman Sobotkab,c, Julie Ková ˇrováa,b, Anna Gnipováa,d, Pavel Flegontova,b, Anton Horváthd,Miroslav Oborníka,b,c, Francisco J. Ayalae,1, and Julius Luke ˇsa,b,1
aBiology Centre, Institute of Parasitology, Czech Academy of Sciences and bFaculty of Science, University of South Bohemia, 370 05 �Ceské Bud�ejovice, CzechRepublic; cInstitute of Microbiology, Czech Academy of Sciences, 379 81 T�rebo�
n, Czech Republic; dFaculty of Natural Sciences, Comenius University, 842 15
Bratislava, Slovakia; and eDepartment of Ecology and Evolutionary Biology, University of California, Irvine, CA 92697
Contributed by Francisco J. Ayala, January 19, 2012 (sent for review December 8, 2011)
Heme is an iron-coordinated porphyrin that is universally essential
aerobic environment (8). In soluble guanylyl cyclase, heme serves
as a protein cofactor for fundamental cellular processes, such as
as the nitric oxide sensor, and thus plays an important role in
electron transport in the respiratory chain, oxidative stress re-
signal transduction. Heme is also an important regulatory mol-
sponse, or redox reactions in various metabolic pathways. Parasitic
ecule because it reversibly binds to certain proteins, such as
kinetoplastid flagellates represent a rare example of organisms
transcription factors and ion channels, and thus modulates their
that depend on oxidative metabolism but are heme auxotrophs.
functions (9).
Here, we show that heme is fully dispensable for the survival of
The central position of heme in a variety of cellular functions
Phytomonas serpens, a plant parasite. Seeking to understand the
makes it essential for the viability of virtually all living systems.
metabolism of this heme-free eukaryote, we searched for heme-
There are only a few examples of facultatively anaerobic or
containing proteins in its de novo sequenced genome and exam-ined several cellular processes for which heme has so far been con-
pathogenic bacteria that do not require heme (10–12), but no
sidered indispensable. We found that P. serpens lacks most of the
eukaryote that can survive without heme has been identified.
known hemoproteins and does not require heme for electron trans-
Most aerobic organisms synthesize heme by a multistep pathway
port in the respiratory chain, protection against oxidative stress, or
that is conserved in all three domains of life: bacteria, archaea,
desaturation of fatty acids. Although heme is still required for the
and eukaryotes. A few eukaryotes that lost this pathway are
synthesis of ergosterol, its precursor, lanosterol, is instead incorpo-
known to scavenge heme from external sources. For example,
rated into the membranes of P. serpens grown in the absence of
ticks have easy access to heme from blood (13), whereas parasitic
heme. In conclusion, P. serpens is a flagellate with unique metabolic
nematodes uptake it either from their host or from endosymbiotic
adaptations that allow it to bypass all requirements for heme.
bacteria (14). The free-living nematode Caenorhabditis eleganslacks the capacity to synthesize heme but is able to take it from the
cytochromes respiration sterols protist
bacteria it feeds on (15). Even the parasitic protists Entamoeba,Trichomonas, and Giardia, which dwell in an anaerobic environ-
Heme is a tetrapyrrole molecule that consists of a porphyrin mentanddonotneedhemeforprocessesconnectedtooxidative
ring coordinated with the iron molecule. It interacts with
metabolism, have retained a few hemoproteins, for which heme is
various apoproteins giving rise to functional hemoproteins,
likely obtained from their hosts (16).
which are ubiquitous in biological systems and exhibit a wide
Flagellates of the order Kinetoplastea, which includes major
range of activities. The oxidation state of the iron is important
human parasites, depend on oxygen but are unable to produce
for most biological roles of heme, but its exact function is ulti-
heme. Media for their cultivation must therefore be supplemented
mately determined by the properties of the polypeptide bound toit (1). Heme can exist in either the oxidized ferric (Fe3+) or
with heme to support their growth (17). Members of the genus
reduced ferrous (Fe2+) state, which enables it to accept or do-
Trypanosoma lost the entire biosynthetic pathway and extract
nate electrons and to function in various redox reactions and
heme from host blood (18, 19), whereas Leishmania spp. have
electron transport.
retained genes for the last three steps of the pathway, allowing
The most abundant group of heme proteins are cytochromes
them to synthesize heme from their host-derived precursors (20).
(2). In aerobic organisms that produce energy mainly through
Some kinetoplastids that parasitize insects obtain heme from their
oxidative phosphorylation, most of the synthesized heme is used
bacterial endosymbionts, which can be eliminated by antibiotic
for the formation of the cytochromes functioning in the electron
treatment, turning these protists into heme auxotrophs (17).
transport respiratory chain. Other cytochromes, such as the
Kinetoplastid flagellates of the genus Phytomonas are impor-
members of the cytochrome b5 or cytochrome P450 family, are
tant yet understudied parasites of plants with a major economic
involved in various redox reactions of specific metabolic path-
impact in Latin America and the Caribbean (21). They reside in
ways, such as desaturation of fatty acids and sterol biosynthesis,
carbohydrate-rich tissues, such as phloem, latex, fruits, and seeds;
and also in drug detoxification (3, 4). In catalases, heme func-
their ATP production is based on glycolysis (22). In the present
tions in the degradation of hydrogen peroxide, whereas in per-
study, we show that Phytomonas serpens does not require heme for
oxidases, it oxidizes a wide variety of organic and inorganic
viability and possesses unique metabolic properties that allow it to
compounds in the presence of hydrogen peroxide. Through the
bypass all functions of this otherwise omnipresent molecule.
consumption of hydrogen peroxide, these enzymes greatly con-tribute to the oxidative stress defense (5, 6). In addition to itsfunction as an electron carrier, heme iron has the capacity to
Author contributions: L.K., A.H., M.O., F.J.A., and J.L. designed research; L.K., R.S., J.K.,
bind diatomic gases. Hemoglobin is well known as the oxygen
and A.G. performed research; L.K., P.F., and M.O. analyzed data; and L.K., F.J.A., and J.L.
transporter in animals, but members of the same protein family
wrote the paper.
are widespread in all groups of organisms, including anaerobes.
