W.emacromall.com

MArch 2015
The 2014 Drug TrenD reporT
Commercially Insured Year in Review
Medicare Year in Review
A Look at Overall Drug Trend for 2014
A Look at Medicare Overall Drug Trend for 2014Medicare: Traditional Therapy Classes and Insights
Therapy class revIew
Top 10 Medicare Traditional Drugs
Comparison of Medicare and Commercial Trend: Traditional Therapy Classes
Commercially Insured: Traditional Therapy Classes and Insights
Medicare: Specialty Therapy Classes and Insights
Top 10 Traditional Drugs
Top 10 Medicare Specialty Drugs
Commercially Insured: Specialty Therapy Classes and Insights
Comparison of Medicare and Commercial Trend: Specialty Therapy Classes
Top 10 Specialty Drugs
MeDIcaID
2015-2017 TrenD forecasT
Medicaid Year in Review
Spend for Traditional Drugs in the Next Three Years
A Look at Medicaid Overall Drug Trend for 2014
Spend for Specialty Drugs in the Next Three Years
Medicaid: Traditional Therapy Classes and Insights Top 10 Medicaid Traditional Drugs
Medicaid: Specialty Therapy Classes and Insights
Patent Expirations
Top 10 Medicaid Specialty Drugs
The Express Scripts Prescription Price Index
The Drug Trend Report MethodologyCitations
coMMercIally InsureD year
In revIew
2014 Drug TrenD requires unpreceDenTeD AlignmenT AnD AcTion
or nearly three decades, Express Scripts has delivered innovative solutions that have helped contain rising medication costs for our clients and members.
F Annual drug spending increases have often been below the annual rate of overall
healthcare inflation in the U.S. Throughout this period, achieving the industry's lowest drug trend while still maintaining patient safety – as Express Scripts has done time and time again – has been neither accidental nor easy. Rather, it was the intentional result of our clients taking action and joining us as partners. The aggregate effect of our collective partnership has been to make the use of prescription drugs safer and more affordable.
In 2014, the pharmacy landscape underwent a seismic change, confronting healthcare payers with the highest annual increase in drug spend since 2003. New treatments for nonorphan conditions like hepatitis C were introduced in the U.S. market at exorbitant, orphan-drug pricing. Compounding pharmacies began exploiting a loophole in a new regulation that made the creation and dispensing of unproven topical creams a lucrative cottage industry. Drug manufacturers – brand and generic alike – continued to consolidate, placing additional strain on the supply chain to handle temporary shortages. The pipeline of
The aggregate effect of our collective
new medications to treat conditions like cancer and high blood cholesterol also threatened to undermine the sustainability of the U.S. pharmacy benefit.
partnership with clients has been to
These challenges are unprecedented, and the need to respond has never been greater.
make the use of prescription drugs
However, plan sponsors can no longer rely on the wave of less-expensive generics to control drug costs. They need to act now to more tightly manage the benefit, implement smarter
safer and more affordable.
formularies, control the use of compounded medications and offer clinical support to ensure that all patients are able to achieve the best health outcome possible.
To sustain a meaningful pharmacy benefit, payers must team with a pharmacy benefit manager (PBM) with the strength and scale to challenge the status quo, the flexibility and thoughtfulness to adapt to the evolving marketplace, the curiosity to explore new solutions, and the drive to make pharmacy care in America smarter. Express Scripts is dedicated to expanding both access and affordability, so that every patient receives the right medicine at the right time and at a fair cost. We are prepared to lead and make a difference.
DynAmic, complex phArmAcy chAllenges loom lArge
To sustain a meaningful pharmacy benefit, payers must team with
The challenges facing plan sponsors include the pipeline of niche drugs, orphan-drug
a PBM with the strength and scale to challenge the status quo,
pricing for nonorphan drugs, new innovations in commonly used therapy classes, cost
the flexibility and thoughtfulness to adapt to the marketplace, the
inflation for compounded medications and industry consolidation.
curiosity to explore new solutions, and the drive to make pharmacy
Pipeline of Niche Drugs
care in America smarter.
Historically, drug manufacturers' business models focused on research and development of key drugs to treat common conditions that affected millions of patients. These branded medications became blockbusters because of the volume of patients treated and a high,
Orphan-Drug Pricing for Nonorphan Drugs
but not exorbitant, price tag. As a result of patent expirations, generic competition and
Orphan drugs are among the most expensive medications in the U.S., often costing tens
a withering pipeline of broad-reaching drugs, manufacturers are shifting their drug
of thousands of dollars per prescription. These medications treat extremely rare conditions
discovery, development and pricing strategies. Now, manufacturers are increasing their
and diseases with very small, very specific populations – typically only several thousand
focus on medications that treat small subsets of patients with diseases like cancer, or
patients or fewer. The high price tag is necessary – and justified – to fund manufacturer
patients with rare diseases such as hereditary angioedema.1
research and development costs for these and future medical breakthroughs that might not otherwise happen.
Manufacturers also are tailoring molecular drugs to patients with specific genetic profiles known to be affected by certain diseases, so the drugs are more effective in treating those
One example, approved by the U.S. Food and Drug Administration (FDA) in late 2014, is
specific patients. For example, Ruconest® (C1 esterase inhibitor [recombinant]), approved
Harvoni® (ledipasvir/sofosbuvir), which is indicated for patients with genotype 1 of the
in July 2014, treats adult and adolescent patients with hereditary angioedema, a condition
hepatitis C virus. In the U.S. alone, there are an estimated three million patients with
that affects fewer than 10,000 Americans. Another such drug, Cerdelga™ (eliglustat),
hepatitis C – far beyond the 200,000-patient threshold that the FDA uses pursuant to the
offers an oral alternative to enzyme replacement therapy for adult patients with Gaucher
1983 U.S. Orphan Drug Act – to qualify an orphan-disease population.2 Despite its potential
disease type 1, an inherited lysosomal storage disorder that affects approximately 6,000
to treat millions of patients, Harvoni is priced at a staggering wholesale acquisition cost of
patients in the U.S.
$1,125 per tablet – more than $33,000 per 30-day prescription.
In addition, more than 1,000 targeted cancer treatments, many genetically guided, are
These new treatments for hepatitis C are just one
in development. One such niche cancer drug that recently received approval is Keytruda®
example of nonorphan drugs with orphan-drug price tags.
(pembrolizumab), an immunotherapy approved in September 2014 to treat a small
Rewarding pharmaceutical breakthroughs is undeniably
subpopulation of patients with certain genetic expressions of advanced, non-small cell
important to the discovery of future treatments and cures;
lung cancer. Keytruda and other niche drugs have the potential to vastly improve outcomes
however, payers and patients have limited resources
and are often clinical game changers. They typically have little or no competition and
and simply cannot afford these prices. Absent more fair
are often much more effective than the broader-spectrum drugs they are replacing as
drug pricing, payers will face half a trillion dollars in
first-line or second-line treatments. Many come with unprecedented price tags, however,
prescription drug costs as soon as 2020.
as pharmaceutical companies try to maintain profit margins and recoup their investments in drug discovery.
New Innovations in Commonly Used Therapy Classes
High blood cholesterol – a therapy class currently dominated by low-cost generics – also
Despite the recent focus on the development and promotion
is poised for significant cost increases in the coming years. New medications that inhibit
of specialty medications, there is still a considerable drug
proprotein convertase subtilisin/kexin type 9 enzyme, known as PCSK9 inhibitors, are
market for medications indicated to treat more common
currently in development with a primary indication for the treatment of a genetic disorder
chronic conditions, such as diabetes and high blood
called familial hypercholesterolemia. This inherited condition leaves the body unable to
cholesterol, which combined affect at least 19 million
remove low-density lipoprotein (LDL) cholesterol from circulation, causing cardiovascular
Americans.3 Although both chronic conditions have well-
disease to develop early in life.
established treatment regimens, many of which have
These new biologic products inhibiting PCSK9 represent a novel way to lower cholesterol
generic equivalents, recent approvals and innovative
and may offer additional treatment options for those patients with very high LDL
pipelines will increase current and future drug spend.
cholesterol levels. Although PCSK9 inhibitors may initially be used in patients with familial
Diabetes medications were the only nonspecialty therapy class to have a significant
hypercholesterolemia and in those who cannot tolerate statins, their potential for expanded
increase in per-member-per-year (PMPY) drug spend in 2014, largely due to two newly
uses as adjunct therapies to lower LDL in general could impact drug spend significantly.
approved medications known as sodium-glucose co-transporter 2 (SGLT2) inhibitors.
Projected to command an annual cost of $10,000 or more per patient, they eventually could
SGLT2 inhibitors work with the body's natural processes to remove excess glucose from the
be used as a chronic therapy for a large portion of the 71 million patients in the U.S. with high cholesterol.5
bloodstream. The FDA approved the first SGLT2 inhibitor in 2013, two more were approved in 2014 and many more are in the development pipeline.
Cost of Inflation for Compounded IngredientsIn 2013 and 2014, spend for compound medications escalated rapidly. Compounded drugs
The diabetes pipeline also includes once-weekly oral and injectable treatments that
now rank as the third-most-expensive therapy class on a PMPY basis, displacing high blood
demonstrate greater clinical efficacy than currently available therapies, as well as drugs
pressure medications, which had ranked among the top three most-expensive traditional
with new mechanisms of action aimed at regenerating insulin-producing cells.4 In addition,
therapy classes for at least a decade. Compounding was the primary manner in which
we anticipate insulin medications such as Lantus® (insulin glargine) will soon compete
prescriptions were prepared until the 1950s, but mass pharmaceutical production had
with next-generation biosimilar insulins from several pharmaceutical manufacturers, likely
supplanted this method by the 1970s. The volume of compounded prescriptions continued
spurring the escalation of costs for insulin medications that occurred in 2014.
to drop over time, representing less than 1% of all prescriptions by the early 2000s,6 and their share is similar today.
Diabetes medications were the only nonspecialty therapy class
The reasons for the current increases in the cost and utilization of compounded medications are complex. Key factors include changes in industry practices that make it easier to
(aside from compounded medications) to have a significant
track the costs of these medications, marketing and billing practices of compounding
increase in PMPY spend. We anticipate that insulin medications
pharmacies, physician prescribing and patient demand. Compounding practices are
such as Lantus® (insulin glargine) will soon compete with next-
regulated under the Food and Drug Administration Modernization Act of 1997 (FDAMA).7
generation biosimilar insulins from several pharmaceutical
Drug compounders are not required, however, to demonstrate the safety, efficacy, strength,
quality or purity of their products – as producers of commercially manufactured drugs must do. Moreover, pharmacies are not required to report compounding-related adverse events to the FDA or, as currently required in only a few states, to the state boards of pharmacy.8
An effort toward increased transparency, in fact, began fueling the current increase in
innovATive heAlThcAre soluTions Drive BeTTer heAlTh AnD
trend for compounded medications. In 2012, an updated version of the Health Insurance
Portability and Accountability Act (HIPAA) standard for pharmacy claims transactions –
Through the technology and talent at work in the Express Scripts Lab, expanded in 2014, we
National Council of Prescription Drug Plans (NCPDP) Telecommunications Standard Version
turn data into insights and insights into proven solutions at an even faster pace. We study,
D.0 – was implemented. One component of this standard was the requirement that all
in real time, how patients interact with their healthcare providers and their medications.
components of compounded drugs be specified and billed using average wholesale price
Using these insights, we rapidly translate research findings into practical applications
(AWP) at the ingredient level, rather than being rolled up under the highest-priced ingredient
and proven solutions that address our members' and plan sponsors' most pressing needs.
according to previous claims and billing standards.9 Since then, bulk manufacturers and
By keeping our clients and members at the center of everything we do, we align our interests
compounding pharmacies have substantially raised AWP prices for the components of
to make the use of prescription medications safer, more affordable and more accessible.
many compounded drugs, creating unsustainable cost increases.
National Preferred Formulary
Today, most therapy classes offer more drug choices than ever. But the downside of today's
The wave of billions of dollars' worth of brand blockbuster medications losing patent
pharmacy climate is that many prescription drugs cost more but deliver no additional
protection in the past few years has led to unprecedented availability of generic drugs, while
health benefit. Smart formulary management is therefore vital to preserve patient access
the resulting competition among manufacturers and suppliers of new generic medications
and choice while ensuring that payers can obtain fair and affordable pricing. By excluding
drove down drug costs substantially in most of the top therapy classes. But, the pace
a group of "me-too" products from the Express Scripts National Preferred Formulary (NPF),
of price reductions has begun to slow: generic prices for the most commonly used drugs decreased 20% from 2013 to 2014, compared to the 30% drop seen from 2012 to 2013. And some generic drugs, for example, doxycycline and oxycodone, available generically for
plan cosT for IMpacTeD Drugs for clIenTs who DID anD DID noT
many years, experienced considerable price increases in 2014.
aDopT The naTIonal preferreD forMulary In 2014
The most significant contributing factor was consolidation in the supply chain as a
PMPM Plan Cost for IMPaCted drugs by Month
result of merger and acquisition activity among pharmaceutical manufacturers and drug
distributors. Fewer manufacturers means less competition and increased prices. There
now is only one – or at most, two – generic manufacturers of some specific products, for example, digoxin, when there used to be five or six producing a medication. According to
analysts, just three generic drug companies were responsible for almost half of the revenue
generated by all generic drugs in 2013.10 This industry consolidation will continue to be a challenge for payers; but the good news is that, on the whole, generic medications continue
to deliver significant cost savings.
