Encyfelobalt mykloprefiks i pregutturale mememerelasjoner

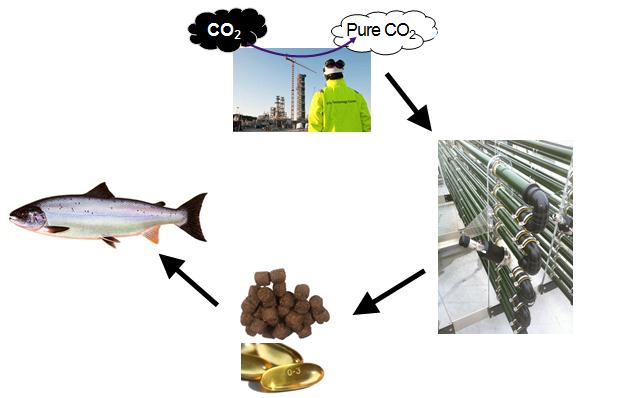

CO2 to Bio
Microalgae as an omega-3 rich feedstock -
integrating CO2 sequestration and aquafeed production
Project final report
January 2012
Prepared by Hans Kleivdal, Uni Miljø Svein M. Nordvik, Nordhordland Handverk- og Industrilag Trond Mork-Pedersen, Nofima Anders Haugland, BTO
CO2 to Bio
In 2010 the new gas-fired power plant at Mongstad (EVM) started the production of power and steam. In this respect, the Norwegian Government also decided to establish a Technology Center
for carbon capture at Mongstad (TCM) in order to test and improve CO2 capture technologies. TCM will open in May 2012, and is designed for the capturing of up to 100.000 tons of CO2. Fur-
thermore, the planning of a full-scale CO2 capture facility at Mongstad scaled to capture one
million tons of CO2 is ongoing, and involves transport and subsurface storage in geological for-
mations in the North Sea. Until further – the captured CO2 from TCM will be released into the
air during the test period.
The unique access to large amounts of pure CO2 can put Mongstad in a valuable position,
as CO2 can be used as a raw material for several applications. The industry consortium "Nord-
hordland Handverk- og Industrilag" (NHIL ) has suggested that using CO2 as a resource is a
more sustainable and economically viable alternative to subsurface storage, and have evaluated
several alternatives for the use of captured CO2 together with research institutions. For this purpose,
NHIL and TCM signed a preliminary agreement for use of up to 30.000 tons of captured CO2.
The global demand for food is increasing together with the world population. Norway is a
valuable contributor of farmed seafood to the global market, and the aquaculture industry expe-
rience an increasing demand. However, while the market demand of omega-3 fatty acids is in-
creasing rapidly, the global resources of fish oil and omega-3 used as aquafeed ingredients are
limited. Therefore, one of the largest challenges for the salmon industry is to develop new and
reliable sources of omega-3 for continued aquafeed production. Our focus has been on microal-
gae as they are the primary producers and main source of omega-3 in the ocean.
The purpose of this preliminary project "CO2 to Bio" has been to provide an as-
sessment of an industrial scale production of omega-3 rich biomass from microal-
gae, based on captured CO2 and available resources at Mongstad, for the production of omega-
3 supplements in aquafeed. The study has been divided in seven work package reports, where
the findings and conclusion is given in the Executive Summary. The pilot project "CO2 to Bio" started in June 2011 in collaboration between NHIL, Uni Research, Nofima and Bergen
Teknologioverføring, and was financed by the above organizations and Sparebanken Vest.
The overall report concludes that the industrial production of microalgae at
Mongstad can be a valuable contribution to the aquaculture industry. CO2 is vital
input for photosynthetic algae production, and will also result in a positive climate effect. By
integrating CO2 sequestration and production of omega-3 rich algae biomass for aquafeed, this
project initiative can make it possible for the Norwegian aquaculture industry to market the salmon as a good health food product also in the future. However, this will require research efforts
to develop biological assets as well as the current production and processing technology. Several
research institutions and industrial companies in Bergen have a well-established competence
within this field and can, through national and international collaborations, provide the re-
search and development required to fulfill this project. The company "CO2Bio AS" is now established as a result of this pilot project with industrial par-ticipation including Ewos AS, Grieg Seafood ASA and Salmon Group AS. The first step will be to
establish a pilot plant and a research group for the production of algae, biomass and processing
before the main target; production of omega-3 fatty acid in large scale at Mongstad.
We wish to thank CO2 Technology Centre Mongstad (TCM) for their valuable cooperation, and
the engineering consultants at Akvator AS for their contribution.
Nordhordland Handverk- og Industrilag (NHIL)
The following have contributed to the preparation of this report "CO2 to Bio":
Steering Committee:
Hans Kleivdal Uni Research/Uni Miljø
Trond Mork-Pedersen Nofima Anders Haugland BTO
Svein M Nordvik NHIL
Other contributors:
Caspar Lund
Marie- Lise Schläppy
Uni Research/Uni Miljø
Friederike Hoffmann
Uni Research/Uni Miljø
UiB, Institutt for Biologi
Svein Rune Erga
UiB, Institutt for Biologi
Katerina Kousoulaki
Øystein Høstmark Nofima
Henning Egede-Nilsen
Eyolf Langmyhr Nofima
Tor Andreas Samuelsen
Einar Wathne Ewos Group
University of Wageningen
We also wish to thank the Board and the Energy and Environment committee in Nordhordland
Handverk- og Industrilag for the valuable contribution to facilitate this report.
CO2 to Bio
Contents
Executive summary 2 pages
Work package 1 - Location 16 pages
Work package 2 – Production methods 23 pages
Work package 3 – Algae 27 pages
Work package 4 – Biomass aspects 32 pages
Work package 5 – Costs and markets 14 pages
Work package 6 – Environmental aspects 9 pages
Work package 7 – Conclusions 5 pages
Executive summary
The purpose of the pre-project "CO2 to Bio" is to provide an assessment of the proposed industrial
production of algal biomass at Mongstad based on captured CO2 and residual heat. These unex-ploited resources are considered as waste, but can be valuable inputs in up-scaled algal production.
The algal biomass is rich in omega-3 and can be a valuable ingredient for the aquafeed market – a
sizeable market where the demand for omega-3 is expected to rise significantly. Our aim was to assess the industrial production of omega-3 fatty acids and other high-value prod-ucts from microalgae, and the work was divided in 7 work packages – including this summary.
WP1 - Location
Mongstad is a suitable location for industrial microalgae production, and will benefit from the ex-
isting industrial infrastructure. A future microalgae production facility will have to be connected to
the current TCM pilot facility and future large scale facilities for the supply of captured CO2. In ad-dition, microalgae facility will need a steady supply of residual heat, cold seawater and freshwater,
and the ability to handle large volumes of waste water at suitable treatment. The weather condi-
tions are challenging with low temperatures and limited solar irradiation. However, the algal pro-
duction will benefit from long daylight hours during the summer period. Recommendations:
The planning of production facilities must be conducted in dialogue with industrial initiatives
at Mongstad, as well as the municipalities and land owners (Statoil).
Production facilities should be inside a greenhouse to protect PBRs and control the temperature.
WP2 – Production methods
The most promising production systems are closed photobioreactors (PBR) that are highly con-
trolled systems made of transparent tubes were the algae is exposed to natural or artificial light. The main challenge is to reduce production costs by lowering the energy demanding steps and in-
crease the photosynthetic efficiency and biomass yield. The existing PBR systems must be im-
proved before they are suitable for large scale production of microalgae for the bulk market. The
current production cost estimate is 4.15 €/kg dry weight biomass, but technologists aim to reduce
cost down towards 1.0 €/kg. The AlgaePARC (NL) focus on cost-efficient production systems suit-
able for industrial purpose, and have a strong international position and scientific competence within this field. We propose to establish a pilot production facility to develop and compare prom-
ising PBR designs, aiming at a cost-effective production system suitable for up-scaled production at
Mongstad. A collaboration with AlgaePARC will therefore be beneficial - both for establishing the
Mongstad pilot production facility and for the following research activity. Recommendations:
Establish a pilot production facility for the development and comparison of industrial microal-
gae production systems at Mongstad.
Enter collaboration with AlgaePARC in the development of the Mongstad pilot facility. Seek national collaborators in the establishment of a national forum for microalgae. Develop an R&D strategy and detailed plan to maximize the efficiency of the future research
efforts and reduce the risks at an early stage.
WP3 – Algae
A comprehensive overview of algae growth requirements and nutritional qualities related to aquafeed
has been given by algal experts at the University of Bergen. The optimization potential of the produc-tion efficiency lies not only on the production systems, but through screening and selection of the
most production-efficient algal strain in collaboration with Bergen Marine Biobank. Furthermore,
the algae can be stressed to produce more omega-3 through just before harvest. Recommendations:
CO2 to Bio
Several algae with high omega-3 levels are suitable as aquafeed ingredients, of which four local
algae are recommended; P. lutheri, P. tricornumtum, I. galbana and Nannochloropsis spp.
Develop industrial scale procedures to induce high omega-3 levels by metabolic stress.
WP4 – Biomass
All aspects of the handling and processing of the algae biomass from harvesting, dewatering, dry-
ing, optimization of nutrients and digestibility, oil extraction procedures up to the stage of incorpo-
ration into the aquafeed has been elaborated in a comprehensive WP-report.
The water content in the algal biomass is very high, and harvesting by centrifugation is a highly
energy demanding step that drives the overall cost picture. A main challenge is therefore to signifi-
cantly reduce the costs associated with water removal. Another challenge is to break open the tough
cell wall of the algal cells during processing, in order to release valuable nutrients and make these available for digestion or further processing. Nofima are highly experienced in marine biomass
processing, and will work to convert their competence towards algal biomass. Once the various
product alternatives are developed, the characteristics during the pellet extrusion will need to be
investigated. Furthermore, the product's nutritional value as an input in fish feed must be fully
documented in feeding experiments with salmon. Based on their nutritional quality, four algae
were recommended and were the same algae that were recommended under WP3.
WP5 – Costs and markets
The price of fish oil is expected to increase significantly in the next coming years due to an upcoming
shortage of fish oils. This is because the production of fish oil, which comes from wild caught fish such
as anchovies, is already at or close to maximum levels. At the same time the demand for omega-3 con-
tinues to rise, much driven by an increasing demand from pharmaceutical industry and the health food sector which uses it as in nutritional supplements. The world market for farmed salmon is also growing (annually about 6%), and the salmon industry has
a large export share (> 95%). The growing salmon market will directly affect the aquafeed industry,
which will challenged by having to increase production and face the expected shortage of fish oil at the
same time. For the long run, new and sustainable omega-3 sources must be developed if the Norwegian salmon industry still wants to market farmed salmon as a good health food product in the future. Omega-3 production based on algal biomass is innovative, sustainable, has a good environmental
profile and will be free of contaminants often associated with fish oil. Our competitive advantage is a
significant and steady supply of CO2, residual heat at Mongstad. Furthermore, we have an ongoing dialogue with the affected aquaculture-industries to ensure that the products are customized to meet
their needs and requirements. Establishing a pilot plant where technology can be tested, develop
optimized algae cultures and production processes for full-scale production, will require an in-
vestment of 11 MNOK. The pilot plant will be ready at the end of 2012 and will be operating for 3
years. Operation of the pilot plant will cost approximate 8 MNOK. The technology development
of cost-effective microalgae production systems at the pilot facility, supported by waste resources, is expected to lower costs and increase productivity to make the algal biomass competitive on world
WP6 – Environmental aspects
The industrial production of microalgae use captured CO2 and reduces waste greenhouse gas emis-
sions. The microalgae omega-3 is therefore a sustainable ingredient in aquafeed, and will be an
ecofriendly alternative to replace currently imported fish oil recovered from wild caught fish. It is recommended to conduct a full scale LCA to document the proposed environmental benefits
WP7 – Conclusion
Based on the "CO2 to Bio" findings and assessments, we recommend establishing of a pilot facility
for industrial production of microalgae at Mongstad, which will provide a basis for competitive
production of omega-3 for use in aquafeed. This should be done in close cooperation between re-
search institutions and the aquaculture industry. It is recommended that further work is being managed by the newly founded company, CO2Bio AS,
where the central industrial stakeholders are shareholders.

CO2 to Bio
Work Package 1 - Location
Ver. HK 280911
Prepared by Hans Kleivdal, Marie-Lise Schläppy, Friederike Hoffmann, Uni Miljø Svein M. Nordvik, Nordhordland Handverk- og Industrilag
Contents
1.1 Mongstad Industrial area _ 5
1.1.1 Mongstad _ 5
1.1.2 Mongstad oil refinery 5
1.1.3 CO2 Technology Centre Mongstad (TCM) 5
1.2 Available resources for exploitation in microalgae cultivation _ 7
1.2.2 Heat/steam 7
1.2.3 Cold seawater intake 7
1.2.4 Water discharge 7
1.2.5 Electricity _ 7
1.2.6 Sun light 7
1.3 Available area and infrastructure facilities 7
1.3.1 Infrastructure assets at Mongstad 7
1.3.2 Area available for pilot algae test facility _ 8
1.3.3 Area available for large-scale algae production facility 8
1.4 Climate data _ 9
1.4.1 General _ 9
1.4.2 Solar irradiation _ 9
1.4.3 Photosynthetical y active radiation (PAR) at Mongstad 9
1.4.4 Hours of daylight _ 11
1.4.5 Cloud cover and precipitation _ 11
1.4.6 Temperature 12
1.4.7 Conclusion on climate data 12
1.5 A biotechnological "moonlanding" at Mongstad _ 13 1.6 Challenges and recommendations _ 13
1.6.1 Integrating microalgae production with existing industrial activity _ 13
1.6.2 Area for microalgae production _ 14
1.6.3 Weather conditions _ 14
1.6.4 Low temperatures 14
1.6.5 Low solar irradiation values 14

CO2 to Bio/WP1 - Location
1.1 Mongstad Industrial area
1.1.1 Mongstad
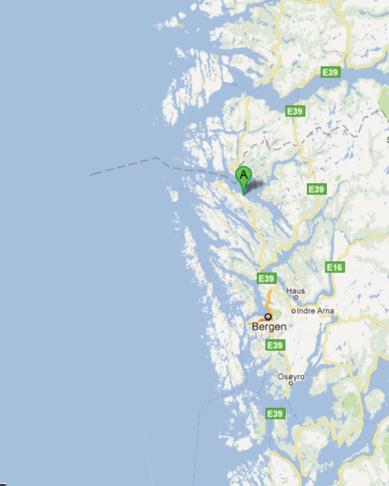
Mongstad (60°48'47" North, 5°1'14" East) is located on the south-west coast of Norway, about
65 km North of Bergen which is the second largest city in Norway (figure 1). Bergen is the ad-
ministrative centre of Hordaland County, recognized as the unofficial capital of Western Nor-
way. At Mongstad, the industrial site is located in the municipalities of Lindås and Austrheim.
The industrial site features an oil refinery for Statoil with port facilities1, with an unexploited
area in the South that is relatively flat and marshy, partly covered with heather and conifers.
1.1.2 Mongstad oil refinery
At Mongstad, Statoil has a crude oil terminal with a capacity of 10 million barrels/year2. The
port at the support facility Mongstad is the largest in Norway, measured in tonnage. The refin-
ery at Mongstad is modern, and has been extensively upgraded, with a capacity of 10 million
tonnes crude oil per year. The refinery is the largest in Norway, though medium sized by Euro-
pean standards. It is owned by Mongstad Refining, in which Statoil has a 79% ownership share
and Shell 21%. All the crude oil refined at Mongstad comes from the North Sea. The largest pro-
duction is petrol, diesel, jet fuel and light petroleum products. The heaviest components are
used to make petrol coke, an important ingredient in anodes for aluminum production. In 2010, Statoil and DONG Energy opened Mongstad power station, a natural gas-fired thermal
power plant, to provide the site with heat energy and electricity, as well as to the Troll gas field.
The station is owned and operated by DONG Energy, and will be integrated in the Statoil refin-
ery. The power station will have an installed effect of 280 MW in electricity production and 350
MW in heat. The energy will be used to operate the Mongstad Refinery as well to supply the
Troll Gas Field with power. The plant will use 0.7 normal cubic meter (BCM) gas per year. Emis-sions of CO2 will be up to 1.2 million tons.
1.1.3 CO2 Technology Centre Mongstad (TCM)
The CO2 capture at Mongstad is central to the Norwegian government's efforts to obtain tech-
nologies that can reduce emissions of CO 3
2 , in line with Norway's commitment to the Kyoto Pro-
tocol4. Development of technologies for CO2 capture is difficult and there will always be uncer-
tainty involved in the development of technologies from the research stage to industrial scale.
Fig 1: Mongstad Industrial site. A) Location of Mongstad, 65 km North of Bergen, on the south-west coast of Norway; 60°48'47"
North, 5°1'14" East, at 10 meters above sea level. B) Aerial photo taken showing Mongstad industrial site from South-East. The
Statoil oil refinery shown in the North (top) and the Mongstad support base in the South, surrounded by undeveloped industrial land.
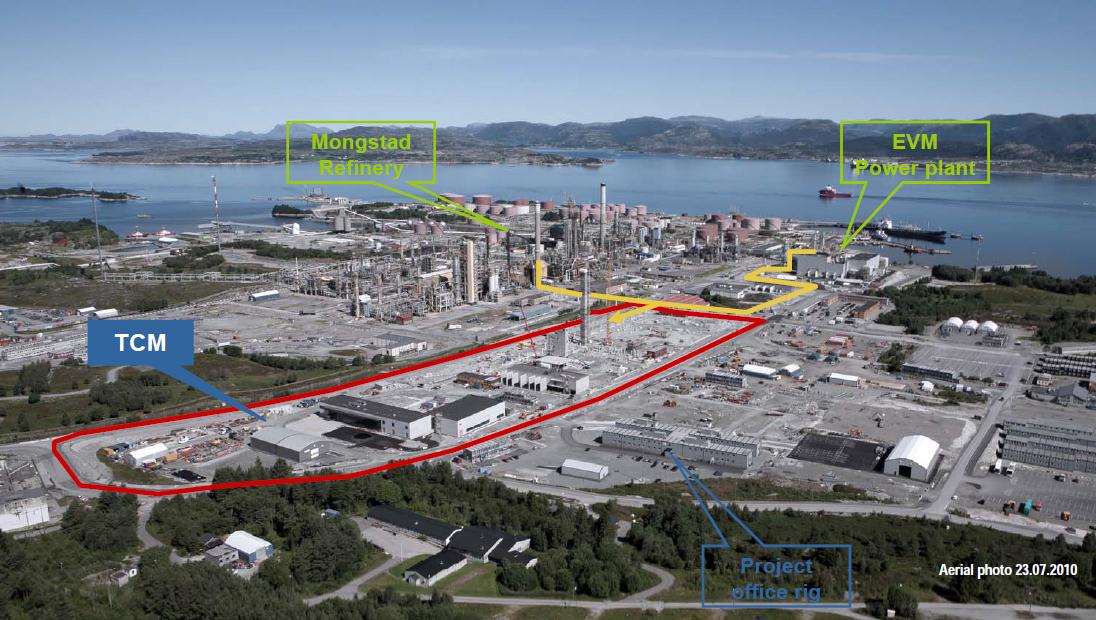
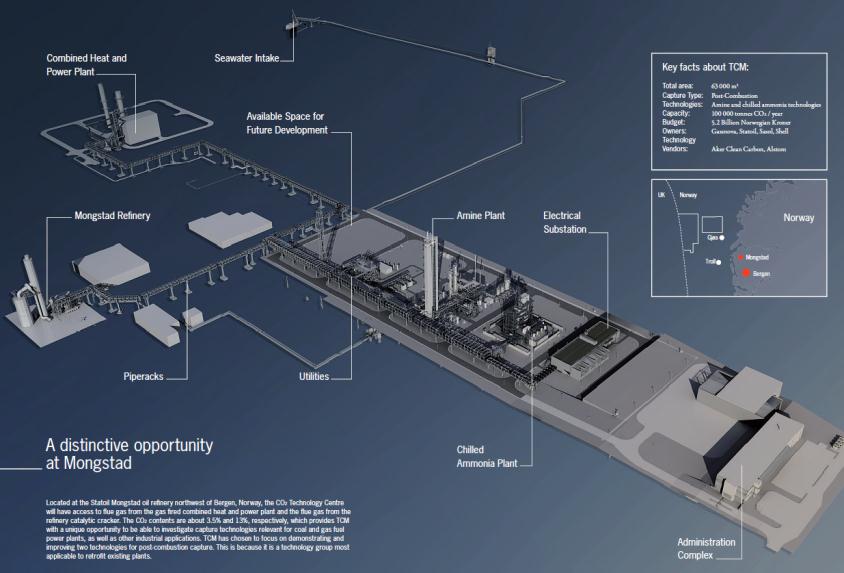
Fig 2: CO2 Technology Center Mongstad seen from the South. Location of the TCM facility receiving flue gas from both the
Mongstad oil refinery and the EVM combined heat and power station. Source
The aim of the Norwegian government with the technology center is to create a venue for target-
ed development, testing and qualification of the technology for CO2 capture. It also aims to con-
tribute to the international dissemination of these experiences so that the cost and risk of full-
scale CO2 capture can be reduced. In 2007 Statoil entered into an implementation agreement in cooperation with the Norwegian
State, to develop a test centre at Mongstad for test of carbon dioxide capture technologies. The
CO2 Technology Centre Mongstad DA (TCM DA) was established in 2009 with the main ambi-
tions being to test, verify and demonstrate CO2 capture technology owned and marketed by ven-
dors, and encourage the development of the market for carbon capture technology5. The CO2 Technology Centre Mongstad will be operational from 2012, and will have access to flue gas from the gas fired combined heat and power plant and the flue gas from the refinery catalyt-
ic cracker (figure 2). The CO2 contents are about 3.5% and 13%, respectively, which provides
Fig 3: Schematic view of CO2 Technology Centre Mongsta The construction of the two capture-technology facilities is close to
being finalized. The available space for future developments is indicated in the centre.
CO2 to Bio/WP1 - Location
TCM with a unique opportunity to be able to investigate capture technologies relevant for coal
and gas fuel power plants, as well as other industrial applications. TCM has chosen to focus on
demonstrating and improving two technologies for post-combustion capture. This is because it
is a technology group most applicable to retrofit existing plants.
The construction work is still ongoing, and recently (June 2011) the construction of the first CO2 capture facility was finalized (figure 3). This is the post-combustion capture technique using
amines, where Aker Clean Carbon is the provider of the amine unit. The second technology that
will be tested is based on separating CO2 from the exhaust gases using chilled ammonia as the
solvent to absorb the CO2. The Chilled Ammonia post-combustion technology is provided and
operated by Alstom.
1.2 Available resources for exploitation in microalgae cultivation
1.2.1 CO2
The TCM will test two different CO2 capture technologies with the deigned capacity to capture
approx 100 000 tons per year, where there is an agreement between NHIL and TCM to deliver up to 30 000 tons capture CO2 for development of CO2 utilizing technologies. The CO2 is esti-
mated to about 99,7% purity with differences in trace elements pending on the capture technol-
ogy used. TCM has applied for a permit to release captured CO2 in to the air until storage solu-
tions is in place.
1.2.2 Heat/steam
The TCM have access to steam from the oil refinery. The steam is delivered to the TCM facility in
pipes (figure 3),.
1.2.3 Cold seawater intake
The TCM have access to seawater from an intake at 40 meter below sea level (4-8°C) that will be
used as cooling water for TCM.
1.2.4 Water discharge
The seawater intake system also has the capacity to return seawater at 30 meter below sea level.
An important issue that requires consideration if pre-treatment is required before used sea wa-
ter drained from algal cultivation reactors can be emitted into the sea.
1.2.5 Electricity
The TCM have an own electrical substation delivering 10 MW to the test technologies, .
1.2.6 Sun light
The solar irradiation factor is dealt with in more detail in section 1.4 Climate conditions.
1.3 Available area and infrastructure facilities
1.3.1 Infrastructure assets at Mongstad
Infrastructure and location makes the Mongstad site particularly suitable for algal production
with supply of large quantities of pure CO2, steam and cooling water. Mongstad can be reached
by car in about 50 minutes from Bergen city. There are also good port facilities for transport of
biomass or algae based products to customers in Norway and abroad at the Mongstad base.
1.3.2 Area available for pilot algae test facility
The TCM facility has available space for future developments, and has set apart space for a pos-
sible third technology, or several smaller test facilities. Part of this area may be made available for a pilot algae test, with a possible direct access point to CO2.
1.3.3 Area available for large-scale algae production facility
There are large open areas of land around the refinery, which is suitable for algae production.
Lindås and Austrheim their new land-use plans for the Mongstad in 2010 arranged for nearly
3,000 acres of new commercial space. In addition, several hundred acres of undeveloped indus-
trial land is available between the oil refinery and the Mongstad base from the previous regula-
tions (figure 4).
Fig 4: Mongstad Industrial site – aerial photo from E. The Statoil Mongstad Oil Refinery is seen in N-W (top rigth), while the
Mongstad Base is seen from the S (bottom left). The surrounding area is largely exploitable for further industrial use.
CO2 to Bio/WP1 - Location
1.4 Climate data
1.4.1 General
The climate at Mongstad is coastal with fronts coming in from the North Sea mainly from the
West and North. The national weather stations with long-time registration of climate data clos-
est to Mongstad are the island of Fedje6 (17 km West of Mongstad) and the village Isdalstø (30
km South of Mongstad). Climate data of these permanent weather stations are available from
the Norwegian Meteorological Institute through their web resources. The production of microalgae at Mongstad will be affected by solar irradiation, temperature,
and precipitation.
1.4.2 Solar irradiation
The production of microalgae will be possible to regulate by the supply of nutrients and CO2 in
relation to the need for growth. The limiting factor is thus in practice the supply of light energy
for photosynthetic microalgae, and the growth rates can be directly proportional to the irradia-
tion intensity7. Only the solar radiation in the visible range from 400-700 nm is absorbed by microalgae pigments, and this wavelength range is therefore called the photosynthetically ac-
tive radiation (PAR). In addition, the irradiation angle is an important factor for the actual ir-
radiation intensity.
1.4.3 Photosynthetical y active radiation (PAR) at Mongstad
From the Photovoltaic Geographical Information System under the EU Joint Research Centre8,
the solar irradiation was calculated for Mongstad showing a strong seasonal variation, with the
lowest in December (124 Wh/m2/day on a horizontal plane) and the highest values in June (5150 Wh/m2/day on a horizontal plane). This is comparable to the base case of Norsker et al
(2010) for Eindhoven in the Netherlands.
Fig 5: Average monthly irradiation at Mongstad. Data, given in kWh/m2/day, calculated from the Photovoltaic Geographical
Information System under the EU Joint Research Centr 7 Norsker et al, (2010)
Fig 6: Height of sun at Mongstad through the year. Data calculated from the Photovoltaic Geographical Information System
under the EU Joint Research Centr
Table 1. Complex data set on the irradiation. Data calculated from the Photovoltaic Geographical Information System under
the EU Joint Research Centr
Hh: Irradiation on horizontal plane (Wh/m2/day)
Hopt: Irradiation on optimally inclined plane (Wh/m2/day)
H(90): Irradiation on plane at angle: 90deg. (Wh/m2/day)
Iopt: Optimal inclination (deg.)
D/G: Ratio of diffuse to global irradiation (-)
TD: Average daytime temperature (°C)
T24h: 24 hour average of temperature (°C)
NDD: Number of heating degree-days (-)
CO2 to Bio/WP1 - Location
Fig 7: Hours of daylight through the year. Data collected from the Gaisma websit
1.4.4 Hours of daylight
The times for sunrise, sunset, dusk and dawn for Bergen (80 km South of Mongstad) show that
there is a strong variation in hours of daylight during the year, with only 1 hour of darkness dur-
ing a daily cycle in June, and only about 6 hours of daylight in December9.
1.4.5 Cloud cover and precipitation
There were no data on cloud cover or hours of sunshine for the weather stations closest to
Mongstad. The precipitation data gives an indirect indication on cloud cover (figure 8), where the precipita-
tion is lowest in June (100 mm/month) and highest in September (275 mm/month) and
throughout autumn.
Precipi 100
Fig 8: Average precipitation at FEdje and Isdalstø through the year. The data points are calculated from monthly normal (aver-
ages from 1961-1990) by the Norwegian Met Institut
Fig 9: Average monthly temperature (red line) at Fedje July 2010 to June 2011. The black line indicates the normal temperature
for comparison10.
1.4.6 Temperature
The normal temperature (average of 30 years, 1961-1990) shows seasonal variations in a rather
small range, with lowest temperatures in January-February of 1-2 ºC and highest in July-August
of 13-14 ºC (figure 9). In the last 12 months, the highest temperature was measured in August
(23.5 ºC) and the lowest in December (-6.6 ºC) (figure 10). The annual average temperature is
Fig 10: Normal temperature (monthly temperature averages from 1961-1990) at Fedje and Isdalstø.
1.4.7 Conclusion on climate data
Due to the location high up in the northern hemisphere, irradiation shows a strong seasonal
variation. This is explained by the seasonal variation of sunrise and sunset with only 1 hour of
darkness during one day and night in June, and only 6 hours of daylight during one day and night in December. In addition the lower solar angle will decrease the irradiation intensity per
m2, compared to areas closer to the equator. Algae cultures will benefit from long hours of day-
light and high irradiance during the summer months. The seasonal temperature varies accordingly, but the total range in average temperature during
the year is low, varying between 2-13 ºC; the annual average temperature is about 7 ºC. The cli-
mate is generally mild, without extreme values. This is beneficial for maintaining stable temper-
ature conditions for algae cultures at the open air. Most precipitation comes in the dark autumn and winter period.
CO2 to Bio/WP1 - Location
Fig 11: Pilot versions of closed industrial cultivation systems. Examples of a horizontal photobioreactor (left) and a vertical
photobioreactor (right) where the microalgae are being circulated in the transparent cultivation tubes – with sunlight and CO2 as the
most important growth factors (source: AlgaePARC).
1.5 A biotechnological "moonlanding" at Mongstad
The Mongstad industrial area has unexploited resources that could benefit a new bioindustry
based on production of microalgae. The purity of the captured CO2 will be most beneficial to the cultivation of microalgae as a direct use of exhaust gases from combustion will also contain oth-
er components (NOx, SOx) that may have a negative effect both on growth effects and the final
product. A cost study from the Netherlands showed that CO2 may represent a significant por-
tion of operating costs - by up to 10% depending on the scale and production system (Norsker et
al, 2011)11. The exploitation of residual heat from cooling water can also be exploited to raise temperatures to the optimum growth temperatures around 20°C, and might also be used as an
asset in dewatering/drying procedures. The industrial infrastructure with port facilities on site, supports good transport alternatives. A large scale production at Mongstad will be based on closed cultivation systems (photobioreac-
tors) – with vertical tubular reactors being the most likely choice (figure 11). The establishment
of a pilot production facility would be the important first step towards an industrial scale pro-
duction of microalgal biomass.