The authors declare no conflict of interest.
The original roles of globins might have been the responses to
1To whom correspondence may be addressed. E-mail: or
nitric oxide and nitrosative stress (7) or sensing of oxygen, which
This article contains supporting information online at
was highly toxic to cells before they managed to adapt to an
3808–3813 PNAS March 6, 2012 vol. 109 no. 10

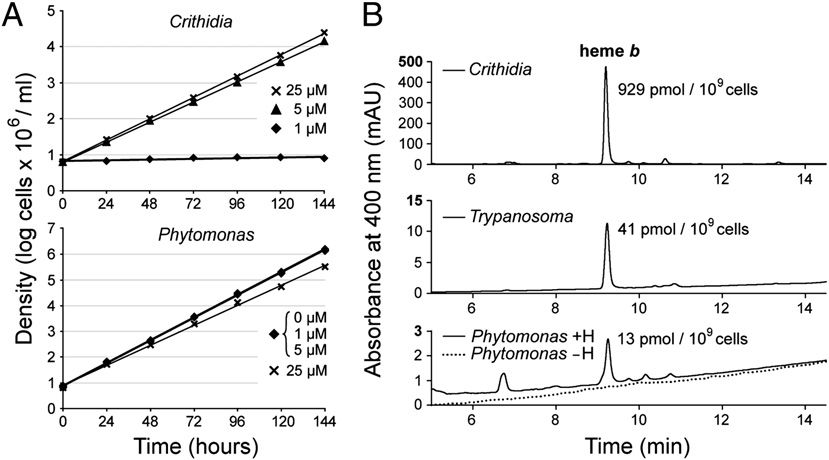
Growth dependence on availability of heme and quantification of heme b in P. serpens and related flagellates. (A) Growth rate of P. serpens is the same
without heme or when heme is supplied up to a concentration of 5 μM; 25 μM heme inhibits growth, likely attributable to toxic effects of free heme. Quite oppositedependence of growth on heme concentration is observed in the closely related C. fasciculata, which grows best when supplied with 25 μM heme and stops growingwhen heme concentration in the media is lowered to 1 μM. (B) Heme b extracted from equal numbers of cells from various kinetoplastids was separated by HPLC anddetected by diode array detector. C. fasciculata was used as a related organism that possesses a complete set of respiratory complexes. It was grown in the samemedium and supplemented with the same amount of heme (5 μM) as P. serpens. The bloodstream stage of T. brucei, which does not express its respiratory complexesIII and IV in this life cycle stage, and thus functionally resembles P. serpens, was used as another control. The absence of respiratory complexes that normally consumemost of heme is reflected in the much lower amount of extracted heme compared with C. fasciculata. The heme content in P. serpens is even lower than in T. brucei,which is in accordance with the lowest number of heme proteins found in the Phytomonas spp. genomes among all kinetoplastids (Table 1). Not even a trace amountof heme is detected in P. serpens grown without heme (dotted line). +H, with heme; −H, without heme.
Results and Discussion
into one lacking it did not alter their growth. This is in contrast to
We cultivated P. serpens strain 9T in a chemically defined medium
related flagellates, such as Crithidia fasciculata, which requires
without heme continuously for over a year without
heme for growth and was used as a control (Fig. 1A).
noticeable decrease of the growth rate (generation time of ∼8 h),
We sought to test the heme biosynthetic capacity of P. serpens
compared with parallel cultures supplemented with heme (Fig.
by measuring the amount of extractable heme. Even using a very
1A). Abrupt transfer of cells grown in heme-containing medium
sensitive HPLC assay, we failed to detect any traces of heme in
Heme proteins of kinetoplastid flagellates
Lanosterol 14α-demethylase (cytochrome P450)
Heme-binding subunit of the respiratory complex II
Soluble cytochrome c of the respiratory chain
Cytochrome b subunit of the respiratory complex III
Cytochrome c1 subunit of the respiratory complex III
Heme a and heme a3 binding subunit of complex IV
Heme-dependent plant peroxidase homolog 1
Heme-dependent plant peroxidase homolog 2
Δ9 Fatty acid desaturase (cytochrome b5 domain)
Δ4 Fatty acid desaturase (cytochrome b5 domain)
Δ5 Fatty acid desaturase (cytochrome b5 domain)
Δ6 Fatty acid desaturase (cytochrome b5 domain)
Nitrate reductase (cytochrome b5 domain)
Fumarate reductase-like (cytochrome b5 domain)
Ferric reductase (cytochrome b561)
Ferric reductase (flavocytochrome b558)
Globin domain of adenylate cyclase-like protein
Cytochromes P450 with unknown function*
Cytochromes b5 with unknown function*
The presence/absence data for PE and PH were kindly provided by Michel Dollet (CIRAD-BIOS, Montpellier,
France) and Patrick Wincker (Genoscope, Evry, France). GenBank accession numbers are quoted for the proteinsof Leishmania major. CF, Crithidia fasciculata; Lei, Leishmania spp.; PE, Phytomonas sp. strain EM1; PH, Phyto-monas sp. strain Hart1; PS, Phytomonas serpens; TB, Trypanosoma brucei; TC, Trypanosoma cruzi.; (−), heme-binding domain is missing, but the rest of the protein is present.
*Proteins that do not have known function but were identified as either cytochrome P450 or cytochrome b5 arenot listed individually. The number of these proteins is shown for each taxon.
Ko�rený et al.