NPF Implementation
Did Not Adopt the National Preferred Formulary
Adopted National Preferred Formulary
we have the necessary leverage to negotiate more effectively with manufacturers and
coMparIson of TraDITIonal Drug TrenD In clIenTs who DID
ultimately achieve lower drug prices for our clients and patients. And in the rare instance
anD DID noT IMpleMenT our coMpounDeD Drug uTIlIzaTIon
when a patient has a medical need for an off-formulary drug, we have a pathway for the
ManageMenT (uM) soluTIon
excluded drug to be covered for the patient.
In January 2014, we moved 48 products to "not covered" status for our 2014 NPF, which is the selected formulary for approximately 30% of our members. The products that
we removed represented only about 1% of all the products currently on our formulary, and
were used by fewer than 3% of our members. Importantly, each of these products had clinically equivalent alternatives on the market that remain on our formulary. This effort at
cost control without sacrificing patient health paid off for plan sponsors who adopted the
2014 NPF: drug costs in the affected therapy classes decreased on average 3.9% over the same time period in 2013 (see chart on previous page). Among plan sponsors who didn't
adopt the new standards, however, drug costs in the same therapy classes increased on
Clients Who Implemented UM
Clients Who Did Not Implement UM
average 7.2% over the first 6 months of 2013.
Our 2015 NPF excludes only 74 drugs out of 4,800 that were available on the market on the
before the compound-management solution was offered, plan sponsor spend for
first day of the year. As a group, plan sponsors who adopt the 2015 NPF will save more than
compounded medications was 276.9% greater than spend for the same period in 2013.
$1 billion for the year. In the U.S. healthcare industry – where government does not set price
By contrast, after the four-month period post implementation of the compound-management
limits on medications and consumers are not exposed to the full price of those medications
solution, the growth in plan sponsor spend had slowed to 128.4%.
– smart formulary management is one of the few ways to swing the balance of power from
The chart above shows the solution's monthly impact by comparing the traditional drug
pharmaceutical manufacturers to those who pay for healthcare: health plans, employers,
trend for commercial plan sponsors that chose to actively manage compounded drugs and
taxpayers and individual patients.
the trend for those that did not. In September, the traditional trend for the sponsors that
Compounded Drugs
began actively managing compounded-drug spend was 6.6%, compared to a trend of 7.5%
Compounded drugs often cost more than similar
for plan sponsors that were either waiting to implement the program or did not intend to
FDA-approved medications, but are not necessarily
implement the program.
more effective. In response to skyrocketing plan costs
Compounded-drug spend continued to decrease among clients that were actively managing
for compounded drugs, Express Scripts developed
their spend for these drugs. By December 2014, the traditional trend for clients who had
a comprehensive compound-management solution
Drug Trend Before
implemented the management solution was 4.5%, a decrease of 2.1 percentage points
that evaluates all ingredients used in compounded
over the four-month period, while it was 7.7% for clients who had not. By continuously
drugs to identify needless cost and waste, actively
monitoring for clinically unproven ingredients used in compounded medications, the
manages the use of compounded drugs and blocks
solution has eliminated the majority of unnecessary spend for these medications with
more than 1,000 clinically unproven ingredients. This solution had a significant and
minimal patient impact.
immediate impact on drug spend in 2014. For the first eight months of the year,
Actions to Establish More Fair Drug PricingThroughout 2014, Express Scripts was largely critical of a recent trend of drug manufacturers: bringing products to market that, while innovative, are priced so high that payers could not sustain the burden. The leading example was the hepatitis C treatment Sovaldi® (sofosbuvir), whose cost of $84,000 threatened to bankrupt both private and public plan sponsors around the United States. Never before had a drug to treat a population so large been priced so high. Clients and patients needed a champion, and Express Scripts took action.
After a year-long campaign advocating for more fair drug pricing, Express Scripts announced in December a new agreement with AbbVie, makers of the new hepatitis C medication Viekira Pak™ (ombitasvir/paritaprevir/ritonavir packaged with dasabuvir). The unprecedented arrangement addresses both affordability for payers and access for patients. The cost to cure is now low enough that plan sponsors can afford to treat all hepatitis C patients with genotype 1, not just the sickest.
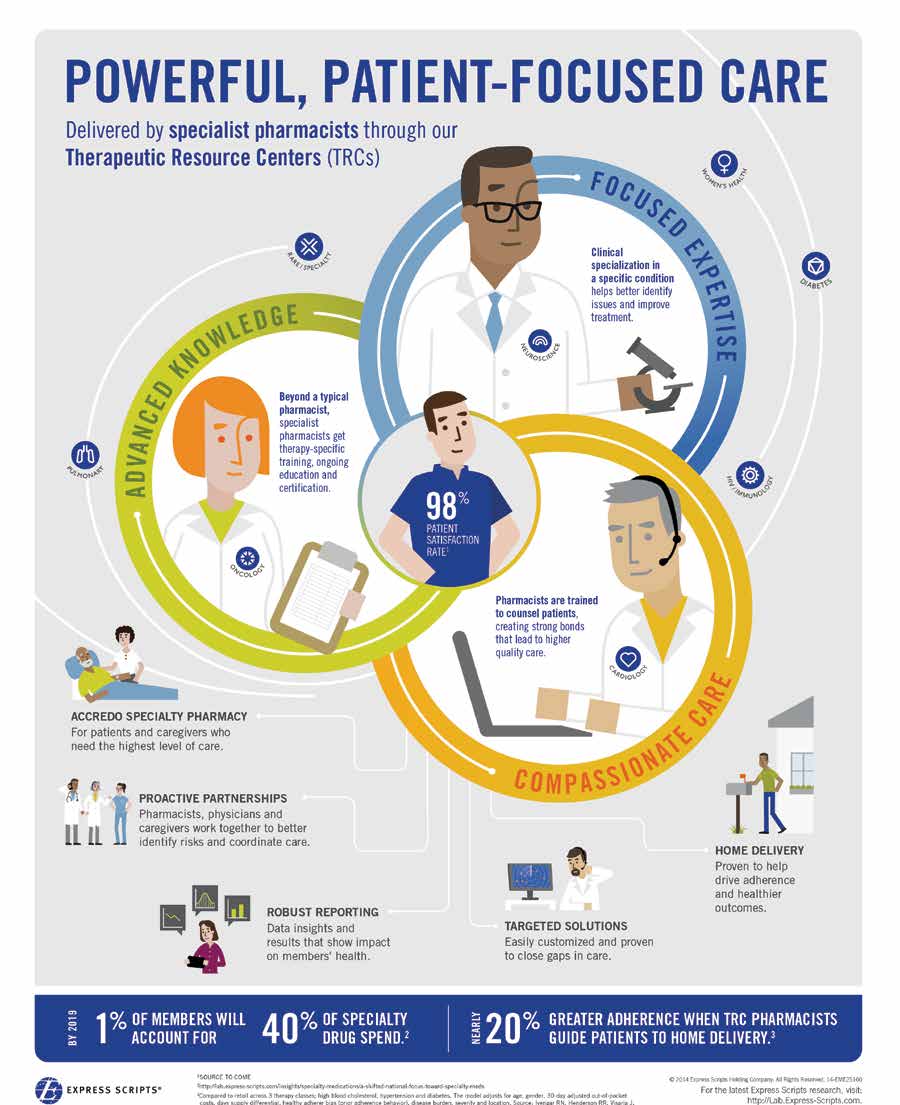
Therapeutic Resource CentersThe clinically specialized Therapeutic Resource Centers (TRCs) of the Express Scripts PharmacySM and Accredo Specialty Pharmacies deliver superior treatment outcomes and cost-effectiveness by supporting patients through the challenges of complex and costly diseases. Our specialist pharmacists and nurses each receive clinically specialized training in one disease state, and the work they do focuses almost exclusively on that clinical condition. This specialized expertise and commitment enables an optimal patient experience and ensures the highest performance in pharmacy safety, improved medication adherence and closing gaps in care. With their highly specialized knowledge of the complex disease states and complicated treatment protocols they manage, these TRC pharmacists and nurses often have more experience in rare conditions than some of the physicians who prescribe the treatments. For example, the Multiple Sclerosis (MS) Therapeutic Resource Center® manages 72,000 patients with MS, whereas the typical neurologist might have only a few MS patients in his or her entire practice.
TRCs are responsible for interacting with millions of members each year, and each interaction is an opportunity to provide expert clinical counseling, reconcile a drug safety issue or close a gap in care. Annually, the TRCs are involved in over 30 million clinical interactions and close more than 4.8 million gaps in care. Accredo's Hepatitis Therapeutic Resource Center®
delivers the industry's highest level of successful therapy completion, which is critical to a patient's avoiding future virus resistance. In addition, Express Scripts research suggests that patients who received care through the Express Scripts Therapeutic Resource Centers had between 11% and 19% better adherence to oral diabetes, hypertension and statin medications than patients at even the highest-performing retail pharmacies.11
Managed vs. Unmanaged ClientsBalancing patient access and costs is a challenge for every payer, given the volume of therapeutic options available and the variety of conditions those medications treat. Leveraging decades of clinical experience and innovation, Express Scripts'
research-driven, state-of-the-art clinical programs and management solutions help optimize drug utilization and costs.
We examined the impact of multiple utilization-management and cost-management strategies on traditional drug spend in 2014.12 Strategies such as drug tier levels, step therapy programs, out-of-pocket pay differentials, formulary management and utilization of home delivery from the Express Scripts Pharmacy were considered. In the study, plan sponsors were categorized into one of three groups based on the type of network-management and utilization-management programs they implemented as part of the pharmacy benefit:
• Unmanaged — Plan sponsors who did not implement any, or who implemented only
one utilization-management or cost-management program
• Managed — Plan sponsors who implemented two or three of the five network-
management or utilization-management programs offered
• Tightly managed — Plan sponsors who implemented four or five of the network-
management or utilization-management programs offered
Compared to unmanaged plans, tightly
The results showed that "unmanaged" plans experienced an annual average increase in PMPY spend for traditional medications of 4.1% in 2014, whereas "tightly managed"
managed plans spent 27.6% less on
plans' traditional drug spend increased only 0.3%. Compared to unmanaged plans, tightly managed plans spent 27.6% less on traditional drugs per member in 2014.
traditional drugs per member in 2014.
a look aT overall Drug TrenD
verall drug spend increased 13.1% in 2014, following several years of declining
coMMercIally InsureD: coMponenTs of TrenD
rate increases. Market forces and changes in patient behavior impacted drug
O expenditures in 2014, but brand drug cost was one of the most important 2014
factors driving trend, especially for specialty medications.
pMpy spenD
unIT cosT
Drug trend comprises two main components: utilization and unit cost. The utilization of
traditional prescription medications decreased marginally (0.1%) from 2013 to 2014, but
the use of specialty medications increased 5.8%. Unit costs – the costs of the medications
themselves – drove spending for both traditional and specialty medications higher by
January-December 2014 compared to same period in 2013
6.5% and 25.2%, respectively. The 13.1% overall trend was composed of a 6.4% increase in spend for traditional (nonspecialty) medications and a 30.9% increase in spend for specialty medications, the highest specialty drug trend ever recorded. Specialty medications
TraDITIonal, specIalTy anD overall TrenD
continued to contribute an ever-increasing share of total spend (31.8%, up from 27.7% in 2013) that is expected to reach 44% in the next three years. (Note: Roughly half of specialty
2006 to 2014
medication drug costs are billed through the medical benefit and therefore are not included
in our trend calculation.)
Overall Drug Trend
Traditional Drug Trend
Specialty Drug Trend
Therapy class revIew
TherApy cl Ass review
coMMercIally InsureD: TraDITIonal Therapy classes anD InsIghTs
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
High Blood Cholesterol
High Blood Pressure/Heart Disease
Heartburn/Ulcer Disease
Attention Disorders
or the fourth consecutive year, medications used to treat diabetes were the most
remained among the top three therapy classes, high blood pressure medications dropped from
expensive traditional therapy class when ranked by per-member-per-year (PMPY)
number three to number five as compounded drugs rose in rank to the third most expensive
F spend. Additionally, the diabetes class had the largest trend with the exception of
therapy class and pain/inflammation moved up from number nine to number four.
compounded drugs – which jumped into third place – largely due to a 128.2% unit cost trend.
Utilization, which increased less than 1.0% in two of the top 10 traditional classes
Total trend was negative for half of the top 10 therapy classes (high blood cholesterol, high
(compounded drugs and pain/inflammation), was up modestly in another three (diabetes,
blood pressure/heart disease, heartburn/ulcer disease, asthma and depression), stemming
attention disorders and depression). At the same time, unit costs decreased in five
from declines in utilization, unit cost or both among the different classes.
traditional classes (high blood cholesterol, high blood pressure/heart disease, heartburn/
The top three therapy classes (diabetes, high blood cholesterol and compounded drugs)
ulcer disease, asthma and depression). This pattern generally reflects the continuing
contributed 28.8% of traditional drug spend in 2014. Although high blood cholesterol drugs
genericization of commonly used therapy classes.
TherApy cl Ass review
• The increase in spend for compounded medications in 2014 represented a staggering
change from 2013, when compounded medications did not appear among the top 10 therapy classes. Compounded drugs strongly drove 2014 traditional trend; if excluded from the analysis, total traditional trend would have been only 2.3%.
