Pathology.xqhospital.com.cn
The American Journal of Pathology, Vol. 174, No. 3, March 2009
Copyright American Society for Investigative Pathology
Growth Factors, Cytokines, Cell Cycle Molecules
The Neuronal Expression of MYC Causes aNeurodegenerative Phenotype in a NovelTransgenic Mouse
Hyoung-gon Lee,* Gemma Casadesus,†
from inappropriate cell cycle control.
(Am J Pathol
Akihiko Nunomura,‡ Xiongwei Zhu,*
2009, 174:891– 897; DOI: 10.2353/ajpath.2009.080583)
Rudy J. Castellani,§ Sandy L. Richardson,*George Perry,*¶ Dean W. Felsher,储
Neurons in the normal brain are viewed as being quies-
Robert B. Petersen,*† and Mark A. Smith*
cent, not advancing past the G period of the cell division
From the Departments of Pathology *
and Neurosciences,†
Case
cycle. However, in Alzheimer's disease (AD), multiple
Western Reserve University, Cleveland, Ohio; the Interdisciplinary
lines of evidence suggest that neurons vulnerable to
Graduate School of Medicine and Engineering,‡
University of
degeneration emerge from this postmitotic state—pheno-
Yamanashi, Yamanashi, Japan; the Department of Pathology,§
typically suggestive of cells that are cycling, rather than
University of Maryland, Baltimore, Maryland; the College of
the normal terminally differentiated nondividing state.1–5
Sciences,¶
University of Texas at San Antonio, San Antonio,
Notably, cell cycle alterations are not limited to AD. For
Texas; and the Department of Medicine and Pathology,储
Division
example, accumulation of hyperphosphorylated pRb (ppRb)
of Oncology, Stanford University School of Medicine,
and altered localization of E2F-1 also occurs in neurons in
Parkinson's disease and amyotrophic lateral sclerosis, sug-gesting that neurons re-enter the G
phase of the cell
cycle.6–8 The successful duplication of DNA, at least inAD,9–11 indicates that some neurons successfully complete
Many different proteins associated with the cell cy-
S phase and precludes the possibility that the re-expression
cle , including cyclins , cyclin-dependent kinases ,
of various cell cycle markers observed is merely an epiphe-
and proto-oncogenes such as c-MYC (MYC) , are in-
nomena caused by reduced proteosomal activity.12
creased in degenerating neurons. Consequently , an
The causes or consequences of neuronal cell cycle
ectopic activation of the cell cycle machinery in neu-
are incompletely understood. Nonetheless, it is well
rons has emerged as a potential pathogenic mecha-
known that the activation of cell cycle processes is part of
nism of neuronal dysfunction and death in many
the mechanism by which loss of trophic support during
neurodegenerative diseases , including Alzheimer's
development leads to neuronal cell death, and there is
disease. However , the exact role of cell cycle re-entry
during disease pathogenesis is unclear , primarily be-
Supported by the National Institutes of Health (grants AG031364, AG030096
cause of the lack of relevant research models to study
and AG028679) and Alzheimer's Association (NIRG-07-60164).
the effects of cell cycle re-entry on mature neurons in
H.L. and G.C. contributed equally to this study.
vivo. To address this issue , we developed a new trans-
genic mouse model in which forebrain neurons
Accepted for publication December 2, 2008.
(CaMKII-MYC) can be induced to enter the cell cycle
Supplemental material for this article can be found on
http://ajp.
using the physiologically relevant proto-oncogene
MYC to drive cell cycle re-entry. We show that such
M.A.S. is, or has in the past been, a paid consultant for, owns equity or
cell cycle re-entry results in neuronal cell death , gli-
stock options in, and/or receives grant funding from Neurotez, Neurop-
harm, Edenland, Panacea Pharmaceuticals, and Voyager Pharmaceuti-
osis , and cognitive deficits. These findings provide
cals. G.P. is a paid consultant for and/or owns equity or stock options in
compelling evidence that dysregulation of cell cycle
Takeda Pharmaceuticals, Voyager Pharmaceuticals, Panacea Pharma-
re-entry results in neurodegeneration in vivo. Our
ceuticals, and Neurotez Pharmaceuticals.
current findings , coupled with those of previous
Address reprint requests to Hyoung-gon Lee, Ph.D., or Mark A. Smith,
reports , strengthen the hypothesis that neurode-
Ph.D., Department of Pathology, Case Western Reserve University, 2103
generation in Alzheimer's disease , similar to cellu-
Cornell Rd., Cleveland, OH 44106. E-mail:
[email protected]
lar proliferation in cancer , is a disease that results
AJP March 2009, Vol. 174, No. 3
also evidence for a role for cell cycle regulators in neu-
mice were established in the FVB strain. A doxycycline-
ronal death evoked by various stressors.13,14 In support
containing diet (200 mg/kg; Bio-Serve, Frenchtown, NJ)
of the importance of cell cycle reactivation in mediating
was provided before weaning (4 to 6 weeks after birth),
cell death, when a powerful oncogene, SV40 T antigen, is
and, thereafter, MYC expression was induced by replac-
expressed specifically in maturing Purkinje cells or in
ing the doxycycline diet with a regular diet for 5 weeks.
