Psychology.cua.edu
Copyright 2008 by the American Psychological Association
2008, Vol. 22, No. 5, 563–570
Intact Implicit Learning of Spatial Context and Temporal Sequences in
Childhood Autism Spectrum Disorder
Kelly Anne Barnes
James H. Howard Jr.
Georgetown University
Catholic University of America and Georgetown University
Darlene V. Howard
Lisa Gilotty and Lauren Kenworthy
Georgetown University
Children's National Medical Center
William D. Gaillard and Chandan J. Vaidya
Children's National Medical Center and Georgetown University
Autism spectrum disorder (ASD) is defined by atypicalities in domains that are posited to rely on implicitlearning processes such as social communication, language, and motor behavior. The authors examined 2forms of implicit learning in 14 children with high-functioning ASD (10 of whom were diagnosed withAsperger's syndrome) and 14 control children, learning of spatial context known to be mediated by themedial temporal lobes (using the contextual cueing task) and of sequences known to be mediated byfrontal–striatal and frontal– cerebellar circuits (using the alternating serial reaction time task). Both formsof learning were unimpaired in ASD. Spatial contextual implicit learning was spared in ASD despiteslower visual search of spatial displays. The present findings provide evidence for the integrity oflearning processes dependent on integration of spatial and sequential contextual information in high-functioning children with ASD.
Keywords: frontostriatal circuits, medial temporal lobes, spatial attention, sequence learning, develop-mental disorders
The ability to learn environmental regularities (e.g., where or when
In the present study, we examined implicit contextual learning in two
events may occur) implicitly, without intention or conscious aware-
domains: In the spatial domain, repeated experience with invariant
ness, is posited to support linguistic and motor skill acquisition (Per-
spatial relationships provides predictive cues that guide visual atten-
ruchet & Pacton, 2006) and social intuition (Lieberman, 2000).
tion during visual search tasks (e.g., contextual cueing [CC] task;
Impairments in these domains characterize children with autism spec-
Chun, 2000). In the perceptual–motor domain, repeated experience
trum disorder (ASD) in whom difficulties with social communication
with invariant sequential structure of stimuli forms the basis for
accompany repetitive behaviors and restricted interests. Implicit learn-
predicting subsequent responses to contiguous (e.g., serial reaction
ing of contextual information guides perception of social cues and
time [SRT] task; Nissen & Bullemer, 1987) or noncontiguous (e.g.,
predicts actions and, therefore, may mediate atypical cognition in
alternating SRT [ASRT] task; Howard & Howard, 1997) stimuli.
ASD. However, investigation of the integrity of learning processes
Learning is implicit because participants cannot recollect or recognize
has not figured centrally in models of cognitive dysfunction in ASD.
the learned spatial context or sequential information. Knowledge ofthese two forms of implicit learning in ASD is necessary for con-straining knowledge about the status of cognition in the disorder.
Examining two forms of implicit learning in ASD provides the
Kelly Anne Barnes and Darlene V. Howard, Department of Psychology,
opportunity to probe the functional integrity of learning mecha-
Georgetown University. James H. Howard Jr., Department of Psychology,
nisms shown to be dissociable in adults. Whether functional spe-
Catholic University of America; Department of Neurology, Georgetown
cialization of memory systems is complete by late childhood is not
University. Lisa Gilotty and Lauren Kenworthy, Department of Neurology,
fully known. Nevertheless, forms of learning that have been dis-
Children's National Medical Center. William D. Gaillard, Department of
sociated in adults provide a heuristic for systematic examination of
Neurology, Children's National Medical Center; Department of Neurol-ogy, Georgetown University. Chandan J. Vaidya, Department of Psychol-
memory systems in childhood (see also Berl, Vaidya, & Gaillard,
ogy, Georgetown University; Department of Neurology, Children's Na-
2006). Spatial contextual learning is hypothesized to involve the
tional Medical Center.
medial temporal lobes (i.e., the hippocampus and entorhinal,
Funding support came from Georgetown University, National Alliance
perirhinal, and parahippocampal cortices) because learning was
for Autism Research, National Institute of Mental Health Grants MH
reduced in patients with extensive medial temporal lobe lesions
065395, NIA R37AG15450, and the Frederick and Elizabeth Singer Foun-
(Chun & Phelps, 1999). Although hippocampal lesions did not
dation. We thank Jennifer Foss-Feig and Margaret Benner for assistance
disrupt learning on the CC task (Manns & Squire, 2001) hip-
with data collection, and Sunbin Song and Marvin Chun for providing the
pocampal involvement was observed using functional brain imag-
ASRT and CC tasks, respectively. Lisa Gilotty is now with the National
ing during CC performance in healthy adults (Greene, Gross,
Institute of Mental Health, the National Institutes of Health.
Elsinger, & Rao, 2007). Furthermore, activations also involve
Correspondence concerning this article should be addressed to Kelly
Anne Barnes, Department of Psychology, 306L White Gravenor, George-
lateral–frontal and temporal cortices projecting to the medial tem-
town University, Washington, DC 20057. E-mail:
[email protected]
poral lobe. Thus, although the role of the hippocampus remains to
BARNES ET AL.
be elucidated, other medial temporal lobe regions and their cortical
uration repeats on some trials and is novel on others. Context-
projections appear to be important for spatial contextual learning.
dependent learning is indexed by faster responding on trials with
In contrast to spatial contextual learning, sequence learning is
repeated than novel distractor configurations. On the ASRT task,
hypothesized to involve striatal circuitry because it is impaired in
participants respond to the location of a visual stimulus by pressing
people with Huntington's and Parkinson's disease (Willingham,
a corresponding key. Unbeknownst to participants, the stimulus
1997), which are characterized by degeneration of basal ganglia
location varies in a fixed sequence involving alternate trials (i.e.,
structures. Functional brain imaging studies also show involve-
item
n predicts item
n ⫹ 2 on these trials); randomly determined
ment of the cerebellum and regions projecting to the striatum such
stimulus locations alternate with sequence trials. Context-depen-
as prefrontal and motor cortices in adults on the ASRT and SRT
dent learning is indexed by faster responding on sequential than on
tasks (Fletcher et al., 2005; Rauch, Whalen, et al., 1997; Willing-
random trials. The ASRT rather than SRT task was used for two
ham, Salidis, & Gabrieli, 2002) and in children on the SRT task
reasons. First, the ASRT task is more resistant to the development
(Thomas et al., 2004). Double dissociations in elderly participants
of conscious awareness of underlying sequential structure and use
further suggest that implicit spatial contextual and sequence learn-
of explicit memory strategies during performance. Therefore, dif-
ing are separable. Specifically, Negash et al. (2007) reported
ferences in explicit memory abilities are less likely to influence
reduced CC but not ASRT learning in individuals with mild
sequence learning. Second, the ASRT task is more sensitive to
cognitive impairment, a condition characterized by medial–tem-
ongoing learning because performance on sequential and random
poral lobe pathology, compared with age-matched controls. In
trials is assessed continuously during learning rather than after
contrast, reduced ASRT but not CC learning was reported in
learning has occurred. Thus, factors affecting expression of learn-
healthy aging (Howard, Howard, Dennis, Yankovich, & Vaidya,
ing, such as fatigue, are minimized for ASRT than they are for
2004), a period characterized by reductions in striatal, cerebellar,
SRT learning.
and prefrontal volumes with relative sparing of the medial–tem-poral lobes (Raz et al., 2005). Thus, brain imaging and neuropsy-
chological findings suggest that medial temporal and frontostria-
tal– cerebellar circuits mediate learning of spatial context andsequential structure, respectively.