• Another driver of positive trend among traditional therapy classes was diabetes. Trend
was 18.0%, primarily from a 16.3% increase in drug costs. Much of the increase was due to continued brand innovation and brand inflation. Drug cost increases have been particularly significant among insulins, such as Lantus® (insulin glargine [rDNA origin] injection) and Levemir® (insulin detemir [rDNA origin] injection). Because insulins are manufactured by biological processes, true generics for them are not possible. But biosimilar formulations being developed are expected to cost between 20% and 40% less than the branded innovators.13 At least some of the increases in price for currently available insulins may have been a result of the upcoming patent expiration for Lantus and the uncertainty around biosimilar activity in the diabetes space.
• Utilization for medications used to treat high blood cholesterol decreased 2.9% after
a 2.1% decrease in 2013. In addition to market saturation of generics for the most commonly used drugs, the decline may also be related to the ongoing impact of low-cost generic programs offered by retail pharmacies.
If compounded medications were
• Total trend for traditional asthma medications was -14.9%, driven by drops in utilization
excluded from the analysis, total
(3.2%) and unit cost (11.6%). The decrease in utilization is likely related to market movement toward specialty asthma medications such as Xolair® (omalizumab). Lower unit
traditional trend would have been
cost is due mainly to lasting impact from the August 2012 patent expiration of Merck's Singulair®. The generic, montelukast, captured 33.4% of the asthma market in 2014.
just 2.3% instead of 6.4%.
• In addition to a small increase in utilization compared to 2013, the 15.7% increase in
unit cost resulted in a 16.0% spend increase for pain/inflammation medications. Large price increases for two commonly used drugs, generic opioid oxycodone/acetaminophen and branded Celebrex® (celecoxib), are driving unit cost trend. The cost of oxycodone/acetaminophen, which captured 7.3% of 2014 market share in the class, likely increased because of generic supply chain issues and new U.S. Food and Drug Administration (FDA) regulations limiting the amount of acetaminophen allowed in combination opioid products. Brand Celebrex increased in price prior to the launch of generic formulations in December 2014.
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
Medications used to treat diabetes were the most expensive for the fourth
year in a row when ranked by per-
pMpy spenD
unIT cosT
member-per-year (PMPY) spend, which
was $97.68, 18.0% higher than in
High Blood Cholesterol
2013. Brand innovation continues in
this traditional therapy class. Four
new diabetes treatments – Farxiga™
High Blood Pressure/Heart Disease
(dapagliflozin), Tanzeum™ (albiglutide),
Heartburn/Ulcer Disease
Jardiance® (empagliflozin) and
Invokamet™ (canagliflozin/metformin) –
Attention Disorders
were approved in 2014. Less than half
of the prescriptions filled for diabetes
treatments were generic in 2014. Despite
the influx of new, branded medications however, three of the most commonly used diabetes treatments – metformin,
Top Drugs
generIc fIll raTe (gfr)
glipizide and glimepiride – are generic drugs with branded formulation patents
by MarKet share
that expired at least a decade ago.
Despite having the highest PMPY cost,
OneTouch® Ultra Test Strips
2014 prevalence of use for diabetes
Lantus® (insulin glargine)
medications was among the lowest for
traditional therapy classes in the top 10.
0.881NUMBER OF PRESCRIPTIONS
$110.86 AVERAGE COST
5.1% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
hIgh BlooD
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
A 3.9% decline in unit cost and a 2.9%
pMpy spenD
unIT cosT
drop in utilization contributed to an overall
6.8% decrease in per-member-per-year
High Blood Cholesterol
(PMPY) spend for high blood cholesterol
treatments in 2014. The market saturation
of generic drugs continues to fuel declines
High Blood Pressure/Heart Disease
in drug prices. In addition, one of the
Heartburn/Ulcer Disease
last remaining brand statins, Crestor®
(rosuvastatin), is set to lose patent
Attention Disorders
protection in 2016. However, a new class
of biologic products known as PCSK9
inhibitors, which is in development,
represents a novel way to lower cholesterol. Although initial approval of these agents likely will be to treat rare forms of hypercholesterolemia, their potential
Top Drugs
generIc fIll raTe (gfr)
expanded use as adjunctive therapies to
by MarKet share
reduce low-density lipoproteins (LDL) for a
wider population could impact drug spend
significantly. This will impact specialty
drug trend in the future. Atorvastatin,
Crestor® (rosuvastatin)
the active ingredient in Lipitor®, is the
most commonly used medication in
1.141 NUMBER OF PRESCRIPTIONS
$42.69 AVERAGE COST
11.0% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMpounDeD
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
Compounded drugs ranked in the top 10
pMpy spenD
unIT cosT
therapy classes for the first time. Since
the implementation of new regulations
High Blood Cholesterol
in 2012 requiring that all components
of compounded drugs be specified and
billed at the ingredient level rather than
High Blood Pressure/Heart Disease
being rolled up under the highest priced
Heartburn/Ulcer Disease
ingredient, bulk manufacturers and
compounding pharmacies have raised
Attention Disorders
prices substantially for the components
of many compounded drugs. The result
has been unsustainable cost increases.
If compounded drugs were excluded, traditional drug trend would have been only 2.3% (vs. 6.4%) and total overall trend would have been 10.4%
Total trend for compounded
(vs. 13.1%). Many of the compounded
by voluMe
drugs – 128.4% – was higher
medications billed under the pharmacy
benefit contained ingredients used to
than trend for any other top 10
baclofencyclobenzaprine
traditional therapy class.
progesterone micronizedpropylene glycol
0.040 NUMBER OF PRESCRIPTIONS
$1,164.12 AVERAGE COST
1.4% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
A marginal increase in utilization (0.3%)
pMpy spenD
unIT cosT
combined with a significant increase in
unit cost (15.7%) contributed to a 16.0%
High Blood Cholesterol
increase in per-member-per-year (PMPY)
spend for pain/inflammation medications
in 2014. Although generic medications
High Blood Pressure/Heart Disease
continue to dominate the class, PMPY
Heartburn/Ulcer Disease
spend has not declined in accordance.
This is because manufacturers of newer
Attention Disorders
versions of branded, tamper-resistant
formulations have been successful in
blocking generic market saturation with
claims of superior safety. Together, the five most commonly used drugs, all generics, captured 55.1% of market share.
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
A study showed that 78.5% of patients
prescribed pregabalin treatment for neuropathic
pain discontinued therapy within one year.14
oxycodone/acetaminophen
1.127 NUMBER OF PRESCRIPTIONS
$40.82 AVERAGE COST
22.7% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
hIgh BlooD
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Per-member-per-year (PMPY) spend for
High Blood Cholesterol
medications used to treat high blood
pressure/heart disease decreased 12.6%,
to $36.06, driven by a 12.2% decrease in
High Blood Pressure/Heart Disease
unit cost. Generic medications made up
Heartburn/Ulcer Disease
93.5% of total 2014 market share, in part
due to the final approval and subsequent
Attention Disorders
launch of generics to brand Diovan®
(valsartan), which legal issues had
delayed more than a year. The number
of PMPY prescriptions for high blood pressure/heart disease medications was the highest among the traditional therapy classes in the top 10.
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
2.493NUMBER OF PRESCRIPTIONS
$14.46 AVERAGE COST
16.9% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
Per-member-per-year (PMPY) spend for
pMpy spenD
unIT cosT
medications used to treat heartburn and
ulcer diseases such as gastroesophageal
High Blood Cholesterol
reflux disease (GERD) decreased 10.6%, to
$33.40, fueled by a 9.2% decrease in unit
cost and a 1.4% decline in utilization. The
High Blood Pressure/Heart Disease
decline in utilization is due primarily to a
Heartburn/Ulcer Disease
shift to the over-the-counter (OTC) version
of Nexium® (esomeprazole magnesium),
Attention Disorders
which became available in May 2014.
With generic medications now making up
77.0% of total market share in the class,
it is not surprising that the average cost per prescription dropped from $62.08 in 2013 to $56.26 in 2014.
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
A recent review of studies examining the
utilization of proton pump inhibitors (PPIs)
Nexium® (esomeprazole magnesium)
suggests that adherence among patients using
pantoprazole ranitidine
PPI ranges between 53.8% and 67.7%.15
0.594NUMBER OF PRESCRIPTIONS
$56.26 AVERAGE COST
8.3% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
Although the generic fill rate (GFR) for asthma medications was the lowest of
any top 10 traditional therapy class,
pMpy spenD
unIT cosT
per-member-per-year (PMPY) spend for
asthma medications dropped 14.9%
High Blood Cholesterol
in 2014, to $29.59, due primarily to an
11.6% decrease in unit cost. Asthma
also dropped in rank from the fifth to
High Blood Pressure/Heart Disease
the seventh most expensive therapy
Heartburn/Ulcer Disease
class from 2013 to 2014. Montelukast,
the generic formulation of Singulair®,
Attention Disorders
continued to capture more market share
than any other medication. Asthma
medications had a relatively high
average cost per prescription in 2014, although two of the five most commonly used drugs in the class were generics.
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
54.9% of adult patients and
montelukast Proair® HFA (albuterol)
78.3% of pediatric patients are
Ventolin® HFA (albuterol) Symbicort (budesonide/formoterol)
nonadherent to medication therapy.
0.431NUMBER OF PRESCRIPTIONS
$68.60 AVERAGE COST
8.8% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
aTTenTIon
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
Per-member-per-year (PMPY) spend for
pMpy spenD
unIT cosT
medications used to treat attention
disorders increased 6.3% in 2014,
High Blood Cholesterol
driven by a 3.4% increase in utilization
and a 2.9% increase in unit cost. A non-
controlled drug, Intuniv™ (guanfacine),
High Blood Pressure/Heart Disease
first approved in 1986 as the blood
Heartburn/Ulcer Disease
pressure medication Tenex®, was
reapproved in September 2009 as an
Attention Disorders
extended-release product for attention
deficit hyperactive disorder (ADHD). The
first generic for Intuniv entered the U.S.
market in December 2014, and others are expected to launch in mid-2015.
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
Recent research suggests that medication
adherence may be less than 12% in adult
ADHD patients taking stimulant medications
Strattera® (atomoxetine)IntunivTM (guanfacine)
such as methylphenidate.16
0.222NUMBER OF PRESCRIPTIONS
$126.11 AVERAGE COST
2.6% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
Utilization of medications used to treat depression increased 2.1%, but a
20.5% decrease in unit cost contributed
pMpy spenD
unIT cosT
significantly to negative overall trend.
Recent literature has noted rising use
High Blood Cholesterol
of antidepressants due to increases
in both the prevalence of depression
and the duration of therapy for the
High Blood Pressure/Heart Disease
condition.17 In 2014 alone, the generic
Heartburn/Ulcer Disease
fill rate (GFR) for the depression therapy
class climbed from 88.4% to 95.9%,
Attention Disorders
and only one brand drug, Viibryd®
(vilazodone), remains among the top
10 most commonly used medications in
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
sertralinebupropion extended release
0.905NUMBER OF PRESCRIPTIONS
$28.72 AVERAGE COST
10.0% PREVALENCE
TrADiTionAl spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
A significant increase in unit cost (9.6%)
High Blood Cholesterol
contributed to the 9.1% increase in
per-member-per-year (PMPY) spend for
mental/neurological disorders treatments
High Blood Pressure/Heart Disease
in 2014. An antipsychotic, Abilify®
Heartburn/Ulcer Disease
(aripiprazole), which captured 16.3%
of market share in 2014, faces the
Attention Disorders
impending loss of patent protection in
April 2015 and experienced a significant
increase in unit cost. Price increases
often are seen in the months before a brand drug's patent expires.
Top Drugs
generIc fIll raTe (gfr)
by MarKet share
Abilify® (aripiprazole)
donepezil lithium
0.118NUMBER OF PRESCRIPTIONS
$209.96 AVERAGE COST
1.5% PREVALENCE
TherApy cl Ass review
Top 10 TraDITIonal Drugs
ine of the top 10 drugs when ranked by PMPY spend in 2014 were branded
Although total trend for Nexium
medications, accounting for 18.4% of total traditional spend. The lone generic,
N duloxetine, the active ingredient in Cymbalta®, climbed to sixth place in its was -5.7%, it remained as the most
first full year of availability. Cymbalta's patent expired in December 2013 and duloxetine's
expensive traditional drug therapy used
total trend was 2,010.7%, reflecting the ongoing utilization switch from the branded formulation of Cymbalta to the generic formulation throughout 2014 (the generic was
by commercially insured beneficiaries.
only available for a short time in 2013). Nexium again had the highest PMPY spend at $24.02, followed by Crestor at $17.89. Among the top 10, utilization trend was marginally or significantly negative for seven drugs, but all except duloxetine showed at least a 3.9% increase in unit cost, with the highest for Humalog, at 36.1%.