1.6 Challenges and recommendations
This study of the Mongstad locality has been conducted to evaluate the feasibility of producing
microalgae in an industrial setting. Some challenges have been identified and recommendations
on how to meet these will be given below.
1.6.1 Integrating microalgae production with existing industrial activity
A large scale microalgae production facility will have to be connected to the current TCM pilot
facility and future large scale facilities for the supply of pure CO2. In addition, microalgae facility
will need a steady supply of heat/steam, cold seawater and freshwater. The handling of large
volumes of waste water will require suitable facilities for treatment and discharge.
Recommendation: The design and planning of a large scale facility must be conducted in tight
dialogue with current and planned industrial activities at Mongstad.
11 Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production — A close look at the economics.
Biotechnology Advances 29:24-27
1.6.2 Area for microalgae production
A large scale microalgae production facility based on photosynthesis will require large land are-
as around Mongstad industrial site. Recommendation: The area required will be determined through the pilot production phase, but
it is recommended to enter a dialogue with the municipalities and land owners (Statoil) at an
1.6.3 Weather conditions
The weather conditions at Mongstad can be harsh and windy, and can be challenging for outside
PBRs. In addition, snowfall and ice can cause damage to outside installations in general.
Recommendation: Production facilities should be inside a greenhouse to protect the PBRs from harsh weather conditions, low temperatures and photoinhibition.
1.6.4 Low temperatures
The average temperature at Mongstad is far below the recommended temperatures for an opti-
mal microalgae production (20-25°C).
Recommendation: The temperature should be kept stable at 20-25°C by keeping the PBRs in-
side a temperature controlled greenhouse, using steam/warm cooling water from the Mongstad
1.6.5 Low solar irradiation values
The photoactive radiation (PAR) values for Mongstad will be clearly the limiting factor, especial-
ly during the winter and autumn period.
Recommendation: The industrial production of microalgae should be conducted with the supply
of an artificial lightsource. The preferred option should be light emitting diodes (LED) to lower
CO2 to Bio
Work Package 2 – Production methods
Ver. 171111
Prepared by Hans Kleivdal and Marie-Lise Schläppy, Uni Miljø
Contents
2.1 Introduction 5 2.2 Open pond phototrophic algae production 6
2.2.1 Open pond advantages 6
2.2.2 Open pond disadvantages 6
2.3 Closed cultivation systems 8
2.3.1 Closed cultivation system advantages _ 9
2.3.2 Closed cultivation system disadvantages _ 9
2.4 Photobioreactor principles and main components 9 2.5 Photobioreactor system requirements 11
2.5.1 Light requirements _ 11
2.5.2 CO2 addition and pH control 12
2.5.3 O2 removal _ 12
2.5.4 Mixing _ 13
2.5.5 Nutrient requirements _ 13
2.5.6 Temperature control 13
2.5.7 Salinity control 13
2.6 Photobioreactor designs _ 14 2.7 Industrial process requirements _ 15 2.8 Comparison of microalgae production systems _ 15
2.8.1 Comparison of system productivity _ 15
2.8.2 Comparison of estimated production costs 16
2.9 The AlgaePARC pilot facility 17 2.10 Challenges and recommendations _ 20
2.10.1 Industrial microalgae production at Mongstad – initial assessment 20
2.10.2 Photobioreactor development – development of detailed R&D plan _ 20
2.10.3 Establish a pilot facility for industrial microalgae production at Mongstad _ 20
2.10.4 Collaboration with national partners 22
2.10.5 Collaboration with Prof Rene Wijffels and AlgaePARC at Wageningen University 22
CO2 to Bio/WP2 – Algae production
2.1 Introduction
Like plants, algae use the sunlight for the process of photosynthesis. Photosynthesis is an im-
portant biochemical process in which plants, algae, and some bacteria convert the energy of sun-
light to chemical energy. The photosynthetic algae can fix and convert inorganic CO2 into simple
sugars in the Calvin cycle using the captured ligh(figure 1). The simple sugar molecules
are then the starting point for the production of all the other molecules (lipids, proteins, carote-
noids etc) required for the algae to grow.
Fig 1: Simplified scheme of the photosynthesis.
Algae that can grow solely on light energy and inorganic CO2 are called photoautotrophes, while
the algae that obtain their energy from organic compounds and do not depend on light energy
are called heterotrophes. In addition, a group called mixotrophes is algae that can utilize both
these metabolism pathways. There are two main methods of phototrophic algae cultivation (table 1, figure 2):
Closed Photobioreactors
Table 1. Main types of photoptrophic microalgae cultivation systems.
Open cultivation
outdoor system
Shallow big pond
Tanks (circular or rectangular)
Deep (circular or rectangular)
Closed cultivation
covered systems
(pump or airlift)
Vertical column
(bubble- or airlift)
Panels/plates
Microalgae cultivation
Horizontal
Alveolar
vertical y
Fig 2. Main types of photoptrophic microalgae cultivation systems.
2.2 Open pond phototrophic algae production
Until the 1940s micro-algae culture was primarily conducted in the laboratory and in the late
1940s began the exploration of outdoor production systems (Terry & Raymond 1985). The in-
tent was to produce food and feed. Another area of interest was the ability of micro-algae to carry out gas-exchange either as an oxygen producer or a CO2 consumer. Prior do that, open
systems had been used in natural setting such as lakes, ponds and lagoons, in Myanmar, for ex-
ample, where there has been a long tradition of cultivating Spirulina sp. in natural open sys-
tems. Existing lakes, ponds and lagoons can be used as they are or the bottom can be lined with
asphalt, concrete, plastic sheets, rubber, or sprayed material to enhance the cleaning possibili-
ties (figure 3). Alternatively, artificial open systems can be constructed that fit the purpose of pro-duction better. Artificial open systems for microalgae cultivation are often made of concrete, lined
with plastic or tanks made of plastic. The vessels can take the shape of a pond or a tank. Raceway
ponds are the most common type of open cultivation method worldwide (Wolkers et al. 2011).
2.2.1 Open pond advantages
Open systems are recognised for being simple, easy to operate and inexpensive (low capital and
operating costs). They are easy to clean up after cultivation (Ugwu 2008) and low process con-
trol is needed. They are good systems for lower value products with large markets. This technol-ogy exists since the 1950s, so extensive experience exists on operation and engineering of race-
ways (Chisti et al. 2007).
2.2.2 Open pond disadvantages
Open systems suffer from a small illumination surface to volume ratio, so that the limited light pene-
tration only reaches the cells near the surface (Ugwu 2008). This results in a low photosynthetic
efficiency at about 1.5% and a low biomass density of about 0.1-0.2 g/l (Pulz 2001, Norsker et al.
2011, Wolkers 2011). Besides poor productivity, a large ground space is needed for such operations (Ugwu 2008). Also, only short cultivation periods are possible and only a few strains of microalgae
can be cultivated in such systems (Ugwu 2008). Additionally, there is a waste of resources as the
CO2 added to the culture escapes and ends up entering the atmosphere and evaporative losses are
large (Ugwu et al. 2008). The expansion of open systems is only possible in 2D, which is a problem
when space is limited or expensive. Since the system is open, there is a high contamination risk (Pulz
2001). A large amount of algae is needed to initially inoculate the pond and there is only a low level
of control over culture conditions (Ugwu 2008). There is possible contamination by heavy metal and by other algae species (Waltz 2009). Microalgae in open systems are subject to predators
(Waltz 2009) but this may be overcome by combining photobioreactors and open ponds. Algae
strain chosen for open systems must be able to cope with extreme temperature conditions and rain-
fall (Chaumont et al. 1993) and is absolutely weather dependant (Pulz 2001). Only a few algal varie-
ties will cope with this setup (Pulz 2001). The processing product harvesting costs are high because
of large volume of flows with a low cell density and concentration of biomass (Pulz 2001, Chen et al. 2009). It takes 6-8 weeks to produce microalgae again after an interruption (Pulz 2001), so batch
production is more common than continuous production.
CO2 to Bio/WP2 – Algae production
igh ) )for
Fig 3. Examples of open cultivation systems. Both natural and artificial cultivation systems are shown. The source is indicated
under each photo.
2.3 Closed cultivation systems
The disadvantages of open systems have led to the development of closed photobioreactors. In the
1970s microalgae cultivation for the production of methane stared in Europe (Terry & Raymond
1985) and both open and closed systems were investigated and the latter were deemed necessary for
the production of high value products. The design of photobioreactors that maximises productivity,
reduce capital and operating costs is not trivial and many designs have been developed (figure 4).
Fig 4. Examples of closed cultivation system designs. The source is indicated under each photo.
CO2 to Bio/WP2 – Algae production
2.3.1 Closed cultivation system advantages
The advantage of closed systems is the possibility to optimize and control the algal growth more
closely than in open systems. This can result in a much higher biomass yield and density (2-8 g
dryweight/l), and the harvesting process will become more efficient due to small volume of fluid
and the high concentration of algae (Pultz 2001). High algae productivity is possible (Waltz
2009) due to a large surface to volume ratio that increases the photosynthetic efficiency. Prod-
uct standardisation is possible because every element of the production can be controlled: CO2
supply, water supply, temperature, light exposure, culture density, pH, mixing regime. Closed
systems offer good control of CO2 transfer and helps minimal CO2 and culture medium loss. De-
pending on the design, closed systems require less ground area as 3D expansion is possible.
There is less contamination and no external predation on the microalgae. There is an insignifi-
cant dependence on the weather which makes it adequate for many algal species. After a pro-
duction interruption, only 2-4 weeks are needed to have fully functioning system again, and in
case of self-cleaning designs, no production interruption is necessary. Recently-developed self-
cleaning systems allow less fouling than in open and early closed systems and allows continuous
production. Unlike open systems, closed systems can be tuned to avoid photoinhibition. Overall
they are better systems for high value products (such as pharmaceuticals and animal feed). If
genetically modified microalgae were to be produced, the use of closed systems would be abso-
lutely necessary to protect the environment (Waltz 2009).
2.3.2 Closed cultivation system disadvantages
Closed systems are far more complex than open ponds and have therefore higher capital and
operating costs than open systems (Waltz 2009). This is the major disadvantages for closed
cultivation systems compared to open ponds. Productivity and production cost are not always
better than open systems so the end product usually determines the type of production unit cho-
sen. Continuous production requires very fine tuning of all the elements to prevent a collapse of
the culture. There are technical difficulties in sterilizing the culture, which makes it problematic
when targeting a specific product. In order to achieve better photosynthetic efficiency, higher
mixing and installation costs are incurred. There still are difficulties associated with the control
of gradients of pH, oxygen removal, fouling and wall growth which is difficult to control but
some designs have been invented to solve those problems. Additional challenges involve the
hydrodynamic stress experienced by the algae (Chen et al 2009) which might lower production
and can make scale up problematic (Waltz 2009). For very high-value products artificial illumi-
nation is often used (Chisti et al. 2007).
2.4 Photobioreactor principles and main components
All photobioreactors are built in similar fashion: a man-made vessel holds the algal culture
which is composed of water, algae CO2 and nutrients. The design principle of a typical tubular
photobioreactor system is shown in figure 5, with the main components listed in table 2. From
the mixing or feeding vessel where the CO2 and nutrients are added, the algal solution enters a
series of pipes of various designs but which ensure adequate amounts of light, and minimises
hydrodynamic stress for the algae. The photobioreactor itself is used to promote biological
growth by controlling environmental parameters including light. The tubes are made of acrylic and are designed to have light and dark intervals to enhance the growth rate. To ensure the
highest possible productivity of microalgae the following factors have to be controlled in the
photobioreactor: light, nutrients, CO2, mixing, culture density, pH, temperature, and flow rate.
Each of these elements will have to be optimised at each step of the scaling up process. Optimi-
sation will be necessary to avoid culture crashes. The composition of the culture medium is not trivial and differs depending on which alga is be-ing cultivated. After the algae have completed the flow through the pho it passes
Fig 5. Design principle of a tubular photobioreactor system. Most photobioreactors differs in the design of the transparent
vessel, which in this example would be closed loop glasstubes with a diameter between 3-5 cm. The light system can be based on
either natural or artificial light – or a combination of the two.
back to the feeding vessel. As it progresses through the tubes, oxygen sensors continuously
monitor how much oxygen has built up in the plant and this oxygen is to be removed (stripped or
degassed) in connection with the return into the feeding vessel. It is also at this stage that an
optical cell density sensor determines the growth rate over time.
Table 2. The main components of a photobioreactor system.
Main components
General function
1. Light system
Ensure sufficient and effective sunlight
Artificial light source
or artificial light
Avoid too much light that can lead to
Sunlight covering system
2. Optical transmission system
Transparent vessel with high surface to
Different designs exist
volume ratio to allow even light distribu-
3. Gas exchange system
Effective control and removal of
O2 and CO2 sensors
O2 release system
Correct addition of CO2
pH sensor CO2 injection valve
4. Mixing system
To ensure even cell density and distribu-
Recirculation pump
tion of CO2 and nutrients
5. Nutrient system
Continuous addition to ensure optimal
level of nutrients
Nutrient pump Water inlet valve
6. Instrumentation system
Ensuring optimal flow
7. Controlling system
Monitoring of critical parameters and
Conductivity sensor
Temperature sensor PLC Control panel
CO2 to Bio/WP2 – Algae production
When the algal density have reached it optimum, a fraction of the total growth volume is tapped
for harvesting and new water and nutrition is added to replace the harvest volume. In some pho-tobioreactor systems the separated water is passed back to the cultivation system again, while
others discharge the water (supernatant) after the algal biomass have been dewatered. When harvesting algae from a photobioreactor, the dry weight of microalgae is approx. 0.5-3.0 g
of algae per L of media. There are several ways of harvesting. Centrifugation is the most effec-
tive separation method, but is also the most energy demanding dewatering technology. Accord-
ing to Chang et al. 2009 belt harvest system based on advanced membrane or flocculation are the best candidates. Some microalgae may also be induced to flocculate (self-aggregate) by using
coagulants (iron, alum, lime, cellulose, salts, polyacrylamide polymers, surfactants, chitosan,
and other man-made fibres) in order to increase harvesting efficiency. Harvesting and dewater-
ing techniques will be dealt with in another report under this project (WP4 – Biomass).
2.5 Photobioreactor system requirements
The key to success for industrial algal production is to maintain all cultures in the exponential
phase of growth (Lavens and Sorgeloos 1996). The specific growth rate is mainly dependent on
algal species, light intensity and temperature. Furthermore, cell division slows down when nu-
trients, light, pH, CO2 or other physical and chemical factors becomes limiting. In order to
achieve a highly efficient production with maximum growth rates, the optimum culture condi-
tions must be understood and controlled. Optimum culture conditions, in terms of high growth rates, are, however, not necessarily the same as optimum conditions for directing the metabolic pathways – for example towards in-
creased omega-3 production. A compromise between the nutritional quality and growth kinetics
will often have to be considered. For instance, it has been shown that the essential fatty acid,
eicosapentaenoic acid (EPA), increase with decreasing light (Sukenik et al. 1989).
2.5.1 Light requirements
Microalgae are photosynthetic organisms; they assimilate inorganic carbon and transform it to
organic matter. Light is the energy source that drives this reaction. The consideration of the light intensity, photoperiod and spectral quality (see pigments and wavelengths) will contribute to
develop an overall light regime in a photobioreactor. After identifying the type of algae culture to
be grown, it is important to identify the right type of light source with appropriate wavelengths
in order to achieve a high level of photosynthetic efficiency. The light requirement for algae is
dependent upon the major pigments present in the algal cell. Chlorophyll a, b and c ab-
sorb/harvest specific regions in the photosynthetic active region (PAR) of visible light. The biomass density will also affect the light intensity and light penetration through the culture
medium (Richmond, 2004). Optimal cell density is specific to each strain and needs to be main-
tained in order for light intensity and light penetration to remain at optimal levels (Kunjapur,
2010). In practical terms, this will lay restrictions on the design as to decrease the light penetra-
tion pathway to only a few centimeters into the algal culture. In addition to the importance of
the photobioreactor design to lower the surface to volume ratio, the mixing regime is also an
important factor to ensure that the algae receive the right amount of light.
Natural sunlight
Since light is the critical factor to achieve a high biomass and production efficiency by photo-autotroph microalgae, the use of natural daylight as the only light sources will the limit pro-
duction efficiency strongly during the winter months and periods in the spring and autumn. On the other hand, longer days and lower light intensity (less photoinhibition) may prove to
be an advantage in the summer. However, it is reasonable to assume that a full-year large-
scale production at Mongstad must be operated with a combination of daylight and artificial
Another option is to pause the algal production in the darkest months (Nov, Dec, Jan, Feb),
as these months represent only 5% of the total annual solar irradiation in southern Norway. A second option is to use a microalgal species suitable for mixotrophic growth, like Phaeo-
dactylum tricornutum and numerous other species, where the addition of glycerol may con-
tribute to increased EPA levels (Ceron Garcia et al, 2006). Such an exchange can thus be used
to "replace" the photoautotrophic production in low light conditions and to drive the metabo-
lism in a desired direction.
Artificial light
When using an artificial light source to replace or complement natural sunlight, the efficiency
of converting electricity into light must be considered. Light systems used for terrestrial plant
and algae production have often been based on high pressure sodium lamps1, but there is also an ongoing development of low-energy solutions using light-emitting diodes (LED)2. Since
LEDs are the most efficient light source for converting electricity into light with the desired
wavelength, they should be given high priority for use. However, LEDs do not produce light
in a broad white light spectrum which may make it necessary to use a combination of light
sources or combination of LEDs.
Light intensities
The intensity of a light source gives the number of photons that are available for the photo-
synthetic process. The energy associated with photons with a wavelength of 680nm is the en-
ergy level required by chlorophyll a to initiate photosynthesis, and most of the visible light
has sufficient energy to support photosynthesis. However, if the wavelength is small the en-ergy associated with the wavelength is high. Light intensity plays an important role, but the
requirements vary greatly with culture depth and cell density. At high depths and densities
the light intensity must be increased (100-200 µmol m-2 s-1 is often required for large vol-
umes). On the other hand, too high light intensities may result in photoinhibition.
Since naturally grown algae have dark times (nighttime), it is assumed by many researchers
that dark periods are required. Several studies report dark periods from a few milliseconds to
several hours with a positive effect on the photosynthetic effectivity (Jansen, 2003), but there
is no consensus on what an appropriate light:dark cycle or photoperiod should be. Long dark periods generally results in biomass loss as well as a decline in growth rates, because the al-
gae undergo photorespiration and consume oxygen and carbohydrates (Molina et al. 2001,
Merchuk and Wu 2003).
2.5.2 CO2 addition and pH control
The addition of CO2 must be carried out in such a fashion as not to change the pH of the culture
dramatically. The algal cells will assimilate the dissolved CO2 and transform it into organic mat-
ter. However, if the growth rate is low and cells do not remove the CO2 at the expected rate, the increasing level of dissolved CO2 will eventually decrease the pH in the culture medium. The pH
range for most cultured species is between 7 and 9, with the optimum range being 8.2-8.7 (Lav-
ens and Sorgeloos 1996). Culture collapse can be the result when failing to keep an acceptable
pH. The CO2 originating from air ( 0.03%) is limiting growth when bubbled through a dense
culture. Pure CO2 may then be supplemented to the air supply usually at a rate of 1-2% of the
volume of air. CO2 addition furthermore buffers against pH changes. CO2 may also be supple-
mented to the inflow of seawater into the cultures (e.g. Jacobsen et al. 2010).
2.5.3 O2 removal
A high presence of oxygen around algae cells is undesirable. High oxygen concentration results
in photooxidative damage to algal cells, so the oxygen concentrations should be maintained be-
1 Gavita 2 Lumnigro
CO2 to Bio/WP2 – Algae production
low 400% of air saturation value (Chisti, 2007). This will also limit the length of tubular reac-
tors, and challenge scale-up. However, in closed loop systems the culture will be lead through an airlift zone where the accumulated oxygen from photosynthesis is stripped by air/degassed
before the O2 stripped cultivation medium returns to the transparent photobioreactors. Optimi-
zation of the degassing is an important feature to improve the design process.
2.5.4 Mixing
The level of mixing strongly contributes to algal growth in two primary ways;
i) to improve productivity by increasing the frequency of cell exposure to light and dark vol-
umes of the reactor
ii) by increasing mass transfer between the nutrients and cells.
In other words, the mixing of the culture medium attempts to distribute radiation evenly to all
cells in the culture and reduce diffusion barriers around the cells (Jansen, 2003). However, the
level of mixing has to be optimized carefully because high levels of mixing will result in cell
death from shear stress. Shear stress is tolerated differently between algal species and strains,
and should be taken into consideration when selecting the algae to be produced. There are several mixing techniques like low-shear pumps and air-lift systems allowing com-
pressed air to be sparged into the bottom of a reactor, but the optimal solution will depend on
the photobioreactor design principle. Mixing is the most energy demanding factor during closed
system production, and contribute to a significant increase of the operational costs.
2.5.5 Nutrient requirements
Cultures of microalgae must be enriched with nutrients in order to sustain growth. Macronutri-
ents include nitrate, phosphate and silicate. Silicate is mainly used by diatoms, which utilize this compound for production of an external cell covering. Micronutrients consist of various trace
metals (Zn, Co, Cu, Mo, Mn, Fe) and vitamins (thiamine, cyanocobalamin and biotin). Two en-
richment media are commonly used for growth of algae in aquaculture; Walne medium (Laing
1991) and f/2 medium (Guillard 1975). However, the complexity and cost of media excludes
their use for large scale production. Alternative enrichment media for large scale production are
often composed of agriculture-grade rather than laboratory-grade nutrients, and they often con-
tain only the most essential nutrients (Superba Rød3).
2.5.6 Temperature control
Optimum temperature for most microalgae (temperate and sub-tropical species) used is gener-
ally between 18 and 24°C. Many of the cultured species tolerate temperatures between 16-27°C.
Temperatures lower than 16°C will most likely result in slow growth, while temperatures above
35°C will lead to culture collaps (e.g. Acien Fernández et al. 2003). In order to keep stable tem-
peratures, cultures can be cooled down by flow of cold water over the surface of the cultures or
by controlling the air temperature with refrigerated air. The challenge of maintaining both a system that can be used for heating during winter and cooling during summer, can be met by
using a temperature controlled greenhouse – or by keeping a strict control of the temperature in
the feeding vessel.
2.5.7 Salinity control
Marine microalgae are in general tolerant to changes in salinity. In culture, most species grow
best at a salinity that is a bit lower than found in their native habitat (diatoms at 20-25 ‰ and
flagellates at 28-30‰). This can be obtained by diluting sea water with distilled water (Lavens and Sorgeloos 1996).
Fig 6. Examples of different closed reactor design principles. a) conceptual tubular reactor. b) conceptual column reactor. c)
conceptual flat panel reactor. Figures collected from Kunjapur (2010).
2.6 Photobioreactor designs
Beyond the surface area and volume, the unique geometry of a photobioreactor influences the
light distribution, which will, together with the design-dependant mixing method, determine its photosynthestic efficiency and productivity. The design of the transparent cultivation vessel dif-
fers between the three main types of closed photobioreactor types, as shown in figure 6. Each of
the reactor types have their benefits and disadvantages (table 3), and it is not possible to say
which system will be the best option in general.
There are several factors that will determine what the best PBR solution is for a particular pur-
The product - which should be on the market (value, volume, market development, side
The algae - which are best to produce this product (and possibly by-product).
The conditions of production - solar / light, temperature, availability of CO2, nutrients,
batch versus continuous, limiting factors
The volume - requirements for scale and production
Maintenance - convenient in FHT cleaning, material life, etc.
Costs - both capital costs and operating costs will be decisive.
With this background, the whole production system had to be tailored for a given product, the
comparison of different PBR system must be part of a pilot or planning stage.
Table 3. Typical advantages and disadvantages of the three main types of closed photobioreactors (Kunjapur, 2010).
Reactor type
Typical advantages
Typical disadvantages
Flat panels
• shortest oxygen path
• low photosynthetic efficiency
• low power consumption
• shear damage from aeration
• high volumetric biomass density
• oxygen accumulation
• photoinhibition
• most land use
Vertical
• greatest gas exchange
• support costs
• best exposure to light/dark cycles
• least land use
• high photosynthetic efficiency
CO2 to Bio/WP2 – Algae production
2.7 Industrial process requirements
Algae@work4 recognizes ten essential requirements for algal cultivation to become a viable
mode of CCR (carbon capture and recycle). A high degree of control yet flexibility is required in
modes of cultivation and harvesting. Uninterrupted production is necessary over long periods of
time and the photobioreactors must be designed to ensure high productivity, profitability and
industrial relevance. The production cannot be stopped by infections (bacteria, virus and other
microalgae) and those threats to production must be managed successfully to ensure a steady supply of the product. Photobioreactors must often be cooled or heated and the efficient man-
agement of water and energy must be achieved. Once the production has been started and is
successful the operations must be scaled up in a sustainable way, and there must be enough
space to ensure the possibility of expansion. Crop value, length of productive season and indus-
trial reliability must be obtained through high technological advances in photobioreactors de-
velopments. Cellular re-suspension must be obtained to ensure access of algae to nutrients, limit cell death and bacterial growth leading to system crashes. Bacterial biofilms growing on the
pipes of the photobioreactors can reduce the access of algae to light and also start bacterial in-
fections. Biofilms management must thus be carried out with high efficiency. The only manner
in which the production of microalgae will be of significance in CCR, is to ensure high industrial
efficiency as many upstream and downstream processes will depend on it when operating at full
scale. The planning of a microalgae production for the purpose of CCR must fall in line with the
goal of regulatory agencies, framework, funding agencies, lending banks etc to be politically de-ployable.
2.8 Comparison of microalgae production systems
The comparison of microalgal production system described in the literature is not an easy task,
because the cultures have been grown under slight different conditions. Several condition pa-rameters will have an effect on the productivity – not only the system design alone. The present
photobioreactor designs have not reached its full optimization potential wrt increasing the pho-
tosynthetic efficiency - defined as the fraction of light energy converted into chemical energy
during photosynthesis (theoretic maximum at 9%). The main focus is to keep costs and energy
demand low during production and increase the photosynthetic efficiency and biomass yield. However, the overall optimization potential of the photosynthetic efficiency lies not only on the
production systems, but there is also a promising potential in the screening and development of production-efficient algal strains, in combination with exploiting physiological properties
through metabolic stress until harvest.
2.8.1 Comparison of system productivity
When searching the literature about the productivity of various microalgae production systems
the difficulty is to obtain estimates in the same units; a summary is provided of the most com-
monly used units to measure production capacity per area (Table 4). Open raceway ponds can
have productivities as low as natural lakes, but as high as flat horizontal photobioreactors. Among the closed photobioreactors vertical columns and horizontal tubular photobioreactors
score the highest.
However, one should keep in mind that a true comparison should be performed side-by-side
under similar conditions in order to be valid. Different growth conditions will affect the out-
come, as will the production scale at which the cultivation studies have been performed. An increasing light intensity will increase the productivity up to a certain point where oversatu-ration and photoinhibition occurs. Cuaresma et al (2011) showed how the productivity increased
with a vertical orientation compared to a horizontal system under light conditions simulating
daily light cycles in southern Spain. The strong irradiance at 1800 umol photons m-2 s-1 on the
4 A2BE Carbon Capture
Table 4. Production efficiency and capacity of various cultivation systems.
Cultivation type
Production capacity
References
efficiency (PE)
ton dry weight/ha/year
Raceway pond
horizontal
Vertical column
Flat panel
horizontal reactor could not be exploited properly due to oversaturation, but with a vertical position-
ing the incident light were reduced to a level (400 umol photons m-2 s-1) where the productivity was
high. This may not be the case for Norway where solar irradiation may not have to be diluted.
2.8.2 Comparison of estimated production costs
The research group of Rene Wijffels at AlgaePARC (see next section) focus strongly on the eco-
nomics of microalgal production. In a recent study, Norsker and co-workers compared the pro-
duction costs of three photobioreactor systems; open pond, tubular bioreactors and flat panel
bioreactors (Norsker et al, 2011). The photosynthetic efficiency and productivity were signifi-
cantly higher for the flat panel bioreactors, with values about 30% higher than those of the tubu-
lar system (table 4). However, while the flat panels were more productive, this production system also proved to be
more costly compared to tubular bioreactors when calculating the production costs (figure 7).
The critical cost contributions (underlined) for both reactor systems are the mixing instrumen-
tation to ensure mass transfer and oxygen removal. While the tubular systems use low-shear
pumps, the flat panels use airflow with an energy demanding screw blower. The production
costs for algal biomass produced with tubular bioreactors was calculated at 4.16 € per kg dry
weight, compared to the flat panels at 5.96 per kg dry weight (table 5).
Fig 7. Unit biomass production cost (in cts, eurocents). Based on various capital and operating cost elements for raceway
ponds, tubular photobioreactors and flat panel photobioreactors. Table view from Norsker et al (2011).
CO2 to Bio/WP2 – Algae production
Table 5. Sensitivity analysis. Biomass cost in € per kg/ with different scenarios (100 ha plant).Source: Norsker et al. (2011).
Flat panel
(€/kg DW)
(€/kg DW)
(€/kg DW)
1. Base case
2. Bonaire location
3. Minimum mixing
4. CO2 is free
(in addition 3)
5. Medium is free
(in addition to 4)
6. PE increase 60%
(in addition to 5)
7. Bonaire location
(in addition to 6)
Norsker and co-workers also performed a sensitivity analysis of the effects of ongoing and fore-seen improvements regarding reduction of mixing costs, improvement of irradiation and photo-synthetic efficiency, free CO2 and reduction of nutrient costs (table 5, points 3-6). These are pro-
duction system improvements considered realistic by the authors in short term (about 10 years
from now). Under these premises, the production costs for algal biomass produced in outside
tubular photobioreactors in the Netherlands was calculated to 1.43 € per kg dry weight, and 1.44
for production in flat panels. In addition, the study also investigated how the light conditions and productivity in the Nether-lands compared with microalgal production at a tropical site (Bonaire at the Dutch Antilles),
and how that would affect the biomass production costs. The simulated production in a tropical
site with much sunlight would drive this down to 0.70 € per kg dry weight – about half the cost
compared to a production relying on natural sunlight in the Netherlands. This is a good indica-
tion of what impact light conditions have. It may also indicate the potential benefits of in-
creased productivity when using optimized artificial light systems, and how it can affect the final
biomass cost level. The technology development will keep driving the productivity up and production cost down -
and as the maturity level is not reached at the moment, it is too early to estimate the area re-
quirements for a given product volume. However, is important to keep working with the existing
production systems and participate in technology drive and system development.