Fourteen children with ASD (13 boys) ages 8 to 14 years with
Cognitive strengths and weaknesses observed in ASD lead to
IQ within the normal range were recruited from Children's Na-
distinct hypotheses about the status of implicit learning. A strength
tional Medical Center (see Table 1). Ten children with ASD had a
observed in ASD is a tendency toward superior processing of local
diagnosis of Asperger's syndrome; of the 4 remaining children
information. Relative to controls, participants with ASD are faster
with ASD, 2 had a diagnosis of high-functioning autism and 2 had
at detecting targets embedded in complex visual figures (Jolliffe &
a diagnosis of pervasive developmental disorder—not otherwise
Baron-Cohen, 1997) and give fewer context-appropriate pronun-
specified. Fourteen control children (13 boys) ages 7 to 14 years
ciations of homographs (Happe´, 1997). The source of this bias,
with IQ within the normal range were recruited from the Wash-
whether due to impaired (Happe´, 1999) or unaffected (Mottron,
ington, DC, area through advertisements. The groups matched for
Burack, Iarocci, Belleville, & Enns, 2003; Plaisted, Saksida, Al-
sex, age (ASD:
M ⫽ 11.57 years,
SD ⫽ 1.65; controls:
M ⫽ 11.00
cantara, & Weisblatt, 2003) global information processing,
years,
SD ⫽ 1.80;
p ⫽ .39), and IQ (ASD:
M ⫽ 110.43
,
remains unresolved. Nevertheless, those findings suggest that con-
SD ⫽ 12.59; controls:
M ⫽ 116.29
, SD ⫽ 13.79;
p ⫽ .25). All
textual information weakly modulates visual–perceptual and lin-
parents or guardians provided informed consent; children provided
guistic processing in ASD. Such a bias could reduce contextual
informed assent and were paid for participation.
encoding, thereby reducing learning dependent on invariant con-
Children were diagnosed with ASD by clinicians using criteria
textual information in ASD, regardless of stimulus domain. Thus,
in the
Diagnostic and Statistical Manual of Mental Disorders (4th
this view hypothesizes reduced learning on both sequence learning
ed., text revision;
DSM–IV–TR; American Psychiatric Association,
and contextual cueing tasks. Consistent with this prediction, se-
2000); diagnosis was confirmed by expert opinion of clinicians
quence learning on the SRT task was reduced in children with
specializing in ASD (LK, LG; see Table 1). The Childhood As-
ASD (Mostofsky, Goldberg, Landa, & Denckla, 2000). Alterna-
perger Syndrome Test (CAST) (Scott, Baron-Cohen, Bolton, &
tively, intact learning on both sequence learning and contextual
Brayne, 2002) was used to objectively screen for ASD symptoms
cueing tasks may be hypothesized in light of one of the core
(cutoff ⫽ 15); all participants with ASD were above the ASD
symptoms of ASD, the need for sameness and regularity. The
cutoff (see Table 1). In addition, a portion of children with ASD
preference for repetition in ASD may promote acquisition of
who had clinical evaluations at Children's National Medical Cen-
invariant contextual information, leading to spared or superior
ter received the Autism Diagnostic Interview—Revised (ADI–R)
learning of spatial and sequential relationships. Thus, there are
and the Autism Diagnostic Observation Schedule (ADOS; Lord
reasonable arguments to hypothesize both impaired and intact
et al., 2000; Lord, Rutter, & Le Couteur, 1994). Seven children
contextual learning in ASD. The present study tested these hy-
received both ADI–R and ADOS, 3 children ADOS only, 1 child
potheses by examining both learning of spatial context and se-
ADI–R only, and 3 children neither ADI–R nor ADOS. All ADOS
quential information in the same children with ASD and matched
Social Domain summary scores (
M ⫽ 8.60, range ⫽ 4 –13, cut-
off ⫽ 4) and all but one of the ADOS Communication Domain
We examined implicit learning of spatial context using the CC
summary scores (
M ⫽ 3.60, range ⫽ 1– 8, cutoff ⫽ 2) were above
task and of sequences using the ASRT task in children with ASD
the ASD cutoff. Restricted and Repetitive Behavioral Domain
and age-, sex-, and IQ-matched controls. On the CC task, partic-
summary scores were consistent with Lord et al.'s (2000) scores
ipants search for a target among distractors whose spatial config-
(
M ⫽ 3.00, range ⫽ 2– 4, no cutoff). ADI scores were above the
IMPLICIT LEARNING IN ASD
Table 1
Demographics of Participants With Autism Spectrum Disorder
FSIQ ⫽ Full-scale IQ determined by Wechsler Intelligence Scale for Children (3rd ed.) or Wechsler Abbreviated Scale of Intelligence; CAST ⫽
Childhood Asperger Syndrome Rating Scale Score (autism spectrum disorder [ASD] diagnosis suggested by scores higher than 15). M ⫽ male; F ⫽ female.
Diagnosis: ASP ⫽ Asperger syndrome; HFA ⫽ high-functioning autism; PDD ⫽ pervasive developmental disorder—not otherwise specified. All childrenwith a diagnosis of ASP had normal onset of language and normal adaptive functioning. Contextual cueing (CC) learning ⫽ faster reaction times to repeatedthan to novel configurations in the last epoch. Alternating serial reaction time (ASRT) learning ⫽ faster reaction times to pattern than to random trials inthe last epoch. S3's CAST score was 1 point below cutoff, but this participant met criteria for ASD on the Autism Diagnostic Interview—Revised or theAutism Diagnostic Observation Schedule.
autism cutoff (Reciprocal Social Interaction:
M ⫽ 21.12, range ⫽
from the screen's center and screen half (left/right); no targets
18 –25, cutoff ⫽ 10; Communication:
M ⫽ 19.25, range ⫽ 14 –24,
appeared in the four center or corner cells. Every element was
cutoff ⫽ 8; Restricted and Repetitive Behaviors:
M ⫽ 8.62,
randomly repositioned by ⫾2 pixels along each axis to avoid
range ⫽ 5–12, cutoff ⫽ 3). Exclusion criteria included other
colinearity. Each block consisted of 24 trials: 12 unique configu-
neurological disorders (e.g., epilepsy), IQ ⬍ 85, or use of antipsy-
rations of distractors (novel) and 12 configurations of distractors
chotic medications. Medications could not be withdrawn in 10
that repeated across the experiment (repeated). Target location, but
children with ASD who participated on antidepressants (9), stim-
ulants (3), nonstimulants (i.e., Strattera; 1), or valproic acid (1); 4
children were unmedicated.