PNAS March 6, 2012 vol. 109 no. 10 3809

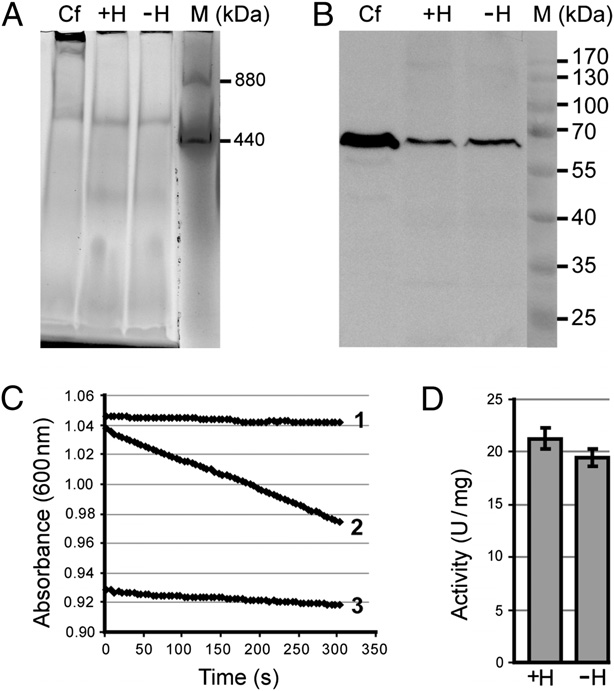
cells grown in its absence (Fig. 1B). This indicates that P. serpensis able to survive without heme, which is further supported by thefact that, with the exception of ferrochelatase, no other genes forheme synthesis were found in the draft genome of P. serpensstrain 9T obtained for this study. On the other hand, we founda small amount of heme in cells growing in the medium sup-plemented with heme (Fig. 1B), which implies that P. serpens isable to uptake this compound from the medium.
To find out how P. serpens can survive without the key heme-
dependent activities and possibly identify any functions still usingheme, we decided to test cellular processes experimentally inwhich heme is known to be involved. A screen for homologs ofheme-containing proteins in the genome produced only a fewhits, compared with the list of hemoproteins from related flag-ellates (Table 1 and ). The same results were obtainedfor two other recently sequenced Phytomonas genomes (Table1). Unlike other kinetoplastids, Phytomonas spp. have an ap-parent lack of respiratory cytochromes, heme-dependent perox-idases, and several enzymes that possess heme-binding domains,such as front-end fatty acid desaturases for the production ofpolyunsaturated fatty acids (23), a nitrate reductase, and twodifferent ferric reductases, one of which was shown to be in-volved in the iron uptake of related Leishmania (24) (Table 1).
The absence of heme peroxidases in P. serpens, exceptional
even among the kinetoplastids, most of which lack catalase (25)(Table 1), corresponds to our finding that heme added to themedium does not increase the resistance of P. serpens against
Respiratory complex II (succinate dehydrogenase) is assembled and
oxidative stress induced by the superoxide generator paraquat
active in P. serpens grown with (+H) or without (−H) heme. (A) Clear native
This is the opposite of what was found for the evolu-
gel (3–12%) after in-gel staining for succinate dehydrogenase activity; C.
fasciculata (Cf) served as a control. Ferritin (monomeric and dimeric forms)
tionarily related Trypanosoma brucei, which needs heme for ox-
was used as a molecular weight marker (M). (B) Lysates from the same cells
idative stress defense (18).
as in A were analyzed by SDS/PAGE and immunoblotted with specific anti-
Although P. serpens lacks the heme-containing respiratory
serum against the T. brucei subunit of complex II, SDH1. (C) Activity of suc-
complexes III and IV (26–28), the mitochondrial respiratory
cinate dehydrogenase in P. serpens grown without heme. The decrease in
chain remains functional, serving to reoxidize NADH produced
absorbance (A600) with time (curve 2) was caused by the addition of ubi-
during glycolysis (22, 29). Complex I is present in P. serpens (27,
quinone to the reaction, which mediated the electron transfer from succi-
30), which, instead of cytochrome c reductase (complex III) and
nate to 2,6-dichlorophenolindophenol. The activity was specifically inhibited
cytochrome c oxidase (complex IV), uses alternative oxidase to
using malonate (curve 3). Curve 1 represents the background without ubi-quinone. (D) Activity did not significantly differ between P. serpens grown
reduce oxygen to water (31). We found that succinate de-
with (+H) or without (−H) heme. Medium values were calculated from three
hydrogenase (complex II) is also present (Fig. 2), with a con-
served histidine residue in its SDH4 subunit, which supposedlybinds heme in the related Trypanosoma cruzi and other kineto-plastids (32). Visualization of the P. serpens complex II by in-gel
as well as the soluble cytochrome c, may be bypassed by using the
staining in clear-native gel revealed that its abundance is not
alternative terminal oxidase, which utilizes nonheme iron to
influenced by the availability of heme in the medium (Fig. 2A).
transfer electrons from ubiquinone directly to oxygen. This is
Moreover, its size of ∼600 kDa is unaltered in the heme-de-
also known for the bloodstream (mammalian) stage of T. brucei,
prived cells, being almost the same as in T. cruzi (32) and the
which, similar to Phytomonas, dwells in a sugar-rich environment,
related C. fasciculata, used as a control (Fig. 2A), suggesting
whereas the T. brucei procyclic (insect) stage has a fully de-
a proper assembly of complex II in the absence of heme. To
veloped mitochondrion equipped with the heme-containing
assess the abundance of its subunits, we generated specific an-
complexes (36). This metabolic switch is impossible in Phyto-
tiserum against one subunit of the kinetoplastid complex II,
monas, which has lost the genes encoding the subunits of these
SDH1. The amount of the target protein was the same in cells
complexes from its genome (26, 28) (Table 1).
grown with or without heme (Fig. 2B). Furthermore, the absence
Because of its capacity to transfer electrons, heme participates
of heme did not affect the capacity of complex II to reduce
in various redox reactions, some of which are virtually universal
ubiquinone (Fig. 2 C and D).
for eukaryotes. One of them is the desaturation of fatty acids. In
These findings are in line with previous reports showing that
heme is not universally indispensable for the function of complex
eukaryotes, this reaction needs electron equivalents that are
II (33, 34). In mammalian cells, the absence of heme disrupts
transferred from reduced cytochrome b5, and thus depends on
proper assembly and inhibits the activity of complex II (35).
heme (37, 38). Many desaturases contain cytochrome b5 as
However, in yeast and Escherichia coli, the homologous com-
a domain conveniently fused to their N- or C-termini, including
plexes retain physiological activity even without heme (33, 34). It
the most widespread one, which creates the double bond in the
has been suggested that although heme does not participate in
Δ9 position (23, 39). Our phylogenetic analyses revealed that this
the electron transfer in complex II and is not necessarily required
fusion took place only once in the evolution of eukaryotic Δ9
for the assembly of the complex, it may provide an electron sink
fatty acid desaturases, specifically at the base of a superclade
to protect against free radical damage during periods of high
comprising fungi, amoebozoans, rhodophytes, choanozoans, and
electron flux (34). However, the presence of heme in the proton-
excavates, including kinetoplastids (Fig. 3A and Re-
pumping complexes III and IV is indispensable, because it di-
markably, Δ9 desaturase in P. serpens is the only member of this
rectly mediates electron transport. However, when enough en-
superclade that conspicuously lacks the cytochrome b5 domain,
ergy is produced by glycolysis, these heme-containing complexes,
apparently as a consequence of its secondary loss, a singular
Ko�rený et al.