2.9 The AlgaePARC pilot facility
The cultivation capacity of microalgae is relatively small and inefficient and there experience
with large-scale, cost-effective production. Therefore, Wageningen UR, together with industry
partners investigate the optimization of algae production in outdoor systems at the recently es-
tablish Algae Production And Research Centre (AlgaePARC). At the AlgaePARC facility teams of
scientists study various aspects of algae cultivation. To make the production of algae competitive
at the bulk products market a strong economic and technological boost is needed5.
5 Source: AlgaePA
Below is an excerpt from the report "Microalgae: the green gold of the future?" describing the
research aims and activities planned at AlgaePARC6:
"Research in AlgaePARC The production costs of algae cultivation must be decreased drastically, to
one-tenth of the current level. Increasing the photosynthetic efficiency is one of the most important stipulations. This can be achieved by applying improved
reactor designs and use more efficient algae. In addition, a substantial saving on nutrients becomes possible by making use of waste and residual flows and
recycling of these nutrients. Furthermore, a considerable reduction of energy
consumption can be reached by means of mixing the algae soup less and the use of energy-efficient pumps. Also better harvest and downstream
processing methods (biorefining) can significantly contribute to reduce costs, but also to improve the final product.
For example, conventional methods to isolate oil from algae cells are quite harsh, i.e. high pressure and temperature disrupt the cell wall causing the oil
to be released. However, because of the harsh conditions the proteins will
denature resulting in a lower value of the biomass. Therefore, mild biorefinery techniques to isolate the algae products are necessary. Finally, shifting the
cultivation to sunnier locations might contribute to a higher efficiency and substantial cost reduction. AlgaePARC must be a bridge between small-scale laboratory research and large-scale production. The research team will verify the results of the small scale
experiments on larger scale in AlgaePARC. In addition, the team will
compare the four major algae cultivation systems regarding costs, growth efficiency and sustainability. An open pond will serve as reference as it is the most common cultivation system worldwide. The researchers will compare the performance of closed
systems, for example, horizontal layers, vertically transparent tubes, flat
plates and a raceway pond, throughout the year. Each cultivation system has specific advantages and disadvantages, but the ultimate goal is maximum
production of high quality algae at the lowest possible price throughout the year. Besides the type of cultivation system, it is also important to design the cultivation conditions in such a way that an optimal production of the desired
algae product, e.g. oil, is obtained. Traditionally the algae are grown until a
certain density. Subsequently they are starved from nutrients; the algae stop growing and start to produce more oil. Usually these two processes are run in
sequence: first the amount of biomass is increased rapidly, and then the oil starts to accumulate slowly. In AlgaePARC the goal is to design a process
aimed at the optimal production of certain metabolites (such as oil or starch) and not of biomass. Because production conditions will continuously change,
production strategies that are aimed at a constant quality of the final product
will be designed, by means of measuring and controlling.
The test facility is just a start of what should become the leading algae testing
centre of Europe. Particularly research and development of methods aimed at making specific end products will be further expanded in the coming years.
Not only reactor design will get attention, but also the search for new algae
species and improvement of existing algae strains by genetic modification.
In addition, there will be a lot of attention for improved biorefinery methods
and sustainability of the entire production chain. Wageningen UR wants to do innovative research on algae cultivation technologies in collaboration with
6 Wolkers et al, (2011)
CO2 to Bio/WP2 – Algae production
other knowledge institutes and industry within the Netherlands and abroad.
This can be both fundamental and applied research. It is also important that
the research on algae is an inspiring learning environment for students. AlgaePARC will be a success if after 5 years we:
Are able to make a good comparison of different production systems
based on the following parameters: photosynthetic efficiency, volumetric productivity, energy use, use of nutrients and water availability,
robustness and scalability
Have achieved and maintained throughout the year, a photosynthetic
efficiency on sunlight outdoors of 5%
Have developed an improved reactor concept and/or process strategy in
which the production costs and energy needs are lower compared to traditional systems
Have obtained sufficient basic information for the design of a large-scale
production facility
Worldwide research on algae is emerging. Companies and governments invest a lot in algae research programmes. In the United States there are large
projects in the field of genetic modification of microalgae, China does a lot in
bioinformatics of algae and in Europe scientists will realise the first demonstration facilities within some years. Wageningen UR distinguishes itself from these activities by working in an integrated, multidisciplinary manner on the improvement of the technology.
This means working at the same time on the improvement of species, on development of efficient production methods and on biorefinery- and
sustainability aspects."
Fig 8. Outdoor facilities at the AlgaePARC seen from the NW. The different pilot production systems are compared side-by-side.
From the left; the vertical tubular reactors, the horizontal tubular reactor, a flat panel system shown in the front and the reference
raceway pond in the far right. Source
2.10 Challenges and recommendations
The main challenge is to reduce investment and production costs by lowering the energy de-
manding steps and increase the photosynthetic efficiency and biomass yield. However, it is important to stress that the overall optimization potential of the photosynthetic
efficiency lies not only on the production systems described in this WP-report. There is also a
promising potential in the screening and development of production-efficient algal strains, in
combination with exploiting physiological properties through metabolic stress until harvest (WP3). In addition, the downstream processing and logistics solutions along the value chain
through to the finished product will be crucial for the cost picture (WP4).
Based on this, some recommendations on how to meet the challenges at this stage is given below.
2.10.1 Industrial microalgae production at Mongstad – initial assessment
Our initial technical assessment is that Mongstad is a suitable location for industrial microalgae
production. Based on this report – together with the reports on WP3 and WP4, some recom-
mendations can be made at this stage for the pilot facility.
Recommendations:
The production should be based on closed photobioreactors.
The best performance and low-cost tubular and flat panel reactors should be compared.
The production area should be covered by a greenhouse.
The production areas should be temperature controlled.
The production should be based on artificial light as a supplement to natural sunlight.
The production area should be flexible to allow development and comparison of photobioreactors.
The pilot facility should establish a photobioreactor similar to an AlgaePARC type as reference.
A water treatment facility is required.
A biomass harvesting facility should be located on site to ensure sustained biomass quality.
A fully equipped laboratory is required for research and maintaining cultures.
2.10.2 Photobioreactor development – development of detailed R&D plan
There are currently no photobioreactor systems that are suitable for large scale production of
microalgae intended for the bulk market. Although the free access to pure CO2 will contribute to
lower the production costs, a technology improvement is required. This will be possible through
a focussed effort together with the University of Bergen, as well as national and international collaborators. However, this is a complex field and this report could not cover a complete review
to evaluate all the efficiency of all methods and technologies. Therefore, an R&D strategy and
detailed plan should be developed to maximize the efficiency of the future research efforts and
reduce the risks at an early stage.
Recommendation:
Conduct in-depth study on the most promising bioreactor systems.
Develop an internal strategy and plan on how to address the R&D challenges. This task should
be conducted immediately together with current partners – and be integrated in any funding proposal where required.
2.10.3 Establish a pilot facility for industrial microalgae production at Mongstad
Only active in-house research and technology development will drive production cost down.
Although several promising technological solutions are described, the field is not yet mature and
requires optimization. It is therefore important to compare technological alternatives at an ac-
tual pilot facility under relevant conditions and in a relevant scale. This will also enable optimi-
zation of a customized production system adapted for the specific algae and target endproducts. The pilot facility at Mongstad will benefit much from a close collaboration with the AlgaePARC
pilot facility, with the same focus on the technology development towards more cost-efficient
production. With a pilot production facility in place, the focussed R&D activity could be funded
by public/private agencies in collaboration with selected national and international partners.
CO2 to Bio/WP2 – Algae production
Fig 9. Proposed pilot with support building and reference PBR set-up. The draft floor plan showing the 180 m2 greenhouse to
the right, with the 90 m2 solid support building to the left. The production area inside the greenhouse will allow flexibility and simulta-
neous comparison of up to 4 medium to large photobioreactors. The water treatment facilities will handle all the water before it is
used for cultivation, and the harvesting facility will high biomass yield from the ongoing cultures. The laboratory will be fully
equipped to handle stemcultures, culture start up and upscale.
Recommendation:
Establish a pilot production facility for industrial microalgae production systems at
Mongstad, to benefit from pure CO2, residual heat and access to cold seawater in sufficient
amounts (figure 9).
The ultimate aim of the pilot production facility will be to pilot production facility is to devel-
op a defined PBR system suitable for full-scale production of microalgae biomass suitable as
ingredients in aquafeed. The budget for the pilot facility is specified in WP5.
The pilot should be organized in four phases over 3-4 years, following the proposed project
Pilot phase 1 – Planning phase
Aim: Design and plan the construction of the pilot facility.
In the planning phase, we will involve all relevant partners, collaborators and sub-contractors,
for the design and construction planning of the pilot facility. This is an important process that
will set the operational framework for the pilot facility, and must therefore involve interna-tional experts in the field that can foresee future challenges and the level of flexibility required
in some areas.
Deliverables: Complete plans for design and construction with estimated cost calculations.
Pilot phase 2 – Pilot facility construction and establishment
Aim: Construction of greenhouse, laboratory, support facility and reference PBRs.
The first implementation phase will focus on the construction of a greenhouse, and adjacent
buildings with laboratory and facilities to support operational pilot PBRs. This phase will also
include the installation of a reference vertical stacked PBR (24 m2) comparable to the AlgaeP-ARC version, and a smaller PBR unit (2,4 m2). Once these units have become operational, they
will be important standards to compare and evaluate the production parameters at the pilot fa-
cility with those at the Algae Park.
Deliverables: Established pilot facility with operational base-case PBRs.
Pilot phase 3 – Research and development of low-cost PBRs
Aim: Develop low-cost PBRs
The third phase will be the first research and development stage where alternative low-cost pro-
duction solutions will be subject to feasibility studies in pilot scale (2,4 m2 up to 24 m2). The al-ternative PBRs will be designed in collaboration with partners and tested under relevant condi-
tions with selected algae to evaluate their cost-effectiveness. In parallel, the challenges of de-watering and biorefining for the aquafeed market will be addressed. Overall, these feasibility
studies will enable better estimations of production costs under the given conditions at Mongstad. The outcome of this phase should be the selection of one clear candidate PBR considered fit for
purpose from a decision process based on predetermined production performance parameters
and product-specific criteria.
Deliverables: Defined PBR system qualified for up-scale studies
Pilot phase 4 – Up-scale of selected low-cost PBR unit
Aim: Verify scale up of identified PBR system
The selected low-cost PBR unit from Phase 3 will be constructed in a near production-scale set up
(50-100m2), to study the effects of scaling and optimization of large-volume systems. In this
phase, we aim to study production stability and gather more precise data about production costs based on an optimized and focused production, dewatering and refinery with the focus on a spe-
cific product. Ultimately, this will contribute to verify fitness for purpose of a specific ‘PBR and al-
gae'-combination in a cost-effective, full-scale production setting.
Deliverables: Defined PBR system suitable for full-scale production at Mongstad
2.10.4 Collaboration with national partners
The current research on industrial microalgae production in Norway is limited to the larger uni-
versities (UiB, UiT, UMB) and research institutes (Uni Research, Sintef). We have a strong col-
laboration with members of the research group on algal physiology at the University of Bergen,
where Dr. Anita Jacobsen have long experience and high competence in the field of commercial
scale production. Jacobsen will be an integrated partner at Uni Environment, together with Dr. Siv Kristin Prestegard who will be responsible for the microalgae collection at the Bergen Ma-
rine Biobank under Uni. However, there are strong researchers on algal physiology in Tromsø,
expertise in reactor optimization at Sintef in Trondheim and the use of commercial Biofence-
reactors at UMB at Ås. During a national workshop on microalgae in Tromsø 11-12th October
2011, a working group memo suggested to Innovation Norway and NFR to establish a national
forum for microalgae. This can be an important arena for the development of good research
collaborations required to support the CO2 to Bio pilot facility.
Recommendation:
Be an active part in the establishment of a national forum for microalgae, and be visible for
potential collaborators.
2.10.5 Collaboration with Prof Rene Wijffels and AlgaePARC at Wageningen University
The AlgaePARC focus on the technology development towards more cost-efficient production sys-
tems suitable for future industrial production, and is therefore very much in line with the CO2 to Bio-
project aims. Prof Rene Wijffels and the AlgePARC team have a strong international position and
scientific competence within this field, and are interested to contribute to the CO2 to Bio-project in
demonstrating the viability of industrial microalgae production. The collaboration can be both on the establishment of a pilot production facility at Mongstad and the following research activity.
Such collaboration may be subject to regulations laid down by the AlgePARC consortium.
Recommendation:
Enter a formal collaboration in the development of a pilot facility at Mongstad.
Enter a research collaboration to ensure high international competence involvement.
CO2 to Bio/WP2 – Algae production
References
Cerón Garcìa MC, Garcìa Camancho F, Sánchez Mirón A, Fernàndez Sevilla JM, Chisti Y, Molina Grima E.
(2006) Mixotrophic production of marine microalga Phaeodactylum tricirnutum on various carbon
sources. J Micorbiol Biotechnol, 16(5); 689-694.
Chaumont D (1993) Biotechnology of algal biomass production: a review of systems for outdoor mass
culture. Journal of Applied Phycology 5:593-604
Chen P, Min Min, Chen Y, Wang L, Li Y, Qin Chen, Wang C, Wan Y, Wang X, Cheng Y, Deng S, Hennessy
K, Lin X, Liu Y, Wang Y, Martinez B, Ruan s (2009) Review of the biological and engineering aspects of algae to fuels approach. International Journal of Agricultural and Biological Engineering 2
Chisti Y (2007) Biodiesel from microalgae. Biotechnology Advances 25:294-306
Cuaresma M, Janssen M, Vilchez C, Wijffels R. (2011) Horizontal or vertical photobioreactors? How to
improve micoralgae photosynthetic efficiency. Bioresource technology 102; 5129-5137.
Jacobsen A, Grahl-Nielsen O, Magnesen T. (2010) Does a large-scale continuous algal production sys-
temprovide a stable supply of fatty acids to bivalve hatcheries? J Appl Phycol 22:769-777.
Kunjapur AM, Eldridge RB. (2010) Photobioreactor Design for Commercial Biofuel Production from Mi-
croalgae. Ind Eng Chem Res 2010, 49, 3516–3526.
Lavens P. and Sorgeloos P. 1996. Manual on the production and used of live food for aquaculture. FAO
Fisheries Technical Paper 361.
Merchuk J.C. and Wu X. 2003. Modeling of photobioreactor: Application of bubble column simulation. –
J Appl Phycol 15:163-169.
Molina Grima E, Acien Fernández F.G., García Camacho F., Rubio F. and Christi Y. 2001. Scale-up of
tubular photobioreactors. – J. Appl. Phycol. 12:355-368.
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production — A close look at the
economics. Biotechnology Advances 29:24-27
Richmond A. 2004. Handbook on Microalgal Culture: Biotechnology and Applied Phycology. Iowa State
Press, Iowa: Blackwell Publishing.
Skrede A, Mydland LT, Ahlstrøm A, Reitan KM, Gislerød HR, Øverland M. (2011) Evaluation of microal-
gae as sources of digestible nutrients for monogastric animals. J Anim Feed Sci, 20; 131–142.
Sukenik A. and Wahnon R. 1991. Biochemical quality of marine unicellular algae with special emphasis on
lipid composition. I. Isochrysis galbana. – Aquaculture 97:61-72
Posten, C. 2009. Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci 9(3)
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Applied
Microbiology and Biotechnology 57:287-293
Terry KL, Raymond LP (1985) System design for the autotrophic production of microalgae. Enzyme and
Microbial Technology 7
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresource
Technology 99:4021-4028
Waltz E (2009) Biotech's green gold? Nature Biotechnology 27:15-18
Wijffels R, Barbosa M (2011) An outlook on microalgal biofuels. Science 329: 796-799.
Wolkers H, Barbose M, Kleinegris D, Bosma R, Wijffels R. (2011) Microalgae: the green gold of the future.
Wageningen University Research
CO2 to Bio
Work Package 3 – Algae
CO2 to Bio/WP3 - Algae
Prepared by Anita Jacobsen and Svein Rune Erga, Department of Biology, University of Bergen
Contents
3.1 Introduction 5 3.2 Essential parameters for growing algae 6
3.2.1 Growth rate _ 6
3.2.2 Pigments and absorption wavelengths 6
3.2.3 Optimum culture conditions _ 7
3.2.4 Temperature _ 8
3.2.5 Salinity _ 8
3.2.6 Nutrients 8
3.2.7 Light intensity and photoperiod 8
3.2.8 pH, dissolved gasses (CO2 and O2) and mixing 10
3.3 Nutritional qualities of algae 10
3.3.1 Gross composition _ 10
3.3.2 Protein and amino acids 11
3.3.3 Carbohydrate _ 11
3.3.4 Lipids _ 12
3.3.5 Pigments 14
3.3.6 Minerals _ 14
3.3.7 Vitamins _ 14
3.3.8 Quality variation _ 15
3.4 Manipulation of biochemical composition 16 3.5 Potential new algal species for cold waters 17 3.6 Overview of algal species used for large scale production of biomass _ 18 3.7 Algal species suitable for production of protein and lipid at Mongstad _ 18 3.8 Short overview over recent research at UiB 18 3.9 R & D challenges and recommendations 18
3.9.1 Production _ 18
3.9.2 Species _ 18
3.10 Conclusion _ 19
CO2 to Bio/WP3 - Algae
3.1 Introduction
Marine microalgae (sometimes referred to as algae in this text) are widely used as food for many
species of larval and juvenile fish, shellfish and shrimps, either as live or dry feed. Many studies
have shown that they are rich sources of marine protein and lipids, particularly the long-chained
fatty acids. In addition, some marine microalgae may also contain other high quality products
such as pigments (chlorophylls, carotens and xanthophylls). With an increasing shortage of protein and lipids in feed for animal production, alternative sources have to be developed. Production of marine microalgae has shown to be a promising
source in this respect. Many studies have been undertaken in order to determine the nutritional value of microalgal
species, their biochemical composition and potential use as a food source (e.g. Volkman et al.
1981, 1989, 1991, 1993; Brown et al. 1989, 1993; Brown 1991; Brown and Jeffrey 1992; Brown
and Miller 1992; Dunstan et al. 1992, 1994; de Roeck-Holtzhauer et al.1993; Brown and Farmer
1994; Brown et al. 1997). The biochemical composition determines the nutritional value of the algae (Webb and Chu
1983, Brown et al. 1989, Brown 1991, Brown et al. 1999), and can be manipulated by changing
the culture medium (Wikfors et al. 1984; Ben-Amotz et al. 1985), temperature (Redalje and
Laws 1983; James et al. 1989), stage of harvest (Chu et al. 1982; Whyte 1987) and light condi-
tions (Caron et al. 1988; Sicko-Goad et al. 1988; Cohen et al. 1988; Thompson et al. 1990). The
total concentrations of protein, lipid and carbohydrate may vary substantially between species (e.g. Brown et al. 1989; Brown et al. 1997). Optimum culture conditions in terms of high growth rates are, however, not necessarily the
same as optimum conditions for nutritional quality (e.g. Sanchez et al. 2000). A compromise
between the nutritional quality and growth kinetics will often have to be considered. For in-
stance, it has been shown that the essential fatty acid EPA increased with decreasing light for
Nannochloropsis sp. and C. gracilis (= C. muelleri) (Sukenik et al.1989; Thompson et al. 1990). The fatty acid (FA) composition is highly dynamic and responds significantly to variation in light intensity (e.g. Thompson et al. 1990; Brown et al. 1993). Other studies have also demon-
strated that changes in culture media (Ben-Amotz et al. 1985), temperature (James et al. 1989,
Thompson et al. 1992, Zhu et al. 1997), pH (Guckert and Cooksey 1990), stage of harvest and
different culture techniques (e.g. Emdadi and Berland 1989; Hodgson et al. 1991; Dunstan et al.
1993; Brown et al. 1997; Pernet et al. 2003) have an impact on FA content and composition. It appears that lipid class and FA composition of microalgae are highly variable during cultur-ing. Dunstan et al. (1993) investigated changes in lipid class and FA composition of P. lutheri
and Isochrysis sp. grown in 100 litre bags, either as logarithmic and stationary phase batch cul-
tures or as semi-continuous cultures, and found that they changed depending culture technique
and growth phase. Pernet et al. (2003) found high variability in both lipid class and FA composi-
tion of C. muelleri and Isochrysis sp. in a semi-continuous system. In a continuous bag culture
system some variability in FA composition were observed between replicate bags of Isochrysis
sp., P. lutheri and C. muelleri, but the production of PUFA increased over time (Jacobsen et al. 2010; 2011).
3.2 Essential parameters for growing algae
3.2.1 Growth rate
Fast growing species is preferred when growing algae for commercial purposes. Growth of algae
is characterized by five phases; 1) lag or induction phase 2) exponential phase 3) phase of declin-
ing relative growth 4) stationary phase 5) death phase (Lavens and Sorgeloos 1996). The key to
success of algal production is maintaining all cultures in exponential phase of growth. During the exponential phase, cell density increases as a function of time t according to a logarithmic
function: Ct = C0 . e µt With Ct and C0 being the cell concentrations at time t and 0, respectively, and µ = specific
growth rate. The specific growth rate can also be expressed as: µ = ln(C1/C0)/t1-to The specific growth rate is mainly dependent on algal species, light intensity and temperature.
Cell division slows down when nutrients, light, pH, CO2 or other physical and chemical factors
becomes limiting. As earlier stated, the growth rate of microalgae differs among the different species depending on
nutrients, light, CO2, pH or temperature. Optimum culture conditions often results in maximum
growth rates. Abu-Rezq et al. (1999) reports on optimum production conditions for Nannochlo-ropsis sp (Kuwaitian strain), Tetrasemis suecica and Isochrysis sp. (T-iso) giving growth rates
of 0.11-0.13 d-1, 0.14-0.15 d-1 and 0.14 d-1, respectively. Maximum cell densities obtained were
24.9-32.4x106 cell ml-1, 4.73-5.06x106 cells ml-1 and 4.47x106 cells ml-1 for N. sp (Kuwaitian strain), T. suecica and I. sp. (T-iso), respectively. However, in Aquatext
maximum specific growth rates of 0.81 d-1 and 0.46 d-1 are reported for I. sp. (T-iso) and T.
suecica, respectively. Sukenik et al. (1989) reports a maximum growth rate of 0.7 d-1 for Nanno-chloropsis oculata and in Aquatext a growth rate of 0.92 d-1 is given. For
Isochrysis galbana a maximum growth rate of 0.55 d-1 have been reported .
Acién Fernández et al. (1998, 2003) reported a maximum growth rate of 1.2 and 1.4 d-1 for
Phaeodactylum tricornutum in an outdoor reactor, while Chismadha and Borowitzka (1994)
reported a maximum value of 0.48 d-1 in an indoor reactor at irradiance on reactor surface of
286 µmol m-2 s-1. Acién Fernández et al. (2003) showed a hyperbolic relationship between
growth rate and average irradiance values below 250 µmol m-2 s-1. Above these values a linear
decrease in growth rate was observed. Although growth rate is an important parameter, the yield of the system is quantified by the biomass concentration and productivity. Maximum bio-
mass concentration reported for photoautotrophic growth of P. tricornutum is 1.5 g l-1 (Acién
Fernández et al. 2003) and was obtained at an average irradiance of 50-150 µmol m-2 s-1, corre-
sponding to growth rates of 0.84-1.04 d-1. However, when grown mixotrophic on glycerol, max-
imum biomass concentration increased to 16.2 g l-1 (Cerón García et al. 2000).
3.2.2 Pigments and absorption wavelengths
Chlorophyll a is the main photosynthetic pigment in all micro-algae (Hoek et al. 1995). The ac-
cessory chlorophyll b is found together with chlorophyll a in the green algae (e.g. Prasi-
nophyceae), whereas chlorophyll c is found with chlorophyll a in the chromophyte algal classes
(e.g. Bacillariophyceae and Prymnesiophyceae). Chlorophyll a and β-carotene are commonly found in the different classes. Chlorophyll a is located as a part of core and reaction center pro-
tein complexes and in the light-harvesting antenna (Richmond 2004). Other important pig-
ments such as chlorophyll b and c, carotenes and xanthophylls act as supplementary pigments
for light harvesting (Richmond 2004).
CO2 to Bio/WP3 - Algae
Figure 1. Absorption spectrum of different pigments. Light is often considered to be one of the most important factors in photobioreactors (Richmond 2004). Different types of algal cultures need different light and nutrient sources. The light re
quirement for algae is dependent upon the major pigments present in the algal cell. A detailed
explanation of pigments and their importance is discussed in Kommareddy and Anderson
(2003). Kommareddy and Anderson (2003) also discuss how different light wavelengths that
are absorbed are converted to energy for the photosynthetic process. Different pigments absorb/harvest different regions of visible light energy (Fig. 1, Table 1).
Richmond (2004) showed the penetration depth of light spectra in Nannochloropsis sp. as a function of cell density. The important pigments of Nannochloropsis are chlorophyll a, and β-
carotene. By comparing Figures 1 and Richmond (2004) it is observed that the light wavelengths
corresponding to the absorption range of these pigments (approximately 400-500nm and 600-
700nm) also corresponds to the light wavelengths with the least penetration depth because they
are absorbed by algae. Another interesting aspect, which can be seen from Richmond (2004), is
that when the concentration of algae in gL-1 is small, there is low absorption by the supplemen-tary pigments. This suggests that supplementary pigments are not used to harvest light until
there is a deficiency of light in the wavelengths absorbed by chlorophyll a. Richmond (2004)
also showed that algal cultures with a density of 3 gL-1 effectively absorb all blue light (300-
400nm). For blue light to have a greater penetration depth, the culture density must be less than
3 gL-1.
Table 1. Light absorption wavelengths of pigments.
Pigments
Wavelengths (nm)
Chlorophyll a
Chlorophyll b
Chlorophyll c
3.2.3 Optimum culture conditions
The most important parameters regulating algal growth are temperature, salinity, nutrients,
light, pH and mixing. These parameters may also be interdependent and a parameter that is
optimal for one set of conditions is not necessarily optimal for another. Optimum culture condi-
tions, in terms of high growth rates, are, however, not necessarily the same as optimum condi-
tions for nutritional quality (e.g. Sanchez et al. 2000). A compromise between the nutritional
quality and growth kinetics will often have to be considered. For instance, it has been shown
that the essential fatty acid, eicosapentaenoic acid (EPA), increase with decreasing light
(Sukenik et al. 1989, Thompson et al. 1990). A summary of optimum culture conditions for
some species is given in Appendix A.
3.2.4 Temperature
Optimum temperature for microalgae (temperate and sub-tropical species) used is generally
between 18 and 24°C, although this may vary within strains and species. Many of the cultured
species tolerate temperatures between 16-27°C. Temperatures lower than 16°C will most likely
result in slow growth, while temperatures above 35°C will lead to culture collaps (e.g. Acien Fer-
nández et al. 2003). In order to keep stable temperatures, cultures can be cooled down by flow
of cold water over the surface of the cultures or by controlling the air temperature with refriger-
ated air. Optimum temperature reported for production is 19-21°C for Nannochloropsis oculata and
Tetraselmis suecica, 20°C for Nannochloropsis sp. ("kuwaitian strain") and 24-26°C for
Isochrysis sp. (T-iso) (James et al. 1989, Abu-Rezq et al. 1999). Optimal temperature reported
for Phaeodactylum tricornutum is 18-22°C (e.g. Molina Grima et al. 1996) and 20°C for
Isochrysis galbana (Molina Grima et al 1994).
3.2.5 Salinity
Marine microalgae are in general tolerant to changes in salinity. In culture, most species grow
best at a salinity that is a bit lower than found in their native habitat (diatoms at 20-25 ‰ and
flagellates at 28-30‰). This can be obtained by diluting sea water with distilled water (Lavens and Sorgeloos 1996).
Optimum salinity reported for production is 20-40‰ for Nannochloropsis sp. 20-35‰ for
Tetraselmis suecica and 25-35‰ for Isochrysis sp. (T-iso) (Abu-Rezq et al. 1999). Fabregas et
al. (1985) and Renaud & Parry (1994) reported that the optimal growth rate for Isochrysis sp.
(T-iso) was achieved when salinity was between 15 and 35‰, whereas Nannochloropsis sp. had
a significant slower growth rate only at 35‰.
3.2.6 Nutrients
Cultures of micro-algae must be enriched with nutrients in order to sustain growth. Macronutri-
ents include nitrate, phosphate and silicate. Silicate is mainly used by diatoms, which utilize this
compound for production of an external cell covering. Micronutrients consist of various trace metals (Zn, Co, Cu, Mo, Mn, Fe) and vitamins (thiamine, cyanocobalamin and biotin). Two en-
richment media are commonly used for growth of algae in aquaculture; Walne medium (Laing
1991) and f/2 medium (Guillard 1975). Various specific recipes for algal culture media are de-
scribed by Vonshak (1986). The complexity and cost of media often excludes their use for large
scale production. Alternative enrichment media for large scale production are often composed of
agriculture-grade rather than laboratory-grade nutrients, and they often contain only the most
essential nutrients (e.g. Palanisamy et al. 1991).
3.2.7 Light intensity and photoperiod
Microalgae are photosynthetic organisms; they assimilate inorganic carbon and transform it to
organic matter. Light is the energy source that drives this reaction. Light intensity, photoperiod and spectral quality (see pigments and wavelengths) need therefore to be carefully considered.
After identifying the type of algae culture to be grown, it is important to identify the right type of
light source with appropriate wavelengths in order to achieve a high level of photosynthetic effi-
ciency. Light may be natural or supplied artificial. Kommareddy and Anderson (2003) discussed energy produced by different light sources in the
visible spectrum. Electricity is used in closed loop photobioreactors to produce light making it essential to ensure that the light source is optimized relative to algae light use and cost of pro-
duction so that a high level of electrical efficiency is obtained. The efficiency at converting elec-
tricity into light varies with different light sources. The efficiency of light sources to convert elec-
tricity to light is further compounded when wavelength of light is considered. Light sources with
descending order of efficiency are light emitting diodes (LEDs), grow flux / fluorescent lights
CO2 to Bio/WP3 - Algae
and incandescent/halogen lamps. Since LEDs are the most efficient light source for converting
electricity into light with the desired wavelength, they should be given high priority for use.