forebrain neurons in transgenic mice, the cells replicate
After behavioral tests, the mice were sacrificed and the
their DNA (ie, initiate cell cycle), but then subsequently
brains were processed for immunocytochemistry and
degenerate and die.15
Unfortunately, studies performed to date in cell culture
do not faithfully reproduce age-related neurodegenera-tive diseases because the cell culture studies use em-
bryo-derived primary neuronal cultures that do not accu-
Immunocytochemistry was performed by the ABC
rately model adult neurons. Furthermore, in traditional
method according to the manufacturer's protocol (Vector
transgenic mouse models,15 the expression of trans-
Laboratories, Burlingame, CA). Formalin-fixed brains
genes during embryogenesis, which results in neurode-
were processed and embedded in paraffin. Six-m-thick
generation at an early age, is more akin to a develop-
serial sections were cut, mounted onto slides, and rehy-
mental error than an age-dependent process associated
drated according to standard protocols. All slides were
with neurodegenerative disease. Although a recently de-
randomized and blinded with regard to genotype before
veloped inducible SV40 T-antigen transgenic model16
staining and subsequent analysis. Briefly, slides were
addressed such developmental issues, SV40 T antigen is
immersed in xylene, hydrated through graded ethanol
not a physiologically relevant entity in any neurodegen-
solutions, and endogenous peroxidase activity was elim-
erative disease. As such, a faithful and physiologically
inated by incubation in 3% hydrogen peroxide for 30
relevant model of neuronal cell cycle activation in mature
minutes. To reduce nonspecific binding, sections were
neurons was lacking. To generate such a faithful model,
incubated for 30 minutes in 10% normal goat serum in
in this study, we used the tetracycline-controlled trans-
Tris-buffered saline (50 mmol/L Tris-HCl, 150 mmol/L
activator (tTA) system to generate bitransgenic mice
NaCl, pH 7.6). After rinsing briefly with 1% normal goat
(CaMKII-MYC) that can be induced to overexpress hu-
serum in Tris-buffered saline, the sections were incu-
man c-MYC (MYC) under the control of the CaMKII pro-
bated overnight at 4°C with one of the following primary
moter that drives high transgene expression in forebrain
antibody; anti-proliferating cell nuclear antigen (PCNA)
neurons.17 MYC is well known for its oncogenic activity
mouse monoclonal antibody (Santa Cruz Biotechnology,
and overexpression of MYC is commonly associated with
Santa Cruz, CA), anti-Ki-67 rat monoclonal antibody
tumorigenesis. Indeed, previously we used the Tet sys-
(DAKO, Carpinteria, CA), anti-cyclin D1 rabbit monoclo-
tem to generate mice that conditionally expressed a hu-
nal antibodies (Lab Vision, Fremont, CA), and anti-GFAP
man MYC transgene in hematopoietic cells and hepato-
mouse monoclonal antibody (Chemicon, Billerica, MA).
cytes18,19 and found that the sustained expression of the
Antibodies were localized using 3–3⬘-diaminobenzidine
MYC transgene induced cell cycle progression and cul-
as a chromogen (DAKO) after incubation with a second-
minated in the formation of malignant T-cell lymphomas,
ary antibody.
acute myeloid leukemias, and hepatomas. Interestingly,
For double immunocytochemistry, the brain sections
and of physiological relevance to AD, phospho-MYC is
were incubated overnight at 4°C with anti-NeuN mouse
increased in dystrophic neurites and neurons with neu-
monoclonal antibody (Millipore, Billerica, MA) or anti-
rofibrillary tangles in AD and MYC is induced in degen-
MAP2 mouse monoclonal antibody (Millipore) in addition
erating neurons in animal models of trauma and isch-
to anti-PCNA rabbit polyclonal antibody (Abcam, Cam-
emia.20 Therefore, MYC expression in transgenic mice in
bridge, MA), anti-Ki-67 rat monoclonal antibody, or anti-
our model is not only a tool for inducing cell cycle re-entry in
BrdU rat monoclonal antibody (Abcam). Alexa Fluor 488-
neurons, but MYC expression itself is pathophysiologically
and 568-coupled secondary antibodies (Invitrogen,
relevant to AD and other neurodegenerative diseases.