Stimuli were presented via Matlab with instruc-
Control children were screened for ASD using the CAST (Scott
tions to locate the
T as quickly and accurately as possible. Fol-
et al., 2002) and psychiatric conditions (e.g., attention problems)
lowing 24 practice trials, participants completed 30 blocks of 24
using the Child Behavior Checklist (Achenbach, 1991). Children
trials each. Trials were randomized within blocks. Blocks were
completed the subtests of the Woodcock–Johnson III Diagnostic
grouped into 6 epochs of 5 blocks (e.g., Blocks 1–5 made up
Reading Battery to screen for reading disorder. No control partic-
Epoch 1). On each trial, a fixation dot appeared for 1 s, followed
ipants had any neurological or psychiatric conditions, including
by a stimulus, which remained until a response was made. If no
ASD. Unpaired
t tests confirmed that symptoms on the CAST were
response was made within 6 s, the trial timed out following an
higher in ASD than those in control participants (ASD:
M ⫽ 19.71,
error tone. Feedback tones were high pitched for correct responses
SD ⫽ 4.20; controls:
M ⫽ 5.00,
SD ⫽ 3.19)
, t(26) ⫽ 10.45,
p ⬍
and low pitched for errors. Following the task, 24 configurations
(12 novel, 12 repeated) were presented for recognition memory;participants pressed a key for familiar configurations.
Design and stimulus materials.
A 2 ⫻ 2 ⫻ 6 mixed design was
used with group (ASD vs. control) as a between-subjects factor
Design and stimulus materials.
A 2 ⫻ 2 ⫻ 5 mixed design was
and configuration (repeated vs. novel) and epoch (1– 6) as within-
used with group (ASD vs. control) as a between-subjects factor
subject factors.
and trial type (pattern vs. random) and epoch (1–5) as within-
Each trial consisted of a 12-element stimulus array of a single
subject factors.
target and 11 distractors presented in white on a gray background
Each trial began with three empty circles displayed horizontally
(Figure 1, upper portion). The target was a horizontal
T rotated left
across a screen (Figure 1, lower portion), mapped to a keyboard key
or right by 90°, to which subjects responded by pressing a key-
(
M and the adjoining symbol keys ⬍ and ⬎). On each trial, one circle
board key (
Z for left, "/" for right). The distractors were
Ls
filled in and remained filled until participants pressed the correct key.
randomly rotated by 0°, 90°, 180°, or 270°. Arrays were generated
The circles remained empty for 120 ms between trials. One of two
by randomly placing the 12 items into cells of an invisible grid (6
patterns was randomly assigned to each participant (either A-r-B-r-C
rows ⫻ 8 columns). Target location was balanced for distance
or A-r-C-r-B-r, where A, B, and C denote the left, central, and right
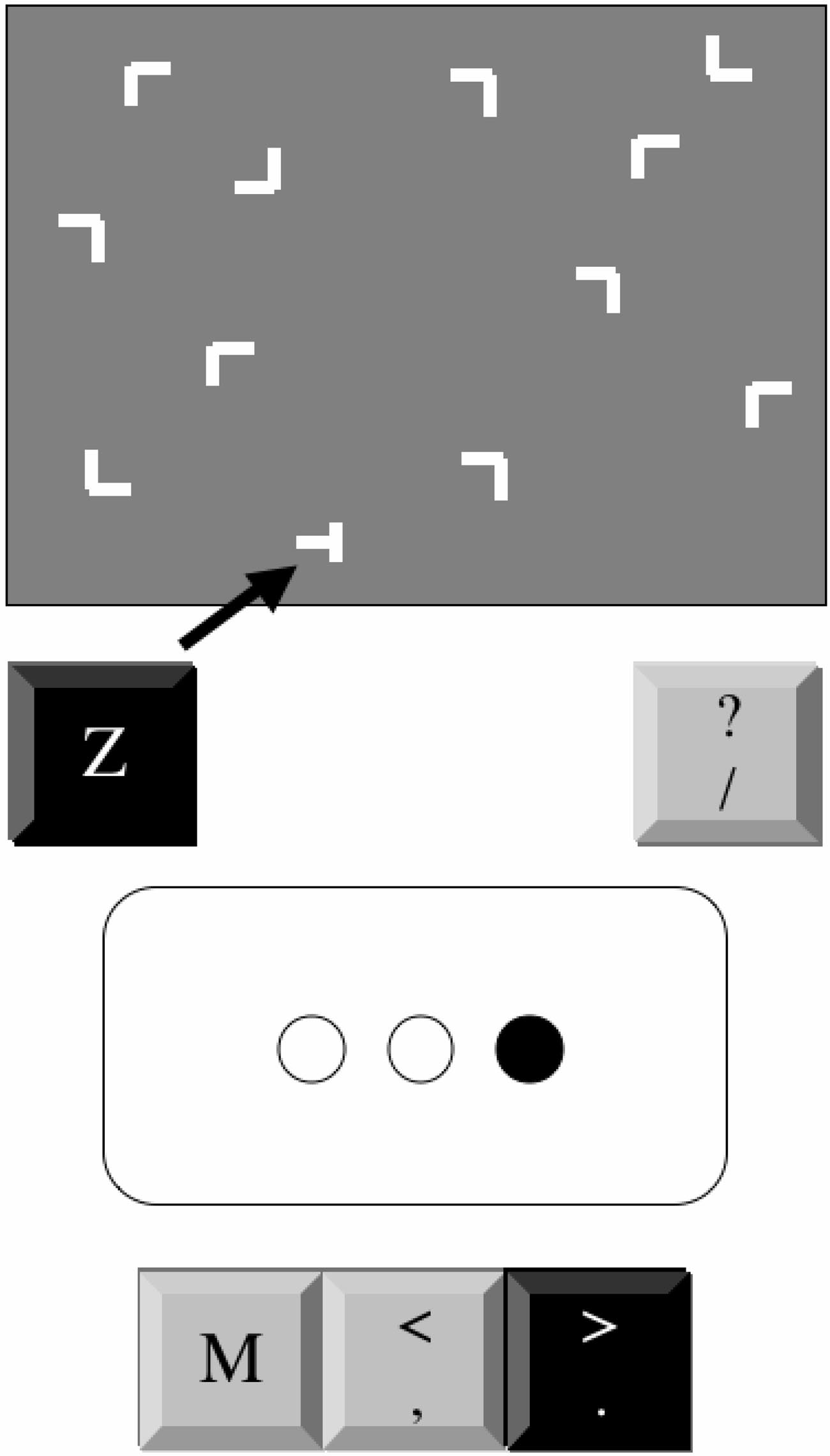
BARNES ET AL.