However, LEDs don't produce light in a broad white light spectrum which may make it neces-
sary to use a combination of light sources or combination of LEDs. Essentially any type of light sources which produces light between 400 nm – 500 nm and
525nm to 680 nm should support growth of algae. For example; prasinophytes (e.g. Tetraselmis spp.) which have chlorophyll a and b, in addition to the light harvesting pigment β-carotene, any
light source which can produce wavelength ranges of 400 nm to 500nm and 620 nm to 680 nm
should be able to support growth of these algae (Fig. 1). The intensity of a light source gives the number of photons that are available for the photosyn-
thetic process. The energy associated with photons with a wavelength of 680nm is the energy
level required by chlorophyll a to initiate photosynthesis. Light with a wavelength of 680nm is
near the longest wavelength of visible light. Therefore, most of the visible light has sufficient energy to support photosynthesis. However, if the wavelength is small the energy associated
with the wavelength is high. Light intensity plays an important role, but the requirements vary
greatly with culture depth and cell density. At high depths and densities the light intensity must
be increased (100-200 µmol m-2 s-1 is often required for large volumes). Too high light intensity
may result in photoinhibition. Since naturally grown algae have dark times (nighttime), it is assumed by many researchers that dark periods are required. The effect of duration of light exposure on the cell is not well under-
stood. Very little information is available as to how much time the cell should be in the light and
dark. Many studies have used growth conditions that have a small dark period, others have used
no dark period, and some have used dark periods up to 18 hours (e.g. Kim et al. 2002). Merchuk
and Wu (2003) suggest that an appropriate dark period is 6 seconds (s). Once a photon is ab-
sorbed, it needs 6s to reset itself (perform photosynthesis) so that it is ready to receive another
photon (Merchuk and Wu, 2003). Richmond (2004) on the other hand states that the rate of photosynthesis is governed by the turn over of electron transport that takes 1-15 milliseconds
(ms). It is further asserted that an algal culture that has adapted to a high light intensity may
require only 2 ms. This short dark period would make it difficult to move algae into and out of
the lit region of a photobioreactor, while the 6s dark period is more manageable. High illumina-
tion of the algal antennas may also damage them necessitating longer dark periods to repair the
damage (Wu and Merchuk 2001). However, there is no consensus on what is an appropriate light:dark cycle. Long dark periods generally results in biomass loss as well as a decline in
growth rates, because the algae undergo photorespiration and consume oxygen and carbohy-
drates (Molina et al. 2001, Merchuk and Wu 2003). Tzovenis et al. (2003) showed the advantage of shorter photoperiods for high biomass produc-
tion of Isochrysis sp. (T-iso). Brown et al. (1993) reported an optimal light intensity of 100 µmol
m-2 s-1 at a12:12 hrs light:dark cycle for I. sp. (T-iso) that gave best nutritional value for animals.
This is also in accordance with Abu-Rezq et al. (1999) who used a light intensity of approx. 100 µmol m-2 s-1 in order to investigate optimum production conditions for I. sp., T. suecica and N
sp. Sukenik et al. (1989) investigated growth-irradiance relationships for N. sp. and found that
growth was light-saturated above 200 µmol m-2 s-1 and that photo-inhibition occurred at levels
above 500 µmol m-2 s-1. For P. tricornutum optimum light intensities of 50-250 µmol m-2 s-1 has been reported (Thomp-
son et al. 1990, Molina Grima et al. 1996, Acién Fernández et al. 1998, Acién Fernández et al. 2003). Caron et al. (1988) showed that P. tricornutum grown in 2:2 hrs light:dark cycle instead
of 12:12 hrs increased the C-assimilation by 1.5. Evidence also suggests that the minimum ac-
ceptable value of the light:dark cycle frequency of P. tricornutum is about 1 s-1 in short light-
path photo-bioreactors (Molina Grima et al. 2001).
3.2.8 pH, dissolved gasses (CO2 and O2) and mixing
The pH range for most cultured species is between 7 and 9, with the optimum range being 8.2-
8.7 (Lavens and Sorgeloos 1996). Culture collapse can be the result when failing to keep an ac-
ceptable pH. This can be prevented be aerating the culture. At high cell densities, addition of
CO2 to the air allows to correct for increased pH due to high primary production. The CO2 origi-nating from air ( 0.03%) is limiting growth when bubbled through a dense culture. Pure CO2
may then be supplemented to the air supply usually at a rate of 1-2% of the volume of air. CO2
addition furthermore buffers against pH changes. CO2 may also be supplemented to the inflow
of seawater into the cultures (e.g. Jacobsen et al. 2010). Mixing is necessary in order to prevent sedimentation, ensure that the cells are equally exposed
to light and nutrients, avoid thermal stratification and to improve gas exchange between culture
and air. Mixing can be achieved by stirring by hand (small volume flasks), aerating (bags, tanks), paddle wheels or jetpumps (ponds). Not all species can, however, tolerate vigorous mixing.
In closed bioreactors over-saturation of O2, due to respiration, can limit the growth of algae.
This can be prevented by ventilation of the cultures. Acién Fernández et al (2003) observed that
P. tricornumtum grew well with dissolved oxygen values <400% Sat. (with respect to air satu-
3.3 Nutritional qualities of algae
3.3.1 Gross composition
Protein, carbohydrate, lipids and minerals make up 90-95% of the dry weight of an algal cell.
The remainder is accounted for by nucleic acids (5-10%) (Becker 1986, Fabregas et al. 1986, Fa-
bregas et al. 1986). Typical values for the gross chemical composition of different micro-algae
are shown in Table 2. Protein is always the major organic constituent, followed by lipid and car-
bohydrate. Expressed as percentage of dry weight, the range of protein, lipid and carbohydrate are 12-35%, 7.2-23% and 4.6-23%, respectively.
Variables such as photoperiod, light intensity, wavelength, temperature, nutrients in culture
media and stage of growth at harvest can influence the gross composition (e.g. Myklestad 1974,
Goldman 1977, 1979, Savidge 1980, Walsh and Legendre 1983, Fabregas et al. 1985, Hitchcock
Table 2. Gross chemical composition of some algae commonly used in aquaculture harvested during
exponential growth phase. Numbers given are pg cel -1 and (% dry weight). Modified from Brown (1991). Algal species
cell-1 pg cell-1 drate
Isochrysis sp. (T-iso)
6.8 (23) 1.8 (6.0)
Isochrysis galbana
0.3 (0.98) 8.8 (29) 3.9 (12.9)
Phaeodactylum tricor- 76.7
Skeletonema costatum
0.63 (1.21) 13.1 (25) 2.4 (4.6)
Tetraselmis suecica
52.1 (31) 20.2 (12.0)
Table 3. Amino acid composition of some microalgae (g/100g of total amino acid fraction in hydrolysate).
n.d.=not detected, +=present. Modified from Brown et al. (1989).
CO2 to Bio/WP3 - Algae
Isochrysis Isochrysis Pavlova Phaeodactylum Skeletonema Tetraselmis
sp. (T-iso) lutheri
Phenylalanine 4.4
Epifanio et al. Enright et al. Epifanio 1979
3.3.2 Protein and amino acids
The nutritional value of protein is determined by the content and availability of its constituent
amino acids. Of the total amino acids in algae, 90-98% occurs in protein (Dortch et al. 1984). A
number of studies have been conducted on total amino acid composition in micro-algae (e.g.
Parsons et al. 1961, Chau et al. 1967, Enright et al. 1986a, Hayashi et al. 1986, Brown 1991). Brown (1991) showed that micro-algae have a well balanced amino acid composition. The pro-
portions of individual amino acids do not vary greatly between different algal species (Table 3).
Differences in the nutritional quality of algae are therefore, mostly, unrelated to amino acid
composition (Webb and Chu 1983, Brown 1991).
The amino acid composition of micro-algae is quite similar to chicken egg protein (high nutri-
tional value for humans), although the latter is richer in methionine and lower in arginine
(Teshima et al. 1986). Some amino acids are unavailable for animal digestion and absorption if sections of the mole-
cule are bound to other molecules (e.g. the free amino group of lysine can sometimes be bound
to carbohydrate, particularly, during processing of harvested algae (like drying)).
3.3.3 Carbohydrate
Micro-algae provide a rich source of carbohydrate (Brown 1991). Few studies have been made
on carbohydrate composition of micro-algae (Parsons et al. 1961, Chu et al. 1982, Whyte 1987,
Brown 1991). The total carbohydrate fraction is composed of the polysaccharide fraction (45-
97% of total carbohydrate fraction, Whyte 1987) and mono-and oligo-saccarides. Carbohydrate
profiles of micro-algae can vary greatly (Table 4). The principal sugars are glucose, galactose, mannose and ribose, with others in varying proportions.
Differences in polysaccharides have been shown between the major groups of micro-algae. Dia-
tom polysaccharides contain mainly chrysolaminarin (ß1-3 glucan) and mannans (Myklestad
1974, Whyte 1987), while flagellates mainly contain glucans like glucose and galactose (Whyte
Table 4. Carbohydrate composition (g/100g of total monosaccharide in hydrolysate fraction) of some
microalgae. n.d.=not detected.
Isochrysis Isochrysis Pavlova Phaeodactylum Skeletonema Tetraselmis
sp. (T-iso) lutheri
Ribitol/xylitol n.d.
Chu et al. Handa and Yanagi Handa
3.3.4 Lipids
The lipids are grouped as polar and neutral lipids depending on their polarity. The polar lipids
include the phospholipids and glycolipids. The neutral lipids include the triacylglycerides, di-
acylglycerides, hydrocarbons, alkenones, sterols and pigments.
Fatty acids
The fatty acids (FA) constitute a major proportion of the lipid fraction in micro-algae (20-40% of total lipid on a weight basis, Cohen 1986). Fatty acids occur mainly in an esterified form with
glycerol, and are found in tri- and di-acylglycerides, phospholipids and glucolipids. Most studies
report only the total FAs, although some report the FA profiles (e.g. Fried et al. 1982, Sheffer et
al. 1986). Data from some micro-algae are shown in Table 5. The different classes of algae show quite dis-
tinct distribution patterens (Chuecas and Riley 1969, Beach et al. 1970, Waldock and Nascimen-to 1979, Webb and Chu 1983, Volkman et al. 1989). Saturated FA constitute about 15-30% of the
total FAs in green algae, while the range is 30-40% in diatoms and prymnesiophytes. Green al-
gae are low in monosaturates (5-20%), but high in polyunsaturates (50-80%), whereas prymne-
siophytes and diatoms have similar levels of both monosaturates (20-40%) and polyunsaturates
(20-50%). The polyunsaturated fraction of the green algae is, however, dominated by 16 and 18
carbon chain length FAs, whereas higher carbon FAs (like 20:5n-3 (eicosapentaenoic acid, EPA)
and 22:6n-3 (docosahexaenoic acid, DHA)) are typical for the other groups of algae. Despite these trends, the levels of specific FA may vary widely in closely related species within the same
class (e.g. Isochrysis galbana and I. sp. (T-iso)). Most species contain moderate to high concentrations of EPA (Table 5). Particularly Pavlova
lutheri, Phaeodactylum tricornumtum and Nannochloropsis spp. are species rich in EPA, while
Isochrysis sp. (T-iso) contains very low levels of this fatty acid. Species that are rich in DHA are
Isochrysis galbana, Isochrysis sp. (T-iso) and Pavlova lutheri. Only one species is rich in both DHA and EPA and that is Pavlova lutheri. Green algae like Tetraselmis suecica are usually rich
in C16 and C18 PUFAs, in addition to ARA (arachidonic acid, 20:4n-6), but low in higher carbon
fatty acids like EPA and DHA, which may contribute to low nutritional value.
CO2 to Bio/WP3 - Algae
Table 5. Fatty acid composition (g/100g of total fatty acid fraction) of some micro-algae. n.d.=not detect-
ed, TR=trace amount detected, -not analysed.
Fatty acid Isochry-
Isochry- Pavlo-
Phaeodacty- Skele-
sis gal- sis
lum tricor- tonema
saturates C16 poly- 0.4
Reference Waldock
Ben-Amotz et Volkman et Volkman et Hu
n et al. al. 1987
Polar lipids
Phospholipids may constitute 5-25% of total lipid weight, but an average of 10% is often seen in most species (Ben-Amotz et al. 1985). Phospholipid subfractions detected in most algae include
phosphatidyl inositol, phosphatidyl choline, phosphatidyl glycerol, phosphatidyl ethanolamine
and diphosphatidyl glycerol. Phosphatidyl choline and inositol are present in prymnesiophytes
and green algae (Ben-Amotz et al. 1985) and cryptophytes contain only phosphatidyl choline
(Beach et al. 1970).
Neutral lipids
Sterols are only a minor component of the lipid fraction (0.5-2.5%) (Orcutt and Patterson 1975,
Ballantine et al. 1979). Marked variations between the total sterol content and the type of sterols
present have been observed in many species (Ocrutt and Patterson 1975, Ballantine et al. 1979,
Volkman et al. 1981, Lin et al. 1982, Nichols et al. 1987). The sterol content of three algae is giv-
en in Table 6. Hydrocarbons and alkenones are two other classes of neutral lipids that may be of nutritionally
importance (Volkman et al. 1980, Marlowe 1984, Ben-Amotz et al. 1985). Saturated or mono-
unsaturated hydrocarbons constitute a minor proportion ( 0.1-2%) of the total lipid component
of micro-algae. A specific lipid fraction enriched in both alkenones and cyclic and polyunsatu-
rated hydrocarbons has also been reported for a number of micro-algae (Marlowe 1984, Ben-
Amotz et al. 1987).
Table 6. Sterol composition of some microalgae (mg/g dry weight). n.d. = not detected. Results from Lin
et al. (1982).
Isochrysis gal- Pavlova
24-methylene cholesterol
Campesterol/24 epicampesterol
Brassicasterol/24 epibrassicasterol
Sitosterol/clionasterol
3.3.5 Pigments
The major pigments of most algae are the green chlorophylls and the yellow, orange and red
carotenoids, which contribute 0.5-5% of the dry weight of the cell (Parsons et al. 1961, Ben-
Amotz et al. 1985). Blue-green algae, red algae and the cryptophytes also contain the red, pro-tein-bound water-soluble, phycoerythrins and/or the blue phycocyanins. Chlorophylls and ca-
rotenoids are contained in the extracted lipid fraction of the cell. Carotenoids are made up of a
number of isoprene units, functioning both as photoprotectants and light-harvesting pigments
in photosyntesis (Cohen 1986). Each algal species may contain between 5 and 10 different carot-
enoids, and more than 60 different carotenoids are known from algae (Cohen 1986). ß-carotene, or provitamin A, is a common constituent of the carotenoid fraction of micro-algae.
It is found in highest concentration in the green algae. Although it generally constitutes less than 1% dry weight, it may accumulate levels up to 10% dry weight in halotolerant algae (Fried
et al. 1982, Ben-Amotz et al. 1985). Algae of interest is prasinophytes (Tetraselmis spp), eustig-
matophytes (Nannochloropsis spp), prymnesiophytes (Isochrysis spp), and bacillariophytes
(Phaeodactylum spp). The various pigments present are shown in Table 7. Some other species
like Euglena, Haematococcus and Chlorella can produce significant quantities of other carote-
noids such as astaxanthin. Astaxanthin and luthein may serve as a vitamin A precursor in fish that are not able to absorb ß-carotene (Torrissen and Christiansen 1995, Rønnestad et al. 1998).
Green algae, like for instance Tetraselmis suecica, have also shown to contain adequate
amounts of luthein (Rønnestad et al. 1998).
3.3.6 Minerals
The mineral fraction of the algal cell can constitute a major proportion of the dry weight, rang-
ing from 6-39%, but there are few detailed analyses. Algae can be a major source of a number of
minerals. They can also accumulate trace- and heavy metals, which can be a disadvantage if the
metals are toxic (Sakaguchi et al. 1981, Fisher 1985, Fabregas and Herrero 1986). Major ions
that are of biologically importance are phosphorous, silica, calcium, sodium, potassium, chlo-
rine, iron, magnesium, and zinc; manganese, copper and cobalt occur in trace amounts.
3.3.7 Vitamins
Algae are a significant source of nearly all the vitamins. However, few studies have been con-
ducted on marine micro-algae (Kanazawa 1969, Aaronson et al. 1971, Dubinsky et al. 1978, Brown et al. 1999). The major vitamins identified are thiamine (vitamin B1), riboflavin (B2),
pyridoxine (B6), cyanocobalamin (B12), biotin, ascorbic acid (vitamin C), nicotinic acid, panto-
thenic acid, choline, inositol, tocopherol (E) and ß-carotene (provitamin A) (Table 8). In addi-
tion, vitamin K has been detected in trace amounts in Porphyridium cruentum when grown
heterotrophically (Antia et al. 1970) and vitamin D precursors have also been isolated from algae
(Hollick 1984). Many algae also have specific vitamin requirements, particularly thiamin, cyano-cobalamin and biotin have shown to be of importance (Provasoli and Carlucci 1974).
CO2 to Bio/WP3 - Algae
Table 7. Major vitamin content of some microalgae (µg/g dry weight). n.d.=not detected, R= vitamin re-
quired for growth.
Nannochloropsis Tetraselmis
Cyanocobalamin (B12)
Pteroylmonoglutamic
Pantothenic acid
Ascorbic acid (C)
ß-carotene (pro A)
Aaronson et al. 1970 Kanazawa 1969
Brown et al. 1999
3.3.8 Quality variation
Algal species vary significantly in their nutritional value under different growth conditions
(Brown et al. 1997). Algae that have been found to have good nutritional qualities include spe-
cies like Chaetoceros calcitrans, C. muelleri, Pavlova lutheri, Isochrysis sp. (T-iso), Tetraselmis
suecica, Skeletonema costatum and Thalassiosira pseudonana (Enright et al. 1986a, Thompson
et al. 1993, Brown et al. 1997) amongst others. The gross composition of micro-algae can influ-
ence the nutritional value (e.g. Enright et al. 1986b), however, it may seem that it is the balance
of other key nutrients that are of more importance. Brown et al. (1989, 1993, 1997) have found many algal species that are rich in polyunsaturated
fatty acids (PUFAs) which are essential to marine larvae, include species from the classes chlo-
rophyceae, prasinophyceae, cryptophyceae, bacillariophyceae, eustigmatophyceae and prymne-
siophyceae. PUFAs, especially docosahexaenoic acid (DHA, 22:6n-3), eicosapentaenoic acid (EPA, 20:5n-3) and arachidonic acid (ARA, 20:4n-6) which may be essential for various larvae
(Langdon and Waldock 1981, Sargent et al. 1997) vary significantly between algal classes and
algal species. Cultures of Phaeodactylum tricornutum and Nannochloropsis sp. has shown to
contain high amounts of EPA (e.g. Yongmanitchai and Ward 1991, Acién Fernández et al. 2000,
Zou et al. 2000). Most species have moderate to high concentrations of EPA, however, few are
rich in DHA. Isochrysis sp. (T-iso), Pavlova lutheri, Micromonas pusilla and Rhodomonas sa-
lina are examples of DHA-rich microalgae (e.g. Brown et al. 1989, Volkman et al. 1989). Isochrysis sp. (T-iso) on the other hand, has shown to contain very low concentrations or even
lack of EPA (Table 6, Liu and Lin 2001). Nannochloropsis spp., Tetraselmis suecica and Chae-
toceros calcitrans have relatively high amounts of ARA (Brown et al. 1989), while many other
species lack or contain trace amounts of this fatty acid (FA). The fatty acid composition is also dependent on the growth conditions and stage of harvest. For
example, Nannochloropsis oculata and I. galbana contained more PUFA cell-1 in logarithmic
phase than in stationary phase (Dunstan et al. 1993, Zhu et al. 1997). Other studies have demon-
strated changes in FAs associated with light intensity (Thompson et al. 1990, 1993, Brown et al.
1993), culture media (Ben-Amotz et al. 1985), temperature (James et al. 1989, Thompson et al.
1992, Zhu et al. 1997) and pH (Guckert and Cooksey 1990). Other important nutrients that have been found to be variable in algae include sugars, vitamins
and sterols. Brown (1991) showed that variation in the sugar composition could contribute to
differences in the nutritional value of some species, since animals digest polysaccharides of dif-
ferent composition at different rates. Algae rich in mannose (such as diatoms) are probably less
digestible to animals (Brown et al. 1997).
Micro-algae may also vary in their vitamin content. Ascorbic acid (vitamin C) shows the greatest
variation, 16–fold (1–16 mg g–1 dry weight; Brown and Miller 1992). Concentrations in other
vitamins typically show a 2- to 4-fold difference between species (Seguineau et al., 1996, Brown
et al., 1999). Brown et al. (1999) showed that some of the micro-algae used in aquaculture (e.g. I.
sp. (T-iso) and N. oculata) contained adequate contents of vitamins for aquaculture food chains.
De Roeck-Holtzhauer et al. (1991) measured the content of ten vitamins in algae and found that they were rich in most vitamins. However, they also found that the algae had low concentrations
of at least one vitamin, which could account for differences in their nutritional value. To put the
vitamin content of the micro-algae into context, data should be compared with the nutritional
requirements of the consuming animal. These data suggest that a carefully selected, mixed-algal
diet should provide adequate concentrations of the vitamins for aquaculture food chains. Sterols
(Knauer et al. 1999), minerals (Fabregas and Herrero 1986) and pigments (Rønnestad et al.
1998) may also contribute to nutritional differences in microalgae. The carotenoid content may vary, especially with the light conditions. When light intensity in-
creases the content of the supplementary pigments like the carotenoids increase (Cohen 1986,
Richmond 2004). It has been shown that the carotenoid content of P. tricornutum increases
linear with external irradiance (Acién Fernández et al. 2000).
3.4 Manipulation of biochemical composition
When the effects of light intensity (irradiance) have been studied, continuous light conditions
have often been used (Thompson et al. 1990, Sukenik and Wahnon 1991), even though many
hatcheries now use light:dark (L:D) cycles (e.g. 12:12 or 16:8 hrs light:dark). The effects of irradiance on the biochemical composition of the prymnesiophyte Isochrysis sp.
(T-iso) was investigated by Brown et al. (1993). Cultures were grown under a 12:12 hrs light:dark
regime at five irradiances ranging from 50-1000 µmol m-2s-1 and harvested at late-logarithmic
phase for analysis of biochemical composition. Highest levels of protein were present in cultures
grown at 100 and 250 µmol m-2s-1. Minimum levels of lipid and carbohydrate were found at 50 µmol m-2s-1. The composition of amino acids did not differ over the range of irradiances. The
proportion of the lipid class components and fatty acids (FAs) showed little variation with irra-
diance. They concluded that on the basis of biochemical composition, an irradiance of 100 µmol
m-2s-1 (12:12 L:D cycle) may provide optimal nutritional value for animals. Thompson et al. (1990) investigated the influence of irradiance on the FA composition of eight species commonly used in aquaculture, and found that for most species there was a trend that
EPA increased with decreasing light intensities and DHA decreased with decreasing light inten-
sities. They concluded that the FA composition is a highly dynamic component and responds
significantly to variation in light intensity. The light conditions which produced the greatest
proportions of the essential FAs were species specific. Based upon their results I. sp (T-iso) had
the best FA profile and growth at 80-125 µmol m-2s-1, and Phaeodactylum tricornutum at 125 µmol m-2s-1. Sukenik et al (1989) showed that irradiance level affected cellular fatty acid composition of
Nannochloropsis sp. (N. oculata). Increasing light intensity (35-550 µmol m-2s-1) imposed an
exponential reduction in the proportion of PUFAs (ARA and EPA), and an increase in the satu-
rated and monosaturated FAs (16:0, 16:1). Cells grown in light-limiting conditions (35 µmol m-2s-1) contained high levels of EPA. Caron et al. (1988) found that reducing the photoperiod from 12:12 to 2:2 hrs light:dark cycles
enhanced the carbon assimilation rate by 1.5 for P. tricornutum. EPA (20:5n-3) is the major PUFA found in P. tricornutum being 40-57% of total FAs (Acién
Fernández et al. 2000). They also found that the external irradiance had little effect on the FA
profile, while the dilution rate did. However, the FA content decreased with increasing irradi-
ance and dilution rate. The maximum EPA content was attained at the lowest dilution rate
(0.025 h-1) and low external irradiance (900 µE m-2 s-1).
CO2 to Bio/WP3 - Algae
James et al. (1989) investigated effects of temperature on growth, FA and amino acid composi-
tion of N. sp. ("Kuwaitian strain"). They found optimum growth at 20°C. Also, the total n-3
highly unsaturated fatty acid (HUFA) content in this strain increased with increasing tempera-
ture up to 25°C and EPA (20:5(n-3)) constituted the major component. The long-chained fatty
acids (e.g. 20:3(n-3), 22:6(n-3)) were observed more at 25°C than at the other temperatures
tested (15, 20 and 35°C). The majority of amino acids and protein content increased up to 30°C. Zhu et al. (1997) found that Isochrysis galbana grown at 15°C contained a higher proportion of
PUFAs (18:3(n-3), 22:6(n-3)) compared to 30°C, and that the exponential growth phase con-
tained higher contents of FAs than the stationary growth phase. Hu and Gao (2003) found that enrichment with 2800 µl CO2 l-1 enhanced growth, total FA con-
tent, PUFAs and EPA in N. sp. This is also in agreement with Roncarati et al. (2004) who
showed that increasing the CO2 concentration from 1% to 2% of the volume of air increased the
content of the long chained fatty acids in N. sp., N. oculata and I. sp. (T-iso). On the other hand,
cultures aerated with 5% (v/v) CO2 had a significant increase in carbohydrate content, but no in
lipids of P. tricornumtum (Chrismadha and Borowitzka 1994). Under nutrient sufficient conditions, cells synthesise mainly proteins to support growth and
division (Myers 1980). However, when a culture is deprived of an essential nutrient, cell division
stop and the fraction of carbon allocated to lipids and carbohydrates can be greatly increased at
the expense of protein synthesis. In most algae, enhancement of lipid accumulation is imposed by nitrogen deficient conditions (Spoehr and Miller 1949, Shifrin and Chisholm 1981, Piorreck
et al. 1984, Cohen et al. 1988). Herrero et al. (1991) showed that the protein content per cell was
more susceptible to medium induced variation than the other cellular constituents. Sukenik and Wahnon (1991) showed that severe nitrogen limitation increased abundance of
saturated (C16:0) and monosaturated (C18:1) fatty acids and decreased the percentage of DHA in I. sp. (T-iso).
3.5 Potential new algal species for cold waters
Temperate and sub-tropical species like Isochrysis sp. (T-iso), I. galbana, Tetraselmis suecica
and Nannochloropsis oculata are widely used feed organisms in cold waters, as well. Today,
algal species isolated from cold waters are hardly in use. Therefore, new and more suitable spe-cies isolated from local areas for use in cold water are highly demanded. Some attempts have
been made on isolating new species from cold waters, but they have so far not been implement-
ed in intensive production. Based on biochemical composition, particularly the fatty acids, some promising candidates have emerged from the algal classes; cryptophyceae, chlorophyceae, dinophyceae, bacillariophyceae,
prasinophyceae and dictyochophyceae (Leirvoll et al. 2001, Solbakken and Johnsen 2004). The-
se species have yet to be tested for their suitability as feed organisms in high density cul-
tures.Leirvoll et al. (2004) used algal species like Phaeodactylum tricornutum and Oocystis sp.
isolated from Sognefjorden (Norway) in their enrichment studies of Brachionus plicatilis. Their
results showed that these cold water strains, grown at 8°C, gave higher contents of n-3 fatty ac-ids than the tropical algae. It was particularly the PUFAs 18:3n-3, EPA and DHA that increased.
The rotifers fed these cold water based algal diets had a better nutrient value for cold water fish
larvae. The cold water strain of P. tricornutum contained particularly high levels EPA and other
n-3 fatty acids, in addition saturated (16:0) and monosaturated (16:1) fatty acids. Solbakken and Johnsen (2004) isolated a range of different cold water algal species and ana-lysed their fatty acid profiles. Their results showed that the dinoflagellate Amphidinium sp. had
a very good fatty acid profile with a DHA/EPA ratio of 1.6. A DHA/EPA ratio between 1 and 2 is
suggested to be optimal for cold water larval fish (e.g. van der Meeren 2003). In general, natural
dinoflagellates have shown to contain high levels of DHA (Mayzaud et al. 1976). Solbakken and
Johnsen (2004) also showed that species from the genuses Pyramimonas, Cryptomonas, Hem-
iselmis, Mantoniella and Pseudoscourfielda are promising candidates with respect to their fatty acid profiles.
3.6 Overview of algal species used for large scale production of
The most common species used for large scale production of biomass are as follows (more de-
tails can be found in Appendix A): Anabaena, Aphanizomenon flos-aquae, Artrospira, Botryococcus braunii, Chaetocero cerato-
sporum, Chaetocero gracilis (= muelleri), Chaetoceros calcitrans, Chlamydomonas reinhard-
tii, Chlorella spp., Chlorococcum spp., Crypthecodinium spp., Cylindrotheca sp., Dunaliella
spp., Haematococcus spp., Isochrysis galbana, Isochrysis sp. (T-iso), Monallanthus salina, Nannochloropsis spp., Nitzschia spp, Odontella aurita, Pavlova lutheri, Phaeodactylum tri-
cornutum, Scenedesmus spp., Schizochytrium spp., Skeletonema costatum, Spirulina spp.,
Tetraselmis spp., Thalassiosira spp.
3.7 Algal species suitable for production of protein and lipid at
Mongstad
Based on the literature there are many species suitable for production of proteins and lipids.
Species such as Nannochloropsis sp, P. tricornutum, Isochrysis sp. (T-iso), I. galbana, P. lu-theri and Chaetoceros spp. are well known species in large scale production and are also suita-
ble candidates for production of protein and lipids.
3.8 Short overview over recent research at UiB
The research at the Department of Biology, University of Bergen that involve algal production
and algae used as feed are located in two different research groups; Marine microbiology (MM) and Fisheries Ecology and Aquaculture(FEA) Researchers from FEA have during recent years worked with large scale algal production in aq-
uaculture; involving developing new production systems for marine hatcheries, optimization of
production systems and nutritional qualities (Briassoulis et al. 2010, Jacobsen et al. 2010; 2011).
Researchers from MM have focused on studying the effects of light, including UV and day
length, and nutrients on growth of phytoplankton and how these factors might influence the
species composition of natural assemblages. In addition work as been done on the importance of mixed-versus stratified water conditions for the growth and vertical distribution of phytoplank-
ton (Erga et al. 2010).
3.9 R & D challenges and recommendations
3.9.1 Production
One of the main challenges in large scale production of microalgae for use as feed will be to in-
crease the dry weight from ca 2% to 20% in order to get a sustainable production. The focus here must be to increase the cell density of the cultures in order to get the maximum dry weight by
optimizing the production. The type of production system and light will in this respect be of im-
Another challenge will be to develop methods for opening the cell wall of the microalgae so that
the proteins and lipids can be easily utilized and digested by fish. Today this is a challenge that
restricts utilization of marine microalgae as feed.
3.9.2 Species
It is recommended to start with a species that is well known, relatively easy to grow with high
growth rates and contains relatively high amounts of protein and lipids. A potential candidate in
this respect is Nannochloropsis sp.