Top 10 TraDITIonal Therapy Drugs
ranKed by 2014 PMPy sPend
% of ToTal
Drug naMe
pMpy spenD TraDITIonal spenD
unIT cosT
Nexium® (esomeprazole magnesium)
Crestor® (rosuvastatin)
High Blood Pressure/Heart Disease
Lantus® (insulin glargine)
Abilify® (aripiprazole)
Humalog® (insulin lispro)
OneTouch Ultra® Test Strips
AndroGel® (testosterone gel)
Hormonal Supplementation
Lialda® (mesalamine)
Inflammatory Conditions
Januvia® (sitagliptin)
TherApy cl Ass review
coMMercIally InsureD: specIalTy Therapy classes anD InsIghTs
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Inflammatory Conditions
Multiple Sclerosis
Miscellaneous Specialty Conditions
Growth Deficiency
Pulmonary Arterial Hypertension
er-member-per-year (PMPY) spend for the top three specialty therapy classes –
from the top 10. For the first time, drugs used for primary prevention and treatment of
inflammatory conditions, multiple sclerosis and oncology – contributed 55.9%
acute bleeding in hemophilia patients were among the most expensive specialty classes
P of the spend for all specialty medications billed through the pharmacy benefit
ranked by PMPY spend.
in 2014. Drugs for inflammatory conditions (such as rheumatoid arthritis and psoriasis) continued to hold the top spot. The class of drugs used to treat hepatitis C was not even among the top 10 specialty classes in 2013. However, just three hepatitis C medications –
The class of drugs used to treat hepatitis C was not even among the
Sovaldi® (sofosbuvir), Olysio® (simeprevir) and Harvoni® (ledipasvir/sofosbuvir) made up
top 10 specialty classes in 2013. However, just three hepatitis C
96.4% of total hepatitis C spend and 11.8% of total specialty spend, propelling the class
medications – Sovaldi® (sofosbuvir), Olysio® (simeprevir) and
to the fourth most expensive specialty therapy class. In 2014, decreased spend for specialty
Harvoni® (ledipasvir/sofosbuvir) – made up 96.4% of total
anticoagulant medications, combined with significant growth in unit cost for drugs that
Hepatitis C spend and 11.8% of total specialty spend.
treat and prevent hemophilia episodes, led to the displacement of specialty anticoagulants
TherApy cl Ass review
• Increases in both utilization and unit cost of oncology medications contributed to a
20.7% increase in PMPY spend for the therapy class. With new, highly targeted therapies increasingly being approved, oncology drugs continue to rank among the most expensive therapies; but growth in spending is tempered by the fact that many of the new drugs are indicated for rare types of cancer. The utilization increase likely is related to advances in cancer survivorship – more patients are living longer with some types of the disease, which now often can be treated like chronic illnesses that require ongoing therapy.
• The 35.6% increase in total spend for medications used to treat miscellaneous specialty
conditions – many of which are orphan conditions, including narcolepsy, nephropathic cystinosis and chorea associated with Huntington disease – is attributable primarily to increases in the costs of individual drugs. Drug costs continue to rise as manufacturers provide products to captive patient populations with few other options.
• Trend for hemophilia medications was 16.9% in 2014, driven by a double-digit increase
in unit costs. Two-thirds of market share in this class is captured by formulations of desmopressin, a generic medication frequently used for milder cases. Brand inflation occurred for clotting factors such as BeneFix® (coagulation factor IX [recombinant)] and
Specialty medications accounted for
Feiba NF (anti-inhibitor coagulation complex), helping drive an increase in unit cost.
more than 31% of total drug spend
• Spend for transplant-rejection medications continued to decline in 2014, dropping
2.3% due in large part to the increasing availability of lower-cost generic formulations.
in 2014, up from 27.7% in 2013.
In January 2014, two of the few remaining brand immunosuppressant drugs for post-transplant use, Myfortic® (mycophenolic acid) and Rapamune® 0.5mg tablets (sirolimus), lost patent protection, contributing significantly to the 86.1% generic fill rate (GFR) in this therapy class.
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
Inflammatory conditions was the most
pMpy spenD
unIT cosT
expensive specialty therapy class for the
Inflammatory Conditions
sixth year in a row, resulting from an 8.5%
Multiple Sclerosis
increase in utilization and a 15.7% increase
in unit cost. The greater utilization is due
to the prevalence of rheumatoid arthritis
(RA), the most common condition these
Miscellaneous Specialty Conditions
medications treat, and an expansion
Growth Deficiency
in approved indications for some of the most commonly used drugs in the class.
The 15.7% increase in unit cost is likely
Pulmonary Arterial Hypertension
related to typical brand inflation. Two
drugs with multiple indications, Humira®
(adalimumab) and Enbrel® (etanercept), continued to account for more than 80% of market share. Doubling its market share from 2013 to 2014 was Xeljanz®
Top Drugs
(tofacitinib), the only oral disease modifying
by MarKet share
anti-rheumatic drug indicated to treat RA
that has not responded to other therapies.
Humira® (adalimumab)
At the time of approval by the FDA in 2012,
Enbrel® (etanercept)
questions concerning its safety profile made
Stelara® (ustekinumab)
its place in therapy unclear. As longer-term
Orencia® (abatacept)Cimzia® (certolizumab)
safety and effectiveness data have become
available, however, prescribers and patients are more accepting of using Xeljanz.
0.027NUMBER OF PRESCRIPTIONS
$2,913.33 AVERAGE COST
0.3% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
MulTIple
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
A 9.7% increase in unit cost was the
pMpy spenD
unIT cosT
key driver of the 12.9% increase in per-
Inflammatory Conditions
member-per-year (PMPY) spend for
Multiple Sclerosis
multiple sclerosis (MS) medications.
New oral medications continue to change
the treatment landscape in this therapy
class. Together, Tecfidera® (dimethyl
Miscellaneous Specialty Conditions
fumarate, approved in 2013) and Gilenya®
Growth Deficiency
(fingolimod, approved in 2010) captured
29.8% of market share in 2014. The new
Pulmonary Arterial Hypertension
longer-acting formulation of Copaxone®
(glatiramer) was the class leader with
29.7% of market share. A new interferon product, Plegridy™ (peginterferon beta-1a), received approval by the FDA in August 2014 as a longer-lasting treatment
Top Drugs
for MS, but it has not impacted trend yet.
by MarKet share
Recommended dosing for Plegridy is once
every two weeks by subcutaneous injection,
Copaxone® (glatiramer)
rather than the weekly intramuscular
Tecfidera® (dimethyl fumarate)
dosing for Avonex
® (interferon beta-1a).
Avonex® (interferon beta-1a)Gilenya® (fingolimod)
The average cost per prescription in
Rebif® (interferon beta-1a)
this class increased 9.2% to $4,510.06 in 2014.
0.012NUMBER OF PRESCRIPTIONS
$4,510.06 AVERAGE COST
0.1% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
Per-member-per-year (PMPY) spend for oncology medications was $41.64
in 2014. Although the increase in
pMpy spenD
unIT cosT
spend for this class of medications
Inflammatory Conditions
has slowed in comparison to the PMPY
Multiple Sclerosis
trend observed in previous years, trend
was still 20.7% in 2014, spurred by
an 8.9% increase in utilization and
an 11.7% increase in unit cost. Unit
Miscellaneous Specialty Conditions
cost increases for Gleevec® (imatinib),
Growth Deficiency
which captured 12.5% of market share,
and Revlimid® (lenalidomide), which
Pulmonary Arterial Hypertension
captured 10.8% of market share, were
prime contributors. Temozolomide, a
generic for Temodar® that launched in 2013, continued to capture market share. In addition, generics to Xeloda®
Top Drugs
38.2% of patients
(capecitabine) that were launched in 2014 were the fourth most commonly
by MarKet share
are nonadherent to
used oncology medications for the year.
However, nine new branded oncology
medication therapy
Gleevec® (imatinib)Revlimid® (lenalidomide)
therapies that were approved for use in
Lupron Depot® (leuprolide)
(oral oncology).
2014 are likely to have an impact on the
class going forward.
0.007NUMBER OF PRESCRIPTIONS
$6,191.29 AVERAGE COST
0.1% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
hepaTITIs c
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
Per-member-per-year (PMPY) spend for hepatitis C medications increased
742.6% to $37.95 in 2014, propelling
pMpy spenD
unIT cosT
this class of drugs to the fourth most
Inflammatory Conditions
expensive specialty therapy class when
Multiple Sclerosis
ranked by PMPY spend. Hepatitis C
medications did not rank in the top 10
list in 2013. These changes were driven
entirely by newly approved clinical
Miscellaneous Specialty Conditions
breakthroughs for the treatment of
Growth Deficiency
hepatitis C. Sovaldi® (sofosbuvir) and
Olysio® (simeprevir) that were approved
Pulmonary Arterial Hypertension
in 2013 along with Harvoni® (ledipasvir/
sofosbuvir), approved in October 2014,
jumped into the top five for the class. This new generation of oral antiviral medications offers clinical benefits that
Top Drugs
are superior to earlier therapies. Their price tags, however, are unsustainable.
by MarKet share
At approximately $84,000 for one
12-week course of treatment, Sovaldi
Sovaldi® (sofosbuvir)ribavirin
alone captured 37.5% of the 2014
Olysio® (simeprevir)
market share for hepatitis C.
Pegasys® (peginterferon alfa-2a)Harvoni® (ledipasvir/sofosbuvir)
0.002NUMBER OF PRESCRIPTIONS
$16,373.40 AVERAGE COST
0.03% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
Per-member-per-year (PMPY) spend for HIV medications increased 14.8% from
2013 to 2014, primarily due to a 10.3%
pMpy spenD
unIT cosT
increase in unit cost. The average cost
Inflammatory Conditions
per prescription for more than two-thirds
Multiple Sclerosis
of the HIV medications on the market in
2014 exceeded $1,000 per prescription,
compared with slightly more than half
of the HIV medications on the market
Miscellaneous Specialty Conditions
in 2013. The 10 most commonly used
Growth Deficiency
medications were all branded drugs;
the top two were combination therapies
Pulmonary Arterial Hypertension
that each contain more than one active
ingredient in a single pill.
Top Drugs
by MarKet share
Isentress® (raltegravir)Viread® (tenofovir)
0.024NUMBER OF PRESCRIPTIONS
$1,138.48 AVERAGE COST
0.14% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
specIalTy
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Inflammatory Conditions
The class of medications used to
Multiple Sclerosis
treat an assortment of specialty
conditions, many of them orphan
diseases, is ranked sixth in the top
10 most expensive specialty therapy
Miscellaneous Specialty Conditions
class for 2014. The class includes
Growth Deficiency
Xenazine® (tetrabenazine), used to treat
Huntington's disease; Xyrem® (sodium
Pulmonary Arterial Hypertension
oxybate), used to treat narcolepsy; and
Arestin® (minocycline), used to treat
periodontal disease. Many medications in this class are only available through a limited network of specialty pharmacies. Several medications in this therapy class
Top Drugs
Nearly 40% of
also have substantial average costs per prescription, but a 27.3% increase
by MarKet share
the drugs in this
in utilization in 2014 mainly drove the
therapy class have
35.6% total trend – the second highest
Xyrem® (sodium oxybate)
of the specialty therapy classes in the
Arestin® (minocycline)
been approved as
top 10. The five most commonly used
medications captured 83.2% of the
market share in this therapy class.
0.002NUMBER OF PRESCRIPTIONS
$4,539.50 AVERAGE COST
0.06% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
The utilization of medications indicated
pMpy spenD
unIT cosT
to treat growth deficiency decreased by
Inflammatory Conditions
almost 1% in 2014, but a 7.5% increase
Multiple Sclerosis
in unit cost resulted in a 6.6% increase
in per-member-per-year (PMPY) spend.
Norditropin® FlexPro® (somatropin),
whose market share continues to grow
Miscellaneous Specialty Conditions
year-over-year, was the most commonly
Growth Deficiency
used medication in this class in
2014. The top two drugs in this class
Pulmonary Arterial Hypertension
accounted for more than two-thirds of
market share. The overall prevalence
of human growth hormone use, however, remained extremely low. Less than 0.05% of commercially insured beneficiaries filled a prescription for one
Top Drugs
of these medications in 2014.
by MarKet share
Norditropin® FlexPro® (somatropin)
Genotropin® (somatropin)Humatrope® (somatropin)
Omnitrope® (somatropin)Norditropin® NordiFlex® (somatropin)
0.003NUMBER OF PRESCRIPTIONS
$3,852.58 AVERAGE COST
0.03% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
The steady increase in spend for hemophilia drugs brought the class into
the top 10 most-expensive specialty
pMpy spenD
unIT cosT
therapy classes when ranked by
Inflammatory Conditions
per-member-per-year (PMPY) spend.
Multiple Sclerosis
With a 16.9% trend, spend for
hemophilia is increasing at a faster
rate than that for drugs used to treat
much more common conditions, such
Miscellaneous Specialty Conditions
as multiple sclerosis and HIV. Trend was
Growth Deficiency
driven by a 17.6% increase in unit costs.
Desmopressin captured two-thirds of
Pulmonary Arterial Hypertension
the market share in this class.
Top Drugs
A review of studies of hemophilia
by MarKet share
patients using prophylactic therapy
suggests that age and the presence
of symptoms were significantly related
Advate [antihemophilic factor (recombinant)] Stimate® (desmopressin nasal spray)DDAVP® (desmopressin)
to medication adherence.18
Kogenate® FS [antihemophilic factor (recombinant)]
0.001NUMBER OF PRESCRIPTIONS
$7,519.16 AVERAGE COST
0.01% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
pulMonary
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
arTerIal
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Inflammatory Conditions
Following a relatively flat trend in
Multiple Sclerosis
2013, spend for pulmonary arterial
hypertension treatments increased
13.8% in 2014, with increases in
utilization and cost contributing equally.
Miscellaneous Specialty Conditions
Much of the increased utilization for
Growth Deficiency
the class is related to the 2012 patent
expiration for Revatio® (sildenafil).