Trials with reaction times (RTs) that were 3 or more standard
deviations from the mean were excluded. The percentage of ex-cluded trials did not differ between groups (CC–ASD: M ⫽ 1.00%,SD ⫽ 0.73, control: M ⫽ 0.81%, SD ⫽ 0.54, p ⫽ .45, d ⫽ 0.30;ASRT–ASD: M ⫽ 1.15%, SD ⫽ 0.49, control: M ⫽ 1.27%,SD ⫽ 0.63; p ⫽ .56, d ⫽ 0.21). Based on past research using theCC task (Chun & Jiang, 1998), trials without a response within 6 swere excluded (total trials: ASD ⫽ 11; controls ⫽ 5). Cohen's dand 2 effect sizes are reported for t tests and analyses of variance
Percentage of correct responses (accuracy) and mean RTs for
correct trials were computed for each participant and were ana-lyzed in Group (ASD vs. control) ⫻ Configuration (repeated vs.
novel) ⫻ Epoch (1– 6) repeated measures ANOVAs (see Figure 2).
Analysis of accuracy revealed no significant main effects or inter-actions, except a trend for higher accuracy for repeated than novelconfigurations (main effect of configuration), F(1, 26) ⫽ 3.89, p ⫽.06, 2 ⫽ .13 (other ps ⬎ .26, 2 ⬍ .05). Overall accuracy was
high (ASD: M ⫽ 97.58%, SD ⫽ 1.88; control: M ⫽ 97.03%,SD ⫽ 2.31).
Analysis of RTs revealed that responses were slower in ASD
than in control children (main effect of group), F(1, 26) ⫽ 5.20,
Schematics of computer displays for the contextual cueing
p ⬍ .03, 2 ⫽ .17. Participants exhibited learning of visual search
(CC; upper portion) and alternating serial reaction time (ASRT; lower
skill because responses were faster with practice (main effect of
portion) tasks. The arrow in CC task display indicates the target's location.
epoch), F(5, 130) ⫽ 43.57, p ⬍ .0001, 2 ⫽ .63. Although overall
For both tasks, the black keys indicate the correct response.
responses were faster to repeated than to novel configurations(main effect of configuration), F(1, 26) ⫽ 17.95, p ⬍ .0001, 2 ⫽
.41, children exhibited context-dependent learning because the
positions and r denotes a random element, constrained so that all
benefits of repetition increased with practice (Configuration ⫻
locations appeared with equal frequency). The three-position long
Epoch interaction), F(5, 130) ⫽ 3.25, p ⫽ .008, 2 ⫽ .11.
pattern repeated throughout the experiment.
Magnitude of learning did not differ between groups (Group ⫻
Stimuli were presented via E-Prime with instruc-
Epoch ⫻ Configuration interaction, p ⫽ .95, 2 ⫽ .01). No other
tions to press the key that matched the filled-in circle's location (M
interactions reached significance (all ps ⬎ .14, 2 ⬍ .06).
and the adjoining symbol keys ⬍ and ⬎ on a keyboard). Partici-
In light of slower visual search in ASD relative to control
pants completed 20 blocks of 60 trials each. Blocks were grouped
children, we determined whether differences in magnitude of
into 5 epochs of 4 blocks (e.g., Blocks 1– 4 made up Epoch 1).
learning were apparent on a measure that equated speed by ex-
Each block began with 8 practice trials and ended with feedback
pressing learning as a proportion of one's baseline speed (i.e.,
encouraging speed and accuracy. Conscious awareness for learnedsequences is commonly tested subjectively with questions such as"Did you notice any regularity in the way the stimulus moved?"We did not include such a test because metacognitive immaturity
in childhood often results in unreliable introspective reports(Kuhn, 2000).
General Procedure
ASD, NovelCON, Repeated
Participants performed the CC and ASRT tasks within a single
session in counterbalanced order. Both tasks were self-paced.
Participants took short breaks between blocks, approximately ev-
ery 60 s on the CC task and every 90 s on the ASRT task. Includingbreaks, total time on the CC task ranged from 30 to 45 min and
total time on the ASRT task ranged from 20 to 25 min. For bothtasks, children were instructed to rest their hands over the relevant
Mean response time (in seconds) on the contextual cueing (CC)
response keys during the experiment. The experimenter confirmed
task as a function of epoch and type of configuration for autism spectrum
that this was done throughout the task.
disorder (ASD) and control groups.
IMPLICIT LEARNING IN ASD
novel – repeated/novel, calculated per epoch). Proportional learn-
other interactions reached significance (all ps ⬎ .26, 2 ⬍ .05).
ing scores computed for each participant were analyzed in a
We examined the three-way interaction for effects of group (with
Group ⫻ Epoch ANOVA. The main effect of group and the
Epoch ⫻ Trial Type ANOVAs for each group) and epoch (with
Group ⫻ Epoch interaction were not significant ( ps ⬎ .43, 2 ⬍
Group ⫻ Trial Type ANOVAs for each epoch). Each group
.02), indicating that measures of proportional learning did not
exhibited sequence learning because the Epoch ⫻ Trial Type
differ between ASD and control children. Thus, the absence of
interaction reached significance: ASD, F(4, 52) ⫽ 2.60, p ⬍ .05,
group differences in learning was not an artifact of speed differ-
2 ⫽ .17; control, F(4, 52) ⫽ 3.60, p ⫽ .01, 2 ⫽ .22. Sequence
ences because group differences were not observed after equating
learning marginally differed between groups in Epoch 5 (Group ⫻
for response speed.