CO2 to Bio/WP3 - Algae
Other species suitable for production and screening of new potential strains will then have to be
tested and developed. Species that can grow mixrotrophic or heterotrophic on glycerol and low
light are potential new candicates.
3.10 Conclusion
a) Optimum culture condition varies between species and strains. Essential parameters
regulating growth are temperature, salinity, nutrients, light, pH, CO2 and mixing.
b) Gross composition differs among micro-algal species, but for many species this is not the
major factor relating to food value. Protein is always the major organic constituent, fol-
lowed by lipid and carbohydrate. Expressed as percentage of dry weight, the range of
protein, lipid and carbohydrate are 12-35%, 7.2-23% and 4.6-23%, respectively.
c) The protein content and quality of all micro-algae is high. Micro-algae have a well bal-
anced amino acid composition and the proportions of individual amino acids do not vary
greatly between different species.
d) Micro-algae are a rich source of carbohydrate and the carbohydrate profiles can vary
greatly between species. The sugar composition is variable; particularly the polysaccha-
rides glucose and mannose, and can in some instances affect the nutritional value.
e) Fatty acids constitute a major proportion of the lipid fraction (20-40% of total lipid). The
essential PUFAs EPA and DHA are key nutrients in animal nutrition, and most algae are
rich in one or both of these acids.
f) Most species contain moderate to high concentrations of EPA. Particularly Pavlova lu-
theri, Phaeodactylum tricornumtum and Nannochloropsis spp. are rich in EPA, while
Isochrysis sp. (T-iso) contains very low levels of this fatty acid.
g) Species that are rich in DHA are I. galbana, I. sp. (T-iso) and P. lutheri. Only one species
is rich in both DHA and EPA and that is P. lutheri.
h) Green algae like Tetraselmis suecica are usually rich in C16 and C18 PUFAs, in addition
to ARA, but low in higher carbon fatty acids like EPA and DHA. This may contribute to
low nutritional value.
i) Micro-algae are rich sources of pigments like chlorophylls and carotenoids. The carote-
noid ß-carotene is a present in most micro-algae. Some other species, like Chlorella spp.
and T. suecica can also produce significant amounts of astaxanthin and luthein, respec-tively.
j) Micro-algae are rich sources of almost all vitamins, particularly the vitamins C (ascorbic
acid) and B (e.g. riboflavin), however some species lack specific vitamins. Because of
this, mixed algal diets are necessary to provide high concentrations of all vitamins.
k) Biochemical composition of micro-algae can be manipulated readily by changing the
growth conditions, but varies between species.
l) The conditions that increase the biomass productivity and the polyunsaturated fatty acid
contents are often in opposition. For instance, optimum production of PUFAs in Phaeo-
dactylum tricornutum is being reached by operating under light limiting conditions.
m) A potential candidate for pilot production at Mongstad could be Nannochloropsis sp.
References
Abu-Rezq T.S., Al-Musallam L., Al-Shimmari J. and Dias P. 1999. Optimum production conditions for
different high-quality marine algae. – Hydrobiologia 403:97-107.
Acién Fernández F.G., García Camacho F., Sánchez Perez J.A., Fernández Sevilla J.M. and Molina Grima
E. 1998. Modelling of biomass productivity in tubular photobioreactors for microalgal cultures: effects of
dilution rate, tube diameter and solar irradiance. - Biotechnol. Bioeng. 58(6):605-616.
Acién Fernández F.G., Hall D.O., Cañizzares Guerrero E., Krishna Rao K. and Molina Grima E. 2003.
Outdoor production of Phaeodactylum tricornutum biomass in a helical reactor. – J. Biotech. 103:137-
Acién Fernández F.G., Sánchez Perez J.A., Fernández Sevilla J.M., García Camacho F. and Molina Grima
E. 2000. Modeling of Eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures
in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. – Biotech. Bio-
eng. 68:173-183.
Antia N.J., Desai I.D. and Romilly M.J. 1970. The tocopherol, vitamin K, and related isoprenoid quinine
composition of a unicellular red alga (Porphyridium cruentum). – J. Phycol. 6:305-312.
Ballantine J.A., Lavis A. and Morris R.J. 1979. Sterols of the phytoplankton – effects of illumination and
growth stage. – Phytochemistry 8:1459-1466.
Beach D.H., Harrington G.W. and Holz G.G.jr. 1970. The polyunsaturated fatty acids of marine and
freshwater cryptomonads. – J. Protozool. 17:501-510.
Becker E.W. 1986. Nutritional properties of microalgae: potential and constraints. In:handbook of micro-
algae mass culture. Richmond A. (ed). CRC Press, Florida, pp.339-419.
Ben-Amotz A., Fishler R. and Schneller A. 1987. Chemical composition of dietary species of marine uni-
cellular algae and rotifers with emphasis on fatty acids. – Mar.Biol. 95:31-36.
Ben-Amotz A., Tornabene T.G. and Thomas W.H. 1985. Chemical profiles of selected species of microal-
gae with emphasis on lipids. – J. Phycol. 21:72-81
Ben-Amotz A., Tornabene T.G., and Thomas W.H. 1985. Chemical profile of selected species of microalgae
with emphasis on lipids. – Journal of Phycology 21:72-81
Boeing P. 2004. Larval feed alternatives. Technical paper. Aquafauna Bio-Marine inc. (USA) 14pp (avail-
Borowitzka M. 1988. Vitamins and fine chemicals from microalgae. In: Micro-Algal Biotechnology. Boro-
witzka M.A. and I.J. (eds). Cambridge University Press, 153-196.
Brown M. R., Mular M., Miller I., Trenerry C. and Farmer C. 1999. The vitamin content of microalgae
used in aquaculture. - J. Applied Phycology, 11: 247–255.
Brown M. R., Skabo S. and Wilkinson B. 1998. The enrichment and retention of ascorbic acid in rotifers
fed microalgal diets. - Aquac. Nutrition, 4: 151–156.
Brown M.R, Graeme A.D., Jeffrey S.W., Volkman J.K., Barrett S.M. and LeRoi J.-M. 1993. The influence
of irradiance on the biochemical composition of the prymnesiophyte Isochrysis sp. (clone T-iso). – Jour-
nal of Phycology 29:601-612.
Brown M.R. 1991. The amino acid and sugar composition of 16 species of microalgae used in mariculture.-
J. exp. mar. Biol. Ecol. 145: 79–99.
Brown M.R. 1995. Effects of storage and processing on the ascorbic acid content of concentrates prepared
from Chaetoceros calcitrans. – J. Appl. Phycol. 7:495-500.
Brown M.R. and Miller K.A. 1992. The ascorbic acid content of eleven species of microalgae used in mari-
culture. – J. Appl. Phycol., 4:205-215.
Brown M.R., Dunstan G.A., Jeffrey S.W., Volkman J.K., Barrett S.M. and LeRoi J.M. 1993. The influence
of irradiance on the biochemical composition of the prymnesiophyte Isochrysis sp. (clone T-iso). –
J.Phycol. 29:601-612.
CO2 to Bio/WP3 - Algae
Brown M.R., Farmer C.L. 1994. Riboflavin content of 6 species of microalgae used in mariculture. - J.
appl. Phycol. 6: 61–65.
Brown M.R., Garland C.D., Jeffery S.W. Jameson I.D. and LeRoi J.M. 1993. The gross and amino acid
compositions of batch and semi-continuous cultures of Isochrysis sp. (clone T.ISO), Pavlova lutheri and
Nannochloropsis oculata. – Journal of Applied Phycology 5:285-296.
Brown M.R., Jeffery S.W. and Garland C.D. 1989. Nutritional Aspects of Microalgae Used in Maricul-
ture; a Literature Review. CSIRO Marine Laboratories Report, Hobart, Australia, 205, 44p.
Brown M.R., Jeffery S.W., Volkman J.K. and Dunstan G.A. 1997. Nutritional properties of microalgae for
mariculture. – Aquaculture 155:315-331.
Brown M.R., Jeffrey S.W. 1992. Biochemical composition of microalgae from the green algal Classes Chlo-
rophyceae and Prasinophyceae.1. Amino acids, sugars and pigments.- J. exp. mar. Biol.Ecol. 161: 91–113.
Caron L., Mortain-Bertrand A. and Jupin H. 1988. Effect of photoperiod on photosynthetic characteristics
of two marine diatoms. – J. Exp. Mar. Biol. Ecol. 123:211-226.
Cerón García M.C., Fernandez Sevilla J.M., Acien Fernandez F.G., Molina Grima E. and Garcia Camacho
F. 2000. Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid pro-
file. – J. Appl. Phycol. 12:239-248.
Chau Y.K., Chuecas L. and Riley J.P. 1967. The component combined amino acids of some marine phyto-
plankton species. – J.Mar Biol. Ass. UK 47:543-544.
Cheng-Wu Z., Zmora O., Kopel R. and Richmond A. 2001. An industrial-sizes flat plate glass reactor for
mass production of Nannochloropsis sp. (Eustigmatophyceae). – Aquaculture 195:35-49.
Chrismadha T. and Borowitzka M.A. 1994. Effect of cell density and irradiance on growth, proximate
composition and eicosapentaenoic acid production of Phaeodactylum tricornutum grown in a tubular
photobioreactor. – J. Appl. Phycol. 6:67-74.
Chu F.E., Dupuy J.L. and Webb K.L. 1982. Polysaccharide composition of five algal species used as food
for larvae of the American oyster, Crassostera virginica. – Aquaculture 29:241-252.
Chuecas L. and Riley J.P. 1969. Component fatty acids of the total lipids of some marine phytoplankton. –
J. Mar. Biol. Ass. UK 49:97-116.
Cohen Z. 1986. Products from microalgae. In: Handbook of microalgal mass culture. Richmond A. (ed).
CRC Press, Florida, pp.421-454.
Cohen Z., Vonshak A. and Richmond A. 1988. Effect of environmental conditions on fatty acid composi-
tion of the red alga Porphyridium cruentum: correlation to growth rate. – J. Phycol. 24:328-332.
Conklin D. E. 1997. Vitamins. In L. R. D'Abramo, D. E. Conklin and D. M. Akiyama (Eds.), Crustacean nu-
trition: Advances in world aquaculture, Vol. 6, World Aquaculture Society, pp. 123–149.
Contreras A., Garcia F., Molina E. and Merchuk J.C. 1998. Interaction between CO2-Mass Transfer, Light
availability, and hydrodynamic stress in the growth of Phaeodactylum tricornutum in a concentric tube air-
lift photobioreactor. – Biotechnology and bioengineering 60(3):317-325.
Cordero Esquivel B. and Voltolina Lobina D. 1996. Nutritional value of preserved microalgae for subadult
Mytilus galloprovincialis. – Journal of the World Aquaculture Society 27:113-118.
Coutteau P. and Sorgeloos P. 1992. The use of alga substitutes and the requirement for live algae in the
hatchery and nursery rearing of bivalve molluscs; An international survey. – J of Shellfish Research
Cowey C.B. and Corner E.D.S. 1966. The amino acid composition of certain unicellular algae and of the
fecal pellets produced by Calanus finmarchicus when feeding on them. In: Some contemporary studies in
Marine science. Barnes H. (ed), George Allen and Unwin, London, pp. 225-231.
D´Souza F.M.L., Lecossois D., Heasman M.P., Diemar J.A., Jackson C.J. and Pendrey R.C. 2000. Evalua-
tion of centrifuged microalgae concentrates as diets for Penaeus monodon Fabricius larvae. – Aquaculture
Research 31:661-670.
De RoeckHoltzhauer Y., Claire C., Bresdin F., Amicel L., Derrien A. 1993. Vitamin, free amino acid and
fatty acid compositions of some marine planktonic microalgae used in aquaculture.- Bot. mar. 36: 321–
De Roeck-Holtzhauer Y., Quere I. and Claire C. 1991. Vitamin analysis of five planktonic microalgae and
one macroalgae. – J. Appl. Phycol. 3:259-264
DePauw N.M., Morales J. and Persoone G. 1984. Mass culture of microalgae in aquaculture systems: pro-
gress and constraints. – Hydrobiologia 116/117:121-134.
Depauw N.M., Persoone G. 1988. Micro-algae for aquaculture. In: Microalgal Biotechnology. Borowitzka
M.A. and L.J. Borowitzka (eds). Cambridge University Press, Cambridge U.K., pp. 197-221.
Dortch Q., Clayton J.R. Jr., Thoresen S.S. and Ahmed S.I. 1984. Species differences in accumulation of
nitrogen pools in phytoplankton. – Mar.Biol. 81:237-250.
Dubinsky Z., Berner T. and Aaronson S. 1978. Potential of large scale algal culture for biomass and lipid
production in arid lands. – Biotech. Bioeng. Symp. 8:51-68.
Dunstan G.A., Volkman J.K., Barrett S.M. and Garland C.D. 1993. Changes in the lipid composition and
maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. –
J.Appl. Phycol. 5:71-83.
Dunstan G.A., Volkman J.K., Barrett S.M., Leroi J.M., Jeffrey S.W. 1994. Essential polyunsaturated fatty
acids from 14 species of diatom (Bacillariophyceae). - Phytochemistry 35: 155–161.
Dunstan G.A., Volkman J.K., Jeffrey S.W., Barrett S.M. 1992. Biochemical composition of microalgae
from the green algal Classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. - J. exp.
mar. Biol. Ecol. 161: 115–134.
Ellertsen B., Solemdal P., Strømme T., Tilseth S., Westgaard T., Moksness E. and Øiestad 1980. Some
biological aspects of cod larvae (Gadus morhua L.) – Fiskeridirektoratets Skrifter, Serie Havundersøkel-
Enright C.T., Newkirk G.F., Craigie J.S. and Castell J.D. 1986a. Evaluation of phytoplankton as diets for
juvenile Ostrea edulis L. – J. Exp. Mar. Biol. Ecol., 96:1-13.
Enright C.T., Newkirk G.F., Craigie J.S. and Castell J.D. 1986b. Growth of juvenile Ostrea edulis L. fed
Chaetoceros calcitrans Schütt of varied chemical composition. – J. Exp. Mar. Biol. Ecol., 96:15-26.
Erga, S.R., Lie, G.C., Aarø, L.H., Aursland, K., Olseng, C.D., Frette, Ø., Hamre, B. 2010. Fine scale vertical
displacement of Phaeodactylum tricornutum (Bacillariophyceae) in stratified waters: Influence of halo-
cline and day length on buoyancy control.- J. Exp. Mar. Biol. Ecol. 384: 7-17
Epifanio C.E. 1979. Growth in bivalve molluscs: nutritional effects of two or more species of algae in diets
fed to the American oyster Crassostrea virginica (Gmelin) and the hard clam Mercenaria mercenaria
(L.) – Aquaculture 18:1-12.
Epifanio C.E., Valenti C.C. and Turk C.L. 1981. A comparison of Phaeodactylum tricornutum and Thalas-
siosira pseudonana as foods for the oyster, Crassostrea virginica. – Aquaculture 23:347-353.
Fabregas J. and Herrero C. 1986. Marine microalgae as a potential source of minerals in fish diets. - Aq-
uaculture 53: 237–243.
Fabregas J., Herrero C., Albade J., Liano R. and Cabezas B. 1985. Biomass production and biochemical
variability of the marine microalgae Dunaliella tertiolecta (Butcher) with high nutrient concentrations. -
Aquaculture 53:187-199.
Fabregas J., Herrero C., Cabezas B. and Abalde J. 1985. Biomass production and biochemical variability
of the marine microalgae Tetraselmis suecica Kylin (Butch) with high nutrient concentrations. - Aquacul-
ture 49:231-244.
Fabregas J., Herrero C., Cabezas B. and Abalde J. 1986. Biomass production and biochemical composition
in mass cultures of the marine microalga Isochrysis galbana Parke at varying nutrient concentrations. –
Aquaculture 53:101-113.
Fabregas J.C., Herrero C., Abalde J. and Cabezas B. 1985. Growth chlorophyll a and protein of the marine
microalgae Isochrysis galbana in batch cultures with different salinities and high nutrient concentra-
tions. – Aquaculture 50:1-11.
Fisher N.S. 1985. Accumulation of metals by marine picoplankton. – Mar. Biol. 87:137-142.
Fried A., Tietz A., Ben-Amotz A. and Eichenbergen W. 1982. Lipid composition of the halotolerant alga,
Dunaliella bardawil. – Biochem. Biophys. Acta 713:419-426.
CO2 to Bio/WP3 - Algae
Gara B., Shields R. J. and McEvoy L. 1998. Feeding strategies to achieve correct metamorphosis of Atlan-
tic halibut, Hippoglossus hippoglossus L., using enriched Artemia.- Aquaculture Research, 29: 935–948.
Goldman J.C. 1977. Temperature effects on phytoplankton growth in continuous culture. – Limnol.
Oceanogr. 22:932-935.
Goldman J.C. 1979. Temperature effects on steady state growth, phosphorus uptake, and the chemical
composition of a marine phytoplankter. – Microbiol. Ecol. 5:153-166.
Guckert J.B. and Cooksey K.E. 1990. Triglyceride accumulation and fatty acid profile changes in Chlorella
(chlorophyta) during high pH-induced cell cycle inhibition. – J. Phycol. 26:72-79.
Guillard R.R.L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: smith W.L., Chanley
M.H. (eds). Culture of Marine Invertebrate Animals. Plenum Press, New York, p. 29-60.
Handa N. and Yanagi K. 1969. Studies on water extractable carbohydrates of the particulate matter from
the northwest Pacific Ocean. – Mar. Bio. 4:197-207.
Hayashi T., Suitani Y., Murakami M., Yamaguchi K., Konosu S. and Noda H. 1986. Protein and amino
acid composition of five species of marine phytoplankton. – Bull.Jpn Soc. Sci. Fish. 52:337-343.
Herrero C., Angeles C., Fabregas J. and Abalde J. 1991. Yields in biomass and chemical constituents of
tour commercially important marine microalgae with different culture media. – Aquacult. Eng. 10:99-110.
Hintz H.F., Heitmann H., Weird W.C., Torrell D.T. and Meyer J.H. 1966. Nutritive value of algae grown
on sewage. – J. Anim. Sci. 25:675-681.
Hitchcock, G.L., Goldman J.C. and Dennet M.R. 1986. Photosynthate partitioning in cultured marine
phytoplankton: metabolic patterns in a marine diatom under constant and variable light intensities. –
Mar. Ecol. Prog. Ser. 30:77-84.
Hoek, C. V. D., D. G. Mann and H. M. Jahns. 1995. Algae: An Introduction to Phycology. New York, N.Y.:
Cambridge University Press.
Hollick M.F. 1984. Isolation and identification of provitamin D2 and previtamin D2. In: Biotechnology in
the marine sciences. Proceedings of the first annual MIT sea grant lecture and seminar. Colwell R.R, Sins-
key A.J. and Pariser E.R. (eds). John Wiley and sons, New york, pp. 197-206.
Hu H. and Gao K. 2003. Optimization of growth and fatty acid composition of an unicellular marine pico-
plankton, Nannochloropsis sp., with enriched carbon sources. – Biotechnology Letters 25:421-425.
James C.M., Al-Hinty S. and Salman A.E. 1989. Growth and ω3 fatty acid and amino acid composition of
microalage under different temperature regimes. – Aquaculture 77:337-357.
Jones D.A., Kurmaly K., Arshard A. 1987. Paneid shrimp hatchery trials using microencapsulated diets. –
Aquaculture 64:133-164.
Kanazawa A. 1969. On the vitamin B of a diatom Chaetoceros simplex as the diet for the larvae of marine
animals. Mem. Fac. Fish. Kagoshima Univ. 18:93-97.
Kim Nag-Jong., Suh I.S., Hur B. and Lee C. 2002. Simple monodimensional model of linear growth rate
of photosynthetic microorganism in flat-plate photobioreactors.- Journal of Microbiology and Biotech-
nology. 12(6):962-971.
Knauer J. and Southgate P.C. 1999. A review of the nutritional requirements of bivalve and the develop-
ment of alternative and artificial diets for bivalve aquaculture. – Reviews in Fisheries Sciences 7:241-280.
Knauer J., Barrett S. M., Volkman J. K. and Southgate P. C. 1999. Assimilation of dietary phytosterols by
Pacific oyster Crassostrea gigas spat. - Aquaculture Nutrition, 5: 257–266.
Kommareddy, A. and G. Anderson. 2003. Study of light as a parameter in the growth of algae in Photo-
Bio Reactor. ASAE Paper No. 034057. Las Vegas, Nevada: ASAE.
Koven W.M., Kissil G.W.M. and Tandler A. 1989. Lipid and n-3 fatty acid requirement of Sarus aurata
larvae during starvation and feeding. – Aquaculture 79:185-191.
Laing I. 1991. Cultivation of marine unicellular algae. MAFF Laboratory Leaflet Number 67. Directorate of
Fisheries Research Lowestoft, UK. 31 pp.
Langdon C.J. and Waldock M.J. (1981). The effect of algal and artificial diets on the growth and fatty acid
composition of Crassostrea gigas spat. – J.Mar. Biol.Ass. U.K. 61:431-448.
Lavens P. and Sorgeloos P. 1996. Manual on the production and used of live food for aquaculture. FAO
Fisheries Technical Paper 361.
Leirvoll J.R., Sørebø K. AND sørheim T. 2001. Ulike anrikningsmedier og innvirkningen de har på næ-
ringssammensetningen og vekstrater hos hjuldyr (Brachionus plicatilis). – Bachelor thesis, Sogn and
Fjordane University College, Norway, 85 p. (in Norwegian).
Lin D.S., Illias A.M., Connor W.E., Caldwell R.S., Cory H.T. and Davis G.D. 1982. Composition and bio-
synthesis of sterols in selected marine phytoplankton. – Lipids 17:818-824.
Liu C.-P. and Lin L.-P. 2001. Ultrastructural study and lipid formation of Isochrysis sp. CCMP1324. – Bot.
Bull. Acad. Sin. 42:207-214.
Marlowe I.T. 1984. Long chain (n-C37-C39) alkenones in the Prymnesiophyceae. Distribution of
alkenones and other lipids and their taxonomic significance. – Br. Phycol. J. 19:203-216.
Mayzaud P., Eaton C.A. and Ackman R.G. 1976. The occurrence and distribution of octadecapentaenoic
acids in a natural plankton population. A possible food chain index. - Lipids 11:858–862.
Merchuk J.C. and Wu X. 2003. Modeling of photobioreactor: Application of bubble column simulation. –
Journal of Applied Phycology 15:163-169.
Moffatt N.M. 1981. Survival and growth of northern anchovy larvae on low zooplankton densities as af-
fected by the presence of a Chlorella bloom. – Rapports et Proces-Verbaux des Reunions Conseil Interna-
tional pour l'Exploration de la Mer 178:475-480.
Molina E., Fernandez J., Acien F.G. and Chisti Y. 2001. Tubular photobioreactor design for
Molina G.E., Sanchez P.J.A., Garcia C.F., Fernandez S.J.M., and Fernandez A.F.G. 1994. Effect of growth
rate on the eicosapentaenoic acid and docosahexaenoic acid content of Isochrysis galbana in chemostat
culture.- Microbiol. Biotechnol. 41: 23-27.
Molina Grima E, Acien Fernández F.G., García Camacho F., Rubio F. and Christi Y. 2001. Scale-up of
tubular photobioreactors. – J. Appl. Phycol. 12:355-368.
Molina Grima E., Sánchez Péres J.A., García Camacho F., Fernández Sevilla J.M. and Acién Fernández
F.G. 1996. Productivity analysis of outdoor chemostat culture in tubular air-lift photobioreactors. – J.
Appl. Phycol. 8:369-380.
Molina Grima E., Sánchez Perez J.A., García Camacho F., Acién Fernández F.G., López Alonso D. and
Segura del Castillo C.I. 1994. Preservation of the marine micro-alga, Isochrysis galbana: influence on the
fatty acid profile. – Aquaculture 134:81-90.
Montaini E., Chini Zittelli G., Tredici M.R., Molina Grima E., Fernández Sevilla J.M. and Sánchez Pérez
J.A. 1995. Long-term preservation of Tetraselmis suecica: influence of storage on viability and fatty acid
profile. – Aquaculture 134:81-90.
Myklestad S. 1974. Production of carbohydrates by marine planktonic diatoms. 1. Comparison of nine
different species in culture. – J. Exp. Mar. Biol. Ecol. 15:261-274.
Nell J.A. 1993. The development of oyster diets. – Aust.J.Agric.Res. 44:557-566.
Nell J.A., Diemar J.A., Heasman M.P. 1996. Food value of live yeats and dry yeast-based diets fed to Syd-
ney Oyster Saccostrea commercialis spat. – Aquaculture 145:236-243.
Nichols P. D., Holdsworth D. G., Volkman J. K., Daintith M. and Allanson S. 1989. High incorporation
of essential fatty acids by the rotifer Brachionus plicatilis fed on the prymnesiophyte alga Pavlo-
va lutheri.- Aust. J. Mar. Freshwater Res., 40:645–655.
Nichols P.D., Volkman J.K., Hallegraeff G.M. and Blackburn S. I. 1987. Sterols and fatty acids of the red
tide flagellates Heterosigma akashiwo and Chattonella antique (Raphidophyceae). – Phytochem.
O Connor W.A. and Nell J.A. 1992. The potential of algal concentrates as food for the production of Aus-
tralian bivalves. In: Proceedings of the Aquaculture Nutrition Workshop, Salamander Bay, NSW, Austral-
ia 15-17 April 1991. Allan G.L. and Dall W. (eds). NSW Fisheries Brackish Water and Shellfish Culture
Research Station, Salamander Bay, Australia, pp 200-201.
Ocrutt D.M. and Patterson G.W. 1975. Sterol, fatty acid and elemental composition of diatoms grown in
chemically defined media. – Comp. Biochem. Physiol. 50B:579-583.
CO2 to Bio/WP3 - Algae
Palanisamy, V., Latif, F.A. and Resat, R.B.M. 1991. A guide on the production of algal culture for use in
shrimp hatcheries. National Prawn Fry Production and Research Centre, Pulau Sayak, Kedah, Depart-
ment of Fisheries, Ministry of Agriculture, Malaysia. 23 pp.
Parsons T.R., Stephens K. and Strickland J.D.H. 1961. On the chemical composition of eleven species of
marine phytoplankton. – J.Fish.Res.Bd.Can. 18:1001-1016.
photoinhibition processes. - Chemical Engineering Science. 56:3527-3538.
Provasoli L. and Carlucci A.F. 1974. vitamins and growth regulators. In: Alga; Physiology and biochemis-
try. Stewart W.P.D. (ed). Blackwell scientific, Oxford, pp.741-787.
Redalje D.G. and Laws E.A. 1983. The effects of environmental factors on growth and the chemical and
biochemical composition of marine diatoms. 1. Light and temperature effects. J. Exp. Mar. Biol. Ecol.
Renaud S.M. and Parry D.L. 1994. Microalgae for use in tropical aquaculture. II: effect of salinity on
growth, gross chemical composition and fatty acid composition of three species of marine microalgae. – J.
Appl. Phycol. 6:347-356.
Renaud S.M., Parry D.L., Luong-Van T., Kuo C. and Sammy N. 1991. Effect of light intensity of the proxi-
mate composition and fatty acid composition of Isochrysis sp. And Nannochloropsis oculata for use in
tropical aquaculture. J. Appl. Phycol. 3:43-53.
Richmond A. 2004. Handbook on Microalgal Culture: Biotechnology and Applied Phycology. Iowa State
Press, Iowa: Blackwell Publishing.
Robinson R.K. and Guzman-Juarez M. 1978. The nutritional potential of the algae. Plant Foods for Man
Rodríguez C., Pérez J. A., Badía P., Izquierdo M. S., Fernández-Palacios H. and Lorenzo Hernández A.
1998.The n-3 highly unsaturated fatty acid requirements of gilthead seabream (Sparus aurata L.) larvae
when using an appropriate DHA/EPA ratio in the diet. - Aquaculture, 169: 9–23.
Roncarati A., Meluzzi A., Acciarri S., Tallarico N. and Melotti P. 2004. Fatty acid composition of different
microalgae strains (Nannochloropsis sp., Nannochloropsis oculata (Droop) Hibberd, Nannochloris
atomus Butcher and Isochrysis sp.) according to the culture phase and the carbon dioxide concentration.
– J.World Aquac. Soc. 35:401-411.
Rønnestad I, Helland S. and Lie Ø. 1998. Feeding Artemia to larvae of Atlantic halibut (Hippoglossus
hippoglossus L.) results in lower larval vitamin A content compared with feeding copepods.- Aquaculture,
165: 159–164.
Sakaguchi T., Nakajima A. and Horikoshi T. 1981. Studies on the accumulation of heavy metal elements in
biological systems. 18. Accumulation of molybdenum by green microalgae. – Appl. Microbiol. Biotech.
Sanchez S., Martinez M. and Espinola F. 2000. Biomass production and biochemical variability of the
marine microalga Isochrysis galbana in relation to culture medium. – Biochem. Eng. J. 6:13-18.
Sargent J.R., McEvoy L.A. and Bell J.G. 1997. Requirements, presentation and sources of polyunsaturated
fatty acids in marine fish larval feeds. Aquaculture 155:117-127.
Savidge G. 1980. Photosynthesis of marine phytoplankton in fluctuating light regimes – Mar. Biol. Lett.
Scura E.D. and Jerde C.W. 1977. Various species of phytoplankton as food for larval northern anchova,
Engraulis mordaz, and relative nutricional value of the dinoflagellates Gymnodinium splendens and Go-
nyaulax polyedra. – Fishery Bulletin U.S. Department of Commerce 75:577-583.
Seguineau C., Laschi-Loquerie A., Moal J. and Samain J. F. 1996. Vitamin requirements in great scallop
larvae. - Aquacult. Int., 4:315–324.
Sheffer M., Fried A., Gottlieb H.E., Tietz A. and Avron M. 1986. Lipid composition of the plasma-
membrane of the halotolerant alga, Dunaliella bardawil. Biochim. Biophys. Acta 857:165-172.
Sicko-Goad L., Simmons M.S., Lazinsky D. and Hall J. 1988. Effect of light cycle on diatom fatty acid
composition and quantitative morphology. – J. Phycol. 24:1-7.
Solbakken J. and Johnsen T. 2004. Development of a production concept for high-concentrated algae
products base don cold water algae species for use in marine larval rearing; selection of species, fatty acid
profiles, conceptual description, profitability and further development. – Akvaplan-Niva Report APN-
Sukenik A. and Wahnon R. 1991. Biochemical quality of marine unicellular algae with special emphasis on
lipid composition. I. Isochrysis galbana. – Aquaculture 97:61-72
Sukenik A., Carmeli Y. and Berner T. 1989. Regulation of fatty acid composition by irradiance level in the
eustigmatophyte Nannochloropsis sp. J. Phycol. 25:686-692.