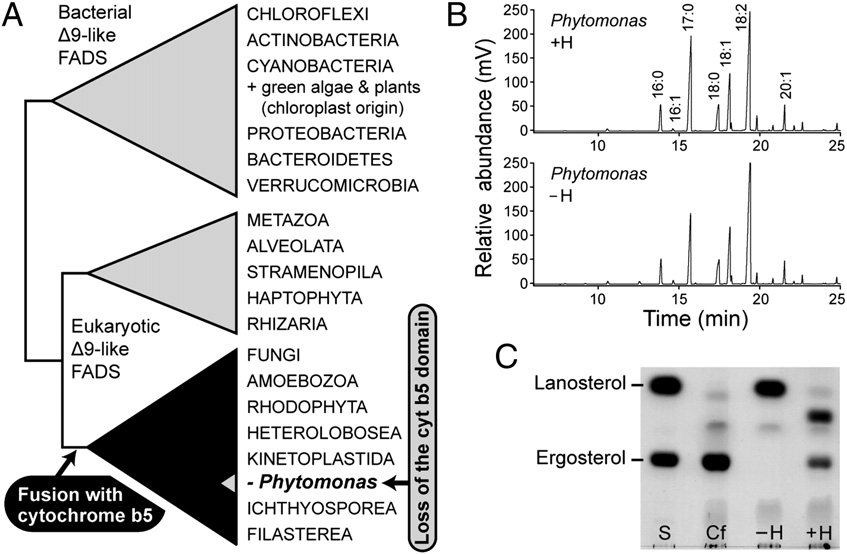
Heme is not needed for desaturation of fatty acids but is required for ergosterol biosynthesis in P. serpens. (A) Schematic phylogenetic tree of Δ9-fatty
acid desaturases (FADS). P. serpens is the only organism that secondarily lost the cytochrome b5 domain. The full phylogenetic tree of Δ9-fatty acid desaturaseis shown in . (B) Analyses of fatty acid composition by gas chromatography demonstrate that in P. serpens, the desaturation of fatty acids is not affectedby the absence of heme. (C) Analysis of sterol composition by TLC. Ergosterol, which is the major membrane sterol of Trypanosomatida, and lanosterol, theprecursor of heme-dependent demethylation, were used as standards (S). C. fasciculata (Cf) served as a control. P. serpens synthesized a sterol that corre-sponded to the ergosterol standard only when heme was added to the growth medium (+H). Cells grown without heme (−H) accumulated lanosterol.
event among all known eukaryotes. To assess the ability of P.
P. serpens possesses this unique capability as well. Based on the
serpens grown in the absence of heme to desaturate fatty acids,
TLC analysis, the cells synthesized a sterol that corresponded to
we analyzed their composition by gas chromatography. We found
the ergosterol standard only when heme was added to the growth
that P. serpens contains unsaturated fatty acids and that their
medium. In contrast, they accumulated lanosterol in the absence
composition is virtually the same regardless of the presence or
of heme with no impact on cell viability (Fig. 3C). The fact that
absence of heme (Fig. 3B). These findings indicate that for the
certain eukaryotes are able to use lanosterol but others are not is
desaturation of fatty acids, P. serpens is able to use an electron
very interesting and implies the existence of some regulatory
donor other than cytochrome b5. It may likely be ferredoxin,
mechanism. Cholesterol-deficient human T cells can adapt to
which serves this role for the desaturases of some bacteria and
growth with lanosterol; the initial growth of these cells dropped
plant plastids. For example, the plastid Δ12 fatty acid desaturase
10-fold when cholesterol was depleted, yet their prolonged cul-
of plants and diatoms depends on ferredoxin as an electron
tivation resulted in a growth rate ∼65% that of the cholesterol-
donor, whereas a homologous desaturase with the same function
supplemented cells (46). A study on yeast revealed that what
in the endoplasmic reticulum of the same organisms, as well as in
regulates the incorporation of lanosterol in the membranes is the
other eukaryotes, uses cytochrome b5 (40). The possibility that
level of synthesized heme (47). The growth of P. serpens in the
these redox molecules could substitute for each other has been
absence of heme precludes the activity of CYP51; thus, this
experimentally demonstrated in E. coli and in yeast expressing
flagellate meets the two conditions that are required in yeast for
cyanobacterial Δ6 fatty acid desaturase (41). Although ferre-
lanosterol utilization (low heme levels and CYP51 inhibition).
doxin is the natural electron donor for this desaturase, cyto-
Overall, there are several cellular processes for which heme is
chrome b5 fully complemented its function when fused or
crucial in a typical eukaryote, yet it is dispensable in P. serpens.
coexpressed with the desaturase enzyme. Three different ferre-
Somewhat lower dependence of a typical kinetoplastid on heme
doxin homologs were identified in the genomic sequences of P.
has been noted when cystathionine-β-synthase, a hemoprotein of
animals and amoebae that is essential for cysteine formation, was
The oxidative 14α-demethylation of lanosterol, another key
reaction in the eukaryotic cell, fully depends on heme. Its sub-
shown to lack heme in kinetoplastids (48). However, P. serpens is
stitution by means of analogous nonheme enzyme has never been
unique, because it lacks most hemoproteins that are present even
documented. This reaction is a crucial step in the synthesis of
in closely related protists. Moreover, the few retained in the P.