Trial Type interaction), F(1, 26) ⫽ 3.84, p ⫽ .06, 2 ⫽ .13, but
For the recognition memory test, d⬘ scores [z (%hits) – z (%false
not in Epochs 1– 4 (all ps ⬎ .11, 2 ⬍ .10). Planned comparisons
alarms)] were computed for each participant. One-sample t tests
indicated that the difference between pattern and random trials was
indicated that d⬘scores did not differ from chance in ASD
larger in ASD than control participants in Epoch 5, t(26) ⫽ 1.96,
(M ⫽ 0.75, SD ⫽ 1.50, p ⫽ .11) and control (M ⫽ 0.28,
p ⫽ .06, d ⫽ 0.74 (other epochs ps ⬎ .11, d ⬍ 0.63). Thus, ASD
SD ⫽ 1.41, p ⫽ .54) children. Furthermore, an unpaired t test
but not control children continued to show learning into the last
indicated that d⬘scores did not differ between groups ( p ⫽ .44,
d ⫽ 0.32). Thus, participants were unable to consciously recognize
It is possible that group differences in magnitude of learning
the repeated configurations.
emerged because the ASD group's response speed appeared toimprove to a greater extent than did controls' response speed. We
therefore determined whether differences in magnitude of learningwere apparent on a measure that equated speed by expressing
Percentage of correct responses (accuracy) and mean RTs for
learning as a proportion of one's baseline speed (i.e., random –
correct trials were computed for each participant and were analyzed in
pattern/random, calculated per epoch). Proportional learning
Group (ASD vs. control) ⫻ Trial Type (pattern vs. random) ⫻ Epoch
scores computed for each participant were analyzed in a Group ⫻
(1–5) repeated measures ANOVAs (see Figure 3). Accuracy did
Epoch ANOVA. Overall measures of proportional learning did not
not differ between ASD (M ⫽ 92.25%, SD ⫽ 3.48) and control
differ between ASD and control children (main effect of group),
(M ⫽ 93.37%, SD ⫽ 3.08) participants (main effect of group, p ⫽
p ⫽ .41, 2 ⫽ .03. Group differences in learning were suggested
.38, 2 ⫽ .03). Participants were more accurate on pattern than
by a significant Group ⫻ Epoch interaction, F(4, 104) ⫽ 2.47, p ⬍
random trials (main effect of trial type), F(1, 26) ⫽ 36.40, p ⬍
.05, 2 ⫽ .09. We examined this interaction to determine whether
.0001, 2 ⫽ .58, and accuracy increased with practice (main
each group demonstrated learning (with one-way ANOVAs for
effect of epoch), F(4, 104) ⫽ 2.42, p ⬍ .05, 2 ⫽ .09. No
each group) and whether magnitude of learning differed between
interactions reached significance (all ps ⬎ .17, 2 ⬍ .06).
the two groups (with unpaired t tests for each epoch). Each group
Overall RTs did not differ between groups (main effect of
exhibited sequence learning because the main effect of epoch was
group, p ⫽ .90, 2 ⫽ .001). Participants exhibited perceptual–
significant: ASD, F(4, 52) ⫽ 3.07, p ⫽ .02,
⫽ .19; control,
motor skill learning because responses were faster with practice
F(4, 52) ⫽ 3.28, p ⫽ .02, 2 ⫽ .20. Unpaired t tests revealed that
(main effect of epoch), F(4, 104) ⫽ 6.59, p ⬍ .0001, 2 ⫽ .20.
proportional magnitude of learning was larger in ASD than control
Although overall responses were faster to pattern than random
children in Epoch 5, t(26) ⫽ 1.99, p ⫽ .06, d ⫽ 0.75 (all other
trials (main effect of trial type), F(1, 26) ⫽ 32.13, p ⬍ .0001,
ps ⬎ .17, d ⬍ 0.54). Thus, group differences in learning persisted
2 ⫽ .55, children exhibited sequence learning because the ben-
after controlling for baseline differences in response speed.
efits of repetition increased with practice (Trial Type ⫻ Epochinteraction), F(4, 104) ⫽ 3.72, p ⫽ .007, 2 ⫽ .13. Group
differences in learning were suggested by a Group ⫻ Epoch ⫻
Trial Type interaction, F(4, 104) ⫽ 2.53, p ⬍ .05, 2 ⫽ .09. No
Two forms of implicit learning, for spatial context and percep-
tual–motor sequences, did not differ between high-functioningchildren with ASD and controls. For spatial contextual learning,learning on the CC task did not differ between groups, despite
slower visual search performance in ASD relative to control chil-
dren. For sequential learning, whereas baseline ASRT perfor-
mance did not differ between the groups, expression of learning
was more prolonged in ASD than control children. Recognition
ASD, RandomCON, Pattern
memory for spatial configurations did not differ between groups,
and therefore, differences in explicit memory ability are unlikely to
account for the observed findings on the CC task. Explicit memory
for sequences on the ASRT task was not tested.
In a disorder characterized by impaired functioning in multiple
behavioral domains, spared learning abilities have important im-
plications for future research and treatment. Nonetheless, accept-ing the null hypothesis requires caution, and we consider several
Mean response time (in milliseconds) on the alternating serial
reaction time (ASRT) task as a function of epoch and type of trial for
alternative explanations: First, it is possible that our measures
autism spectrum disorder (ASD) and control groups.
lacked sensitivity to detect group differences in learning. However,
BARNES ET AL.
previous studies have found reduced magnitude of learning on the
Despite no group differences in implicit spatial contextual learn-
ASRT task in healthy aging (Howard & Howard, 1997; Howard
ing, the ASD group's performance differed from that of controls in
et al., 2004) and dyslexia (Howard, Howard, Japikse, & Eden,
two ways. First, overall response speed on the CC task was slower
2006) and on the CC task in childhood (Vaidya, Huger, Howard,
in children with ASD than it was in controls, a finding that is
& Howard, 2007) and mild cognitive impairment (Negash et al.,
inconsistent with reports of superior visual search in ASD
2007), suggesting that these tasks are sensitive to group differences
(O'Riordan, Plaisted, Driver, & Baron-Cohen, 2001; Plaisted,
in learning. Second, the small sample size could result in reduced
O'Riordan, & Baron-Cohen, 1998). Superior visual search in ASD
statistical power, thereby reducing our ability to detect group
has been posited to arise from weak central coherence (Happe´
differences in learning. Effect size for a group difference in total
1999) or a preference for visual details (O'Riordan et al., 2001).
magnitude of learning (sum of the difference between trial types
However, past studies have noted that superiority in ASD may not
across epochs) was moderate for the ASRT task (d ⫽ 0.43) and
extend to all visual search tasks (Kenworthy et al., 2005; Klein-
small for the CC task (d ⫽ 0.16); the larger effect size for the
hans, Akshoomoff, & Delis, 2005). Our finding of slower visual
ASRT task reflects greater rather than reduced learning in ASD
search in ASD is consistent with at least one previous study
relative to control children. The power to detect these effect sizes
examining visual search for a target letter (T or F) surrounded by
is low (ASRT: .17–.25; CC task: .06 –.08). More than 70 subjects
similar distractors (letters that were halfway between Ts and Fs;
would be needed for group differences of the obtained effect sizes
Edgin & Pennington, 2005). The present CC task also required
to be significant at ␣ ⫽ .05 with power ⫽ .80. Third, similar ASRT
searching for a target (T) among similar distractors (L). In the
learning in the two groups may result from differential explicit
present task, targets were rotated and the response required an
awareness for sequential information between the two groups. In
orientation judgment for the long arm of the T (left/right). This
past studies using a variety of recognition measures, adult partic-
added perceptual demand may have made visual search more
ipants did not develop explicit awareness on the CC and ASRT
effortful, enhancing the task's sensitivity to group differences.
tasks (Chun & Jiang, 2003; Song, Howard, & Howard, 2007).