Pulmonary Arterial Hypertension
Top Drugs
by MarKet share
Adcirca® (tadalafil)Tracleer® (bosentan)
Letairis® (ambrisentan)Revatio® (sildenafil)
0.001NUMBER OF PRESCRIPTIONS
$4,023.23 AVERAGE COST
0.01% PREVALENCE
speciAlT y spenD rAnk
TherApy cl Ass review
coMponenTs of TrenD for The Top 10 specIalTy Therapy classes
ranKed by 2014 PMPy sPend
Per-member-per-year (PMPY) spend for medications used to prevent organ-
transplant rejection decreased 2.3%
pMpy spenD
unIT cosT
between 2013 and 2014. The unit cost
Inflammatory Conditions
trend was the primary reason, stemming
Multiple Sclerosis
from market saturation of generic
drugs in the class. Generic medications
captured more than 85% of total market
share in 2014. Accordingly, the average
Miscellaneous Specialty Conditions
cost per prescription for transplant
Growth Deficiency
medications was lower than that of
any other specialty therapy class. A
Pulmonary Arterial Hypertension
new extended-release formulation of
tacrolimus, Astagraf XL® (tacrolimus
extended release), was approved in July 2013 for use in kidney transplant patients, but it had little impact in
Top Drugs
the class. Generics to Rapamune® 0.5mg tablets (sirolimus) and Myfortic®
by MarKet share
(mycophenolic acid delayed-release
tablets) were also launched in 2014.
Prograf® (tacrolimus)cyclosporine modified
0.025NUMBER OF PRESCRIPTIONS
$208.00 AVERAGE COST
0.2% PREVALENCE
TherApy cl Ass review
Top 10 specIalTy Drugs
espite a decrease in utilization for half of the drugs in the top 10 when ranked by per-member-per-year (PMPY) spend, the unit cost for all top-10 drugs increased
D at least 6%. Two of the new hepatitis C drugs ranked in the top 10 most-expensive
drugs, even though each had been on the market for only a little over a year. Sovaldi and Olysio had the largest increases in utilization, unit cost and total trend. Part of the magnitude of increase is related to these two drugs having only been available for a single month in 2013.
Tecfidera, an oral MS treatment,
In these two cases, just one month's utilization in 2013 was compared to 12 months' worth of utilization in 2014. Sovaldi treats far fewer patients than the most-expensive and second-
had a total trend of 152.1%, driven
most-expensive specialty drugs when ranked by PMPY spend, Humira and Enbrel, which also
by a triple-digit increase in utilization.
ranked among the costliest specialty medications. Olysio ranked 10th. Three drugs used to treat multiple sclerosis (MS) were also among the most-expensive single therapies in 2014, ranked by PMPY spend. Tecfidera, an oral MS treatment, had a total trend of 152.1%, driven by a triple-digit increase in utilization. Two oncology drugs and one drug for HIV rounded out the most-expensive specialty drugs for 2014.
Top 10 specIalTy Therapy Drugs
ranKed by 2014 PMPy sPend
% of ToTal
Drug naMe
pMpy spenD
unIT cosT
Humira® (adalimumab)
Inflammatory Conditions
Enbrel® (etanercept)
Inflammatory Conditions
Sovaldi® (sofosbuvir)
Copaxone® (glatiramer)
Multiple Sclerosis
Tecfidera® (dimethyl fumarate)
Multiple Sclerosis
Avonex® (interferon beta-1a)
Multiple Sclerosis
Gleevec® (imatinib)
Olysio® (simeprevir)
2015-2017 TrenD forecasT
2015-2017 TrenD ForecAsT
spenD for TraDITIonal Drugs
In The nexT Three years
n 2014, the trend for traditional drugs was 6.4%, with per-member-per-
2015-2017 TrenD forecasT
year (PMPY) spend at $668.75. We anticipate that traditional trend will
I decline modestly in 2015 and then increase moderately in both 2016 and
2017. The significant increase in spend for traditional drugs in 2014 was driven by
an unprecedented explosion in spend for compounded medications. The utilization of
traditional medications is likely to increase, but the continuing decline in overall costs related to an abundance of generics and a relative lack of brand innovators in the pipeline for the most commonly used therapy classes (aside from diabetes) will keep traditional
Top TraDITIonal Therapy classes
drug spend from increasing substantially. In fact, trend for five of the top therapy classes – high blood cholesterol, compounded medications, high blood pressure/heart disease,
2015 - 2017
heartburn/ulcer disease and depression – is expected to be negative in 2015. The class
of mental/neurological disorders will have a negative total trend in 2016 and 2017. The
largest increases in the next three years are expected for only two classes – diabetes, which
will continue to experience a slight increase in utilization along with escalating brand
High Blood Cholesterol
inflation, and anticoagulants, which will continue to capture market share from specialty
Compounded Medications
anticoagulants such as enoxaparin and fondaparinux.
High Blood Pressure/Heart Disease
Heartburn/Ulcer Disease
PMPY drug spend for diabetes medications is expected to increase 18.3% in each of the
Attention Disorders
next three years. Although only slight year-over-year increases in utilization are projected,
substantial continued increases in unit cost are likely to come from brand innovation,
steady inflation for branded drugs and switches from older generic monotherapies to newer
combination products. In addition, new, longer-acting dipeptidyl peptidase-4 (DPP-4) and
human glucagon-like peptide (GLP-1) formulations are in the development pipeline. Although
Other Traditional Classes
they will carry lower risks of causing hypoglycemia at night, they may be priced higher than
similar drugs that are currently available. Finally, biosimilars to some types of insulin also are likely to enter the market in the near future, but the impact will not be felt immediately because physicians may be reluctant to change insulins for patients whose diabetes is under control. No significant patent expirations are expected in the next few years.
2015-2017 TrenD ForecAsT
high BlooD cholesTerol
Per-member-per-year (PMPY) spend for the class is forecast to decline for the next several
For medications used to treat pain and inflammation, PMPY spend is forecast to rise
years as a result of decreases in both drug costs and utilization. The January 2016 expiration
moderately in each of the next several years, driven almost entirely by an increase in unit
of the patent for Crestor® (rosuvastatin) is likely to drive down PMPY spend in 2016.
cost. Short-term use of opioids has shown a steady pattern of decline over the past five
Patent expirations for Zetia® (ezetimibe) and Vytorin® (ezetimibe/simvastatin) in 2017 are
years. The Drug Enforcement Administration (DEA) reclassification of all hydrocodone
expected to spur an even faster decline in spend. Although guidelines for the primary and
combination products to schedule II controlled substances in October 2014 also may
secondary prevention of atherosclerotic cardiovascular disease and events, published in
contribute to lower utilization. Drops in utilization are attenuated by the influx of branded,
late 2013, call for treatment based on risk factors rather than for the achievement of
tamper-resistant formulations of older medications, such as OxyContin® (oxycodone), that
specific cholesterol levels, significant changes in prescribing patterns have yet to be seen.
replace or compete with non-abuse-deterrent generically available options. In addition,
Although expensive new treatments known as proprotein convertase subtilisin/kexin type 9
shortages of oxycodone/acetaminophen products may continue to elevate generic prices.
(PCSK9) inhibitors that are in the development pipeline may be an alternative to statins,
Celebrex® (celecoxib), a nonsteroidal anti-inflammatory drug, lost patent protection in
they will be considered specialty medications. Therefore, they will not have a significant
2014. Because it captured so much market share, the lower cost of generic celecoxib
direct impact on traditional spend for high blood cholesterol therapies. A focus on cost
compared to brand Celebrex is likely to decrease spend for the class as a whole; but the
when PCSK9 inhibitors are introduced, however, may lead to an increase in the utilization of
drop will be offset fully by increases in prices for other drugs in this class.
traditional cholesterol-lowering drugs among patients who are not currently being treated. However, any increases in utilization as a result will not offset the steep drops in drug costs for existing traditional high blood cholesterol treatments.
The lower cost of generic celecoxib is likely to decrease spend for
the class, but the drop will be offset fully by increases in prices
for other drugs in the class.
Spend for compounded medications is expected to decline sharply in 2015, and then to increase again to a much more sustainable rate of 8% growth in both 2016 and 2017.
high BlooD pressure/heArT DiseAse
Clients that implemented the compound-utilization management solution in 2014 were able to substantially control the continuing increase in cost. However, only about 60% of
The forecast decline in PMPY spend for medications used to treat high blood pressure is
clients implemented the program by the end of 2014. The negative trend in 2015 is expected
expected to result from stagnant utilization and the release of more generics in the class.
because clients that implemented the program in 2014 will realize more than a full-year
Generics to Diovan® (valsartan), which lost patent protection in 2012, were delayed for
reduction in spend by the end of 2015, and additional clients are expected to implement
nearly two years due to legal issues. The release of the first Diovan generic in June 2014,
the program by the end of the year. The drop in spend is likely to be driven exclusively by a
followed by several others in December, is expected to have a continuing impact on spend
drop in utilization, as there is no expectation that the price increases seen for compounded
throughout 2015. Four of the remaining brand drugs – Azor® (amlodipine/olmesartan),
medications will abate. The utilization-management program is expected to have maximum
Benicar® (olmesartan), Benicar HCT® (olmesartan/hydrochlorothiazide) – and Tribenzor®
impact before 2016 and 2017, when PMPY drug spend is expected to increase moderately,
(amlodipine/olmesartan/hydrochlorothiazide) are set to lose patent protection late in 2016.
primarily as a result of drug inflation.
The resulting decline in spend is likely to be small, however, because together they account
2015-2017 TrenD ForecAsT
for less than one percent of market share. A new drug containing the angiotensin receptor
blocker valsartan and sacubitiril, the first in a new class called neprilysin inhibitors,
PMPY spend for medications used to treat attention disorders is forecast to increase in
could be submitted to the FDA for approval in 2015. Currently known as LCZ696, the drug
each of the next three years. Utilization will increase for adults diagnosed with attention
may have blockbuster potential and larger-than-expected adoption of it could impact
disorders and adults seeking improved ability to focus and concentrate, and utilization
trend considerably.
will continue for children and adolescents already using attention-disorder therapy. In February 2015, a new indication to treat binge-eating disorder was granted for Vyvanse®
(lisdexamfetamine), one of the most commonly used drugs in the class; as a result, utilization of Vyvanse is expected to increase. However increasing generic availability is
The large year-over-year negative trend forecast for medications used to treat
expected to slow the rate of growth in unit costs. Intuniv™ (guanfacine) went generic in
heartburn/ulcer disease is driven primarily by many less-expensive generic and over-the-
late 2014, and the patent for Strattera® (atomoxetine) will expire in 2017. Still, several of
counter (OTC) versions of the most commonly used medications in the class. Continuing
the convenient, once-daily attention disorder drugs will remain under patent protection for
decreases in PMPY spend from 2015 through 2017 are expected after the May 2014
several more years.
launch of Nexium® 24HR – an OTC version of the last remaining blockbuster drug in the class, Nexium® (esomeprazole magnesium) – began to erode prescription market share. Decreasing utilization of prescription medications as patients shift to OTC Nexium will have a significant impact on trend in 2015, followed by even larger impacts in 2016 and 2017.
The utilization of traditional medications is likely to increase, but
Generic Nexium, delayed by regulatory issues for eight months, finally reached the market
the continuing decline in overall costs related to an abundance of
in early 2015. No new drugs for heartburn/ulcer disease are in development and the few
generics and a relative lack of brand innovators in the pipeline for
remaining brands, such as Dexilant (dexlansoprazole), will have difficulty competing with
the most commonly used therapy classes (aside from diabetes) will
the many popular generic and OTC options.
keep traditional drug spend from increasing substantially.
PMPY spend for asthma medications is expected to increase again after the cost lowering
PMPY spend for medications used to treat depression is expected to decrease for the next
impact of the 2012 patent expiration for Singulair® (montelukast) abates in 2015. Brand
several years, due mainly to slight decreases in utilization and declines in drug costs as
inflation among inhalers will push up cost, while increased diagnosis and subsequent
generics become more available. The December 2013 launch of generics for one of the last
initiation of treatment for asthma and chronic obstructive pulmonary disease (COPD)
remaining brand serotonin and norepinephrine reuptake inhibitors, Cymbalta® (duloxetine),
will combine to raise PMPY spend. In addition, increased utilization is expected as new
will continue contributing to a significant decline in spend through 2015. But by 2016, the
indications for existing therapies, such as Spiriva® Respimat® (tiotropium), expand use
market will have absorbed most of the savings offered by generic medications in the class.
among additional populations who were not previously using medications.
Few new antidepressant therapies are in the pipeline, though, so in 2017 trend should essentially be flat.