Carlsbad, CA) were used for detection. To exclude the
Our findings reported in this study show that MYC
possibility of nonspecific reaction, all of the immunocyto-
induction in CaMKII-MYC transgenic mice induced
chemistry experiments contained at least one sample
neuronal-specific cell cycle re-entry, neurodegenera-
without a primary antibody. Images were acquired
tion, and, importantly, significant cognitive deficits. These
through an AxioCam camera on an Axiovert 200M micro-
findings continue to strengthen our novel hypothesis that
scope (Zeiss, Thornwood, NY). Images were then ana-
neurodegeneration in AD, like cellular proliferation in can-
lyzed with the Axiovision software (Zeiss). Hematoxylin
cer, is a disease of inappropriate cell cycle control.
and eosin (H&E) staining and Nissl stains were also per-formed for routine histochemical and morphologicalanalyses.
Materials and Methods
BrdU Incorporation Analysis
CaMKII-tTA17 and tet-o-MYC mice18 were mated to gen-
DNA synthesis was directly examined by analysis of
erate CaMKII-MYC mice. Both CaMKII-tTA and tet-o-MYC
BrdU incorporation. For this experiment, 50 mg/kg of
Neurodegeneration and MYC
AJP March 2009, Vol. 174, No. 3
the thymidine analog 5-bromo-2⬘-deoxyuridine (BrdU)
(Sigma, St. Louis, MO) was intraperitoneally injected intoeach mouse. A day after injection, mice were anesthe-
All mice (n ⫽ 8 in each group) were tested in a modified
tized and perfused with phosphate-buffered saline (PBS)
version of the T-maze essentially as previously de-
followed by 4% paraformaldehyde. Brains were removed,
scribed.21 This behavioral task, used to test working
cut in the sagittal plane, embedded in paraffin, sec-
memory, is based on spontaneous alternation behavior,
tioned, and mounted on slides. The sections were treated
which describes the innate tendency of mice to visit arms
with 2 N hydrochloric acid for 2 hours and then washed
that were not previously explored. All animals were al-
two times with 1⫻ Tris-buffered saline (3 minutes each) to
lowed a 5-minute free run in the maze before testing to
neutralize the acid. Incorporated BrdU was detected us-
familiarize them with the apparatus and to foster alterna-
ing an anti-BrdU rat monoclonal antibody.
tion behavior. On the testing day, mice were placed in thestart arm for 60 seconds before the gate was opened.
Once the mouse entered an arm, the door was closed,and the animal was confined in that arm for 30 seconds;
Reverse Transcriptase-Polymerase Chain
thereafter it was returned to the start arm for a new trial.
Reaction (RT-PCR)
Six consecutive trials were performed and alternationrates across the six trials were expressed as a relative
The brain region was dissected immediately after collec-
percentage based on the maximal alternation rate of
tion and used for mRNA isolation using an RNeasy kit
100%, which occurred when a mouse never entered a
(Qiagen, Valencia, CA). RT-PCR was performed using
repeated arm. Task achievement time (how long it took
the RETROscript RT-PCR kit (Ambion, Austin, TX) ac-
the animal to enter a goal arm after the gate opened) was
cording to the manufacturer's instructions. Briefly, the
also calculated.22
purified total RNA (100 ng) was reverse-transcribed with
Statistical comparisons were performed by parametric
cloned MMLV reverse transcriptase (50 U) by incubating
analysis using a one-way analysis of variance and/or
at 44°C for 60 minutes, followed by heating at 92°C for 10
Student's t-test, as appropriate, to determine significant
minutes. The resulting single-stranded cDNA was then
differences across genotypes. The null hypothesis was
amplified using a pair of primers for each target gene (25
rejected at P ⬍ 0.05.
pmol) and TaqDNA polymerase (2.5 U; Roche, Indianap-olis, IN) for the indicated number of cycles of amplifica-tion (30 seconds at 94°C for denaturing, 30 seconds at
61°C for primer annealing, and 30 seconds at 72°C for
Bitransgenic animals (CaMKII-MYC) that were main-
primer extension). The RT-PCR products were then sub-
tained on the doxycycline diet to suppress MYC trans-
jected to electrophoresis in a 1.5% agarose gel. The
gene expression (MYC-Off mice) showed minimal ex-
nucleotide sequence used for each primer was as
pression of MYC (Figure 1). In marked contrast,
follows: for c-myc: 5⬘-primer, 5⬘-TCTGGATCACCTTCT-
increased expression, as determined by RT-PCR, of the
MYC transgene was significantly increased after the
and for GAPDH: 5⬘-primer, 5⬘-ATGTTCCAGTATGACTC-
doxycycline-containing diet was replaced by doxycy-
cline-free regular diet (MYC-On mice) and, as expected,
CACGACA-3⬘. The specificity of PCR was confirmed by
was specifically increased in the cerebral cortex and
measuring the size of PCR product. In addition, the negative
hippocampus (Figure 1).