sterols, such as cholesterol in animals or ergosterol in fungi, as
serpens genome are not crucial for its survival, at least under
well as in protists, including kinetoplastid flagellates (42). It is
culture conditions. In addition to CYP51 and the SDH4 subunit
catalyzed by lanosterol 14α-demethylase (CYP51), which belongs
of respiratory complex II, we identified 13 proteins that sup-
to the cytochrome P450 family, found in most eukaryotes, in-
posedly bind heme, because they are homologous to cytochrome
cluding Phytomonas spp. (Table 1). No eukaryotic cell can
b5 (Table 1). Their functions are unknown, however, and 5 of
function without sterols or their analogs in its membranes; in-
them lack the HPGG heme-binding motif typical for cytochrome
hibition of this enzymatic step is thus frequently lethal (43).
b5 (41). Thus, it is by no means certain that these proteins ac-
Consequently, CYP51 is a popular target of fungicides and other
tually bind heme in vivo. One of them is a protein recently
drugs, which are also effective against kinetoplastids (44). Until
identified in the flagellar proteome of T. brucei, shown to be
now, the only kinetoplastid known to be naturally resistant to
indispensable for the bloodstream stage but nonessential for the
inhibitors of CYP51 is Leishmania braziliensis, a flagellate closely
procyclic stage (49). Therefore, the only process for which heme,
related to Phytomonas, which seems to be able to incorporate
if present, was found to be actively used by P. serpens, is the
14-methyl sterols into its membranes (45). We have found that
14α-demethylation of lanosterol in the ergosterol biosynthetic
Ko�rený et al.
PNAS March 6, 2012 vol. 109 no. 10 3811

pathway (Fig. 3C). Surprisingly, however, in vitro growth remains
(80 μg of proteins per line) by incubating in a staining solution [50 mM NaPi
unaffected by the lack of this activity.
(pH 7.4), 84 mM sodium succinate, 0.2 mM N-methylphenazonium methyl
It is conceivable that some anaerobic eukaryotes possessing only
sulfate, 4.5 mM EDTA (pH 8.5), 10 mM potassium cyanide, 2 mg/mL Nitro-
a few of the known hemoproteins may survive without heme as
tetrazolium blue chloride) for 3 h at room temperature in dark. Nitro-tetrazolium blue chloride changes color on accepting electrons from succinate
well; however, this will be hard to test, because, so far, none of
via N-methylphenazonium methyl sulfate, a process catalyzed by complex II.
these anaerobic protists can be grown in a chemically defined
SDH1 subunit of complex II was detected by Western blot analysis using 10%
medium. Furthermore, anaerobic protists need to obtain some
(wt/vol) SDS/PAGE and a specific polyclonal antiserum generated against the
products of heme-dependent enzymes, such as cholesterol and
oligopeptide SHLSKAYPVIDHTFDC [SDH1 subunit of T. brucei (Tb927.8.6580)
fatty acids from their environment; thus, their existence cannot be
in a rabbit].
considered to be independent of heme (50). To the best of our
Specific succinate dehydrogenase activity was measured using the fol-
knowledge, P. serpens is the only eukaryote that can survive
lowing protocol: 5 μL of mitochondrial protein lysate was incubated with
without heme and yet depends on oxidative metabolism. This
1 mL of succinate dehydrogenase solution [25 mM KPi (pH 7.2), 5 mM MgCl2,
unique metabolic property, a feature likely developed as an ad-
20 mM sodium succinate] for 10 min at 30 °C. This mixture was transferred in
aptation to the carbohydrate-rich environment of plant sap, makes
the cuvette, and antimycin A (2 μg/mL), rotenone (2 μg/mL), potassium cy-
it an ideal model to study different cellular functions in a heme-
anide (2 mM), and 2,6-dichlorphenolindophenol (50 μM) were added.
free background, which may shed further light on the exact roles
Background absorbance at 600 nm was then measured for 5 min. The re-action was triggered by adding 65 μM coenzyme Q
and essentiality for life of the otherwise omnipresent heme.
2, and the absorbance at
600 nm was measured every 20 s for 5 min. Change in absorbance was
Materials and Methods
caused by the electron transfer from succinate via coenzyme Q2 to 2,6-dichlorophenolindophenol. The activity was specifically inhibited by the
Cultivation Conditions and Growth Curves. Both P. serpens and C. fasciculata
addition of 1 mM sodium malonate.
were grown in a chemically defined medium (supplemented withdifferent concentrations of hemin at 27 °C and shaking at 80 rpm, daily
Genome Sequencing, Assembly, and Protein Search. P. serpens nuclear DNA
diluted with fresh media to the density of 6 × 106 cells per milliliter. Cell
fraction was sequenced using Illumina technology at BGI-Hong Kong (HiSeq
concentration was measured daily using a Beckman Coulter Z2 counter.
2000 sequencing system, average insert size of 500 bp, read length of 90 bp). Adataset of 1.62 Gbp was obtained after basic filtering of low-quality reads.
Quantification of Heme b. In total, 2 × 109 cells of P. serpens, C. fasciculata, and
Genome assembly with MIRA 3.4rc2 (54) produced 5,399 contigs longer than
the bloodstream form of T. brucei were filtrated through a DEAE-cellulose
500 bp (N50 contig size of 6,781 bp) with average coverage 60 (genome as-
column and washed five times with PBS buffer to remove all traces of heme
sembly deposited in National Center for Biotechnology Information BioProject
from the media. The cell pellets were extracted with methanol/0.2% NH4OH,
database under accession no. PRJNA80957). Translated reads and contigs were
and heme was extracted from the delipidated cells with acetone/2% HCl (vol/
screened using tblastn 2.2.24+ with e-value cutoffs at 10−3 and 10−10, re-
vol) and separated by HPLC on a Nova-Pak C18 column (4-μm particle size, 3.9 ×
spectively, against Leishmania major heme-binding proteins (Table 1) and
150 mm; Waters) using linear gradient 25–100% (vol/vol) acetonitrile/0.1% tri-
heme-synthesis enzymes. Conserved protein domains were identified using
fluoroacetic acid at a flow rate of 1.1 mL/min at 40 °C. Heme b was detected by
InterPro database. Draft genome sequences of C. fasciculata were kindly
diode array detector (Agilent 1200; Agilent Technologies) and quantified using
provided by Stephen M. Beverley (Washington University School of Medicine,
authentic hemin standard (Sigma–Aldrich) and extinction coefficient as de-
St. Louis, MO), produced by The Genome Center at Washington University
scribed previously (51).