2015-2017 TrenD ForecAsT
AnTicoAgulAnTs: A clAss To wATch
The forecast for medications used to treat mental/neurological disorders hinges on the
PMPY spend for newer oral traditional anticoagulants like Xarelto® (rivaroxaban) and
introduction of generic competition to the blockbuster atypical antipsychotic, Abilify®
Eliquis® (apixaban) is expected to grow significantly in the next few years. The rise will be
(aripiprazole), in May 2015. Abilify captured more market share than any other drug
driven by increased utilization in the traditional anticoagulants class from 2015 forward,
in this class, and its price has been increasing in advance of the revenue loss to the
as patients continue to switch from less convenient injectable specialty medications
manufacturer. The effect of generics to the Alzheimer's disease medication, Namenda®
like enoxaparin and fondaparinux. Costs are expected to increase significantly, however,
(memantine), expected in July 2015, will be tempered because the manufacturer already
because physicians are more likely to prescribe new patients one of the newer, brand-only
has switched a majority of patients to a newer once-daily formulation, Namenda XR®
oral anticoagulants rather than warfarin, which although vastly less expensive, is much
(memantine extended release). Because Namenda XR is patent-protected until 2029, the
less convenient. In addition, approvals of new anticoagulant reversal agents in the pipeline
profits of the brand franchise will be extended despite generic competition. Still, other
for the newer anticoagulants are expected to shift current users of warfarin, which can be
major generic medications – such as quetiapine and olanzapine, which will continue
reversed, if needed, to the newer medications.
to account for significant market share in the class – will contribute to negative trend beginning in 2016. Utilization is expected to increase marginally from increased use of atypical antipsychotics to treat bipolar disorder and treatment-resistant depression. The new drugs in the pipeline, if approved, will have as competitors the many commonly used generic medications available within this class.
The year-over-year trend forecast for contraceptives is influenced heavily by the Patient
The trend for five of the top therapy
Protection and Affordable Care Act's (ACA) mandate that patients be provided some form of contraception at zero cost to them. Surprisingly, no significant increase has been seen
classes – high blood cholesterol,
in utilization of contraceptive methods commonly billed through the pharmacy benefit.
compounded medications, high blood
The increases in utilization that have occurred have been for long-acting, reversible contraceptives, typically billed through the medical benefit. Unit cost increases, which are
pressure/heart disease, heartburn/ulcer
expected as a result of the continued cost-share shift to payers, likely will taper off after
disease and depression – is expected to
2015. Increased drug spend anticipated for 2016 and 2017 will result from brand inflation, in as much as the many generic options available in the class are likely to compete with
be negative in 2015.
new strengths, dosage forms and branded reformulations of older drugs.
2015-2017 TrenD ForecAsT
spenD for specIalTy Drugs
In The nexT Three years
n 2014, the trend for specialty drugs was 30.9%, with per-member-per-year
TrenD forecasT for key specIalTy Therapy classes
(PMPY) spend at $311.11. PMPY spend was higher than what was predicted
I in 2014 because of the significant impact of the new hepatitis C treatments. 2015 - 2017
Although specialty trend will slow to more sustainable levels in the next three years, it still
is expected to experience fairly stable double-digit growth in 2015, 2016 and 2017 (22.6%,
22.3% and 21.3% respectively). Continuing increases in utilization are likely as indications
Inflammatory Conditions
expand for existing drugs and specialty therapies are prescribed more often. However, the
Multiple Sclerosis
major contributors to rising PMPY spend for specialty medications are brand inflation and
the accelerating development of expensive, highly targeted therapies.
Miscellaneous Specialty Conditions
Growth Deficiency
The basis for the per-member-per-year (PMPY) trend forecast for medications used
Pulmonary Arterial Hypertension
to treat inflammatory conditions – such as rheumatoid arthritis, psoriasis and Crohn's disease – is sustained increases in both utilization and treatment costs. The class will
continue to be dominated by expensive branded, biologic medications. Utilization will
Hereditary Angioedema
increase due to the expansion of indications for current therapies, movement of therapy
Other Specialty Classes
from medical settings to pharmacy and increasing numbers of patients newly diagnosed
with inflammatory conditions. Generally, prevalence of certain autoimmune conditions is
*Trend is forecast only for specialty medications billed through the pharmacy benefit.
going up, but the reasons are not understood. Although some of the market share in the class may shift to new treatments that are expected to be available in 2015 and 2016, price parity with existing drugs is expected. Most current medications in this class will retain patent protection for the foreseeable future, with the exception of the scheduled Dec. 31, 2016, patent expiration for Humira® (adalimumab). Although several companies are in late stages of clinical trials for a biosimilar to Humira, it is unclear if these therapies will be able to mitigate trend growth in 2017.
2015-2017 TrenD ForecAsT
hepATiTis c
The biggest driver of the PMPY trend forecast for medications used to treat multiple
In the next three years, further significant increases in the PMPY trend for hepatitis C
sclerosis (MS) over the next three years is continued brand inflation, offset to some extent
treatments will result from increases in utilization and brand inflation. Most individuals
by generics to Copaxone® 20mg (glatiramer). Moderate inflation rates in this class will
with hepatitis C either do not know they have the virus or have not yet been treated for it.
contribute to the rising costs of treating MS in the next few years. Copaxone 20mg will
This suggests an increase in the utilization of treatments over the next few years is likely,
likely represent approximately 25% of the Copaxone market share by the time generics for
as newly diagnosed patients seek therapy. Although immediate treatment is not critical for
it are available in September 2015. However, limited generic competition is expected at
newly diagnosed patients, both lowered costs for the drugs and more attention paid to this
first, which may limit uptake and cost relief in the short-term after generics launch. An
therapy class are expected to contribute to increased utilization. Also, as a result of more-
oral medication, Tecfidera® (dimethyl fumarate), still is capturing market share from older,
affordable pricing for newer therapies, physicians are expected to increase prescribing,
injectable medications. Ponesimod, a new oral pipeline drug that might be launched in
returning from the slowdown in prescribing which occurred in mid-2014. Still, large cost
2017, may increase spend as well.
increases in 2014 were associated with the introduction of four highly effective therapies to the U.S. market. The addition of Viekira Pak™ (ombitasvir/paritaprevir/ritonavir with dasabuvir) to formularies, however, will limit near-term changes in unit cost to typical
Moderate inflation rates for multiple sclerosis (MS) drugs
brand inflation.
will contribute to the rising cost of treating the disease in the next
few years.
Most individuals with hepatitis C either do not know they have
the virus or have not yet been treated for it, suggesting an increase
in the utilization of treatments over the next few years is likely as
newly-diagnosed patients seek therapy.
The year-over-year trend forecast for oncology medications is based on continuations of brand inflation and brand-drug innovation; both also will increase utilization. Increasingly, many cancers can be treated as chronic conditions, requiring longer and sometimes more complex treatment. Multiple therapies can be used sequentially or as combination treatment
– leading to increased utilization. In addition, more cancer treatments are being managed
The year-over-year trend forecast for medications used to treat HIV is based on modest
through the pharmacy benefit rather than the medical benefit, which will be reflected in both spend and utilization because of improved visibility. Newer, often more-expensive
utilization growth along with double-digit increases in unit cost. Because screening for HIV
oral oncology treatments continue to be approved and capture market share. Generics to
is more accessible and a greater percentage of patients are surviving, utilization continues
Gleevec® (imatinib) are expected in 2016 and their competition will slightly mitigate trend
to increase slightly. The greatest HIV market share is held by newer, branded medications
growth; however, high prices for new and existing branded drugs will minimize the impact
that combine several medications into one dose, minimizing pill burden and helping increase
adherence. Only limited utilization of generic HIV medications is expected because some are poorly tolerated and many are inconvenient, single-drug products that require numerous daily doses, must be taken with other drugs or have complicated dosing patterns. Although strong
2015-2017 TrenD ForecAsT
brand innovation is not expected, new, single-tablet regimens without the inconveniences of
many generics will continue to play an important role in this market. In addition, no abatement
The PMPY spend for medications used to treat hemophilia and other bleeding disorders is
is expected for typical brand inflation of the already-expensive medications in the class.
expected to increase modestly for the next few years. The forecast is based on declining utilization offset by brand inflation. With regard to price, however, a return to the doubling
miscellAneous speciAlTy conDiTions
of price seen in the past is not expected in the next three years. Many of the most recently approved treatments are recombinant factors with increased purity, and they are expected
Medications used to treat less-common specialty conditions, many of them orphan
to remain as market leaders. Although, longer-acting recombinant factors indicated for
diseases, are forecast to increase significantly in the next few years, driven by both brand
the treatment of hemophilia A and hemophilia B that were launched in 2014 are expected
inflation and increased utilization. Medications for miscellaneous specialty conditions
to capture some market share, they require less frequent dosing resulting in lower overall
include Xyrem® (sodium oxybate), which treats cataplexy and excessive daytime sleepiness
PMPY utilization.
in narcolepsy, and Xenazine® (tetrabenazine), which treats involuntary movements (chorea) for patients with Huntington's disease. Most drugs in the class have little or no competition for market share and patients generally have no alternative options. The pharmaceutical
Medications used to treat less-common specialty conditions, many
landscape will be brighter for patients with presently untreatable orphan conditions in the
of them orphan diseases, are forecast to increase significantly in
next few years because new, targeted therapies for these conditions are expected to be
the next few years, driven by both brand inflation and increased
approved and incorporated into the class. The growing number of treatable conditions and the anticipated increase in newly diagnosed patients also will impact trend in this class.
pulmonAry ArTeriAl hyperTension
Driven primarily by brand inflation, PMPY spend for growth hormone products is expected
Increases in the PMPY spend for medications used to treat pulmonary arterial hypertension
to increase in the next few years. Utilization is expected to increase marginally in 2015 and
(PAH)are expected in each of the next three years. Because survival rates for PAH patients are
then to remain flat in 2016 and 2017, partly because utilization management programs are
improving and they're continuing therapy longer, utilization will increase. New diagnoses of
helping to decrease off-label use. In addition to price increases for existing medications
the disease are not expected to increase significantly, though, and utilization is expected to
in this class, there are no biosimilar growth hormones in the development pipeline for
increase at a stable rate. Although generic formulations of Tracleer® (bosentan) are due in
launch in the next few years. Some brand innovation is occurring as well. A long-acting
late 2015, some of the newer therapies, such as Opsumit® (macitentan), have better safety
growth hormone is on course to launch in 2015 and an oral medication, macimorelin,
profiles and simpler dosing. As a result, the decreased prices expected from Tracleer‘s
that is in development to diagnose growth hormone deficiency among adults, is up for
patent expiration are not likely to counteract brand inflation for other existing medications.
FDA consideration in 2016. If approved, both may contribute to increased PMPY spend for the class.
2015-2017 TrenD ForecAsT
The PMPY spend for medications used to prevent organ-transplant rejection is expected to decline even further in the next few years, making this the only specialty therapy class projected to have negative trend in each of the next three years. The expectation is based on the current 86.1% generic fill rate (GFR) for the class and the expectation of a slight decline in utilization. Although supply shortages increased costs for azathioprine in 2014, more increases are not expected to occur in the near future. In addition, Myfortic® (mycophenolic acid) and Rapamune® 0.5mg tablets (sirolimus), two of the remaining branded immunosuppressant medications, became available as generics in January 2014. No brand innovation is anticipated during the next three years.
hereDiTAry AngioeDemA – A clAss To wATch
The year-over-year trend forecast for specialty medications used to treat hereditary angioedema is based on the continued movement of medication billing from the medical benefits to pharmacy benefit. Changes in billing likely will be reflected as a significant increase in utilization for each of the next three years, due to improved visibility of utilization in the pharmacy benefit. Further, three new medications, including one oral drug, in the development pipeline are likely to be launched in 2017. Their releases may impact utilization, drug spend and trend for the class.
Specialty trend will slow to more
sustainable levels, averaging
22.1% over the next three years.
2014 paTenT expIraTIons
esTIMaTeD annual sales
paTenT expIraTIon DaTe
BranD naMe (generIc naMe)
Vivelle-Dot® (estradiol transdermal system)
Estrogen Replacement
Orapred ODT® (prednisolone sodium phosphate orally disintegrating tablets)
Intuniv™ (guanfacine)
Attention Deficit Hyperactivity Disorder
High Blood Pressure
Protopic® (tacrolimus ointment)
High Blood Pressure
Fortesta® (testosterone gel)
Testosterone Replacement
Baraclude® (entecavir)
Megace® ES (megestrol oral suspension)
Klor-Con® (potassium chloride extended-release tablets 8mEq, 10mEq)
Electrolyte Replacement
Diovan® (valsartan)
High Blood Pressure
Testim® (testosterone gel)
Testosterone Replacement
Actonel® (risedronate 150mg tablets)
Celebrex® (celecoxib)
Pennsaid® (diclofenac sodium topical solution 1.5%)
Exalgo® (hydromorphone extended release 8mg, 12mg, 16mg)
Rhinocort Aqua® (budesonide nasal spray)
Astepro® (azelastine nasal spray 0.15%)
Differin® (adapalene 0.3% topical gel)
Ortho Evra® (Xulane™)
Lunesta® (eszopiclone)
Lovaza® (omega-3-acid ethyl esters)
High Blood Triglycerides
Taclonex® (calcipotriene/betamethasone ointment)
Mepron® (atovaquone)
Lodosyn® (carbidopa)
Parkinson's Disease
Cipro® (ciprofloxacin oral suspension )
Evista® (raloxifene)
Micardis® HCT (telmisartan/hydrochlorothiazide)
High Blood Pressure/Heart Disease
Mycobutin® (rifabutin)
Mycobacterium Avian Complex
Avelox® (moxifloxacin)
Boniva® Injection (ibandronate)
Hectorol® (doxercalciferol capsules)
Bromday™ (bromfenac ophthalmic solution 0.09%)
Vanos® (fluocinonide 0.1% cream)
High Blood Pressure/Heart Disease
Micardis® (telmisartan)
High Blood Pressure/Heart Disease
Myfortic® (mycophenolic acid delayed release tablets)
Rapamune® 0.5mg (sirolimus)
Detrol® LA (tolterodine extended release)
Overactive Bladder
highlighTs
• On Dec. 1, 2014, Actavis announced the launch of its generic to Shire's Intuniv™
• On gaining approval from the FDA on Sep. 3, 2014, Teva Pharmaceutical Industries began
(guanfacine), one of the few non-controlled drugs used to treat attention deficit
shipping entecavir tablets, a generic for Bristol-Myers Squibb's Baraclude® tablets. The
hyperactivity disorder (ADHD). It is taken once a day as monotherapy or as an add-on to
oral solution form of Baraclude (entecavir oral solution 0.05mg/mL) remains brand only. Entecavir is indicated for patients at least two years of age and at least 10 Kg
stimulant medications. Actavis has 180 days of generic exclusivity, keeping competition
(22 pounds) who have active hepatitis B and liver damage.
from additional generics away until early June 2015.