control (ie, brain tissues from single transgenic mice) and
To test whether MYC expression induces cell cycle
positive control (ie, SHSY5Y human neuroblastoma cell line)
re-entry in neurons, we measured both the expression
were tested to confirm the specificity of PCR reaction.
level of PCNA and Ki-67, well-established markers of cellcycle activity, which increase in nuclei during the cell
Terminal dUTP Nick-End Labeling (TUNEL)Analysis
Detection of 3⬘-OH termini of DNA strand breaks wasperformed on paraffin sections using an in situ cell deathdetection kit (Roche) following the recommendations ofthe manufacturer. Briefly, the tissue sections were treatedwith proteinase K (20 g/ml in 10 mmol/L Tris-HCl, pH7.4) for 30 minutes at 37°C after rehydration. After rinsingslides with PBS, TUNEL reaction mixture containing ter-
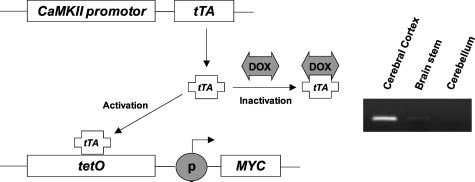
Figure 1. Inducible expression of MYC in hippocampus by Tet regulatory
minal deoxynucleotidyl transferase and fluorescence-la-
system. Expression of the tTA peptide is driven by a CaMKII promoter that isactive in the forebrain neurons. In its active form, tTA binds to the TetO
beled nucleotide was applied for 1 hour at 37°C. Positive
sequence to drive expression of MYC. Doxycycline (Dox) can inhibit expres-
signals were observed directly using fluorescence mi-
sion of MYC by binding to tTA, rendering it inactive. In RT-PCR analysis ofdissected brain tissues, MYC is only detected in cerebral cortex, including
croscopy. The recommended positive and negative con-
hippocampus, after Dox-free regular diet in double-transgenic mice (CaMKII-
trols were applied in adjacent sections.
AJP March 2009, Vol. 174, No. 3
Figure 3. DNA replication in the hippocampal neurons in MYC-On mice.
Incorporation of BrdU during DNA replication is visualized using an anti-
BrdU antibody (red). The number of BrdU-positive nuclei is dramatically
increased in the hippocampal pyramidal neuron layer (CA1) of MYC-On
mice. Double staining with anti-MAP2 antibody (green) clearly demonstrates
that the BrdU-positive cells are neurons. In MYC-Off mice, virtually no
BrdU-positive nuclei are observed. Scale bar ⫽ 50 m.
MAP2, a specific marker for neurons. In contrast, noBrdU-positive cells were found in the pyramidal neuronsin MYC-Off mice (Figure 3). That PCNA, Ki-67, cyclin D1,and BrdU immunoreactivity were all observed in the nu-clei of the population of hippocampal neurons that ex-press MYC (ie, forebrain neurons), suggests that the cellcycle machinery was switched on and that the neuronsprogress through S phase. Therefore, the CaMKII-MYC
Figure 2. Cell cycle markers increase in the hippocampal neurons in
mice appear to be an excellent model for the analysis of
MYC-On mice. A: The expression of PCNA is dramatically increased in the
the specific events occurring after MYC induction and the
nuclei of hippocampal neurons (CA1 region) from MYC-On mice. B: In
MYC-Off mice, a basal level of PCNA is observed with a diffuse staining
subsequent re-entry of postmitotic neurons into the cell
pattern in the nucleus and cytoplasm. C–E: Double staining with anti-NeuN
antibody (red) clearly demonstrates the expression of PCNA (green) in
The pathological effects of MYC expression were also
neurons. F: Similar to PCNA, Ki-67 is strongly increased in the nuclei of
pyramidal neurons of the hippocampal CA1 region in MYC-On mice, but not
examined. Degenerating eosinophilic neurons with a
observed in MYC-Off mice. Scale bars ⫽ 50 m.