Thus, slower performance on tasks requiring visual search in ASD
Although CC recognition was at chance in the present study, the
may be more apparent under certain experimental conditions.
influence of explicit awareness on the ASRT task cannot be
Slower visual search in children with ASD was not due to general
conclusively ruled out because it was not measured. Fourth, there
motor impairments because baseline response speed on the ASRT
were children in the ASD group who did not demonstrate learning
task did not differ between groups. Task selectivity of performance
in the last epoch (see Table 1), suggesting that there may be some
differences suggests that slower visual search in ASD reflects
children with ASD who showed impaired implicit learning. How-
atypical properties of spatial attention, possibly mediated by ocu-
ever, lack of implicit learning on the last epoch at the individual
lomotor dysfunction (Sweeney, Takarae, Macmillan, Luna, &
level is not unusual because it was apparent in some control
Minshew, 2004) rather than perceptual–motor dysfunction. How-
children (ASRT task: 5/14; CC task: 1/14).
ever, motivation levels could have also differed across tasks.
While considering our observation of lack of group differences,
Second, learning on the ASRT task did not differ between the
it is important to note that several characteristics of our sample
groups but its expression was more prolonged in ASD than in control
constrain interpretation and generalization of the present findings.
children. Studies with adults indicate that the expression of sequence
First, IQ was matched across groups, and therefore, the present
learning in performance can be dissociated from the acquisition of
findings are limited to intellectually high-functioning children with
sequence knowledge. For example, participants' response latencies
ASD. Second, the present findings are limited to Asperger's syn-
were modulated by task characteristics (e.g., stimulus context) and
drome, the diagnosis for 10 of the 14 children with ASD. It is also
performance demands (e.g., inclusion of a secondary task), even
important to note that ADOS and ADI scores were unavailable
though the structural knowledge of sequences they gained was un-
on 3 children with ASD. Third, the present findings extend pri-
changed (Jimenez, Vaquero, & Lupianez, 2006; Willingham, Green-
marily to boys with ASD because only 1 girl was included in the
berg, & Thomas, 1997). It is possible that prolonged expression of
ASD sample. Fourth, only 2 children with ASD were left-handed.
sequence learning in ASD reflects cognitive inflexibility that is known
Although hand assignment for the tasks was not changed for these
to characterize the ASD phenotype (Hill, 2004). Cognitive inflexibil-
participants, exclusion of their data from analyses did not influence
ity may promote expression of learning pertaining to invariant stim-
the results. Fifth, psychotropic medications that could not be
ulus-response contingencies, due to an inability to discard the adopted
withheld during testing in some children could have influenced
task set. Indeed, the tendency for more expression of sequence learn-
learning. Four of these children were on medications for attention
ing was observed in another psychiatric condition that is characterized
problems that are most likely to influence learning. However,
by stereotypical behaviors and cognitive inflexibility, obsessive– com-
magnitude of learning did not differ between children medicated
pulsive disorder. Patients with obsessive– compulsive disorder
for attention problems, unmedicated children with ASD, and con-
showed numerically, albeit not statistically, greater SRT improvement
trols on either task (unpaired t tests, all ps ⬎ .31). Furthermore,
relative to controls (Rauch, Savage, et al., 1997). The small sample
magnitude of learning for these children was within 95% confi-
size in the present study precludes examination of the relation be-
dence intervals for mean magnitude of learning in control children
tween magnitude of sequence learning and cognitive inflexibility in
for each task. Sixth, differences in fatigue did not appear to
ASD. However, this hypothesis can be tested in future studies.
influence the results because both groups responded faster as
Unimpaired learning of a complex sequential structure (i.e.,
epochs progressed. Faster performance, particularly on random
involving second-order regularity) in children with ASD is sur-
and novel trials, is inconsistent with fatigued performance. Thus,
prising in light of impaired learning of a simpler sequential struc-
the present findings most directly extend to right-handed, intellec-
ture on the SRT task (i.e., containing zero-order regularity where
tually high-functioning boys diagnosed with Asperger's syndrome.
some positions occur more frequently than others; Mostofsky
IMPLICIT LEARNING IN ASD
et al., 2000). Two factors could have contributed to these differ-
learning in childhood ASD provides a basis for investigating the
ences: First, characteristics of performance differed between the
nature of frontal–striatal– cerebellar involvement that characterizes
groups in the study by Mostofsky et al. (2000). Overall response
preserved learning.
speed was slower in ASD than in control children, perhaps because
In sum, the present findings indicate that two dissociable forms
of motor impairments that are common in ASD. Thus, nonmne-
of learning, of spatial context and perceptual–motor sequences,
monic aspects of SRT performance may have reduced the expres-
were intact in ASD children with a diagnosis of Asperger's syn-
sion of learning in Mostofsky et al.'s ASD participants. Second,
drome. If the present findings are replicated in future studies, they
ASD is characterized by highly heterogeneous symptom expres-
could be harnessed for treatment purposes. Future research could
sion. Perhaps differences in findings between the studies simply
study interventions that encourage children to focus on the degree
reflect distinct cohorts of children with ASD. Our sample consisted
to which social cues and contextual information co-occur and how
primarily of children diagnosed with Asperger's syndrome (10/
that relates to the status of implicit learning. Furthermore, findings
14), whereas participants in the study by Mostofsky et al. were
from the ASRT task suggest that ASD may promote longer ex-
diagnosed with high-functioning autism. Among the 4 non-As-
pression of learning based on invariant sequential information.
perger's children in the present study, learning was not below the
Functional imaging studies of sequence learning are required to
95% confidence interval in any child for the ASRT task but was
elucidate the neural basis of the current findings. The ASRT task
below the 95% confidence interval in 2 children (1 with high-
is an optimal probe for those studies because it taps a well-
functioning autism, 1 with pervasive developmental disorder—not
operationalized learning mechanism that is rooted in frontal–stria-
otherwise specified) for the CC task. Thus, future studies that
tal– cerebellar anatomy.
compare ASD cohorts are needed to clarify the extent of sparing orimpairment in implicit learning.
These results provide new knowledge about the functional in-
tegrity of neural systems that subserve implicit learning in ASD.
Achenbach, T. M. (1991). Manual for the Child Behavior Checklist/4–18
First, the finding that children with ASD did not differ from
and 1991 Profile. Burlington: University of Vermont.