Tacon A. G. J. 1991. Vitamin nutrition in shrimp and fish. In D. M. Akiyama and R. K. H. Tan (Eds.), Proc.
of the Aquaculture Feed Processing and Nutrition Workshop, Thailand and Indonesia, September 1991
pp. 10–41. Singapore: American Soybean Association.
Tamaru C. S., Murashige R. and Lee C.-S. 1994. The paradox of using background phytoplankton during
the larval culture of striped mullet, Mugil cephalus L.-Aquaculture, 119: 167–174.
Teshima S. Kanazawa A. and Yamashita A. 1986. Dietary value of several proteins and supplemental ami-
no acids for larvae of the prawn Penaeus japonicus. – Aquaculture 51:225-235.
Thompson P.A., Guo M.-X. and Harrison P.J. 1992. Effects of variation in temperature. I. On the bio-
chemical composition of eight species of marine phytoplankton. – J. Phycol. 28:481-488.
Thompson P.A., Guo M.-X. and Harrison P.J. 1993. The influence of irradiance on the biochemical com-
position of three phytoplankton species and their nutritional value for larvae of the Pacific oyster
(Crassostrea gigas). – Mar.Biol., 117:259-268.
Thompson P.A., Harrison P.J. and Parslow J.S. 1991. Influence of irradiance on cell volume and carbon
quota for ten species of marine phytoplankton. – J. Phycol. 27:351-360.
Thompson P.A., Harrison P.J. and Whyte J.N.C. 1990. Influence of irradiance on the fatty acid composi-
tion of phytoplankton. – J. Phycol. 26:278-288.
Torrisen O.J. and Christiansen R. 1995. Requirements for carotenoids in fish. – Appl. Ichtyol. 11:225-230.
Tzovenis I., De Pauw N. and Sorgeloos P. 2003. Optimisation of T-ISO biomass production rich in essen-
tial fatty acids I. Effect of different light regimes on growth and biomass production. – Aquaculture
Van der Meeren T. 1991. Algae as first food for cod larvae, Gadus morhua L.: filter feeding or ingestion by
accident? – Journal of Fish Biology 39:225-237.
Volkman J.K., Brown M.R., Dunstan G.A., Jeffrey S.W. 1993. The biochemical composition of marine
microalgae from the Class Eustigmatophyceae. - J. Phycol. 29: 69–78.
Volkman J.K., Dunstan G.A., Barrett S.M., Nichols P.D. and Jeffrey S.W. 1992. Essential polyunsaturated
fatty acids of microalgae used as feedstocks in aquaculture. In: G.L. Allan and W. Dall (Eds), Proceedings
of the National Aquaculture Workshops, Pt. Stephens, NSW Australia, April 1991, pp. 180-186.
Volkman J.K., Dunstan G.A., Jeffrey S.W., Kearney P.S. 1991. Fatty acids from microalgae of the genus
Pavlova.- Phytochemistry 30:1855-1859.
Volkman J.K., Eglinton G., Corner E.D.S. and Forsberg T.E.V. 1980. Long chain alkenes and alkenones in
the marine coccolithophorid Emilinaia huxleyi. Phytochemistry 19:2619-2622.
Volkman J.K., Jeffrey S.W., Nichols P.D., Rogers G.I.,Garland C.D. 1989. Fatty acid and lipid composition
of 10 species of microalgae used in mariculture.- J. exp. mar. Biol. Ecol. 128: 219–240.
Volkman J.K., Smith D.J., Eglinton G., Forsberg T.E.V., Corner E.D.S. 1981. Sterol and fatty acid composi-
tion of four marine haptophycean algae. - J. mar. biol. Ass., U.K. 61: 509–527.
Vonshak, A. 1986. Laboratory techniques for the cultivation of microalgae. In: CRC Handbook of microal-
gal mass culture. Richmond A. (Ed.). CRC Press, Inc., Boca Raton, Florida, USA, pp 117-145.
Waldock M.J. and Nascimento I.A. 1979. The triacylglycerol composition of Crassostrea gigas larvae fed
on different diets. – Mar. Biol. Lett. 1:77-86.
Walsh P. and Legendre L. 1983. Photosynthesis of natural phytoplankton under high frequency light fluc-
tuations simulating those induced by sea surface waves. – Limnol. Oceanogr. 28:688-697.
CO2 to Bio/WP3 - Algae
Webb K.L. and Chu F.E. 1983. Phytoplankton as a source for bivalve larvae. In: Pruder G.D., Langdon C.
and Conklin D. (Eds). Biochemical and Physiological Approaches to shellfish Nutrition. Proceedings of
the Second International Conference on Aquaculture Nutrition, Louisiana State University, Baton Rouge,
Whyte J.N.C. 1987. Biochemical composition and energy content of six species of phytoplankton used in
mariculture of bivalves. – Aquaculture 60:231-241.
Wikfors G.H., Twarog J.W. and Ukeles R. 1984. Influence of chemical composition of algal food sources
on growth of juvenile oysters, Crassostrea virginica. – Biol. Bull. 167:251-263.
Wu X. and Merchuk J.C. 2001. A model integrating fluid dynamics in photosynthesis and and photoinhi-
bition processes. – Chemical Engineering Science 56:3527-3538.
Specific growth rates of algae (available online). Zhu C.J., Lee Y.K. and Chao T.M. 1997. Effects of temperature and growth phase on lipid and biochemical
composition of Isochrysis galbana TK1. – J. Appl. Phycol. 9:451-457.
Aaronson S., De Angelis B., Frank O. and Baker H. 1971. Secretion of vitamins and amino acids into the
environment by Ochromonas danica. – J. Phycol. 7:21
CO2 to Bio
Work Package 4 - Biomass applications Ver 141111
CO2 to Bio/WP4 – Biomass applications
Katerina Kousoulaki, Øistein Høstmark, HenningEgede-Nilssen, Åge Otterhals, Eyolf Langmyhr, Tor Andreas Samuelsen, Gjermund Vogt & Trond Mork Pedersen
Contents
4.1 Introduction _ 4
4.2 Evaluation of the alternative utilisation of nutrients from micro-algae biomass 5
4.2.1 Micro-algal biomass as aqua-feed raw material _ 5
4.3 Microalgal lipids for use in aqua feeds & the production of ω-3 8
4.3.1 Algal oils for aqua feeds _ 8 4.3.2 Algal oil for EPA/DHA concentrates & nutritional supplements 9
4.4 Algae as a nucleotide source 10
4.5 Algae as a source of fine chemicals: Pigments & antioxidants 10
4.6 Algae and environmental pol utants _ 12
4.7 Harvesting and concentration technology 12
4.7.1 Micro-algae harvesting and dewatering _ 12 4.7.2 Micro-algae biomass preservation and storage _ 13
4.8 Micro-algae biomass processing _ 13
4.8.1 Effects of drying method on end product quality 15 4.8.2 Integrated harvesting & processing (dewatering) of micro algae biomass 16
a) Harvesting methodology: The Flottweg process _ 16
b) Harvesting and dewatering methodology: The Algaetech process _ 17
4.9 Micro-algal oil production 18
4.9.1 Algal oil extraction technologies 18
a) Mechanical cel disruption _ 18 b) Solvent extraction 18 c) Supercritical fluid extraction 18 d) Pulse electric field technology (PEF) _ 19 e) Ultrasound/ sonnication _ 19
4.9.2 Suggestion of best up-scalable oil extraction technology _ 19 4.9.3 Micro-algal oil technology platform 20
4.10 Comparison of different algal species & priority suggestion _ 21
4.11 Chal enges & Recommendations _ 27
4.11.1 Reduction of algae biomass harvesting and processing costs _ 27 4.11.2 Maintain balance between production cost & product quality 27
CO2 to Bio/WP4 – Biomass applications
4.1 Introduction
Five decades ago, the mass production of protein-rich micro-algae was considered as a possi-
bility to close the predicted so called "protein gap". Comprehensive analyses and nutritional
studies demonstrated that these algal proteins can be of high quality and comparable to con-
ventional vegetable proteins. However, due to high production costs as well as technical diffi-
culties to incorporate the algal material into palatable food preparations, the propagation of
algal protein is still in its infancy whereas the majority of micro-algal preparations are mar-keted as health food, as cosmetics or as juvenile animal feed. To date, a large number of algae production endeavours around the world are in development
with main scope the production of bio-fuels. Moreover, there is an urgent need for the estab-
lishment of sustainable sources of highly unsaturated fatty acid (HUFA) rich oils for the food
and aqua-feed markets. The food, food additives and aqua feed industries, are nowadays very
advanced technologically and base their business profitability and sustainability to in depth scientific knowledge of human and animal physiology, nutrition, health and raw material and
process quality. As fish oil resources are limited, it is a common understanding among the
food production chain stakeholders that micro-algal biomass and/or algal oils will play an
important role as raw materials in particular for the production of DHA/EPA concentrates
and/or healthy fish, rich in ω-3 fatty acids. Micro-algae biomass is not a defined raw material of a standard range of qualities and the
limited products currently available do not necessary represent the future markets' raw mate-rials. Today's production includes small quantities, of different species and characteristics
more or less suitable for the different applications. The scale is low compared to the needs
and ambitions and the production technology not yet cost efficient. Products with very high
protein quality but low amounts or of low nutritive value oil, or the other way around cannot
be cost efficient. As micro-algae biomass production moves towards large scale efficient modules, unpredicta-ble changes may occur to the end products' characteristics. In order to meet the challenge of
including micro-algae biomass as a significant raw material in fish feed and in particular
salmon and trout feed formulations, it is a prerequisite to provide extensive documentation
of a number of welfare, quality and efficiency aspects related to each product. It is wise to
promptly evaluate the desired characteristics by further screening existing micro-algae raw
materials in order to be early on in position to steer large scale micro-algae production focus-ing on desired end product quality. Following micro-algae production the research and de-
velopment challenges to be met with regard to micro-algae biomass product development
Optimisation of biomass harvesting, separation, dewatering, processing and drying
which are technologically complicated processes involving considerable costs and af-
fecting the end product quality
Optimisation of nutrient and micronutrient levels and digestibility of micro-algae bi-
omass end products (omega-3 fatty acids, protein, vitamins, pigments, antioxidants)
Establish the nutritional and toxicological guidelines for the use of algal biomass as
raw material in animal/fish feeds
Development, adaptation and optimisation of the oil extraction methodologies for the
different micro-algal biomass products
Establish economic cost-benefit parameters based on full size long-term operated sys-
4.2 Evaluation of the alternative utilisation of nutrients from
micro-algae biomass
Micro-algae biomass is an interesting raw material for a number of different purposes. How-
ever, given the existing technology and the current micro-algae biomass production costs,
high value products such as EPA/DHA concentrates and protein and oil rich dry or semi
moist products for aquaculture feeds have the greatest commercial potential in the short
4.2.1 Micro-algal biomass as aqua-feed raw material
Micro-algae biomass can serve as both a protein and omega-3 rich oil raw material in aqua-
feeds with special properties such as antioxidant, immune stimulation, colour enhancement
& feeding stimulation. Thus, there is a significant potential for substitution of both fish meal
and fish oil in aqua-feeds by the use of micro-algae biomass. However, before the algal prod-
uct suppliers and aqua-feed producers can be in position to market and further include mi-
cro-algal products in fish feed formulations, documentation must become available proving
that these products are comparable to fish meal and fish oil, in terms of nutritional quality, technical properties and price. NFR (Norskforskningsrådet) recently funded the project ALGAFEED "Potential of using mi-
cro-algae to partially replace fish oil and fish meal in aquaculture fish feeds", where several
Norwegian organisations were involved:
Institutt for Plante og Miljøvitenskap, Universitetet for Miljø og Biovitenskap, Ås Aquaculture Protein Centre NOFIMA Institutt for biologi, Norges teknisk Naturvitenskapelige Universitet, Trondheim SINTEF Fiskeri og havbruk AS, Trondheim).
The project concluded that micro-algae have low to very low (ref. 25) protein-(18.8-79.9%)
and oil digestibility measured by mink. Moreover, reduced growth performance in both
salmon and cod was observed when algal biomass was included at 6% and 12% in the diets.
The participants concluded that there is a need for pre-processing before dewatering if algae
based products are to be used as raw materials in fish feed. Currently micro-algae are used solely in fish larvae nutritional applications which represent
low volumes and different economic considerations as compared to grower fish feed applica-tions (Table 4.1). In the year 1999, the production of micro-algae for aquaculture reached
1,000 tonnes (29, 30).
Table 4.1 Commercial algal culture and its commercial applications today (ref 28)
Purpose Growing rotifers and in fin fish hatcheries, used in reef tanks for feeding
Nannochloropsis Small green algae
corals and other filter feeders, very high EPA level
Small golden-brown flagellate, Used to increase the DHA/EPA levels in broodstock, oysters, clams, mus-
very difficult to grow so it is not sels and scallops, sterol composition so it is popular with cold water fish
produced by many hatcheries hatcheries (cod) for enriching rotifers
Enrichment of zooplankton such as Artemia, used in shellfish hatcheries
and used in some shrimp hatcheries, good size for feeding brine shrimp
Small golden-brown flagellate and copepods, oysters, clams, mussels, and scallops
Excellent feed for larval shrimps and contains natural amino acids that
stimulate feeding in marine animals, used in conjunction with Nannochlo-
ropsis for producing rotifers, good size for feeding brine shrimp, standard
feed for oysters, clams, mussels, and scallops, excellent feed for increas-
Large green flagellate
ing growth rates and fighting zoea syndrome Used in the shrimp and shellfish larviculture, considered by several hatch-
eries to be the single best alga for larval shrimps, also good for feeding
copepods and brine shrimps, post-set (200 l and larger) oysters, clams,
mussels, and scallops for broodstock conditioning
Small green flagellate
Used to increase vitamin levels in shrimp hatcheries and for the coloration
CO2 to Bio/WP4 – Biomass applications
Fig 4.1: Nofima biolab offers analytical services in the fields of chemistry, microbiology, biology and physical properties and is a
member of the Nofima lipid platform.
a) Chemical composition
In the future, micro-algal biomass products targeting fish feed market have to meet a series of quality criteria including protein content and quality, carbohydrate content and quality,
ligno-cellulosics content, dry matter, anti-nutritional factors, presence and levels of toxic
b) Nutritional value
The biological value of micro-algae biomass in terms of: protein quality, amino acid profile
and essential amino acid composition, lipid quality, essential/non-essential fatty acid and lipid class profile are raw material quality parameters that need to be evaluated. The protein digestibility of micro-algal biomass products must be tested in the targeted
farmed aquatic species (e.g. salmon, trout, tilapia, sea bass, sea bream, pangasius etc.), chem-
ically or in the mink digestibility test. The performance of fish fed increasing levels of the new
raw material in the feed must be evaluated in terms of fish health, growth and feed conver-
sion efficiency. The final product quality in terms of fish fillet yield, physical quality and composition must also be evaluated. Quality parameters of the fish such as texture, gaping,
pH, condition factor, fat and pigment should also be recorded when new plant protein and oil
raw materials are used in feeds for fish. Chemical analysis and nutritional characteristics of
the fish muscle must also be recorded.
True protein digestibility in mink, % (N=10)
Fig 4.2: The correlation between protein digestibility in mink and salmon are higher than 0.95
Fig 4.3: Nofima fish trial facilities at Sunndalsøra.
c) Pigment content
Some pigments present in micro-algal products are desired: e.g. astaxanthin, whereas some
others may be detrimental to end product quality and should be avoided or removed: e.g.
lutein like pigments (ref.1-2 & 9).
d) Technical properties in the extrusion process & pellet quality
The extrusion of fish feed is a thermal process involving complex chemical and physical
changes in the feed raw materials. In order for the feed mass to be pressed through the die in
the extruder, it must be transformed into a liquid mass (melt) by supplying mechanical and
thermal energy. The melt expands after passing through the die creating a pellet with a
"locked" structure. Pellet durability and the degree of expansion is controlled by a combina-
tion of the amount of energy supplied to the feed mass via the extruder in the form of heat from steam and friction, the amount of water added and the functional properties of the feed
mass. In the extrusion process lipids (>13 – 15%) will prevent water uptake to the dry raw
material and also "oil" the system. This will lower viscous heat dissipation and result in low
expansion and poor durability of the feed product. As a consequence most of the lipids there-
fore have to be coated after drying of the extruded feed pellet. This is done in a vacuum coater
(36, 37, 38 & 39). Algal biomass is a promising new ingredient with potential pellet binding properties. The
quality and quantity of carbohydrates as well as the protein and peptide properties in the
algal products may be decisive for the rheological properties of the feed ingredient mixtures. Some species may have good gel formation properties whereas others cause competitive in-
teractions and thermodynamic incompatibility effects with other proteins, e.g. pea protein
(ref 4). The physicochemical and rheological properties of the algal biomass will vary related
to species and processing methods and conditions. This variation may impact the extrusion process and the physical pellet quality. Basic knowledge of the technical properties of algal
biomass is vital in order to secure uniform and high pellet quality. This will have a positive
economical and environmental effect due to reduced product loss and lower discharge of nu-
trients to the aqueous environment.
a)
b)
c)
CO2 to Bio/WP4 – Biomass applications
Fig 4.4: Nofima feed technology center. a: Pilot scale extruder, b & c: Aqua feed pellet quality evaluation equipment.
e) Cost efficiency
The micro-algal products should be economical to use in fish feed formulations. Feasibility
studies involving the different production and processing technologies must be in place be-
fore commercialisation.
4.3 Microalgal lipids for use in aqua feeds & the production of
4.3.1 Algal oils for aqua feeds
Highly unsaturated fatty acids of the ω-3 family are increasingly in focus regarding the
interaction between nutrition and good health (41, 42, 43, 44, 45, 46, 47, and 48). Humans
and marine fish species possess very low capacity of producing DHA and EPA (40) and thus
need to get these essential fatty acids from the diet. Fish, and in particular fatty fish, such as salmon and trout are a very important source of ω-3 fatty acids for human nutrition. Fish get
the ω-3 fatty acids from their pray organisms (smaller fish, crustaceans, molluscs or fish feed
containing fish oil in aquaculture) which in turn are enriched by consuming micro-algae
which are the primary natural producers of long chain polyunsaturated fatty acids (LC-
PUFA) including EPA and DHA. Micro-algae are in the bottom of the food pyramid and very
high photosynthetic capacity. In that sense, harvesting ω-3 fatty acids directly from micro-algae is most environmentally efficient and sustainable production practice. Fish oil from fisheries, is a limited resource and cannot support any further growth of the
salmon industry. Other future potential sources of ω-3 fatty acids are krill, other non-
exploited fish resources, transgenic (GM) producing plants (49, 50, 51 and 52) and yeast
(53). Krill harvesting and processing for the production of krill oil results in a raw material
which is at this stage too costly for being incorporated in fish diets. Moreover, there is a very
long way including both technological and legislative/administrative challenges, from genetic engineering first findings to full size crop cultivation and the global raw material markets.
Thus, micro-algae offer a promising vegetative and non-polluted resource for bio-technology
and bio-engineering of LC-PUFA production as an alternative to fish oil. Today, a significant number of microalgal culture endeavours are in progress around the
world and they are getting closer to cost efficiency and business profitability. Based on the
current level of knowledge, efforts and activities as a whole, it is believed that the commer-cialisation of algae as an oil resource will be a reality within 10 or 15 years (55).
Direct supply of ω-3
Fig 4.5: Schematic representation of the potential for direct supplementation of aquatic animals and humans with omega-3 rich
oils produced by microalgae.
4.3.2 Algal oil for EPA/DHA concentrates & nutritional supplements
Oleo-chemical consumer products are an emerging trend in com-
mercial and privately funded algae projects. Increasingly, the focus
among start-up and venture capital (VC)-backed algae ventures is on
high-value products including: omega-3 fatty acids, livestock and
fish meal, and health, cosmetic and pharmaceutical products. Rat-ledge and Cohen (2008 ref 54) suggested that current prospects in
micro-algal biotechnology should focus on algae as sources of LC-
PUFA rather than for bio-diesel production. As oil production for bio-diesel using micro-
algae is expensive (56), bio-refinery is an approach to maximizing the exploitation of valuable
algal components with the aim of increasing commercial potential. The potential of micro-
algae to synthesise long chain poly unsaturated fatty acids (LC-PUFA), in addition to their
use for energy, should be integrated into a production concept (57). Heterotrophic LC-PUFA production by micro-algae was valued at $195 million in 2004. The global market for ω-3
PUFA is increasing at an average growth rate of 8% from 2004 to 2010
The Norwegian bio-marine industry is by volume the No. 1 producer of Omega-3
oils/ingredients for supplements, foods and medical purposes in the world. 70 per cent of the
omega-3 and omega-6 market in Europe is dominated by six companies: Pronova, Epax,
ONC, Denomega, Croda, and DSM. According to market analyst Frost & Sullivan, the Euro-
pean market was valued at USD$ 296 million (€187.8 million) in 2007, and is expected to be worth USD$ 1.3 billion (€0.82 billion) by 2014, equivalent to a compound annual growth rate
(CAGR) of 23.6 per cent. In addition to Europe, the U.S. and Japanese market are considered
to be most important whereas an overview of the Norwegian omega-3 market is not available.
The European food safety authority (EFSA) recommends a daily intake of marine omega-3
fatty acid of 0.25 g/day. Nifes suggest 0.65 g/day whereas the American Heart Association
recently recommended for people with moderately increased levels of blood triglycerides
(>150-200 mg/l) a daily intake of 0.5-1 g EPA+DHA and for people with 200-500 mg/l, 1-2 g/day. Only these 2 groups represent more than 47% of all American citizens above 20 years old. Dietary supplements are the vehicle of choice in Europe, but functional foods and beverages
represent the fastest growing application section within the omega ingredients market. By the
end of 2014 Frost & Sullivan do anticipate that functional beverages will have a much larger
share in the omega-market, and they will be more or less equal to the dietary supplements
market, if not more. There were 723 omega-3 containing products launched in Europe in 2008 compared with 541 in the US. In 2006 there were 562 launches in Europe and 584 in 9
CO2 to Bio/WP4 – Biomass applications
the US. Based on the huge and growing market for Omega-3 supplements and functional
foods, the Norwegian bio-marine industry has a huge potential for driving innovation and
create value within the Omega-3 business globally. The core knowledge of Norwegian pro-ducers of Omega-3 products is related to refining and adding value to marine oils. This posi-
tion is established through extensive knowledge, but has a significant potential for further
value creation through developments in processing, better utilisation of existing and new raw
materials and by developing new and more sophisticated products/applications. By develop-
ing new documentation and novel sets of standards for high quality Omega-3 oils. Diatoms (micro-algae) contain high relative amounts of EPA whereas other micro-algal spe-cies such as members of the genus Chlorella contain significantly higher amounts of DHA. In
order to efficiently produce high quality EPA/DHA concentrates of algal origin industrial
cultures of more than one micro-algal species will be required.
Existing product examples:
Functional biscuits with PUFS-ω3 from Isochrysis galbana (7).
Future commercial aspects:
"Omega-3 from Norway" building the brand: The required research work will be based on
theoretical platforms and methodology for value chain mapping (13), industrial organisation
(14) and branding (15, 16). In this case the B2B-applications will be the most adequate theo-
ries. Research focus will be placed on building common brands in clusters, building B2B-
brands in global value chains, cost-benefit analysis of investments in branding in omega-3 industry.
4.4 Algae as a nucleotide source
Microalgae contain high amounts of nucleotides, about 4%. As regular constituents of nucleic
acids, purine compounds are always present in the human diet and mostly metabolised to
yield uric acid. If the concentration of the latter exceeds the normal range, stones may
develop in the kidney or/ and gut. Shelef & Soeder (1980) stated that in Europeans, a total
intake level of 15 g microalgae per day, or 0.6 g nucleic acids, would never pose any uric acid problem. In fish, low dietary levels of nucleotides have been used as immune system
stimulators and growth promoters. However, testing of the effects of the presence of nucleic
acids at higher dietary levels due to dietary microalgae needs to be evaluated. Should the
moderate nucleic acid content of microalgae ever become an obstacle, a number of easy
methods are available for the reduction of nucleic acid concentration by additional
processing (17).
4.5 Algae as a source of fine chemicals:
Pigments & antioxidants
The salmon and trout farming industry is a major consumer of a number of synthetic
chemicals, such as antioxidants, pigments and amino acids. The production of bio-chemicals
using renewable instead of fossil resources as practiced today, is a strategic priority in today's
European and world wide research & technological development. Microalgae are a natural
source of a large range of biomolecules such as carotenoids, sterols, vitamins,
polysaccharides, hydrocolloids, proteins, phycobilins and other biologically active
compounds with health protective effects such as natural antioxidants (ref 4-6 & 8). Such compounds should be identified in the produced algal cultures and evaluated in terms of
antioxidant effectiveness and efficiency in different food and feed commodities such as fish
fillet, fish feed, oils and beverages. Haematococcus pluvialis is an alga species rich in the carotenoid pigment astaxanthin ( 4%
in wet biomass). The lipid content of this species can reach very high levels, (41%), while
protein and ash levels are 10% and 9%, respectively. The production of this alga species is already practiced since 1998 by Algatechnologies, Ltd. in Israel. 10
Table 4.2. Natural sources of the pigment astaxanthin and concentrations of astaxanthin in the respective raw materials.
Astaxanthin natural sources
Astaxanthin concentration (ppm)
Phaffia yeast
Haematococcus pluvialis
Monoraphidium sp GK12 is another potential astaxanthin-producing micro-algal species,
tested as potential functional aqua-feed for prawns (32).
a)
b)
Fig 4.6: Commercial algae species.a. Chlorella vulgaris; b. Haematococcus pluvialis
Different algae species produce and contain different pigments some of which may be antag-
onistic to astaxanthin in diets for salmonids (e.g. lutein & zeaxanthin) (33). Eventual algal
biomass products for use in salmon feeds may require additional processing (refining and
rinsing) in order to remove components that would reduce its value in markets of significant
size such as aquaculture. Most microalgae are rich in chlorophyll, carotenes, xanthophyll and
phycocyanin. Green and yellow pigments may have positive or negative effect on fish skin
and muscle pigmentation and this effect must be evaluated case by case for the different fish species produced. Particularly algal biomass use in salmon and trout culture should be
regarded in the scope of the effect of the presence of increasing dietary amounts of
carotenoids on astaxanthin assimilation efficiency in the fillet. Facilities and analytical
methods for the evaluation of pigmentation efficiency in salmonids as well as for the chemical
characterisation of different pigments are available within the Nofima laboratories and fish
trial facilities.
Product examples:
a) Chlorella vulgaris biomass used as colouring source in traditional butter cookies (8). b) Goldfish pigmentation using microalgal biomass (8, 62)
The commonly used in aquaculture green algae species Chlorella vulgaris is also rich in
extractable antioxidants with high ADC (antioxidant activity coefficient), e.g. higher than that
of the chemical antioxidant BHT (ref 3). This property of microalgae has great potential for
their use as food and feed additive (oils & emulsions) increasing product shelf life, quality, safety and consumer acceptability. According to a report from Frost and Sullivan, the market
for synthetic antioxidants is decreasing while the market for natural antioxidants such as
CO2 to Bio/WP4 – Biomass applications
from plant extracts, tocopheroles (Vit E) and ascorbates (Vit C) is increasing due to consum-
ers demand and food safety regulations. Algal extracts have apparently a good potential as food and feed additives but no overview of the size of such markets or existing competing products, in Norway or abroad is currently
available. Assuming significant growth of the algae biomass production industry it will
become feasible in certain applications to phase out from using chemical antioxidants and
preservatives which have unknown effects to human and livestock or aquatic animal health
4.6 Algae and environmental pollutants
Persistent organic pollutants (POPs) bio-accumulate in the marine food chain and are ubiqui-
tously found in wild pelagic fish species. Fish oil produced from these species is the largest
contributor of POPs in salmon feeds (18, 19) which have a potential negative effect to fish
performance (20). Micro-algae contain definitely lower amounts of environmental contami-
nants and pollutants than fish oil or other vegetable raw materials. This property alone, or in combination with the potential high natural antioxidant level, as well as the potential growth
promoting effect makes this raw material of great interest in food and feed products.
4.7 Harvesting and concentration technology
The total micro-algal product cost will be defined by the sum of production, harvesting and
dewatering, processing and administrative costs. Available technologies and investment and operative cost estimates for the different processing alternatives are presented below.
4.7.1 Micro-algae harvesting and dewatering
The term Algae Harvesting refers to concentration of diluted algae suspension until a thick
algae paste is obtained. The concentration of dry matter from photosynthetic growth of mi-
cro-algae is known to be rather low. Thus vast amounts of water have to be removed before
further processing. Harvesting of micro-algae from cultivation ponds or photo-bioreactors
employs several techniques to concentrate the algae followed by de-watering. Normally harvesting of micro-algae can be a single step process or a multi step process which
involves harvesting and dewatering. Harvesting micro-algae is difficult because of the small
size of the algae. Choosing the effective harvesting process for a particular strain depends on
size and properties of the algae strain. As shown in the table below, water removal from the algal biomass accounts for a large per-
centage of the production costs.
Table 4.3 – Historical relative algae production, harvesting and drying costs.
Variable Total price % of price
Fully equipped plant
Most harvesting techniques have been developed for the removal of algal biomass from waste
water (63, 64, 67) and involve:
Specific gravity based harvesting Filtration Chemical flocculation &, Electro-flocculation.
Harvesting and concentration of the algal culture by specific gravity is historically practiced
with centrifuges (21) or by sedimentation. Centrifugation involves high investment and op-
eration costs and is inefficient when the raw material dry matter content is low as in micro-
algal cultures. It may inflict physical damage to fragile species and either increase or reduce their nutritional quality in both cases however reducing viability and quality during storage.
Sedimentation is more commonly used for harvesting filamentous and colony forming algal
species. Most algae settle when aeration is stopped and thus can be separated from the su-
pernatant water. This is however a too slow process for industrial harvesting that can last for
up to several days in order to achieve high harvesting efficiency. Concentration of algae cells by flocculation can be performed using flocculants such as cati-
onic polymers, aluminium oxide, etc. Concentration of algae by filtration may be a successful dewatering method, but only once
cost efficient species specific technology and equipment are developed that operate with lim-
ited filter clogging and increased micro-algae cell recovery. Flocculation is a micro-algae harvesting technique commonly used in sewage treatment. Ap-
plying this method algal blooms were attained by aluminium sulphate (alum) flocculation
and flotation (both dissolved-air and electrolysis-induced flotation are equally successful and result to a raw material with up to 5% dry matter). Chitosan has also been used as it is an
edible, non toxic flocculant of micro-algae which however may be less efficient in salt water.