School of Medicine in St. Louis, and can be obtained from tritryp database.
Two of the heme-proteins of C. fasciculata (lanosterol 14α-demethylase and Δ6
Analysis of Fatty Acids. Lipids were extracted from P. serpens cell pellets by
fatty acid desaturase) were not identified in the draft genome sequences, but
a modified method of Bligh and Dyer (52) with dichloromethane used instead
their partial sequences were amplified by PCR assay from C. fasciculata and
of chloroform. The methyl esters were prepared by trans-esterifying the lipid
extract with BF3-CH3OH at 85 °C for 1 h and analyzed using a gas chromato-graph (HRGC 5300; Carlo Erba) equipped with a flame ionization detector and
Phylogenetic Analysis. Amino acid sequences of Δ9 fatty acid desaturases
TR-FAME capillary column for the separation of Fatty Acid Methyl Esters
from different eukaryotic lineages and bacteria were aligned using MAFFT
(FAMEs) (60-m, 0.25-mm inner diameter and 0.25-μm film thickness; ThermoScientific). Hydrogen was used as the carrier gas with a pressure of 200 kPa.
6.717b (55) and manually edited using BioEdit (56). A maximum likelihood
The following temperature ramp was used: 140 °C to 240 °C with a rate of
tree was constructed with RAxML 7.0.3 using the PROTGAMMALG model
4 °C per min−1 and holding at 240 °C for 10 min. The flame ionization detector
(57) (1,000 replications). The bootstrap supports of individual branches were
was isothermal at 260 °C, and the injector was set to 250 °C. Separated fatty
calculated using the same model after 1,000 iterations.
acids were identified by comparison of their retention times with knownstandards (37-component fatty acid methyl ester mix 47885-U, Supelco;
Oxidative Stress Assay. The sensitivity of cells to oxidative stress was measured
polyunsaturated fatty acid no. 3, menhaden oil).
by exposing them to paraquat added to the cultivation medium in a widerange of concentrations, ranging from 10−8 to 100 mM. After 44 h of in-
Analysis of Sterols. Sterols were extracted and separated on TLC silica gel
cubation, resazurin was added to each culture, and after 4 h, the viability of
plates as described previously (43) and visualized by spraying the plates with
cells was established by measurement of fluorescence. Obtained data were
a water solution of 0.05% ferric chloride/5% (vol/vol) acetic acid/5% (vol/vol)
analyzed by GraphPad Prism software using nonlinear regression (curve fit)
sulfuric acid and heating to 100 °C for 15 min.
with a sigmoidal dose–response analysis (58, 59).
Detection and Activity Measurements of Respiratory Complex II. Mitochondria
ACKNOWLEDGMENTS. We thank Martin Luke�s for his help with gas chro-
were isolated by hypotonic lysis as described previously (53). Protein lysates
matography analysis of fatty acids. Michel Dollet and Patrick Wincker kindlyprovided the absence/presence data for selected genes in the Phytomonas
were prepared by digitonin lysis (4 mg of digitonin per 1 mg of proteins, 1 h
EM1 and Hart1 genomes. This work was supported by the Grant Agency of
on ice) for native gel electrophoresis and histochemical staining and by
the Czech Republic (Grants 204/09/1667, 206/08/1423, and P305/11/2179),
dodecylmaltoside lysis (40 μL of 0.5 M aminocaproic acid and 10 μL of 10% (wt/
a Praemium Academiae award (to J.L.), the Algatech Project (CZ.1.05/
vol) dodecylmaltoside, 1 h on ice) for spectroscopic activity measurements and
2.1.00/03.0110), and the Scientific Grant Agency of the Slovak Ministry of
SDS/PAGE. Whole-complex II was detected in 3–12% (wt/vol) clear native gel
Education and the Academy of Sciences (Grant 1/0393/09).
1. Frankenberg N, Moser J, Jahn D (2003) Bacterial heme biosynthesis and its bio-
5. Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among
technological application. Appl Microbiol Biotechnol 63:115–127.
catalases. Cell Mol Life Sci 61:192–208.
2. Panek H, O'Brian MR (2002) A whole genome view of prokaryotic haem biosynthesis.
6. Bonifacio A, et al. (2011) Role of peroxidases in the compensation of cytosolic ascorbate
peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ 34:1705–1722.
3. Schenkman JB, Jansson I (2003) The many roles of cytochrome b5. Pharmacol Ther
7. Poole RK, Hughes MN (2000) New functions for the ancient globin family: Bacterial
responses to nitric oxide and nitrosative stress. Mol Microbiol 36:775–783.
4. Anzenbacher P, Anzenbacherová E (2001) Cytochromes P450 and metabolism of
8. Green J, Crack JC, Thomson AJ, LeBrun NE (2009) Bacterial sensors of oxygen. Curr
xenobiotics. Cell Mol Life Sci 58:737–747.
Opin Microbiol 12(2):145–151.
Ko�rený et al.
9. Hou S, Reynolds MF, Horrigan FT, Heinemann SH, Hoshi T (2006) Reversible binding of
35. Lemarie A, Grimm S (2009) Mutations in the heme b-binding residue of SDHC inhibit
heme to proteins in cellular signal transduction. Acc Chem Res 39:918–924.
assembly of respiratory chain complex II in mammalian cells. Mitochondrion 9:
10. Brooijmans R, et al. (2009) Heme and menaquinone induced electron transport in
lactic acid bacteria. Microb Cell Fact 8(1):28.
36. Bringaud F, Rivière L, Coustou V (2006) Energy metabolism of trypanosomatids: Ad-
11. Lechardeur D, et al. (2011) Using heme as an energy boost for lactic acid bacteria. Curr
aptation to available carbon sources. Mol Biochem Parasitol 149:1–9.
Opin Biotechnol 22:143–149.