• Par Pharmaceutical Companies, Inc. announced the approval of its generics to all four
• After the FDA granted 180 days of exclusivity to Ranbaxy for a generic of Genentech's
strengths of Exforge® (amlodipine/valsartan — Novartis) on Sep. 30, 2014. A single
Valcyte® (valganciclovir) tablets in 2008, FDA inspectors found violations at Ranbaxy's
pill combining a calcium channel blocker with an angiotensin II receptor blocker (ARB),
manufacturing facilities that were serious enough to ban imports of drugs from those
Exforge was FDA approved for treating high blood pressure.
sites. As a result, Ranbaxy was not allowed to ship generic valganciclovir, but no other generic company was allowed to produce it either. On Nov. 4, 2014, FDA reversed its
• The FDA approved TWI Pharmaceutical's generic to Megace® ES (megestrol) oral
approval, removed the exclusivity and allowed two companies to introduce valganciclovir
suspension 125mg/mL on Aug. 27, 2014. However the brand manufacturer, Par
in the U.S. Valganciclovir is an antiviral drug used to treat retinitis caused by
Pharmaceuticals, appealed the approval. Megestrol is a synthetic steroid that stimulates appetite, helping to relieve malnutrition and slow unwanted weight loss for patients with
cytomegalovirus (CMV) for patients with acquired immunodeficiency syndrome (AIDS),
AIDS, anorexia, cancer and other conditions. It also may be used as adjunctive therapy
and to prevent CMV infection in certain transplant patients.
for patients with advanced cancers of the breast, endometrium or kidneys.
• Zydus Cadila received FDA approval for its generic to Pfizer's Rapamune® 0.5mg tablets
The FDA approved the first generics for all four strengths
(siroliumus) on January 8, 2014. Sirolimus is an immune-system suppressant used to
(50mg, 100mg, 200mg and 400mg) of Celebrex® capsules on
prevent rejection after kidney transplants. Rapamune 1mg tablets, 2mg tablets and
May 30, 2014. It is the only cyclooxygenase-2 (COX-II) inhibitor
1mg/mL oral solution remain brand-only. Generics to another anti-kidney transplant
still available on the U.S. market.
rejection medication, Myfortic® (mycophenolic acid delayed-release tablets), were also launched in January.
• On Jun. 26, 2014, Ranbaxy finally gained full FDA approval for the generic to Novartis'
Diovan® (valsartan) tablets. As the first generic to be approved, Ranbaxy gained exclusive
After settlement agreements, Dr. Reddy's, Mylan and Teva
rights to manufacture its generic version of Diovan for the first six months of generic
all released generics for Sunovion's Lunesta® (eszopiclone) on
availability when the patent first expired in September 2012. However, after receiving
Apr. 15, 2014. Eszopiclone, a sedative-hypnotic drug approved
FDA approval, Ranbaxy's manufacturing facilities in India failed FDA inspections, so
to treat insomnia, competes with several other brand and generic
they could not produce a generic product. Due to Ranbaxy's exclusivity, no other generic company could produce valsartan either – giving Novartis nearly two extra years of
medications, including Ambien® (zolpidem – Sanofi, generics).
full brand revenue estimated at over $2 billion. A Ranbaxy subsidiary in the U.S., Ohm Laboratories, plans to begin shipping valsartan as soon as enough is available to supply the market.
• The FDA approved the first generics for all four strengths (50mg, 100mg, 200mg and
400mg) of Celebrex® capsules on May 30, 2014. The only cyclooxygenase-2 (COX-II) inhibitor still available on the U.S. market, celecoxib is an oral nonsteroidal anti-inflammatory drug used long term to treat osteoarthritis (OA), rheumatoid arthritis (RA) and ankylosing spondylitis. It also is used to manage acute pain in adults.
• Actavis announced on May 12, 2014, that the FDA had approved its abbreviated new
drug application (ANDA) for hydromorphone extended-release tablets in 8mg, 12mg and 16mg strengths. The 32mg strength remains brand only. Neither the brand, Exalgo®, nor the new generic is abuse resistant. However, a risk evaluation and mitigation strategy (REMS) is in place.
• After settlement agreements, Dr. Reddy's, Mylan and Teva all released generics for
Sunovion's Lunesta® (eszopiclone) on Apr. 15, 2014. Eszopiclone, a non-benzodiazepine sedative-hypnotic drug approved to treat insomnia, competes with several other brand and generic medications, including Ambien® (zolpidem – Sanofi, generics).
approval or acTIon DaTe BranD (generIc naMe)
Dyloject™ (diclofenac sodium injection)
Existing product with new dosing form
Alzheimer's Disease
New combination of existing products
Saxenda® (liraglutide)
Existing product with new dosing form
Opdivo® (nivolumab)
Similar to existing products
Rapivab™ (peramivir)
Similar to existing products
Similar to existing products
Soolantra® (ivermectin 1% cream)
Existing product with new dosing form
Similar to existing products
Lynparza™ (olaparib)
New mechanism of action
Xtoro™ (finafloxacin otic suspension)
Similar to existing products
Signifor® LAR (pasireotide long acting)
Existing product with new dosing form
New combination of existing products
New mechanism of action
Onexton™ (clindamycin/benzoyl peroxide gel)
New combination of existing products
Hysingla™ ER (hydrocodone )
Existing product with new dosing form
Multiple Sclerosis
Refinement of an existing product
New combination of existing products
Xigduo™ XR (dapagliflozin/metformin)
New combination of existing products
Obizur (antihemophilic factor [recombinant], porcine sequence)
Acquired Hemophilia A
Similar to existing products
Sotylize™ (sotalol oral solution)
Existing product with new dosing form
Ofev® (nintedanib)
Idiopathic Pulmonary Fibrosis
New mechanism of action
Esbriet® (pirfenidone)
Idiopathic Pulmonary Fibrosis
New mechanism of action
New mechanism of action
New mechanism of action
Iluvien™ (fluocinolone intravitreal implant)
Diabetic Macular Edema
Similar to existing products
New combination of existing products
Tybost® (cobicistat)
Existing product with new dosing form
Spiriva® Respimat® (tiotropium)
Existing product with new dosing form
Similar to existing products
Movantik™ (naloxegol)
Similar to existing products
HyQvia (immune globulin/hyaluronidase)
Primary Immunodeficiency
Refinement of an existing product
New combination of existing products
Metastatic Melanoma
New mechanism of action
New combination of existing products
Arnuity™ Ellipta® (fluticasone furoate)
Refinement of an existing product
Cerdelga™ (eliglustat)
New mechanism of action
Plegridy™ (peginterferon beta-1a)
Multiple Sclerosis
Refinement of an existing product
Belsomra® (suvorexant)
New mechanism of action
New combination of existing products
New mechanism of action
New mechanism of action
Striverdi® Respimat® (olodaterol)
New mechanism of action
Acticlate® (doxycycline hyclate)
Existing product with new dosing form
Zydelig® (idelalisib)
New mechanism of action
Targiniq™ ER (oxycodone extended release/naloxone)
Existing product with new dosing form
Ruconest® (C1 esterase inhibitor [recombinant])
Hereditary Angioedema
Similar to existing products
Ryanodex® (dantrolene)
Malignant Hyperthermia
Existing product with new dosing form
Rasuvo® (methotrexate auto-injector)
Inflammatory Conditions
Existing product with new dosing form
Kerydin™ (tavaborole)
Similar to existing products
Beleodaq™ (belinostat)
New mechanism of action
Low Blood Pressure
Refinement of an existing product
Afrezza® (inhaled insulin)
Existing product with new dosing form
Sivextro® (tedizolid)
Similar to existing products
Eloctate™ (antihemophilic factor [recombinant], Fc fusion protein)
Similar to existing products
Bunavail™ (buprenorphine/naloxone buccal film)
Opioid Dependence
New combination of existing products
Jublia® (efinaconazole topical solution)
Similar to existing products
Vogelxo™ (testosterone nasal gel)
Testosterone Replacement
Existing product with new dosing form
Omidria™ (phenylephrine/ketorolac injection)
New combination of existing products
Natesto™ (testosterone nasal gel)
Testosterone Replacement
Existing product with new dosing form
Similar to existing products
QVAR® with Dose Counter (beclomethasone aerosol)
Refinement of an existing product
Entyvio® (vedolizumab)
Inflammatory Bowel Disease
New mechanism of action
Zontivity™ (vorapaxar)
New mechanism of action
Epanova® (omega-3-carboxylic acids)
Similar to existing products
Incruse™ Ellipta® (umeclidinium)
Similar to existing products
Zykadia™ (ceritinib)
Similar to existing products
Purixan™ (mercaptopurine oral suspension)
Existing product with new dosing form
Sylvant™ (siltuximab)
Castleman's Disease
New mechanism of action
Cyramza® (ramucirumab)
Similar to existing products
Ragwitek® (short ragweed pollen allergen extract)
Similar to existing products
Tanzeum™ (albiglutide)
Similar to existing products
Grastek® (Timothy grass pollen allergen extract)
Similar to existing products
Evzio™ (naloxone autoinjector)
Existing product with new dosing form
Oralair® (mixed grass pollens allergen extract)
New mechanism of action
Alprolix® (coagulation factor IX [recombinant] Fc Fusion protein)
Similar to existing products
Metronidazole 1.3% Vaginal Gel (metronidazole 1.3% vaginal gel)
Refinement of an existing product
Otezla® (apremilast)
Psoriatic Arthritis
New mechanism of action
Impavido® (miltefosine)
New mechanism of action
Hemangeol™ (propranolol oral solution)
Infantile Hemangioma
Existing product with new dosing form
Noxafil® (posaconazole)
Similar to existing products
Qudexy™ XR (topiramate)
Existing product with new dosing form
Xartemis™ XR (oxycodone/acetaminophen)
Similar to existing products
Aveed® (testosterone undecanoate)
Similar to existing products
Refinement of an existing product
Myalept™ (metreleptin)
New mechanism of action
Northera™ (droxidopa)
Neurogenic Orthostatic Hypotension
New mechanism of action
Vimizim® (elosulfase alfa)
Morquio A Syndrome
New mechanism of action
Hetlioz® (tasimelteon)
Non-24-Hour Sleep-Wake Disorder
Similar to existing products
Pennsaid® 2% (diclofenac topical solution 2%)
Refinement of an existing product
Similar to existing products
highlighTs
• On Nov. 14, 2014, Genzyme, a Sanofi company, received FDA approval for Lemtrada™
(alemtuzumab) to treat relapsing forms of multiple sclerosis (MS). It will be third-line
• Bristol-Myers Squibb received accelerated approval from the U.S. Food and Drug
therapy for patients whose condition has not responded to two or more other MS therapies.
Administration (FDA) on Dec. 22, 2014 for its breakthrough cancer therapy, Opdivo®
Because it has the potential for very severe side effects, Lemtrada's prescribing and
(nivolumab). A human programmed death receptor-1 (PD-1)-blocking antibody, it is
dispensing is restricted to certified providers. In the U.S., alemtuzumab originally was
indicated for the treatment of patients with unresectable or metastatic melanoma that
approved in 2001 (under the brand name Campath®) for treating chronic lymphocytic
has progressed despite treatment with Yervoy® (ipilimumab – Bristol-Myers Squibb).
leukemia. Its marketing for the cancer indication was terminated by the manufacturer
• On Dec. 19, 2014, AbbVie received FDA approval of Viekira Pak™ (ombitasvir/paritaprevir/
in Sept. 2012.
ritonavir tablets co-packaged with dasabuvir) for the treatment of chronic hepatitis C
• On Oct. 15, 2014, the FDA approved Esbriet® (pirfenidone – Genentech) and Ofev®
virus (HCV) genotype 1 infection. Viekira Pak contains three new antivirals: ombitasvir,
(nintedanib – Boehringer Ingelheim), the first two drugs to treat idiopathic pulmonary
an NS5a inhibitor; paritaprevir, a protease inhibitor; and dasabuvir, a non-nucleoside
fibrosis (IPF). A fatal lung disease, IPF progressively scars the lungs, eventually ending
polymerase inhibitor. Ritonavir, also a protease inhibitor, is used as a "booster" to
their ability to absorb oxygen. Approximately 100,000 Americans, mostly older men, have
increase the effects of paritaprevir.