Moreover, dissociation and efficient re-suspension of the algal cells from the flocs has been
proven difficult, and the biomass has bad digestion properties (67). Electro-flocculation is
highly inefficient in salt water. The investment or operating costs of the different harvesting methods applied have not been
estimated by the authors of the different studies. However, it is generally accepted that the use of two step or multi-step separation procedures are economically more favourable than
one step centrifugation or filtration. Such methods include combinations of sedimentation,
nozzle/disc stack centrifugation, screw centrifugation, vacuum drum filtration, and chamber
filter pressing (22).
4.7.2 Micro-algae biomass preservation and storage
During storage micro-algae will be susceptible to quick loss of their nutritional value and
must be thus protected in an appropriate way following harvesting and before further pro-cessing. For this purpose antioxidants (ascorbic acid, BHT), food acids (citric acid) and cryo-
protecting agents (glycerol) may be used to reduce the rates of lipid and vitamin oxidation,
autolysis, microbial growth and intercellular crystal formation. A combination of the use of
the above mentioned preservatives can result in retention of algal cell viability in a cooler
following several weeks' storage.
4.8 Micro-algae biomass processing
Post harvesting processing involves heat treatment, pH change, enzymatic hydrolysis and
dewatering. Nofima has a long processing/drying technology experience, applied in raw ma-
terials for feed and food applications, such as fish meal, marine hydrolysates, fish by-
products, krill, and agricultural by-products. Based on this experience it is feasible to esti-
mate the expected algae biomass drying costs at pilot and large scale, using today's technology.
CO2 to Bio/WP4 – Biomass applications
Besides sun and wind drying in open air, mechanical de-watering is the most inexpensive
way of water removal. Sieving, pressing and membrane filtration are among the different
methods of the mechanical dewatering. However, by mechanical dewatering of algae it is not really possible to go beyond 30 % dry matter. Further water removal above 30 % dry matter
must be done through heat treatment. Thermal dewatering includes evapora-
tion/concentration of liquids and drying to a more or less stable material. Freeze drying
technology application also leads to a dry powder from liquid raw materials, but is very ex-
pensive. Industrially, both convection dryer and contact dryers are used industrially. Convection dry-ers have usually higher energy requirement than contact dryers, but are more suitable for
heat labile products as the temperature in the drying goods is significantly lower than in the
contact dryers. The energy consumption of convection dryer under atmospheric conditions
can vary between 0.9 to 1.5 kWh/kg water depending on the temperature choice in the drying
air. The energy consumption of a contact dryer (example: indirect steam dryer) is about 0.9
kWh/kg water. The exhaust steam from a contact dryer can be utilised as energy source in the
process (waste heat evaporators). Convection drying in combination with a heat pump can bring the dewatering energy consumption down to 0.3 kWh/kg water, but due to low temper-
atures (< 100 °C) in the drying gas from the heat pump, large amounts of air are necessary to
increase drying capacity. This fact increases the investment and operating costs. It is also possible to remove water under pressure or vacuum in a steam atmosphere. Under
pressure the volume of the dewatering unit could be reduced for the same dewatering capaci-
ty, but the drawback in this case is the higher temperature in the drying goods and thus the risk of heat damages of heat labile products. Due to increase in the gas volume under vacu-
um, the volume of a dewatering unit increases with dewatering capacity. The positive aspect
of a vacuum dryer lays in the fact that dewatering can be realised at lower temperatures in
the drying goods than in an atmospheric contact dryer. If the drying media of convection dryers is changed from air to superheated steam then the
energy consumption under atmospheric conditions could be 0.8 kWh/kg water or lower. The
drawback in this case is that the drying goods are exposed to a temperature of about 100 °C. The steam that is produced under drying can be utilised for other heating purposes. A con-
vection dryer of superheated steam is currently under development. In such dryers it is pos-
sible to reduce the product temperature down to 60-70 °C. Freeze drying provides the lowest temperature charge of the drying goods. The drawback in
this case is that such dryers have very low capacity. Drying time is long, and the energy costs
increase to 7 kWh/kg water, (apart from the variant with heat pump). The efficiency and applicability of the different drying methods will further vary according to
types of dryers but also to raw material composition and rheological properties. Very fatty,
colloidal or algal biomass raw materials with high carbohydrate content and varying lipid
qualities (lipid class composition neutral: triglycerides / polar lipids: phospholipids) will re-
quire different process adjustments and instrument setup in order to reach cost efficient
functions as compared to already known raw materials. The Nofima processing group has
already faced such challenges when different plant raw materials, animal and marine by-products or krill were to be used in large scale processing applications in feed or food. Nofima
was a major R&D actor in the development of raw material processing modules on board in-
dustrial krill harvesting vessels which are currently in operation, and is therefore one of the
best suited environments to take over the challenges involved in algae biomass process and
product development.
a)
b)
Fig 4.7: Nofima raw material processing facilities in Kjerreidviken, Bergen, Norway. A: Processing hall with drying technology
equipment (including spray-, freeze-, steam-, air- dryers). B: Raw material processing pilot plant including fishmeal and oil pro-
duction, micronisation, hydrolysis, reverse osmosis and membrane filtration.
4.8.1 Effects of drying method on end product quality
The algal biomass composition and mainly the micronutrient content and digestibility may
be significantly affected by the drying method applied. The cellulosic cell wall of micro-algae,
which represents about 10 % of the algal dry matter, poses a serious problem in digest-
ing/utilizing the algal biomass, since it is not digestible for humans and other non-
ruminants. Hence, effective treatments are necessary to disrupt the cell wall to make the pro-tein and other constituents accessible for digestive enzymes. Several authors have studied the
effect of different post-harvesting treatments on the digestibility of various algal species by
evaluating the PER of the treated biomass, demonstrating the important role of proper pro-
cessing the algal biomass. In a pepsin pancreatin enzyme system protein digestibility of drum
dried algae reached 78% while fresh algae only 34% (E.W.Becker, 1978). The most promising drying applications should be established and tested both close to the microalgae biomass production facilities in Mongstad as well as at the raw material and feed
processing facilities. The processing costs, the mass balance and productivity must be evalu-
ated in relation to the obtained product quality and composition.
Table 4.4: Comparative data on biological value (BV), digestibility coefficient (DC) net protein utilization (NPU) and protein
efficiency ratio (PER) in rat, of differently processed algae (Becker, 1976; 2004 and Richmond, 2004)
Algae: AD: air dried; DD: drum dried; PER
SD: sun dried Scenedesmus obliquus DD
1.93 - 1.99 65.8 - 67.3
75.0 - 80.8 81.4 - 88.0
Scenedesmus obliquus SD
Scenedesmus obliquus Cooked-SD
Chlorella sp. AD
Chlorella sp. DD
Spirulina sp. SD
Spirulina sp. DD
Coelastrum proboscideum DD
1.68 - 2.10 57.1 - 68.0
Casein (Standard)
Table 4.5: Apparent digestibility of green algae (Scenedesmus or Chlorella) dependent on processing as determined in rat
balance tests.
CO2 to Bio/WP4 – Biomass applications
Processing
Apparent Digestibility Reference
Untreated fresh algae
Meffert & Pabst 1963/Kraut et al. 1966
Disintegrated fresh algae
Kraut et al. 1966
(Glass-bead homogenisator) Freeze-drying
Erchul & Isenberg 1968
Bock & Wünsche 1967/Nakamura &
Air-current drying
Meffert & Pabst 1963
Boiling (6-8 min)
Cook 1962/Meffert & Pabst 1963
Microwave cooking (2x4 min)
Pabst unpublished
Meffert & Pabst 1963/ Pabst
4.8.2 Integrated harvesting & processing (dewatering) of micro algae biomass
a) Harvesting methodology: The Flottweg process
The separation technology company FLOTTWEG AG has together with the Dutch algae spe-
cialist Ingrepro b.v. developed the Enalgy process. The principle is to treat the micro-algae
suspension in a flotation process called DAF (dissolved air flotation). In offshore installations
the DAF principle is used with dissolved natural gas instead of oxygen because of explosion hazard to remove organic pollutants from waste water. For flotation of micro-algae the use of
dissolved CO2 may protect the biomass from oxidation. The Enalgy process consists of a flo-
tation tank where the low density micro-algal cells are fixed to the fine gas bubbles giving
concentrated biomass at the top of the tank. As an option chemicals can be added to give floc-
culation and thus larger "clusters" of micro-algal cells before flotation. This is reported by Lin
et al., 2011. The algae concentration from a photo-bio-reactor is typically 0.7-0.8 g/l, rising to maximum 30-50 g/l (3-5%) during pre-concentration with DAF. The second step in the
Enalgy process is the final concentration in a decanter centrifuge called Sedicanter. The Sedi-
canter is specialized to efficiently thicken the soft and fine algal cells after flotation to a dry
matter concentration of 22-25% w/w. The possibility of pre-concentration by flotation depends on the properties of the algal bio-
mass. The principle is known from the clarification of waste water where biomass and bad
settling particles has to be removed (65). In some cases micro-algal cells will float without using chemicals. FLOTTWEG says that the Enalgy process will save up to 25% of the invest-
ment cost compared to single centrifugation, while reduced cost for energy will give up to
60% less operational cost. The most rapidly growing algal species are very small and often motile uni-cells. These are
the most difficult to harvest. Thus, it is necessary to maintain an effective interaction between the
development of harvesting technologies and the selection of micro-algal species for mass culture. Membrane filtration by specific micro filtration equipment and rotating screens like the
"Jesma-sikt" are alternative pre-concentration steps to consider.
b) Harvesting and dewatering methodology: The Algaetech process
The dry matter (dm) of the micro-algae biomass suspension from the Algaetech facilities
(raceway ponds) in Brazil is only 0.01 %. However, as described above, higher micro-algae biomass dry matter content can be reached with the use of photo bioreactors (PBR). In all
cases large amounts of water needs to be removed from the raw material to get to a dry prod-
uct. The GEA-process at Algaetech involves the use of pre-filters, hydro cyclones and nozzle
separators to concentrate the algae biomass before drying. From about 30 million tons of
algae in liquid the outcome of dry material is only 3 tons. Because of the high investment and
running costs involved in the use of industrial separators based on g-forces the possibilities for more use of different types of filtration methods should be investigated to lower the high
dewatering costs. A specific recommendation on the reduction of investment and running costs in the initial
harvesting and up-concentrating of algae cells is made in section 4.3.1., above. Moreover,
mechanical dewatering of algae biomass will be more inexpensive by reducing further the
requirement of centrifuges. The possibility of changing the applied separation methods from
g-forces to other forms of filtration must be explored. According to the ALGAFEED project report (2009) centrifuge systems can concentrate the
micro-algae biomass up to 20-25 % dm. This water content is equivalent to that of fish,
meaning that it is possible to apply the methodology and calculate costs as practiced at de-
watering during the fishmeal production processes. Thus, based on a product with 25% dry
mater of which 15 % is fat free dm (ffdm) and 10 % is fat, the dewatering costs are calculated
from 0.187 to 0.222 €/kg powder produced using a mechanical recompression evaporator or a 3-step evaporator. These cost estimates are similar to the productions costs in the fishmeal
industry. The process is based on heating up the algae in a similar way as practiced in fish-
meal production. The main processing costs are linked to the initial up concentration of the dry matter content
in the algal biomass. These production cost has to be drastically reduced in order to make the
algae products suitable for use in the salmon feed industry.
CO2 to Bio/WP4 – Biomass applications
4.9 Micro-algal oil production
Generally, large scale oil and highly unsaturated fatty acids extraction and concentration rep-
resent a considerably high investment as well as complicated operations. This is particularly relevant in terms of new raw material applications. It is however necessary to develop and
evaluate the efficiency of lipid extraction and concentration methods in laboratory and pilot
scale for the different algae species and products.
4.9.1 Algal oil extraction technologies
Alternative micro-algal oil extraction and purification technologies include:
mechanical milling and pressing solvent extraction enzymatic extraction supercritical fluid extraction
a) Mechanical cell disruption
This method minimises contamination from external sources while maintaining the chemical
integrity of the oils. Mechanical disruption methodologies include:
pressing which ruptures cell walls and releases the oil
bead milling which results in damaged cells and is generally used in conjunction with
solvents to recover oil. This method is more economical when cell concentrations are
significant (100-200 g/l) and when the extracted products are easily separated after
disruption, and,
homogenisation, where by forcing the biomass through an orifice, results in a prompt
pressure change and shearing action releasing the oil from the algal cells.
In terms of finding an effective and efficient mode of disrupting algal cells, there are multiple
options when it comes to using mechanical cell disruption technology, some of which include
the identification of biological features of the organisms that make it possible to weaken the
cell wall prior to mechanical disruption, such a pre-treatments with acid/alkali or enzymes,
thus potentially minimising the use of solvents (68).
b) Solvent extraction
Effective organic solvents used for the extraction of oil from micro-algae paste are: benzene,
cyclohexane, hexane, acetone and chloroform. Hexane is the most typical choice of solvent as
besides being a suitable solvent it is also insoluble in water with considerably different densi-
ty than water, has low boiling point which facilitates its removal after extraction and is easily
sourced, inexpensive and reusable. Common solvent extraction methodologies of algal oils involve some kind of cell pre-
treatment. However, continuous lipid production without damaging the cells is shown to be possible with Botryococcus braunii by in situ extraction in an aqueous-organic bioreactor
using the biocompatible solvent tetradecane which is not damaging the algal cells (66).
c) Supercritical fluid extraction
This method is used for the extraction of high-value products from micro-algae. It produces
highly purified extracts free from potentially harmful solvent residues. Moreover the extrac-
tion and separation are quick and safe for thermally sensitive products. During supercritical fluid extraction fractionation is possible which may reduce the separation costs. CO2 can be
used in some of the alternative processes due to its relatively low critical temperature and
d) Pulse electric field technology (PEF)
PEF is a mild non thermal process used in several applications such as oil recovery from
maize, olive, soy bean and rapeseed (70, 71 & 72). In PEF cells are processed by being ex-
posed to brief pulses of a strong electric field. The electric pulses permeabilise the cell walls
enhancing mass transfers across the cell membranes. This fact makes PEF a promising pre-
treatment prior to solvent or mechanical extraction methods.
e) Ultrasound/ sonnication
This technology is more efficient than hexane extraction (69). However to date it has not been determined if it in-
duces negative impacts to oil quality or stability of the pol-
yunsaturated fatty acid rich oils. Moreover, this technology
may be difficult to scale up and is thus appropriate for
small scale applications. Nofima has recently purchased an ultrasonic processor UP200S fitted with a cell for continuous flow of the raw
material, which can process up to 180 l / day. This equip-
ment will be used for sample and raw material preparation
for pilot scale fish and mink feeding trials.
4.9.2 Suggestion of best up-scalable oil extraction technology
Algae organisms are protected by a tough cell wall. If the oil is going to be extracted, the wall
must be opened before extraction of the oil. The challenge is to maximize oil yield by cracking as many of the algae cells as possible with the smallest amount of energy. If the oil is going to
be used for humans, the process has to be optimized for this purpose. Different algae can store the lipids a little differently, and the lipid content varies a lot. Be-
sides this, the cell walls can have different structures and constituents. Because of this there
is no universal method to extract the lipid phase from the algae in a gentle way. Different
extraction procedures give different lipid composition and quality. This has to be tailor-made
to the end product.
Examples:
Conventional extraction with hexane on milled, dry algae give a good result, but the
method use hexane which is not environmental friendly.
Depending on the drying process, the end product can be more or less oxidized. Freeze
drying is best, but have the highest cost. High temperature dryer will give a lower end quality product.
Supercritical CO2 extraction is gentle but also depending on the quality of the dried al-
gae mass. Supercritical CO2 extraction does not extract phospholipids quantitatively.
Traditional pressing leave some oil left in the press cake and give a lower yield. The
press cake can further be used as feed or extracted to take out residual oil.
CO2 to Bio/WP4 – Biomass applications
Depending on the segment in the algae oil market the oil is intended to meet, different pro-
duction methods for oil production are possible. The best way to optimize the extraction
equipment is to decide which algae species is to be used and then decide equipment based on
pilot scale testing. Today, the most cost effective technique to extract oil is probably to use pressing equipment, like a screw press, which is commonly used in the fish meal industry. Further refining of the
oil has to be considered depending on the customers specifications.
4.9.3 Micro-algal oil technology platform
A novel technology platform needs to be developed in order to develop the appropriate lipid
extraction and purification methodologies adapted to large scale specific micro-algal species
production operations. In general, most micro-algae contain LC-PUFA as constituents of their polar lipids, while the accumulation of LC-PUFA in TAG is very rare (59). Laboratory and pilot plant process
equipment must be used in order to quantify lipid content and study crude lipid extraction
from different algae biomass raw materials, crude and refined lipid quality, process optimiza-
tion and novel processing routes. The technology platform required includes the following
a) jacketed stirred tank reactors (2-50 L scale) with temperature and vacuum
b) scraped heat exchanger c) double screw press, hydraulic press d) 3-phase decanter e) disc centrifuge f) filter press and notch g) short-path distillation unit and, h) vacuum steam deodorizer
The oil extraction and purification process optimization must be based on designed experi-
ments and response surface methodology. Trapping mechanisms can be modelled using the
molecular dynamics software MDynaMix. Physicochemical properties can be studied utilising
correlations to GC and LC partition coefficients of well described chemical compounds.
Extraction, further processing and storage will challenge the quality of the end
oil products. Thus the establishment at an early stage of appropriate and standardised pro-
cedures which will safeguard the valuable health stimulating properties of micro-algal oils is
required. Biochemical analysis which will be necessary in order to characterise and further define or
develop the preservation methods to be used for the different algal oil products include anal-
yses of fatty acids, lipid classes, volatile oxidation products, non-volatile oxidation products,
peroxide value, peroxides, aldehydes, anisidine value, TBARS, conjugated dienes and trienes, tertiary oxidation products, superoxide dismutase, caspase 3, glutathione peroxidise, cata-
lase, apoptosis and cytotoxicity and can be studied by several methods: LC-MS, LC-
CORONA, LC-MS-MS, LC-QTOF-MS, GC-MS, CE-MS, HPTLC, HPLC, colorimetric assays,
TUNNEL, MTS assay, accelerated tests, oxypress, oxidograf, rancimat, shaal oven-GCMS. To
quantify all the different neutral and polar lipid classes HPLC "mass" detector can be used.
The selection of different oil qualities may be evaluated by sensory analysis. The product's appearance, smell and taste should also be evaluated.
4.10 Comparison of different algal species &
priority suggestion
Based on the amount of the already existing knowledge and technology developed four algal
species are chosen for further quality evaluation. These are: Nannochloropsis sp., Isochrysis
galbana, Phaeodaktylum tricornutum and Pavlova lutheri and their chemical composition
is presented below against that of fish meal.
Isochrysis galbana and Pavlova lutheri are highly nutritional for fish and other marine
organisms, but are very fragile rendering industrial harvesting or preserving complicated and
impractical.
Phaeodaktylum tricornutum is highly a productive diatom species, resilient, well suited
to production in photo-bioreactors, has high EPA content and its nutritional quality can be
enhanced following centrifugation.
Nannochloropsis oculata is the most extensively used species today in aquaculture.
To date, based on the existing knowledge and technology and also based on the different mi-
cro algae species protein content and technical properties, Nannochloropsis sp., which has a
robust 4 μm diameter cells and can withstand handling, harvesting and transport conditions,
is the microalgae species suggested as the most suitable for advancing to pilot and large scale
production at Mongstad. The post harvesting nutritional quality of the microalgae will be improved through applica-
tion of cost efficient processing technologies in order to provide semidry or dry products that
will be price competitive for inclusion in salmon feeds.
Alternative oil extraction processes for the production of ω-3 highly unsaturated fatty acids
will be performed and the costs efficiency of the different methods will be evaluated. Last, but not least, the cost efficiency of using wet products, such as pasta or slurry will also be evaluated as a function of storage and product quality.
CO2 to Bio/WP4 – Biomass applications
Prymnesiophyceae Bacillariophyceae
Nannochloropsis oceania/sp. Isochrysis galbana Phaeodactylum tricornutum Pavlova lutheri
Total AA, % of 79
Amino acid composition (water-corrected AA, g/kg & g/16gN)*
*Asp=asparagine+aspartic acid; cys= cysteine+cystine; glu=glutamine+glutamic
Carotenoids (M=major, m=minor, ()=occasional or under particular growth conditions: nitrogen starvation)
CO2 to Bio/WP4 – Biomass applications
vaucheriaxanthin -
myxoxanthophyll -
Lipid productivity mg/l/day
acids Nannochloris atomus
Isochrysis galbana Phaeodactylum tricornut- Pavlova lutheri
CO2 to Bio/WP4 – Biomass applications
4.11 Challenges & Recommendations
4.11.1 Reduction of algae biomass harvesting and processing costs
According to Yeh, (2011 ref. 73), the capital cost for the necessary harvesting and processing
equipment amounts to less than 4.5 % of the estimated total capital lost for algae biomass
production whereas the operational costs for running these processes can exceed 25% of the
total production costs. Optimisation of harvesting and processing of biomass algae will lead to significant reduction of the total cost. This can be achieved by incorporation of a flotation
processing step (DAF: dissolved air flotation Flottweg process) which will lead to increased
algal cell concentrations prior to centrifugation (with optional addition of chemicals to in-
duce flocculation and thus larger "clusters" of micro-algal cells before flotation). Thus, the
decanters' dewatering efficiency will increase reducing the related investment and running
costs resulting in a final product of 22-25% dry matter. Following harvesting and preliminary concentration, the dewatering costs will be further re-
duced to 0.187 to 0.222 €/kg powder produced using a mechanical recompression evaporator
or a 3-step evaporator, as in best industrial practice fish meal production process where both
costs are kept at low levels and the product quality safeguarded.
4.11.2 Maintain balance between production cost & product quality
The total production costs, including processing, will always compete with the finally
achieved product quality. The higher the product quality the higher the production costs.
This is not true however the other way around. High production costs do not always lead to
products of high quality for the targeted purpose. Such an example can be found in a recent
study (ALGAFEED 4.1.1) were the produced micro-algae biomasses targeted as raw materials
for fish feed had very low protein digestibility in mink. The processes followed were partly
standard also combined with advanced processing technologies ending up into products with
low value as fish feed raw materials. The failure can be attributed to both the original raw material (live algal cell composition) and the processing techniques used. The present consortium consists of the leading environments in algae production and fish the
feed raw material processing and technology field. It is feasible to stress the algal cells into
desirable final chemical compositions and apply appropriate processing methods that will
ensure proper cell wall disruption and high nutrient availability to the fish. The recognised
expert groups in our consortium will fully evaluate and document the potentials and best practices for the processing and incorporation of algae biomass as sustainable and cost effi-
cient raw material in salmon feeds.
CO2 to Bio/WP4 – Biomass applications
Bibliography
1. J. of Agricultural & Food Chemistry. Vol 54. pp 4593-4599 2. Gouveia, L., & Rema, P., 2005. Effect of microalgal biomass concentration and tem-
perature on ornamental goldfish (Carassius auratus) skin pigmentation. Aquaculture
Nutrition 11, 19-23
3. Guil-Guerrero, L. & Rodriguez-Garcia. Food Chemistry 4. Batista, A.P. et al. Microalgae biomass as a novel functional ingredient in mixed gel
systems. In Gums and Stabilisers for the Food Industry 14, pp: 497-494
5. Borowitzka, M.A. In Micro-algal biothechnology, eds. M.A. Borowitzka and L.J. Bor-
owitzka, Cambridge University Press, Cambridge, 1988, pp: 153-196
6. Pulz, O., & Gross, W., Appl. Microbiol. Biot. 2004, 65, 635 7. Gouveia, L. et al. 2008. Functional biscuits with PUFA-ω3 from Isochrysis galbana.
Journal of the Science of Food and Agriculture 0022-5142/2008
8. Gouveia, L., et al., 2007. Chlorella vulgaris biomass used as colouring source in tradi-
tional butter cookies. Innovative Food Science and Emerging Technologies.
9. Gouveia, L. et al., 2002. Colouring ornamental fish (Cyprinus carpio and Carassius
auratus) with microalgal biomass. Aquaculture Nutrition 8, 1-7.
10. Gouveia et al. 2009. 11. Li et al., 2007. 12. Mata et al., 2010. 13. Porter (1990) The Competitive Advantage of Nations, Macmillan, London. 14. Tirole (1988) The Theory of Industrial Organization, MIT Press, Massachusetts. 15. Saunders & Watt (1979) Industrial Marketing Management 8:114– 123. 16. Kotler & Pfoertsch (2007) J Bus Ind Market 22(6):357-362. 17. Hedenskog, G. (1978). Properties and composition of single-cell protein, influence
and processing, Proc. 11th FEBS-Meeting Copenhagen 1977, Pergamon Press, Oxford,
18. Jacobs, M.N., Covaci, A. & Schepens, P. (2002) Investigation of selected persistent
organic pollutants in farmed Atlantic salmon (Salmo salar), salmon aquaculture feed,
and fish oil components of the feed. Environ. Sci. Technol., 36, 2797–2805.
19. Hites, R.A., Foran, J.A., Carpenter, D.O., Hamilton, M.C., Knuth, B.A. & Schwager,
S.J. (2004) Global assessment of organic contaminants in farmed salmon. Science,
20. Removal of persistent organic pollutants from Atlantic salmon (Salmo salar L.) diets:
Influence on growth, feed utilization efficiency and product quality. Jan Josef Olli, Harald Breivik, Turid Mørkøre, Bente Ruyter, Johan Johansen, Patric Reynolds, Olav
Thorstad and Gunnar Berge. Aquaculture 310, 22 December 2010, 145-155.
21. E.W.Becker, Tübingen, 1978 22. Friedrich Helmuth Mohn, Dortmund. Improved technologies for the harvesting and
processing of microalgae and their impact on production costs. Dezember 1978. Arch.
Hydrobiol. Beih. Ergebn. Limnol. 11, 228-253
23. Ch. Meske & E. Pfeffer, Ahrensburg & Göttingen. Growth experiments with carp and
grass carp. Stuttgart, Dezember 1978. Arch. Hydrobiol. Beih. Ergebn. Limnol. 11, 98-
24. E. Sandbank & B. Hepher, Haifa. The utilisation of microalgae as a feed for fish. De-
zember 1978. Arch. Hydrobiol. Beih. Ergebn. Limnol. 11, 108-120.
25. Skrede, A., Mydland, L. T., Ahlstrom, O., Reitan, K. I., Gislerod, H. R., Overland, M.,
2011. Evaluation of microalgae as sources of digestible nutrients for monogastric an-
imals. JOURNAL OF ANIMAL AND FEED SCIENCES 20, 131-142.
26. Sebastián Sánchez, M Eugenia Martínez and Francisco Espinola, 2010. Biomass pro-
duction and biochemical variability of the marine microalga Isochrysis galbana in re-
lation to culture medium. Biochemical Engineering Journal 6, 13-18.
27., 1992. Algal Biotechnology products and processes – Matching sci-
ence and economicsSource: Journal of Applied Phycology 4, 267-279.
28. Hemaiswarya S., R. Raja, R. Ravi Kumar, V. Ganesan, C. Anbazhagan, 2011. REVIEW.
Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol
27, 1737–1746.
29. Spolaore P, Joannis-Cassan C, Duran E, Isambert A, 2006. Commercial applications
of microalgae. J Biosci Bioeng 101, 87–96.
30. Gagneux-Moreaux S, Moreau C, Gonzalez JL, Cosson RP, 2007. Diatom artificial me-
dium (DAM): a new artificial medium for the diatom Haslea ostrearia and other ma-
rine microalgae. J Appl Phycol 19:549–556.
31. Walker, Abigail B., Berlinsky, David L., 2011. Effects of Partial Replacement of Fish
Meal Protein by Microalgae on Growth, Feed Intake, and Body Composition of Atlan-
tic Cod. NORTH AMERICAN JOURNAL OF AQUACULTURE 73, 76-83.
32. Fujii Katsuhiko; Nakashima Hisatoshi; Hashidzume Yumiko; Uchiyama, Terumasa;
Mishiro, Kenzo; Kadota, Youji, 2010. Potential use of the astaxanthin-producing mi-
croalga, Monoraphidium sp GK12, as a functional aquafeed for prawns. JOURNAL
OF APPLIED PHYCOLOGY 22: 363-369.
33. Saez Patricio, Abdel-Aal M. El-Sayed and Dominique P. Bureau, 2011. Effects of corn
gluten Meal on flesh pigmentation of rainbow trout. Aquafeed international 14, 32-34.
34. E.W.Becker, Tübingen. Major results of the Indo-German Algal Project (C.F.T.R.I.,
Mysore, India). Stuttgart, Dezember 1978. Arch. Hydrobiol. Beih. Ergebn. Limnol. 11,
35. H. Brune and OP. Walz, Grießen. Studies on some nutritive effects of the green alga,
Scenedesmus acutus with pigs and broilers. Stuttgart, Dezember 1978. Arch. Hydro-
biol. Beih. Ergebn. Limnol. 11, 79-88.
36. Mitchell JR, Fan J, Blanshard JMV, 1994. The shrinkage domain. Extrusion Commu-
niqué, March p. 10-12.
37. Strahm B, 1998. Fundamentals of polymer science as an applied extrusion tool. Cereal
Foods World. 43(8):621-625.
38. Strahm B, Plattner B, Huber G and Rokey G, 2000. Application of food polymer sci-
ence and capillary rheometry in evaluating complex extruded products. Cereal Foods
World 45(7): 300-302.