37. Jeffcoat R, Brawn PR, Safford R, James AT (1977) Properties of rat liver microsomal
12. Sambri V, Cevenini R, La Placa M (1991) Susceptibility of iron-loaded Borrelia burg-
stearoyl-coenzyme A desaturase. Biochem J 161:431–437.
dorferi to killing by hydrogen peroxide and human polymorphonuclear leucocytes.
38. Uttaro AD (2006) Biosynthesis of polyunsaturated fatty acids in lower eukaryotes.
FEMS Microbiol Lett 65(1):67–71.
IUBMB Life 58:563–571.
13. Braz GRC, Coelho HSL, Masuda H, Oliveira PL (1999) A missing metabolic pathway in
39. Mitchell AG, Martin CE (1995) A novel cytochrome b5-like domain is linked to the
the cattle tick Boophilus microplus. Curr Biol 9:703–706.
carboxyl terminus of the Saccharomyces cerevisiae delta-9 fatty acid desaturase. J Biol
14. Ghedin E, et al. (2007) Draft genome of the filarial nematode parasite Brugia malayi.
40. Domergue F, et al. (2003) New insight into Phaeodactylum tricornutum fatty acid
15. Rao AU, Carta LK, Lesuisse E, Hamza I (2005) Lack of heme synthesis in a free-living
metabolism. Cloning and functional characterization of plastidial and microsomal
eukaryote. Proc Natl Acad Sci USA 102:4270–4275.
δ12-fatty acid desaturases. Plant Physiol 131:1648–1660.
16. Hjort K, Goldberg AV, Tsaousis AD, Hirt RP, Embley TM (2010) Diversity and reductive
41. Hongsthong A, et al. (2006) Revealing the complementation of ferredoxin by cyto-
evolution of mitochondria among microbial eukaryotes. Philos Trans R Soc Lond B
chrome b (5) in the Spirulina- (6)-desaturation reaction by N-terminal fusion and co-
Biol Sci 365:713–727.
expression of the fungal-cytochrome b (5) domain and Spirulina- (6)-acyl-lipid desa-
17. Chang KP, Chang CS, Sassa S (1975) Heme biosynthesis in bacterium-protozoon
turase. Appl Microbiol Biotechnol 72:1192–1201.
symbioses: Enzymic defects in host hemoflagellates and complemental role of their
42. Zhou W, Lepesheva GI, Waterman MR, Nes WD (2006) Mechanistic analysis of
intracellular symbiotes. Proc Natl Acad Sci USA 72:2979–2983.
a multiple product sterol methyltransferase implicated in ergosterol biosynthesis in
18. Vanhollebeke B, et al. (2008) A haptoglobin-hemoglobin receptor conveys innate
Trypanosoma brucei. J Biol Chem 281:6290–6296.
immunity to Trypanosoma brucei in humans. Science 320:677–681.
43. Lamb DC, et al. (2001) Plant sterol 14 α-demethylase affinity for azole fungicides.
19. Lara FA, et al. (2007) Heme requirement and intracellular trafficking in Trypanosoma
Biochem Biophys Res Commun 284:845–849.
cruzi epimastigotes. Biochem Biophys Res Commun 355:16–22.
44. Lepesheva GI, et al. (2007) Sterol 14α-demethylase as a potential target for anti-
20. Korený L, Luke�s J, Oborník M (2010) Evolution of the haem synthetic pathway in
trypanosomal therapy: Enzyme inhibition and parasite cell growth. Chem Biol 14:
kinetoplastid flagellates: An essential pathway that is not essential after all? Int J
45. Rangel H, Dagger F, Hernandez A, Liendo A, Urbina JA (1996) Naturally azole-re-
sistant Leishmania braziliensis promastigotes are rendered susceptible in the presence
21. Muller E, et al. (1994) Variability in the phloem restricted plant trypanosomes (Phy-
of terbinafine: Comparative study with azole-susceptible Leishmania mexicana pro-
tomonas spp) associated with wilts of cultivated crops; Isoenzyme comparison with
mastigotes. Antimicrob Agents Chemother 40:2785–2791.
the lower trypanosomatids. Eur J Plant Pathol 100:425–434.
46. Buttke TM, Van Cleave S (1994) Adaptation of a cholesterol deficient human T cell
22. Sánchez-Moreno M, Lasztity D, Coppens I, Opperdoes FR (1992) Characterization of
line to growth with lanosterol. Biochem Biophys Res Commun 200:206–212.
carbohydrate metabolism and demonstration of glycosomes in a Phytomonas sp.
47. Gachotte D, et al. (1997) A yeast sterol auxotroph (erg25) is rescued by addition of
isolated from Euphorbia characias. Mol Biochem Parasitol 54:185–199.
azole antifungals and reduced levels of heme. Proc Natl Acad Sci USA 94:
23. Tripodi KE, Buttigliero LV, Altabe SG, Uttaro AD (2006) Functional characterization of
front-end desaturases from trypanosomatids depicts the first polyunsaturated fatty
48. Nozaki T, Shigeta Y, Saito-Nakano Y, Imada M, Kruger WD (2001) Characterization of
acid biosynthetic pathway from a parasitic protozoan. FEBS J 273:271–280.
transsulfuration and cysteine biosynthetic pathways in the protozoan hemoflagel-
24. Flannery AR, Huynh C, Mittra B, Mortara RA, Andrews NW (2011) LFR1 ferric iron
late, Trypanosoma cruzi. Isolation and molecular characterization of cystathionine
reductase of Leishmania amazonensis is essential for the generation of infective
β-synthase and serine acetyltransferase from Trypanosoma. J Biol Chem 276:
parasite forms. J Biol Chem 286:23266–23279.
25. Vonlaufen N, Kanzok SM, Wek RC, Sullivan WJ, Jr. (2008) Stress response pathways in
49. Farr H, Gull K (2009) Functional studies of an evolutionarily conserved, cytochrome b5
protozoan parasites. Cell Microbiol 10:2387–2399.
domain protein reveal a specific role in axonemal organisation and the general
26. Nawathean P, Maslov DA (2000) The absence of genes for cytochrome c oxidase and
phenomenon of post-division axonemal growth in trypanosomes. Cell Motil Cyto-
reductase subunits in maxicircle kinetoplast DNA of the respiration-deficient plant
trypanosomatid Phytomonas serpens. Curr Genet 38(2):95–103.