American Psychiatric Association. (2000). Diagnostic and statistical man-
controls in spatial contextual learning suggests preservation of at
ual of mental disorders (4th ed., text revision). Washington, DC: Author.
least one mnemonic process supported by the medial temporal
Berl, M. M., Vaidya, C. J., & Gaillard, W. D. (2006). Functional imaging
lobes. Volumetric and histological studies have noted differences
of developmental and adaptive changes in neurocognition. Neuroim-
between individuals with ASD and controls in the hippocampus
age, 30, 679 – 691.
(Raymond, Bauman, & Kemper, 1996; Salmond et al., 2005;
Brambilla, P., Hardan, A., di-Nemi, S. U., Perez, J., Soares, J. C., & Barale,
Schumann et al., 2004). Spatial contextual learning appears to rely
F. (2003). Brain anatomy and development in autism: Review of struc-
on cortical regions surrounding the hippocampus because it was
tural MRI studies. Brain Research Bulletin, 61, 557–569.
intact in amnesic patients with lesions restricted to the hippocam-
Buckmaster, C. A., Eichenbaum, H., Amaral, D. G., Suzuki, W. A., &
pus (Manns & Squire, 2001). These surrounding cortices were also
Rapp, P. R. (2004). Entorhinal cortex lesions disrupt the relationalorganization of memory in monkeys. Journal of Neuroscience, 24,
involved in learning of hierarchical relations among elements on a
transitive inference task in monkeys (Buckmaster, Eichenbaum,
Chun, M. M. (2000). Contextual cueing of visual attention. Trends in
Amaral, Suzuki, & Rapp, 2004). These findings suggest that me-
Cognitive Science, 4, 170 –178.
dial–temporal cortices are involved in relational organization of
Chun, M. M., & Jiang, Y. (1998). Contextual cueing: Implicit learning and
spatial information. It would be useful to examine whether these
memory of visual context guides spatial attention. Cognitive Psychol-
cortical areas develop typically in ASD.
ogy, 36, 28 –71.
Second, the finding that sequence learning in ASD did not differ
Chun, M. M., & Jiang, Y. (2003). Implicit, long-term spatial contextual
from controls suggests spared frontal–striatal– cerebellar function.
memory. Journal of Experimental Psychology: Learning, Memory, and
No consistent finding has emerged from volumetric studies of
Cognition, 29, 224 –234.
frontal–striatal– cerebellar structures in ASD (Brambilla et al.,
Chun, M. M., & Phelps, E. A. (1999). Memory deficits for implicit
contextual information in amnesic subjects with hippocampal damage.
2003) as both larger and smaller volumes have been reported.
Nature Neuroscience, 2, 844 – 847.
Although there is agreement that these structures are involved in
Edgin, J. O., & Pennington, B. F. (2005). Spatial cognition in autism
sequence learning and that their maturation supports its develop-
spectrum disorders: Superior, impaired, or just intact? Journal of Autism
ment (Thomas et al., 2004), the specific contribution of each
and Developmental Disorders, 35, 729 –745.
structure is not fully known even in intact sequence learning.
Fletcher, P. C., Zafiris, O., Frith, C. D., Honey, R. A. E., Corlett, P. R.,
Functional imaging in ASD adults showed that despite comparable
Zilles, K., & Fink, G. R. (2005). On the benefits of not trying: Brain
sequence learning with controls, activation was reduced in pre-
activity and connectivity reflecting the interactions of explicit and im-
frontal cortex and increased in premotor cortex (Mu¨ller, Cauich,
plicit sequence learning. Cerebral Cortex, 15, 1002–1015.
Rubio, Mizuno, & Courchesne, 2004). Thus, involvement of dif-
Greene, A. J., Gross, W. L., Elsinger, C. L., & Rao, S. M. (2007).
ferent cortical regions in adults with ASD and matched controls
Hippocampal differentiation without recognition: An fMRI analysis ofthe contextual cueing task. Learning and Memory, 14, 548 –553.
may support intact sequence learning in ASD. However, partici-
Happe´, F. (1997). Central coherence and theory of mind in autism. British
pants learned the sequence explicitly rather than implicitly in
Journal of Developmental Psychology, 15, 1–12.
Mu¨ller et al.'s (2004) study. Prefrontal involvement in sequence
Happe´, F. (1999). Autism: Cognitive deficit or cognitive style? Trends in
learning appears to depend on the extent of explicit awareness of
Cognitive Science, 3, 216 –222.
sequential structure in both SRT and ASRT tasks (Fletcher et al.,
Hill, E. L. (2004). Evaluating the theory of executive dysfunction in
2005; Willingham et al., 2002). The present finding of intact ASRT
autism. Developmental Review, 24, 189 –233.
BARNES ET AL.
Howard, J. H., Jr., & Howard, D. V. (1997). Age differences in implicit
Plaisted, K., O'Riordan, M., & Baron-Cohen, S. (1998). Enhanced dis-
learning of higher order dependencies in serial patterns. Psychology and
crimination of novel, highly similar stimuli by adults with autism during
Aging, 12, 634 – 656.
a perceptual learning task. Journal of Child Psychology and Psychia-
Howard, J. H., Jr., Howard, D. V., Dennis, N. A., Yankovich, H., &
try, 39, 765–775.
Vaidya, C. J. (2004). Implicit spatial context learning in healthy aging.
Plaisted, K., Saksida, L., Alca´ntara, J., & Weisblatt, E. (2003). Towards an
Neuropsychology, 18, 124 –134.
understanding of the mechanisms of weak central coherence effects:
Howard, J. H., Jr., Howard, D. V., Japikse, K. C., & Eden, G. F. (2006).
Experiments in visual configural learning and auditory perception. Phil-
Dyslexics are impaired on implicit higher-order sequence learning, but
osophical Transactions of the Royal Society of London Series B, Bio-
not on spatial context learning. Neuropsychologia, 44, 1131–1144.
logical Sciences, 358, 375–386.
Jimenez, L., Vaquero, J. M. M., & Lupianez, J. (2006). Qualitative differences
Rauch, S. L., Savage, C. R., Alpert, N. M., Dougherty, D., Kendrick, A.,
between implicit and explicit sequence learning. Journal of Experimental
Curran, T., et al. (1997). Probing striatal function in obsessive– compul-
Psychology: Learning, Memory, and Cognition, 32, 475– 490.
sive disorder: A PET study of implicit sequence learning. Journal of
Jolliffe, T., & Baron-Cohen, S. (1997). Are people with autism and
Neuropsychiatry and Clinical Neurosciences, 9, 568 –573.
Asperger syndrome faster than normal on the Embedded Figures Test?
Rauch, S. L., Whalen, P. J., Savage, C. R., Curran, T., Kendrick, A.,
Journal of Child Psychology and Psychiatry, 38, 527–534.