shrunken or pyknotic appearance and condensed chro-matin were readily detected in every MYC-On mouse
cycle, and cyclin D1, a key cell cycle regulator. The
(Figure 4, A and B). Indeed, Nissl stain confirmed mas-
expression of PCNA, Ki-67, and cyclin D1 is strongly
sive and selective neuronal loss in the CA1 region in
induced in the CA1 region of hippocampus after removal
MYC-On mice, but not in MYC-Off mice (Figure 4, C and
of doxycycline for 5 weeks (Figure 2, A–F; and see Sup-
D). To expand on these findings, we assessed TUNEL
plemental Figure S1 at http://ajp.amjpathol.org); the most
staining in MYC-On and MYC-Off mice to measure apo-
intense staining was observed in the nuclei of pyramidal
ptotic changes. Consistent with the histological data, in-
neurons. In contrast, the hippocampal neurons in the
creased numbers of TUNEL-positive neurons were found
CA1 region in MYC-Off mice showed very low levels of
in the CA1 region of the hippocampus of MYC-On mice
both PCNA and cyclin D1, and undetectable levels of
but not in MYC-Off mice (Figure 5, A–C). Furthermore, an
Ki-67. To further confirm the specific expression of these
antibody to glial fibrillary acidic protein (GFAP) used to
cell cycle markers in neurons, double immunocytochem-
detect astrocytes revealed intensely reactive astrocytosis
istry was performed with an anti-NeuN antibody that spe-
in the hippocampal regions (Figure 5D) in MYC-On mice,
cifically labels neurons. The immunoreactivity of both cell
but not in other unaffected regions such as the cerebel-
cycle markers, PCNA and Ki-67, is clearly observed in
lum (not shown). In contrast to MYC-On mice, reactive
NeuN-positive neurons (Figure 2). These data strongly
astrocytosis was not present in the hippocampus of MYC-
indicate a reactivation of the cell cycle in neurons in
Off mice (Figure 5E).
MYC-On mice.
To determine whether the expression of MYC in corti-
To extend these data, DNA replication was assessed
cal/hippocampal neurons resulted in cognitive deficits,
by analysis of BrdU incorporation. BrdU, which is incor-
we tested the spatial working memory of CaMKII-MYC
porated during DNA replication, was injected 1 day be-
mice using a modified version of the T-maze. The
fore sacrificing the mice. After 5 weeks of MYC expres-
MYC-On mice were significantly impaired compared with
sion, the number of hippocampal neurons showing
MYC-Off mice (P ⬍ 0.002). In support of the specificity of
nuclear incorporation of BrdU is dramatically increased in
our transgene, there was no difference in behavior be-
the MYC-On mice (Figure 3); the BrdU-positive cells were
tween single transgenic mice (ie, MYC or CaMKII single-
mostly localized in the pyramidal cell layer, especially the
transgenic mice) or MYC-Off double-transgenic mice
CA1 region, which is confirmed by the co-localization of
(Figure 6A). Interestingly, the length of time all animals
Neurodegeneration and MYC
AJP March 2009, Vol. 174, No. 3
Figure 5. Neurodegeneration and astrocytosis in the hippocampus in
MYC-On mice. TUNEL-positive nuclei are specifically localized in the hip-
pocampal CA1 region in MYC-On mice (A, B) but not in MYC-Off mice (C).
Figure 4. Selective neuronal cell loss in the CA1 region of the hippocampus
B: High magnification of the framed portion in A. Immunocytochemistry with
in MYC-On mice. A and C: H&E staining and Nissl staining with cresyl violet
an anti-GFAP antibody demonstrates the extensive astrocytosis in the hip-
show a significant level of neurodegeneration in the CA1 region in MYC-On
pocampus in MYC-On mice (D) compared with MYC-Off mice (E). DG,
mice. The characteristics of cell death, including condensation of chromatin,
dentate gyrus. Dotted lines outline the hippocampal subfields. Scale bars: 200
pyknotic appearance, and eosinophilic cytoplasm (arrows) are significant in
m (A, C); 50 m (B, D, E).
the CA1 region in MYC-On mice (A) whereas no such change is observed in
MYC-Off mice (B). Nissl staining clearly demonstrates the selective neuro-
degeneration in the CA1 region in MYC-On mice (arrowheads, C) but not
in MYC-Off mice (D). Scale bars ⫽ 100 m.
pocampal pyramidal neurons. Although BrdU data inisolation should be interpreted with caution because itcan also reflect other processes, in light of the other cell
took to enter a desired arm after each consecutive trial
cycle markers, it is unlikely that the BrdU data represents
was measured and the MYC-On animals showed much
anything other than cell cycle activity. As such, this is the
shorter latencies than MYC-OFF mice (Figure 6B). These
first physiologically relevant model of cell cycle re-entry in
data suggest that MYC-On mice did not learn the para-
mature neurons and it allowed us to determine the effect
digm and/or lack the cognitive flexibility needed to re-
of cell cycle re-entry on postmitotic neurons.
member the previously visited arm, incorporate that, and
The pathological effect of MYC expression was neuro-
consequently switch to the alternate, nonvisited arm. Ad-
degeneration. In all of the MYC-On mice, eosinophilic
ditionally, these findings may also indicate a lack of in-hibitory processes, although further testing should beperformed to establish this possibility. Of importance, wealso used a Rotarod to measure motor function acrossgroups and found no differences between MYC-On andMYC-Off mice. The data suggests there is no significantdifference in motor function between MYC-On and MYC-Off mice.