39. Walstra P, 2003. Physical Chemistry of Foods. Marcel Dekker, Inc., New York, pp.
40. Sprecher H (2000) Metabolism of highly unsaturated n-3 and n-6 fatty acids. Bio-
chim Biophys Acta 1486:219–231
CO2 to Bio/WP4 – Biomass applications
41. Bairati I, Roy L, Meyer F (1992) Double-blind, randomized, controlled trial of fish oil
supplements in prevention of recurrence of stenosis after coronary angioplasty. Circu-
lation 85:950–956
42. Connor WE, Prince MJ, Ullmann D, Riddle M, Hatcher L, Smith FE, Wilson D (1993).
The hypotriglyceridemic effect of fish oil in adult-onset diabetes without adverse glu-cose control. Ann NY Acad Sci 683:337–340
43. Eritsland J, Arnesen H, Gronseth K, Gronseth KD, Fjeld NB, Abdelnoor M (1996) Ef-
fect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft
patency. Am J Cardiol 77:31–36
44. Horrobin DF, Huang YS (1987) The role of linoleic acid and its metabolites in the
lowering of plasma cholesterol and the prevention of cardiovascular disease. Int J Cardiol 17:173–180
45. Kalmijn S, van Boxtel MP, Ocké M, Verschuren WM, Kromhout D, Launer LJ (2004)
Dietary intake of fatty acids and fish in relation to cognitive performance at middle
age. Neurology 62:275–280
46. Le HD, Meisel JA, deMeijer VE, Gura KM, Puder M (2009) The essentiality of arachi-
donic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids
47. Bell JG, Sargent JR (2003) Arachidonic acid in aquaculture feeds: current status and
future opportunities. Aquaculture 218:491–499
48. Harel M, Koven W, Lein I, Bar Y, Behrens P, Stubblefield J, Zohar Y, Place AR (2002)
Advanced DHA, EPA and ArA enrichment materials for marine aquaculture using
single cell heterotrophs. Aquaculture 213:347–362
49. Napier JA (2007) The production of unusual fatty acids in transgenic plants. Ann Re-
view Plant Biol 58:295–319
50. Petrie JR, Shrestha P, Mansour MP, Nichols PD, Liu Q, Singh SP (2010b) Metabolic
engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an
acyl-CoA Δ6-desaturase with ω-3-preference from the marine microalga Micromonas
pusilla. Metab Eng 12:233–240
51. Qi B, Fraser T, Mugford S, Dobson G, Sayanova O, Butler J, Napier J, Stobart A, Laza-
rus C (2004) Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol 22:739–745
52. Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X
(2005) Stepwise engineering to produce high yields of very long-chain polyunsaturat-
ed fatty acids in plants. Nat Biotechnol 23:1013–1017
53. Zhu Q, Xue Z, Yadav N, Damude H, Pollak DW, Ruppert R, Seip J, Hollerbach Macool
D, Zhang H, Bledsoe S, Short D, Tyreus B, Kinney A, Picataggio S (2010) Metabolic engineering of an oleaginous yeast for the production of omega-3 fatty acids. In: Co-
hen Z, Ratledge C (eds) Single cell oils: microbial and algal oils. AOCS, Urbana, pp
54. Ratledge C, Cohen Z (2008) Microbial and algal lipids: do they have a future for bio-
diesel or as commodity oils? Lipid Technol 20:155–160
55. Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–
56. Lundquist TJ, Woertz IC, Quinn NWT, Benemann JR (2010) A realistic technology
and engineering assessment of algae biofuel production. Energy Biosciences Institute
University of California, Berkeley
57. Morweiser M, Kruse O, Hankamer B, Posten C (2010) Developments and perspectives
of photobioreactors for biofuel production. Appl Microbiol Biotechnol 87:1291–1301
58. Inna Khozin-Goldberg & Umidjon Iskandarov & Zvi Cohen (2011). MINI-REVIEW:
LC-PUFA from photosynthetic microalgae: occurrence, biosynthesis, and prospects in
biotechnology Appl Microbiol Biotechnol 91:905–915
59. Cohen Z, Khozin-Goldberg I (2005) Searching for PUFA-rich microalgae. In: Cohen
Z, Ratledge C (eds) Single cell oils. Amer Oil Chem Soc, Champaign, pp 53–72
60. Sukenik A, Takahashi H, Mokady S (1994) Dietary lipids from marine unicellular al-
gae enhance the amount of liver and blood omega-3 fatty acids in rats. Ann Nutr
Metab 38:85–96
61. Nitsan Z, Mokady S, Sukenik AJ (1999) Enrichment of poultry products with omega3
fatty acids by dietary supplementation with the alga Nannochloropsis and mantur oil.
Agric Food Chem 47:5127–5132
62. Gouveia L., Rema, P., 2005. Effect of microalgal biomass concentration and tempera-
ture on ornamental goldfish (Carassius auratus) skin pigmentation. Aquaculture Nu-
trition 11, 19-23.
63. Michael Phillip Heasman, Tanya Maree Sushames, John Alfred Diemar, Wayne An-
drew O'Connor and Lynne Adele Foulkes. Production of Micro-algal Concentrates for Aquaculture. Part 2: Development and Evaluation of Harvesting, Preservation, Stor-
age and Feeding Technology. FRDC Project No. 93/123 & 96/342. August 2001. NSW
Fisheries Final Report Series No. 34. ISSN 1440-3544.
64. Lin, Z., Kuang, Y. L., Leng, Y. W., 2011. Harvesting Microalgae Biomass by Instant
Dissolved Air Flotation at Batch Scale. Advanced Materials Research, 236-238, 146
65. Rubio, J., Carissimi, E., Rosa, J. J., 2007. Flotation in water and wastewater treat-
ment and reuse: recent trends in Brazil: INTERNATIONAL JOURNAL OF ENVI-
RONMENT AND POLLUTION 30, 197-212.
66. Zhang, G., Cheng, L.H., Gao, W.L., Xu, X.H., Zhang, L., Chen, H.L., 2011. Mechanism
of lipid extraction from Botryococcus braunii FACHB 357 in a biphasic bioreactor.
Journal of Biotechnology 154, 281-284.
67. Heaseman, M.P., Sushames, T.M., Diemar, J.A., O'Connor, W.A., Foulkes, L.A., 2001.
Production of micro-algal concentrates for aquaculture Part 2: Development and evaluation of harvesting, preservation, storage and feeding technology. FRDC Project
No. 93/123& 96/342. NSW fisheries final report series 34 ISSN 1440-3544, Australia.
68. Mercer, P. and Armenta, R.E., 2011. Review Article. Developments in oil extraction
from microalgae. Eur. J. Lipid Sci. Technol. 113, 539-547.
69. Cravotto, G., Boffa, L., Mantegna, S., Perego, P. et al., 2008. Improved extraction of
vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sono-chem. 15, 898-902.
70. Guderjan, M., Elez-Martínez, P., Knorr, D., 2007. Application of pulsed electric fields
at oil yield and content of functional food ingredients at the production of rapeseed
oil. Innovations Food Sci. Emerg. 8, 55-62.
71. Ramaswamy, R., Jin, T., Balasubramaniam, V.M., Zhang, H., in: Extension FactSheet,
Ohio State University Columbus 3.
72. Guderjan, M., Töpfl, S., Angersbach, A., Knorr, D., 2005. Impact of pulsed electric
field treatment on the recovery and quality of plant oils. J. Food Eng. 67, 281-287.
73. Yeh, B., 2011. Commercializing algae-challenges and opportunities. Inform 22, 485-
CO2 to Bio/WP4 – Biomass applications
CO2 to Bio
Work Package 5 – Costs and Market
Ver 061211
Caspar Lund, Bergen Teknologioverføring
Trond Mork Pedersen, Nofima
Hans Kleivdal, Uni Miljø
Svein M. Nordvik, Nordhordland Handverk- og Industrilag
CO2 to Bio/WP5 - Costs and Market potential
Contents
Summary 4 5.1 Business idea 5 5.2 CO2Bio - ownership and network _ 5
5.2.1 Ownership of CO2Bio 5
5.2.2 Nordhordaland Handverk- og Industrilag - 45% 5
5.2.3 Bergen Teknologioverføring AS - 25% _ 6
5.2.4 Grieg Seafood - 10% 6
5.2.5 Salmon Group -10% _ 6
5.2.6 EWOS - 10% 6
5.3.1 Uni Research AS _ 7
5.3.2 Nofima _ 7
5.3.3 University of Bergen _ 7
5.3.4 Professor Rene Wijffels (Wageningen University) 7
5.3.5 AlgaePARC 7
5.4 Technology 8
5.4.1 Algae production technology 8
5.4.2 Algae biomass processing 9
5.5 Inputs _ 10 5.6 Technological risk 10 5.7 Market _ 11 5.8 Competitors _ 11
5.8.1 MBD Energy 12
5.8.2 Carbon Capture Corporation _ 12
5.8.3 The Algae at Work Network 12
5.9 Milestone plan _ 13
Summary
Fish oil is currently used as a fat and omega-3 source in feed for salmon, but the aquaculture
industry is facing a global shortage of fish oil. The implication of this is that the salmon farming
industry's expansion is currently limited. The supply of fish oil from conventional sources is limited by the traditional fishery of pelagic fish species. At the same time the demand for omega-
3 continues to rise, much driven by an increasing demand from the health food sector as a nutri-
tional supplement. As the global demand is expected to exceed supply within the next 3-5 years,
new and sustainable omega-3 sources must be developed if the Norwegian salmon industry still
wants to market farmed salmon as a good health food product in the future. On this basis, Uni
Research, Nofima, NHIL, and BTO, with the contribution from the University of Bergen and Wageningen University, launched a development project to produce omega-3 for use in fish feed
with an alternative method.
This project aims to establish a pilot manufacturing facility that can produce omega-3 and other
high-value plus products from algal biomass, based on pure CO2 from Technology Centre
Mongstad (TCM) and residual heat from the oil and gas refinery at Mongstad. The focus will be
on the development of a low-cost production system, with cheap inputs and the use of low-cost photo bioreactors (PBR), in order to develop recommendations about the establishment of a
full-scale production facility.
The result is a sustainable solution to an environmental problem and a proactive alternative to
the passive, subsurface deposition of C02.
Establishing a pilot plant where technology can be tested, develop optimized algae cultures and
production processes for full-scale production, will require an investment of 11 MNOK. Opera-
tion of the pilot plant will cost approximate 8 MNOK. The pilot plant will be ready at the end of
2012 and will be operating for 3 years.
The Technology Center Mongstad will provide free CO2, steam and facilities. The rest of the
costs must be covered by public research and development grants and from key industrial play-ers.
CO2 to Bio/WP5 - Costs and Market potential
5.1 Business idea
The business idea is to manufacture and sell Omega-3, proteins and other ingredients produced
by algal biomass to the fish farming industry. The main focus will be on industrial production of
omega 3 to feed, but other high-value plus products for better paying markets will also be an
opportunity for increased earnings. The market for fish oil for fish feed is currently very tight. According to a "precautionary report"
from the feed and salmon fish-farming industry (2011), the global demand for fish oil will be
greater than the supply in 3-5 years. The salmon farmers may replace fish oil with even larger
amounts of vegetable oil, as their incentive to follow the omega-3 price increase is less than
manufacturers of health products for human consumption. The consequence of reduced omega-3 levels in salmon may be reduced quality and health benefits for the consumer, which can harm
Norwegian salmon as a brand. This will affect the industry as a whole.
The business idea is to cover a significant proportion of the omega-3 need in fish feed through
alternatives other than fish oil from pelagic fishing.
Omega-3 in microalgae has been identified as a potential source for alternative, industrial pro-duction. As the marine microalgae are the primary producers of omega-3, they also represent
the biggest source globally. Algal biomass has a good amino acid profile and content of astaxan-
thin, which is the desired carotenoid in salmon farming, and the content of natural antioxidants
is also interesting inputs in fish feed. So there are, in addition to the appropriate fatty acids, two
other advantages of the use of algal biomass in the fish feed.
The production of algal biomass is to be done at or close to the Mongstad oil refinery, where we
will have access to pure CO2 and plant heat from the existing activity on TCM. This will provide
a better and cheaper final product. The heating costs will be low, and we will get a cleaner final
product than other algae plants that use exhaust as its C02 component. We also believe that the
optimal location of production will allow for competitive prices on fish oil from pelagic fish.
From the algal biomasse, we will produce and sell fatty acids to feed in a local and cost effective manner, as an alternative to fish oil. An added bonus with this production method is that it is
environmentally friendly due to its consumption of CO2 in the production and storage of CO2 in
the final products. Our competitive advantage is a significant and steady supply of CO2, residual
heat at Mongstad. Furthermore, we have an ongoing dialogue with the affected aquaculture-
industries to ensure that the products are customized to meet their needs and requirements. The
technology development of cost-effective microalgae production systems at the pilot facility Mongstad, supported by waste resources, is expected to lower costs and increase productivity in
order to make the algal biomass competitive on world markets.
5.2 CO2Bio - ownership and network
5.2.1 Ownership of CO2Bio
Five different companies own the company, where each partner represent a valuable contribu-
tion to increase the possibility to implement the project.
5.2.2 Nordhordaland Handverk- og Industrilag - 45%
Nordhordaland Handverk- og Industrilag (NHIL) has a vision to be a solution-oriented promot-
er to make the whole Nordhordland region to a preferred place to live and work. NHIL want to create a good and lasting effect of the large investment in CO2 capture at Mongstad. NHIL has
total approx. 250 companies in Nordhordland and Gulen as members. Key focus areas are in
transport, skills and regional development.
5.2.3 Bergen Teknologioverføring AS - 25%
Bergen Teknologioverføring AS (BTO) is an affiliate of research institutions in Bergen. BTO
helps researchers to commercialize their research results to ensure that knowledge and inven-
tions benefit individuals, society and industry.
In addition to the management BTO counts 5 full-time business developers, a legal adviser and
3 interns that help projects to:
Develop and implement a commercialization strategy for innovation projects
Provide funding
Legal advisory services in industrial cooperation
Managing Intellectual Property (IP)
Develop business ideas
Manage licensing technology or the establishment of a new company
5.2.4 Grieg Seafood - 10%
Grieg Seafood ASA is one of the world's leading fish farming companies within salmon and
trout. The group has an annual production capacity exceeding 90,000 tones slaughtered weight.
They have farms in Finnmark, Rogaland, British Columbia (Canada) and Shetland. Grieg Sea-
food ASA is headquartered in Bergen. Altogether over 600 people work in Grieg Seafood. The
business development of Grieg Seafood has a focus on results-based growth and sustainable use
of natural resources.
5.2.5 Salmon Group -10%
Salmon Group is the world's largest network of small, family-owned fish farming companies.
Based in Bergen, Norway, they provide the service for its 48 shareholders, who collectively pos-
sesses 88 licenses for salmon and trout along the Norwegian coast, and production of approx. 26
million smolts. Together with their owners they negotiate terms on feed, vaccinations, insurance
and other joint agreements that the individual farmers can benefit from. In their negotiations,
they focus on predictability, and that they should maintain the "Salmon Group-quality" of the
fish produced. By using the best inputs, this quality is a particularly nutritious and good fish, applicable in all niches of the market. Their customers request the Salmon Group-quality of fish
exporters. Salmon Group is also an active part in the aquaculture industry's key issues. They
follow the developments, and seek to be ahead of the demands that the industry is faced with.
Together with their owners on different sites, they measure the results against the demands of
the world market. The main office of the Salmon Group acts as a liaison and service for the own-
ers. Here they address the challenges that may arise, and helps the individual fish farmer to reach the best solution for their business. They make joint agreements, but maintain the princi-
ples of freedom of choice. Thus, you get economies of scale, as well as individual adaptability.
This gives the fish farmer's strength in competition with large, centrally controlled players.
5.2.6 EWOS - 10%
EWOS is one of three major salmon feed companies globally, with a market share of around 33
percent. EWOS delivers extruded fish feed for the entire life cycle of salmon from hatching to
slaughter. EWOS is also a supplier of feed for many marine and freshwater species and is in a position to take part in the expected growth of these species. Today we produce feed for 28 dif-
ferent species. EWOS is present in all the four major salmon-producing countries, with three
production facilities in Norway, and one each in Chile, Canada and Scotland. EWOS also exports
fish to a number of countries in Europe and Asia, in addition to supplying the local markets.
EWOS is recognized in all markets for the five key characteristics: Performance, fish health and
fish welfare, experience, service and partnerships. In 2009, EWOS sold worldwide a total of
787,600 tons of fish feed, had a turnover of 6.2 billion and delivered an operating profit before fair value adjustment of biomass of 379.6 million NOK. EWOS has a total of 643 employees,
CO2 to Bio/WP5 - Costs and Market potential
including research and development company EWOS Innovation. The various EWOS companies
have a special responsibility for the market in their respective areas - both in production and
sales. But companies have also developed close cooperation in the overarching functions such as
procurement, product development, R & D, marketing and information systems. EWOS AS has
its headquarters in Bergen. The company's other activities are mainly concentrated around the
three plants in Florø (Sogn og Fjordane), Halsa (Nordland) and Bergneset (Troms), as well as various sales offices along the coast from Bergen in the south to Tromsø in the north. EWOS has
offices in Scotland, Chile and Canada.
5.3 Network
The development of the project will be conducted in collaboration with multiple stakeholders
with expertise in marine research to ensure the optimal production method, both with regards to costs and quality.
5.3.1 Uni Research AS
Uni Research AS is a research institute engaged in contract research. It is owned by the Univer-
sity of Bergen (UiB) and delivers 430 million NOK in annual sales. Uni Research has 500 em-
ployees, of whom 260 are scientists, in 8 departments. At Uni Research, the department Uni
Environment with the Centre for Applied Biotechnology is particularly relevant in this context,
as they have considerable expertise within biotechnology, marine microbiology and methanogen microorganisms. Hans Kleivdal is the leader of the Centre for Applied Biotechnology at Uni Re-
search and has been the project leader of the CO2 to Bio preproject. He is responsible for the
establishment of a Bergen Marine Biobank together with the University of Bergen, where marine
microorganisms will be collected, characterized and made available for research and industry
collaborators. Through this, Uni Research has an ongoing collaboration with UoB researchers
on algae, which will be extended to involve algae strain screening and development.
5.3.2 Nofima
Nofima is an industry-oriented research institute that conducts research and development of
aquaculture, fisheries and food industries. They deliver internationally recognized research and
solutions that give businesses a competitive edge along the entire value chain. Nofima was es-
tablished on 1 January 2008 and has about 470 employees. Headquartered in Tromsø, and re-
search activities take place at six different locations: Ås, Stavanger, Bergen, Sunndalsøra, Averøy
and Tromsø. In 2009 Nofima has a turnover of approx. 470 million NOK.
5.3.3 University of Bergen
Dr. Anita Jacobsen is a researcher at the Institute of Biology and Microbiology with interest for
the application of microalgae, and has for many years also worked as an industry consultant for
the industrial production of microalge as live feed to scallop larvae. She has contributed to the
prestudy reports in writing a summary of the current algal species for use in algal biomass pro-
duction of fish feed, and made recommendations about their industrial use. Dr. Siv Kristin Pres-
tegard will contribute with her valuable experience on screening and selection of local microal-
gal strains to the Bergen Marine Biobank operated by Uni Research .
5.3.4 Professor Rene Wijffels (Wageningen University)
Professor Rene Wijffels is head of the Department of Bioprocess Engineering and he possesses
great expertise in industrial production and bioraffinering of microalgae. Prof Wijffels research
group have a considerable scientific merit in all aspects of microalgae research. He is also the
co-founder and scientific director of the pilot production plant called AlgaePARC (see below).
5.3.5 AlgaePARC
AlgaePARC is a center for the development of industrial algae production methods. AlgaePARC
is initially a 5-year pilotproject with 18 industry partners in a consortium. CO2Bio have been
invited into the consortium and the invitation is open until 31.12.11. Wageningen University
owns the equipment and infrastructure at the plant, but it is the consortium that owns the IPR
related to procedures and methods. The focus of AlgaePARC have many of the same issues that
a pilotplant at Mongstad will encounter, and we see great learning potential of having them as
partners. AlgaePARC will be valuable as a partner because they want to contribute to a direct
application and demonstrate the industrial use of algae cultivation facilities. Secondly, they are
interested in establishing research collaborations around the progress and development of the plant.
5.4 Technology
The production of fatty acid will take place through nature's own method of C02 storage, the
photosynthesis. If you use the algae for this purpose, one of the main products of industrial pro-
cessing of algae will be marine omega-3 fatty acid.
5.4.1 Algae production technology
The intention is to use closed photobioreactors (PBR) with a short path length through the me-
dium and with a good supply of pure C02 and nutrition to get controlled, ideal conditions for
algae cultivation (figure 1). This will allow for continuous production and harvesting. We will
begin by setting up a vertical PBR unit of 1500 liters, equivalent to that contained in the Al-
gaePARC project. This will be a reference device that alternative low-cost PBR systems, e.g. in
the form of a plastic tube, will be compared to. When it comes to the plastic tube-method it may be of particular interest since the costs are significantly lower and the final product does not
need to be of high value.
Fig 1. Design principle of a tubular photobioreactor system. Most photobioreactors differs in the design of the transparent
vessel, which in this example would be closed loop glasstubes with a diameter between 3-5 cm. The light system can be based on
either natural or artificial light – or a combination of the two.
CO2 to Bio/WP5 - Costs and Market potential
5.4.2 Algae biomass processing
After the algae are harvested must the algal biomass go through an industrial de-watering pro-
cess. The different parts of the production will be processed into omega-3 and protein flour. The
raw materials used in fish feed is currently characterized by the fact that they are dry and stored
in silos or tanks, as is the case when it comes to fish oil. Expertise related to the industrial pro-cessing of a variety of marine, vegetable and animal raw materials is present in Nofima. Fur-
thermore, it will be possible to use the residual heat in the form of steam, as the illustration from
the Mongstad refinery shows.
All fish feed for salmon and trout are currently extruded (figure 2). Before the extrusion process
is the dry ingredient mixed together in a mixing silo. The products powder technological charac-teristics must be tested and documented to avoid problems in the silos. The next step is that the
feed goes on to "pre-conditioner". There are clearly limitations when it comes to the fat content
in ingredients used in the extrusion process.
Nofima has a pilot extruder, and expertise related to powder technology and extrusion, which
will be of great importance to optimize the technological characteristics of the products based on
the algal biomass.
Fig 2. Processing of algal biomass and use as feed ingredient. Upper panel: a schematic view of the processing of algal bio-
mass exemplified by a typical flow sheet for the production of dry algae powder. Lower panel: algae biomass as an added ingredient
must also pass through an extruder to produce feed pellets.
Fig 3. Schematic overview of the mass balance involved in algae cultivation. The normal lipid production of algal cells can be
stimulated by metabolic stress procedures, in order to promote a higher lipid fraction.
5.5 Inputs
An important aspect of the whole idea is that the plant can be placed directly where the CO2 is
available in large quantities, namely at Mongstad. TCM are generally positive to the project and
are considering alternative locations for a pilot facility. Here we will have access to CO2, cooling
water, electricity and heat / steam from the refinery. Figure 3 illustrates a conservative estimate of the inputs needed to produce 0.4 t lipid at optimum conditions.
Furthermore, one will need to identify the most suitable algae type and strain with an optimal
set of properties. We hope we can get this from Bergen Marine Biobank. The Biobank have a
startup in the first quarter of 2012 and will be a culture collection of algal strains and species.
One will screen and select for an alga species and strain that is:
Easy to grow in large scale
Easy to harvest
Has a high content of omega-3 (DHA and/or EPA)
It will also be taken into account that it is optimal with local algal species to be able to handle
the large amounts of water by drainage without having to sterilize several cubic daily before dis-charge into the fjord. A study of local algal strains and a summary of the work that is done na-
tionally and at the University of Bergen is a key delivery in the pilot project.
5.6 Technological risk
We have identified the following technological challenges:
Upscaling: to what extent can output be upscaled
Reduce costs: we must find cost-effective solutions within large-scale algal production. We
have access to free CO2 and residual heat which should be positive for the project accounts
Choosing the right type of algae: with a tight connection to the national database of algae, we
will benefit from the screening and selection program there.
Reduce de-watering costs
Crushing algal cells to release nutrients
If there is a need for extracting fatty acids from the algae, the challenge will be to reduce
costs for the extraction process.
CO2 to Bio/WP5 - Costs and Market potential
Fig 4. Graphic presentation of the global demand (left) and price (right) of fish oils.
5.7 Market
Global production of fish oil is under considerable price pressure, which is expected to increase
further (figure 4). The production of farmed fish has increased considerably since 2000, while production of fish oil has almost stood still. In addition, the human consumption of Omega-3
This will lead to a shortage of fish oil in the market shortly. The business idea is that marine fat-
ty acids from algal biomass can meet this demand. This is the main market.
The current industry norm is to have a minimum 10% EPA/DHA fats in fish feed. In compari-son, the oil that is used to feed today has an average of 20% EPA/DHA fats. If one assume that
the salmon industry adheres to current standards by at least 10% EPA/DHA the lack of fish will
be about 700 000 tones by 2020. Shortage will already occur in 2013 and this will be the market
that omega-3 produced by algal biomass can cover.
It is also highly likely that prices will rise, as they did in 2008 when the market was pressured. According to a report by the International Food Policy Research Institute and World Fish Center
the prices of fish oil are expected to rise by 18%1.
5.8 Competitors
We have done a search on other projects based on the cultivation of microalgae using industrial-
ly captured C02 as an additive to produce aquafeed, and found that there are few competitors in this industry. A series of partnerships have tried to grow micro-algae "fed" with carbon dioxide
from exhaust to produce useful products, but still no one has stepped forward from the pilot
project stage. The companies often consist of an energy company and a biotechnology company,
merged, and the funding comes from venture capital or capital from large companies in the
partnership. In the U.S., the Department of Energy provided substantial grants to micro-algal
projects. Neither company has IP rights that mention the method of producing algae through the use of
carbon dioxide in the exhaust / flue gas, but most companies have IP rights related to photo
bioreactors, carbon storage devices or methods to convert algae into end products.
The most notable companies are:
1 http://www.ifpri.org/sites/default/files/pubs/pubs/fpr/pr15.pdf
5.8.1 MBD Energy2
MBD has successfully partnered with a leading algal based research group from Australia's
James Cook University to develop a 5,000-square test facility capable of producing 14,000 gal-lons of oil and 25 000 pounds of algal flour from every 100 tons of CO2 consumed. These days
MBD is moving from a test plant to full-scale display projects on the production site, at a num-
ber of Australia's largest coal-burning power plants. 10,000 m2 of display projects will be initiat-
ed. The "Proof of concept" project starting in 2010 aimed to take greenhouse gases from the
power plants emissions and produce oil and flour. The projects should run for 6 -12 months be-
fore they eventually were scaled up to commercial production. This has not yet happened.
5.8.2 Carbon Capture Corporation3
Carbon Capture Corporation manufactures Aqua Feed from Spirulaalgen, fed with CO2 emis-
sions. The company operates open algae ponds with a total capacity of 8 million gallons, located
at the existing 40-acre Algae Research Center ("ARC"). ARC is part of a 326-acre research and
development facility in Imperial Valley, California. The facility includes water, electricity and
infrastructure to operate the dams, and a 13,000 square meter building hosting laboratories,
processing plants, offices and storage space. Seven 150-gallon solar tube photo bioreactors are
located indoors. Current production capacity is approx. 300kg per day, however CCC has facili-ties that can be scaled up to produce 1 ton of dried algal biomass by 7% or less moisture content.
Carbon Capture Corporation was formed in 2006 and has successfully completed over $ 100
million dollars in federal contracts issued by various government agencies between 1993 and
The dams were designed by Dr. William Oswald, known as one of the "fathers" for the produc-tion of algae, and his designs have been copied throughout the world. The pilot plant uses two
Capstone C330 (30 kW each) that runs on propane, which is used to generate emissions like
those from a natural gas-fired power plant. These are fed into one of the two dedicated 240,000-
gallon "raceways" with Spirulina algae is produced and harvested. The company plans to build a
50 MW power plant to evaluate the thresholds to capture or control the emission of carbon diox-
ide by this method.
5.8.3 The Algae at Work Network4
Members: the A2BE Carbon Capture LLC, AccelergyCorp., Raytheon, Siemens, grant from the
Pennsylvania Commonwealth Financing Authority for feasibility study.
The Alliance Network consists of companies, researchers and institutions. The Algae @ Work
Alliance Network are collaborating to develop and commercialize etbio-secure, scalable, climate
adaptive and cost-effective system to recycle industrial CO2 emissions. The core of the technolo-
gy is the A2BE carbons capture photo-bioreactor (The PBR) system for the production of algal biomass. The PBR is designed to capture and recycle CO2 emissions without releasing the gas
into the atmosphere. An alga in the PBR absorbs CO2 and converts it to biomass during photo-
synthesis. This biomass is then used to produce fuel, food, fertilizer and other useful products
worldwide. On a commercial scale, each PBR machine is 350 inches long and 50 inches wide,
and consist of two 20 'wide x 10 "deep x 300' long, transparent and growing tubes. Accelergy
provides an alliance with coal-biomass-to-liquid-technology, Siemens offers biofuels automation and control technologies, Raytheon provides product development, system integration, carbon
sequestration, and Six Sigma driven program management. Alliance is expected to move from
pilot scale to full-scale carbon capture for 48 months from August 2010.
2 http://www.mbdenergy.com/ 3 http://www.carbcc.com/21323/ 4 http://www.algaeatwork.com/
CO2 to Bio/WP5 - Costs and Market potential
5.9 Milestone plan
The planned milestones are listed in the table below.
Milestone
Preliminary studies Finalize pre project studies and report is accepted by
Steering Committee
Finalize business plan 1.0
Finalize required LCA studies
Grant application is sent to Innovation Norway
Grant application accepted by Innovation Norway
Close agreements with research institutions
Detail R&D strategy in collaboration with partners
Close agreements with Univ Wageningen and AlgaePark
regarding knowledge transfer
Planning Facilities
Close agreements with suppliers
Planning Facilities
Close agreements with TCM regarding facili-
ties/CO2/heat/water
Planning Facilities
Finalize design of facilities with TCM
Planning General
Communication Plan is approved
Planning General
Approval from local government (building permission)
Planning General
Competencies (startup and production) are hired
Planning General
Finalize business plan 2.0
Building pilot plant
Pilot plant is finished and approved by CO2Bio AS
Scale up of master cultures to PBR inoculant volumes
Startup lab-scale PBRs (10l)
Pilot PBRs are ready for startup
Startup small PBRs (250l)
Startup second PBR (1250l)
Separator is up and running
Pilot development
Development of low-cost PBR systems (1250l)
Pilot development
Optimized production processes
Pilot production
Optimized culture running for a long period of time
Pilot production
Startup from wet to dry biomass processing (Nofima)
Pilot production
Finalize business plan 3.0
Source: http://polsak.ivest.no/polsak_filer/0%5CVEDLEGG%5C2012044384-823220.pdf
MINISTRY OF EDUCATION AND MINISTRY OF HEALTH NATIONAL INSTITUTE OF HYGIENE AND EPIDEMIOLOGY NGUYEN THI UT EPIDEMIOLOGICAL AND CLINICAL CHARACTERISTICS AND OUTCOMES OF TREATMENT REGIMENS FOR PEDIATRIC GASTRITIS AND PEPTIC ULCER DISEASE CAUSED BY DRUG-RESISTANT HELICOBACTER PYLORI AT NATIONAL PEDIATRIC
Vol 23 Number 1 2013 Fiji Medical Journal Instructions to Authors Foreword byFiji National University Vice Chancellor Dr Ganesh Chand Foreword by Fiji Medical Association President Dr. James Fong 9 Evaluation & Assessment of Diabetes Knowledge Among Trainee Teachers Alka Sewram 10-13 Original ResearchSome common barriers to self-care amongst Type 2 diabetic patients in Sigatoka medical area Dr. Sravaniya Dasi