Brown, H. D., et al. (1997). Striatal recruitment during an implicit
Kenworthy, L. E., Black, D. O., Wallace, G. L., Ahluvalia, T., Wagner,
sequence learning task as measured by functional magnetic resonance
A. E., & Sirian, L. M. (2005). Disorganization: The forgotten executive
imaging. Human Brain Mapping, 5, 124 –132.
dysfunction in high-functioning autism spectrum disorders. Develop-
Raymond, G. V., Bauman, M. L., & Kemper, T. L. (1996). Hippocampus
mental Neuropsychology, 28, 809 – 827.
in autism: A Golgi analysis. Acta Neuropathologica, 91, 117–119.
Kleinhans, N., Akshoomoff, N., & Delis, D. C. (2005). Executive functions
Raz, N., Lindenberger, U., Rodrigue, K. M., Kennedy, K. M., Head, D.,
in autism and Asperger's disorder: Flexibility, fluency, and inhibition.
Williamson, A., et al. (2005). Regional brain changes in aging healthy
Developmental Neuropsychology, 27, 379 – 401.
adults: General trends, individual differences and modifiers. Cerebral
Kuhn, D. (2000). Metacognitive development. Current Directions in Psy-
Cortex, 15, 1676 –1689.
chological Science, 9, 178 –181.
Salmond, C. H., Ashburner, J., Connelly, A., Friston, K. J., Gadian, D. G.,
Lieberman, M. D. (2000). Intuition: A social cognition neuroscience ap-
& Vargha-Khadem, F. (2005). The role of the medial temporal lobe in
proach. Psychological Bulletin, 126, 109 –137.
autistic spectrum disorders. European Journal of Neuroscience, 22,
Lord, C., Risi, S., Lambrecht, L., Cook, E. H., Jr., Leventhal, B. L.,
DiLavore, P. C., et al. (2000). The Autism Diagnostic Observation
Schumann, C. M., Hamstra, J., Goodlin-Jones, B. L., Lotspeich, L. J.,
Schedule—Generic: A standard measure of social and communication
Kwon, H., Buonocore, M. H., et al. (2004). The amygdala is enlarged for
deficits associated with the spectrum of autism. Journal of Autism and
children but not adolescents with autism; the hippocampus is enlarged at
Developmental Disorders, 30, 205–223.
all ages. Journal of Neuroscience, 24, 6392– 6401.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism Diagnostic Inter-
Scott, F. J., Baron-Cohen, S., Bolton, P., & Brayne, C. (2002). The CAST
view—Revised: A revised version of the diagnostic interview for care-
(Childhood Asperger Syndrome Test): Preliminary development of a
givers of individuals with possible pervasive developmental disorders.
UK screen for mainstream primary-school-age children. Autism, 6,
Journal of Autism and Developmental Disorders, 24, 659 – 685.
Manns, J. R., & Squire, L. R. (2001). Perceptual learning, awareness, and
Song, S. S., Howard, J. H., Jr., & Howard, D. V. (2007). Implicit proba-
the hippocampus. Hippocampus, 11, 776 –782.
bilistic sequence learning is independent of explicit awareness. Learning
Mostofsky, S. H., Goldberg, M. C., Landa, R. J., & Denckla, M. B. (2000).
and Memory, 14, 167–176.
Evidence for a deficit in procedural learning in children and adolescents
Sweeney, J. A., Takarae, Y., Macmillan, C., Luna, B., & Minshew, N. J.
with autism: Implications for cerebellar contribution. Journal of the
(2004). Eye movements in neurodevelopmental disorders. Current Opin-
International Neuropsychological Society, 6, 752–759.
ions in Neurology, 17, 37– 42.
Mottron, L., Burack, J. A., Iarocci, G., Belleville, S., & Enns, J. T. (2003).
Thomas, K. M., Hunt, R. H., Vizueta, N., Sommer, T., Durston, S., Yang,
Locally oriented perception with intact global processing among ado-
Y., & Worden, M. S. (2004). Evidence for developmental differences in
lescents with high-functioning autism: Evidence from multiple para-
implicit sequence learning: An fMRI study of children and adults.
digms. Journal of Child Psychology and Psychiatry, 44, 904 –913.
Journal of Cognitive Neuroscience, 16, 1339 –1351.
Mu¨ller, R. A., Cauich, C., Rubio, M. A., Mizuno, A., & Courchesne, E.
Vaidya, C. J., Huger, M., Howard, D. V., & Howard, J. H., Jr. (2007).
(2004). Abnormal activity patterns in premotor cortex during sequence
Developmental differences in implicit learning of spatial context. Neu-
learning in autistic patients. Biological Psychiatry, 56, 323–332.
ropsychology, 21, 497–506.
Negash, S., Peterson, L. E., Geda, Y. E., Knopman, D., Boeve, B. F.,
Willingham, D. B. (1997). Systems of memory in the human brain.
Smith, G. E., et al. (2007). Effects of ApoE genotype and mild cognitive
Neuron, 18, 5– 8.
impairment on implicit learning. Neurobiology of Aging, 28, 885– 893.
Willingham, D. B., Greenberg, A. R., & Thomas, R. C. (1997). Response-to-
Nissen, M. J., & Bullemer, P. T. (1987). Attentional requirements for
stimulus interval does not affect implicit motor sequence learning, but does
learning: Evidence from performance measures. Cognitive Psychol-
affect performance. Memory & Cognition, 25, 534 –542.
ogy, 18, 1–32.
Willingham, D. B., Salidis, J., & Gabrieli, J. D. E. (2002). Direct compar-
O'Riordan, M., Plaisted, K., Driver, J., & Baron-Cohen, S. (2001). Supe-
ison of neural systems mediating conscious and unconscious skill learn-
rior visual search in autism. Journal of Experimental Psychology: Hu-
ing. Journal of Neurophysiology, 88, 1451–1460.
man Perception and Performance, 27, 719 –730.
Perruchet, P., & Pacton, S. (2006). Implicit learning and statistical learn-
Received June 12, 2007
ing: One phenomenon, two approaches. Trends in Cognitive Science, 10,
Revision received March 17, 2008
Accepted March 18, 2008 䡲
Source: http://psychology.cua.edu/res/docs/howardpdfs/barnesneuropsychology08.pdf
Prenatal Guide for a Healthy Pregnancy Congratulations on your pregnancy! We are honored you have chosen us to provide your prenatal care. We are dedicated to making your pregnancy a positive and enjoyable experience. Although pregnancy is a very natural process in life, it can sometimes be difficult and complications can occur. This guide is provided as a resource to help you during those difficult times and an attempt to
A Comparative study of the Anticonvulsant effect of Nimodipine andKetamine combination with standardanticonvulsant drug in Rodents Prasanand S1, Pushpalatha C2, Mohsin MD3, Sam Pavan Kumar G4, Gundappa Rao S5 Aim of the study: To evaluate and compare the anticonvulsant property of nimodipine andketamine combination with a standard drug like Sodium valproate in electrically and chemically