The underlying cause of the neuronal damage that oc-curs in AD and other neurodegenerative diseases is notfully understood. However, one likely contributor is thecell cycle re-entry found in the vulnerable neurons inpostmortem brain of AD patients.23–25 Unfortunately, de-termining the consequence of cell cycle re-entry in post-mitotic neurons has been technically challenging and themodels used to date have not been pathophysiologicallyrelevant. To explore this phenomenon and to establishsuch a relevant model system, we developed a trans-
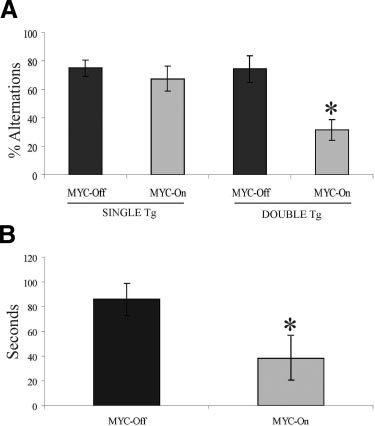
Figure 6. Working memory function is decreased in MYC-On mice (n ⫽ 8).
genic mouse model in which cell cycle re-entry could be
Mice were placed in the start arm for 60 seconds before the gate was opened.
induced in mature neurons. Using the MYC oncogene,
Once the subject entered an arm the door was closed and the animal was
which is expressed in neurons in AD,20 we were able to
confined for 30 seconds; thereafter it was returned to the start arm for a new
trial. A: MYC-On mice had significantly more trouble remembering the arm
induce cell cycle re-entry as determined by the expres-
that they had previously visited and re-entering it (*P ⬍ 0.002). B: The time
sion of classical cell-cycle marker proteins (ie, PCNA,
elapse was measured from when the door in the start box was opened untilthe animal entered the chosen arm. MYC-On mice took significantly less time
Ki-67, and cyclin D1), as well as DNA replication as
to enter the chosen arm indicating lack of cognitive flexibility (*P ⬍ 0.05).
evidenced by BrdU incorporation, specifically in hip-
Mean percentage of alternations over six trials.
AJP March 2009, Vol. 174, No. 3
neurons that were shrunken and pyknotic with con-
gene in forebrain neurons leads to cell cycle re-entry,
densed chromatin were readily observed. In addition,
neurodegeneration, gliosis, and cognitive deficits. The
there were increased numbers of TUNEL-positive neu-
establishment of this model provides a working platform
rons as well as marked astrocytosis. Although the expres-
to test genetic and pharmacological approaches to block
sion of MYC mRNA is found in all forebrain neurons, the
cell cycle re-entry.34
morphological correlates were confined to the CA1 re-gion of the hippocampus suggesting either a restrictedexpression of MYC protein in the CA1 region, similar to
other CamKII transgenic lines,26 or that CA1 neurons aremore susceptible to neurodegeneration than other fore-
We thank Dr. Eric Kandel (Columbia University, New
brain neurons as shown in many studies.27 Of impor-
York, NY) for providing CamKII-tTA transgenic mice.
tance, the pathological changes found in MYC-On trans-genic animals were correlated with cognitive deficits. In amodified T-maze test, there was clear evidence of a
decline in spatial working memory in the MYC-On micecompared with the single transgenic or MYC-Off mice.
1. Arendt T, Holzer M, Grossmann A, Zedlick D, Bruckner MK: In-
Traditional transgenic models that have been used to
creased expression and subcellular translocation of the mitogen ac-
study the role of oncogenes in tumorigenesis continu-
tivated protein kinase kinase and mitogen-activated protein kinase inAlzheimer's disease. Neuroscience 1995, 68:5–18
ously overexpress the transgenes. For example, when a
2. Vincent I, Jicha G, Rosado M, Dickson DW: Aberrant expression of
powerful oncogene, the SV40 T antigen, is expressed
mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's
specifically in maturing Purkinje cells in transgenic mice,
disease brain. J Neurosci 1997, 17:3588 –3598
the cells replicate their DNA, but subsequently degener-
3. Nagy Z, Esiri MM, Smith AD: Expression of cell division markers in the
hippocampus in Alzheimer's disease and other neurodegenerative
ate and die.15 Similarly, expression of the SV40 T antigen
conditions. Acta Neuropathol 1997, 93:294 –300
driven by the rhodopsin promoter causes photoreceptor
4. McShea A, Harris PL, Webster KR, Wahl AF, Smith MA: Abnormal
degeneration, again associated with cell cycle reactiva-
expression of the cell cycle regulators P16 and CDK4 in Alzheimer's
tion and DNA synthesis.28 Although these data are con-
disease. Am J Pathol 1997, 150:1933–1939
sistent with our conclusion that cell cycle reactivation in
5. Zhu X, McShea A, Harris PL, Raina AK, Castellani RJ, Funk JO, Shah
S, Atwood C, Bowen R, Bowser R, Morelli L, Perry G, Smith MA:
neurons leads to neurodegeneration, the effect of the
Elevated expression of a regulator of the G2/M phase of the cell cycle,
SV40 T antigen and dysregulation of cell cycle re-entry
neuronal CIP-1-associated regulator of cyclin B, in Alzheimer's dis-
during development cannot be excluded in those stud-
ease. J Neurosci Res 2004, 75:698 –703
ies. Furthermore, although a new inducible SV40 T anti-
6. Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim
CL: Expression patterns of retinoblastoma protein in Parkinson dis-
gen transgenic model was recently developed16 to avoid
ease. J Neuropathol Exp Neurol 2003, 62:68 –74
those problems, SV40 T antigen is not a physiologically
7. Thakur A, Siedlak SL, James SL, Bonda DJ, Rao A, Webber KM,
relevant entity in neurodegenerative disease and further
Camins A, Pallas M, Casadesus G, Lee HG, Bowser R, Raina AK,
study is required to delineate its exact mechanism of
Perry G, Smith MA, Zhu X: Retinoblastoma protein phosphorylation at
action. Importantly, our CaMKII-MYC mice preclude initial
multiple sites is associated with neurofibrillary pathology in Alzheimerdisease. Int J Clin Exp Pathol 2008, 1:134 –146
and developmentally specific consequences of onco-
8. Ranganathan S, Bowser R: Alterations in G(1) to S phase cell-cycle
gene expression and hence represent a valuable model
regulators during amyotrophic lateral sclerosis. Am J Pathol 2003,
for studying the pathogenesis of age-related neurode-
generative diseases. In contrast to other approaches for
9. Yang Y, Geldmacher DS, Herrup K: DNA replication precedes neuronal
cell death in Alzheimer's disease. J Neurosci 2001, 21:2661–2668
induction of cell cycle re-entry in neurons such as the
10. Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T: Aneu-
SV40 T antigen,16 MYC is a physiologically relevant entity
ploidy and DNA replication in the normal human brain and Alzhei-
that has been shown to be increased in vulnerable neu-
mer's disease. J Neurosci 2007, 27:6859 – 6867
rons in patients with AD.20 Like our studies, this AD-
11. Zhu X, Siedlak SL, Wang Y, Perry G, Castellani RJ, Cohen ML, Smith
related expression of MYC is restricted to increases in
MA: Neuronal binucleation in Alzheimer disease hippocampus. Neu-ropathol Appl Neurobiol 2008, 34:457– 465
specific neuronal populations, dystrophic neurites, and
12. Szweda PA, Friguet B, Szweda LI: Proteolysis, free radicals, and
neurofibrillary tangles without global increases in expres-
aging. Free Radic Biol Med 2002, 33:29 –36
sion by immunoblot.20,29 The latter is perhaps not sur-
13. Giovanni A, Wirtz-Brugger F, Keramaris E, Slack R, Park DS: Involve-
prising given that any neuron expressing MYC would,
ment of cell cycle elements, cyclin-dependent kinases, pRb, and E2Fx DP, in B-amyloid-induced neuronal death. J Biol Chem 1999,
based on our study, rapidly degenerate.
Indeed, it is important to bear in mind that pathological
14. Park DS, Obeidat A, Giovanni A, Greene LA: Cell cycle regulators in
studies represent the terminal stages of disease. As such
neuronal death evoked by excitotoxic stress: implications for neuro-
it does not exclude the possibility that MYC might be
degeneration and its treatment. Neurobiol Aging 2000, 21:771–781
temporally induced in the early stage of disease. In this
15. Feddersen RM, Ehlenfeldt R, Yunis WS, Clark HB, Orr HT: Disrupted
cerebellar cortical development and progressive degeneration of
regard, it should be noted that only 5 weeks induction of
Purkinje cells in SV40 T antigen transgenic mice. Neuron 1992,
MYC is enough to induce cell cycle re-entry and neuro-
degeneration in our mice. In support of this, MYC is
16. Park KH, Hallows JL, Chakrabarty P, Davies P, Vincent I: Conditional
temporally induced in the early stage during traumatic
neuronal simian virus 40 T antigen expression induces Alzheimer-liketau and amyloid pathology in mice. J Neurosci 2007, 27:2969 –2978
brain injury30 and in cerebral ischemia.31–33
17. Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER:
In conclusion, using these CaMKII-MYC animals, we
Control of memory formation through regulated expression of a
demonstrated that activation of an AD-associated onco-
CaMKII transgene. Science 1996, 274:1678 –1683
Neurodegeneration and MYC
AJP March 2009, Vol. 174, No. 3
18. Felsher DW, Bishop JM: Reversible tumorigenesis by MYC in hema-
26. Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ,
topoietic lineages. Mol Cell 1999, 4:199 –207