50. Das S, et al. (2002) Lipid metabolism in mucous-dwelling amitochondriate protozoa.
27. González-Halphen D, Maslov DA (2004) NADH-ubiquinone oxidoreductase activity in
Int J Parasitol 32:655–675.
the kinetoplasts of the plant trypanosomatid Phytomonas serpens. Parasitol Res 92:
51. Rieske JS (1967) The quantitative determination of mitochondrial hemoproteins.
Methods Enzymol 10:488–493.
28. Maslov DA, Zíková A, Kyselová I, Luke�s J (2002) A putative novel nuclear-encoded
52. Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification.
subunit of the cytochrome c oxidase complex in trypanosomatids. Mol Biochem
Can J Biochem Physiol 37:911–917.
53. Horváth A, et al. (2005) Downregulation of the nuclear-encoded subunits of the
29. Chaumont F, Schanck AN, Blum JJ, Opperdoes FR (1994) Aerobic and anaerobic glu-
complexes III and IV disrupts their respective complexes but not complex I in procyclic
cose metabolism of Phytomonas sp. isolated from Euphorbia characias. Mol Biochem
Trypanosoma brucei. Mol Microbiol 58:116–130.
54. Chevreux B, Wetter T, Suhai S (1999) Genome sequence assembly using trace signals
30. Cermáková P, Verner Z, Man P, Luke�s J, Horváth A (2007) Characterization of the
and additional sequence information. Proceedings of German Conference on Bio-
NADH:ubiquinone oxidoreductase (complex I) in the trypanosomatid Phytomonas
informatics, GCB ‘99 (German Research Centre for Biotechnology, Braunschweig,
serpens (Kinetoplastida). FEBS J 274:3150–3158.
Germany), pp 45–56.
31. Van Hellemond JJ, Simons B, Millenaar FF, Tielens AG (1998) A gene encoding the
55. Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence align-
plant-like alternative oxidase is present in Phytomonas but absent in Leishmania spp.
ment program. Brief Bioinform 9:286–298.
J Eukaryot Microbiol 45:426–430.
56. Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and
32. Morales J, et al. (2009) Novel mitochondrial complex II isolated from Trypanosoma
analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98.
cruzi is composed of 12 peptides including a heterodimeric Ip subunit. J Biol Chem
57. Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analy-
ses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690.
33. Tran QM, Rothery RA, Maklashina E, Cecchini G, Weiner JH (2007) Escherichia coli
58. Gould MK, Vu XL, Seebeck T, de Koning HP (2008) Propidium iodide-based methods
succinate dehydrogenase variant lacking the heme b. Proc Natl Acad Sci USA 104:
for monitoring drug action in the kinetoplastidae: Comparison with the Alamar Blue
assay. Anal Biochem 382(2):87–93.
34. Oyedotun KS, Sit CS, Lemire BD (2007) The Saccharomyces cerevisiae succinate de-
59. Räz B, Iten M, Grether-Bühler Y, Kaminsky R, Brun R (1997) The Alamar Blue assay to
hydrogenase does not require heme for ubiquinone reduction. Biochim Biophys Acta
determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gam-
biense) in vitro. Acta Trop 68(2):139–147.
Ko�rený et al.
PNAS March 6, 2012 vol. 109 no. 10 3813
Source: http://www.paru.cas.cz/Cds/Download/?filename=2766_62_Koreny_PNAS_2012
Guatemala, viernes 21 de marzo de 2014 SIGLO 21 Fiscal General lanza política de género Coralia Orantes Claudia Paz y Paz, fiscal general, y María Machicado representante de ONU-Mujeres, presentaron ayer la política para la igualdad entre hombres y mujeres del Ministerio Público (MP). La iniciativa tiene como objetivo que la mitad del capital humano del ente investigador sea mujeres. Paz y Paz dijo que el objetivo es asegurar y proteger el derecho a la igualdad. De acuerdo con la jefa del MP, en los últimos años el porcentaje de mujeres a cargo de fiscalías distritales se ha incrementado del 2 al 36 por ciento, también se ha buscado mayor participación de la mujer en las áreas de investigación y seguridad. Congreso elige relatores contra la tortura Jessica Osorio Como titulares fueron elegidos: Otto Marroquín Guerra, Mario Enrique Carrera, Lucrecia Villalta Martínez, Carlos Alberto Solórzano e Hilario Roderico Pineda Sánchez. Los suplentes: Anthony Giovanni Pivaral de León, María Elizabeth Ramos Aguilar, Iracema Palacios Franco, José Antonio Meléndez Sandoval y Sandra Stephenie Shaw Díaz. La elección se concretó un día después de que la Corte de Constitucionalidad (CC) ordenara al Legislativo cumplir con el convenio ratificado ante la Asamblea General de las Naciones Unidas (ONU), el 10 de diciembre de 1984. Con dicha adhesión, el Estado de Guatemala había manifestado su compromiso para adoptar medidas legislativas, administrativas, judiciales o de otra índole "eficaces para prevenir los actos de tortura en todo el territorio nacional". El amparo fue concedió al diputado de la Unidad Nacional de la Esperanza (UNE), Julio César Villatoro, quien accionó el año pasado para lograr que el Parlamento cumpliera con ese requisito. Recientemente, el representante de la Oficina del Alto Comisionado de Naciones Unidas Alberto Brunori, acudió al Legislativo y planteó a Arístides Crespo, presidente de ese organismo, cumplir con la elección de los relatores. Guatemala, entre países con más políticas de seguridad EFE Esta es la principal conclusión extraída de una nueva plataforma virtual e interactiva, que recopiló cerca de 1 mil 300 políticas de seguridad puestas en marcha en 40 países de América Latina y el Caribe desde 1990, que fue presentada hoy en Río de Janeiro.
Dawood Public School Course Outline 2016-17 Book: International primary Science 5 Work Book-5 Ho Peck Leng- Marshall Cavendish Education AIMS: The Science Syllabus aims to: The Science Syllabus aims to: Provide students with experiences which build on their interest in and stimulate their