Mayford M, Kandel ER, Tonegawa S: Subregion- and cell type-re-
19. Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S,
stricted gene knockout in mouse brain. Cell 1996, 87:1317–1326
Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, Yang Q,
27. Mattson MP, Guthrie PB, Kater SB: Intrinsic factors in the selective
Bishop JM, Contag CH, Felsher DW: MYC inactivation uncovers
vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res
pluripotent differentiation and tumour dormancy in hepatocellular
1989, 317:333–351
cancer. Nature 2004, 431:1112–1117
28. al-Ubaidi MR, Hollyfield JG, Overbeek PA, Baehr W: Photoreceptor
20. Ferrer I, Blanco R, Carmona M, Puig B: Phosphorylated c-MYC ex-
degeneration induced by the expression of simian virus 40 large
pression in Alzheimer disease, Pick's disease, progressive supranu-
tumor antigen in the retina of transgenic mice. Proc Natl Acad Sci
clear palsy and corticobasal degeneration. Neuropathol Appl Neuro-
USA 1992, 89:1194 –1198
biol 2001, 27:343–351
29. Ferrer I, Blanco R: N-myc and c-myc expression in Alzheimer dis-
21. Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bo-
ease, Huntington disease and Parkinson disease. Brain Res Mol
wen RL, Smith MA: Luteinizing hormone modulates cognition and
Brain Res 2000, 77:270 –276
amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim
30. Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B,
Biophys Acta 2006, 1762:447– 452
Faden AI: Cell cycle inhibition provides neuroprotection and reduces
22. Pie´rard C, Liscia P, Valleau M, Drouet I, Chauveau F, Huart B, Bon-
glial proliferation and scar formation after traumatic brain injury. Proc
neau D, Jouanin JC, Beaumont M, Beracochea D: Modafinil-induced
Natl Acad Sci USA 2005, 102:8333– 8338
modulation of working memory and plasma corticosterone in chron-
31. Huang CY, Fujimura M, Noshita N, Chang YY, Chan PH: SOD1
ically-stressed mice. Pharmacol Biochem Behav 2006, 83:1– 8
down-regulates NF-kappaB and c-Myc expression in mice after tran-
23. McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ,
sient focal cerebral ischemia. J Cereb Blood Flow Metab 2001,
Funk JO, Shapiro RA, Smith MA: Neuronal cell cycle re-entry medi-
ates Alzheimer disease-type changes. Biochim Biophys Acta 2007,
32. Nakagomi T, Asai A, Kanemitsu H, Narita K, Kuchino Y, Tamura A,
Kirino T: Up-regulation of c-myc gene expression following focal
24. Evans TA, Raina AK, Delacourte A, Aprelikova O, Lee HG, Zhu X,
ischemia in the rat brain. Neurol Res 1996, 18:559 –563
Perry G, Smith MA: BRCA1 may modulate neuronal cell cycle re-entry
33. McGahan L, Hakim AM, Robertson GS: Hippocampal Myc and p53
in Alzheimer disease. Int J Med Sci 2007, 4:140 –145
expression following transient global ischemia. Brain Res Mol Brain
25. Raina AK, Garrett MR, Previll LA, Obrenovich ME, Hartzler AW, Webber
Res 1998, 56:133–145
KM, Casadesus G, Lee HG, Perry G, Zhu X, Smith MA: Oncogenic
34. Woods J, Snape M, Smith MA: The cell cycle hypothesis of Alzhei-
parallels in Alzheimer disease. Int J Neuroprotec Neuroregen 2006,
mer's disease: suggestions for drug development. Biochim Biophys
Acta 2007, 1772:503–508
Source: http://pathology.xqhospital.com.cn:8050/uploadfile/2009/5/6/20090506100138.pdf
Alastair Baker 9/7/2013 Investigation and treatment of liver disease with acute onset – Local hospital protocol Defined as EITHER sudden onset of jaundice with evidence of liver aetiology OR incidental discovery of raised transaminases in association with symptoms suggesting acute onset Age of onset >3 months
www.rsc.org/csr Chemical Society Reviews Artemisinin and its derivatives: a novel class of anti-malarialand anti-cancer agentsw Devdutt Chaturvedi, Abhishek Goswami, Partha Pratim Saikia, Nabin C. Barua*and Paruchuri G. Rao Received 3rd February 2009First published as an Advance Article on the web 24th August 2009DOI: 10.1039/b816679j In this tutorial review, an effort towards presentation of a comprehensive account of the recentdevelopments on various kinds of artemisinin derivatives including artemisinin dimers, trimersand tetramers has been made and their efficacy towards malaria parasites and different cancercells lines was compared with that of artemisinins, and various other anti-malarial and anti-cancerdrugs. It is expected that this review will provide first-hand information on artemisinin chemistryto organic/medicinal chemists, and pharmacologists working on anticancer and anti-malarial drugdevelopment.