IPF. The median survival time from diagnosis is only two years to five years, and the
• AstraZeneca's Lynparza™ (olaparib) received FDA approval on Dec. 19, 2014. It is
five-year survival rate is less than 40%. Before the approvals of Esbriet and Ofev, the only
indicated as monotherapy to treat patients with advanced ovarian cancer that has or is
therapies for IPF were breathing treatments, supplemental oxygen and lung transplants.
suspected to have deleterious germline BRCA mutations (gBRCAm). Because the specific
• Gilead received FDA approval on Oct. 10, 2014, for Harvoni® (ledipasvir/sofosbuvir)
mutations must be confirmed by an FDA-approved test before Lynparza treatment is
tablets to treat adults with genotype 1 chronic hepatitis C virus (HCV). Launched within
initiated, the FDA also approved Myriad Genetics' BRACAnalysis CDx™. It is a companion
days of approval, Harvoni is a single tablet that contains Gilead's Sovaldi® (sofosbuvir)
diagnostic blood test that identifies the 10% to 15% of ovarian cancers carrying the
and ledipasvir, the first FDA-approved drug in a new class known as NS5a inhibitors.
mutations. Additionally, to be eligible to use Lynparza, patients must have already been
Harvoni is taken once daily, with no need for patients to use ribavirin or interferon. It is
treated with at least three other types of chemotherapy.
priced at $1,125 per tablet.
• The FDA announced its approval of Purdue Pharma's Hysingla™ ER, a controlled-release
• On Sept. 4, 2014, Merck received accelerated approval from the FDA for its breakthrough
form of hydrocodone, on Nov. 20, 2014. Taken once every 24 hours, it is indicated to
therapy Keytruda® (pembrolizumab). It was the first FDA-approved human programmed
manage pain that is severe enough to require continuous, long-term opioid treatment
death receptor-1 (PD-1) blocking antibody. Keytruda is indicated for the treatment of
and that has not been controlled by prior therapy. At the time of its approval, Hyslngla
inoperable or metastatic melanoma that has progressed despite treatment with Yervoy®
ER was the only abuse-deterrent, single-agent hydrocodone product approved in the U.S.
(ipilimumab – Bristol-Myers Squibb). If the cancer is positive for BRAF V600 mutations, a BRAF inhibitor, such as Tafinlar® (dabrafenib – GlaxoSmithKline) also must be tried first.
On Dec. 19, 2014, AbbVie received FDA approval of Viekira
Pak™ (ombitasvir/paritaprevir/ritonavir and dasabuvir) for the
• The FDA approved Contrave® (naltrexone/bupropion) extended-release tablets on
treatment of chronic hepatitis C virus (HCV) genotype 1 infection.
Sept. 10, 2014. Contrave is indicated for chronic weight management in addition to reduced-calorie diet and exercise. It is approved for use by adults who are obese – those
with an initial body mass index (BMI) of 30 Kg/m2 or higher. Adults with BMIs between 27
• On April 3, 2014, the FDA approved a one-use naloxone auto-injector for emergency
Kg/m2 and 30Kg/m2 and with one or more weight-related conditions, such as high blood
treatment of known or suspected opioid overdose. Evzio™ (naloxone injection) is to be
pressure, type 2 diabetes or high cholesterol, also are candidates for its use.
used by caregivers as soon as signs of an opioid overdose are suspected. Each Evzio dose is prepackaged in a small container on which clearly written and pictured directions
• The FDA approved Jardiance® (empagliflozin) on Aug. 1, 2014. Along with diet and
for use appear. The package also includes a speaker that delivers audible step-by-step
exercise, Jardiance is indicated to improve blood sugar control for adults with type 2
instructions for use.
diabetes. Jardiance is the third sodium-glucose co-transporter 2 (SGLT2) inhibitor to be approved in the U.S. Invokana® (canagliflozin – Janssen) was approved in March 2013
• On March 11, 2014, the FDA gave Mallinckrodt approval for Xartemis™ XR Extended-
and Farxiga™ (dapagliflozin – AstraZeneca) followed in Jan. 2014.
Release Capsules (C-II), the first extended-release formulation of oxycodone and acetaminophen. It is indicated for managing severe acute pain requiring an opioid, after
• Biogen Idec announced on Aug. 15, 2014, that the FDA had approved Plegridy™
previous treatment options have been inadequate.
(peginterferon beta-1a) to treat relapsing forms of multiple sclerosis (MS). Plegridy is a pegylated formulation of the same drug in Biogen's Avonex®. However, pegylation
• After priority review, the FDA approved Hetlioz™ (tasimelteon) capsules on
allows the medication to stay in the body longer, so Plegridy needs to be administered
Jan. 31, 2014. Hetlioz is the first treatment for Non-24-Hour Sleep-Wake Disorder
only once every two weeks, compared to once weekly for Avonex. Additionally, Plegridy
(Non-24). A chronic circadian rhythm disorder, Non-24 keeps patients from aligning their
is injected subcutaneously (subQ), which generally is less painful and easier for patients to
body clocks with the 24-hour day-night cycle. Most totally blind patients (about 80,000
self-administer than the intramuscular (IM) injections needed for Avonex.
in the U.S.) have Non-24, which disrupts sleep and causes stress that often results in social and occupational problems.
• After two previous tries, MannKind's inhaled insulin, Afrezza® (insulin human) inhalation
powder won FDA approval on June 27, 2014, for treating adults with diabetes, either type 1 or type 2.
On Oct. 15, 2014, the FDA approved Esbriet® (pirfenidone –
• The FDA approved Biogen Idec's Eloctate™ (antihemophilic [recombinant], Fc fusion
Genentech) and Ofev® (nintedanib – Boehringer Ingelheim),
protein) on June 6, 2014. Eloctate is indicated to control and prevent bleeding episodes,
the first two drugs to treat idiopathic pulmonary fibrosis (IPF).
manage bleeding during surgical procedures and prevent or reduce bleeding episodes
A fatal lung disease, IPF progressively scars the lungs, eventually
in adults and children with hemophilia A. It lasts longer in the body than other antihemophilia factors, so it can be dosed less often for prevention.
ending their ability to absorb oxygen. Approximately 100,000
Americans, mostly older men, have IPF.
• Greer Laboratories announced on April 1, 2014, that the FDA had approved
Oralair® (mixed grass pollens allergens extract). The first FDA-approved sublingual immunotherapy (SLIT) agent for allergies in the U.S., it contains pollen extracts from a variety of grasses. Approval for a second SLIT, Grastek® (Timothy grass pollen extract – Merck), followed two weeks later. And a third, Ragwitek® (short ragweed pollen allergen extract – Merck), was approved on April 17, 2014. These new SLIT drugs may replace allergy shots for some patients.
The express scrIpTs
prescrIpTIon prIce InDex
ompared to the prices of generic drugs and drugs a year earlier, in December
The express scrIpTs prescrIpTIon prIce InDex
2014 generic drug prices were 20.0% lower, whereas brand prices were 15.4%
C higher. The magnitude of change in generic drug prices has decreased since
2013, following the dramatic deflation seen in 2012 – when billions of dollars' worth of
brand blockbuster medications lost patent protection in what was dubbed "the patent cliff," paving the way for unprecedented generic competition. In addition, price increases
for several commonly used generics have had an offsetting effect, contributing to a slowing
of the decline in prices. This isolated inflation in generic-drug pricing is related to a range
of market factors, such as fewer manufacturers of those generics and shortages of active
ingredients. Even so, generic medications overall continue to deliver significant savings
over brand-name alternatives.
The gap between brand inflation and generic deflation has increased slightly, from 29.8
percentage points in December 2013 to 35.5 percentage points in December 2014. From
the base price of $100.00 set in January 2008, in December 2014 prices for the most
Brand Prescription Price Index
Generic Prescription Price Index
Consumer Price Index (BLS)
commonly used generic medications decreased to $37.13 (in 2008 dollars), and prices for the most commonly used brand medications increased to $227.39 (in 2008 dollars). In contrast, a market basket of commonly used household goods costing $100.00 in 2008, as measured by the Bureau of Labor Statistics (BLS) Consumer Price Index, grew to only $111.24 (in 2008 dollars) by December 2014.
meDicAre yeAr in review
MeDIcare year In revIew
n 2014, for the second year in a row, Medicare saw booming growth. Two million more Americans became eligible for the federal program, bringing the number
I of Medicare Part D beneficiaries to 37 million.19 At the same time, Medicare plan
sponsors faced the ongoing challenge of maintaining costs and quality for a growing population while remaining compliant with Centers for Medicare & Medicaid Services (CMS) requirements.
Express Scripts' Medicare plans faced stiff competition as a result of considerable downward pressure on beneficiary premiums and copayments. Plans also faced higher-than-ever stakes around CMS's Star Ratings. The end of the Quality Bonus Payment Demonstration, along with continued shifts in regulation parameters for adherence and diabetes treatments, increased this pressure. The industry also witnessed steep inflation for compounded ingredients. The most challenging issue for Medicare plans in 2014, however, was the management of the skyrocketing trend for specialty drugs, driven primarily by new, expensive treatments for hepatitis C.
To manage these diverse challenges and remain competitive, several trends were common
The total spend for Medicare plans rose
among Medicare Part D plans in 2014:
13.8% in 2014, as a result of a modest
• Adoption of a more aggressive benefit design and a tightly managed formulary
increase in utilization combined with a
• An increase in the number of plans offering five-tier cost-sharing structures
significant increase in unit costs.
• A rapid increase in the number of Prescription Drug Plan (PDP) plan sponsors using
preferred pharmacy networks, allowing penetration to reach 72%
whAT's Driving meDicAre Drug TrenD
The total spend for Medicare plans rose 13.8% in 2014, to $2,987.36, as a result of a modest increase in utilization (0.5%) combined with a significant increase in unit costs (13.3%). Traditional drug spend increased 6.4%, driven by relatively stable utilization and a 5.9% increase in unit costs. Although specialty medications represented only about one-quarter of total Medicare drug spend, their contribution to trend was significant: specialty spend increased 45.9% in 2014, following a much more modest increase of 14.7% in 2013.
meDicAre yeAr in review
Specialty Utilization and Hepatitis C
Quality and Star Ratings Measures
Perhaps the largest impact for Medicare plan sponsors in 2014 arose as a result of new,
CMS continues to emphasize the importance of clinical-outcome measures through the
expensive treatments for hepatitis C, namely Sovaldi® (sofosbuvir), Olysio® (simeprevir)
Medicare Part D Star Rating system. The financial benefits of the highest rated 4-Star or
and Harvoni® (ledipasvir/sofosbuvir). After the approval and marketplace launch of Sovaldi
5-Star plan ratings are key for plans' long-term solvency and competitiveness.
and Olysio in late 2013, treatment guidelines were quickly updated to recommend the use of these novel agents in the treatment of patients with hepatitis C (genotype 1), instead of
Even though the financial incentives that encourage better adherence are aligned with
older treatments on the market.20 Harvoni became available in October 2014.
Medicare Advantage Part D (MAPD) plans, 2014 was the first year in which Part D plan sponsors also demonstrated an overall market improvement in Star Rating measures
Treatment with one of these three new drugs is among
related to adherence. Still, better adherence came with the added cost that naturally
the most-expensive therapeutic regimens available, with
ensues from the use of more prescription drugs. Medicare plan sponsors were focused
the cost of a course of therapy as high as $150,000. Plan
on achieving the increasingly difficult 5-Star threshold for diabetes treatments and the
sponsors spent an average of $102.83 per-member-per-year
three adherence measures – measures that are particularly hard to maintain and even
(PMPY) on these three drugs alone in 2014 – more than the
possible cost of a
PMPY cost for the entire class of inflammatory drugs that
more difficult to improve. For instance, the 2015 5-Star adherence threshold for cholesterol
course of therapy
treat more common conditions, such as rheumatoid arthritis
medication adherence will increase to 83% in 2015, from 75% in 2014. The anticipation of
and psoriasis. In contrast, sponsors of Express Scripts
these types of shifts in Star Rating measures caused Medicare plan sponsors to implement
commercial plans spent only $37.95 PMPY for all hepatitis
more aggressive and creative clinical solutions in 2014 to drive greater member adherence,
C drugs in 2014.
a trend whose success played out in Medicare spend and utilization increases.
The reason for this disparity is that – unlike commercial health plans, which could use
The hypertension measures provided a particularly complex Star Ratings challenge in 2014.
utilization-management tools such as prior authorization and even formulary placement
In February 2014, the Eighth Joint National Committee (JNC 8) released starkly different
to manage the costs of these expensive new drugs – without prior CMS approval,
guidelines for the treatment of hypertension,22 a prevalent condition affecting an estimated
Medicare health plans could not remove the new drugs from their 2014 formularies
58% of Medicare beneficiaries.23 The changed guidelines still advocate tight management
mid-year, hampered from taking proper advantage of innovative cost-containment
of high blood pressure in certain patients, but they now recommend that adults over the
solutions that commercial clients employed successfully. Medicare health plans witnessed
age of 75 with chronic kidney disease not be treated for hypertension with an angiotensin-
a dramatic increase in specialty drug spend.
converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). The use of
Looking ahead, however, Medicare plans can expect improved trend in 2015 as plan sponsors
diuretics, calcium channel blockers (CCB) and beta blockers, when appropriate, is
successfully leverage more utilization-management tools for these drugs, such as prior
still recommended.
authorization criteria. This is welcome news for plan sponsors and the Part D industry, as the impact of these new hepatitis C drug therapies will be felt more fully in 2015 and beyond.
Industry research estimates new therapies for hepatitis C,
The Pharmacy Care Management Association (PCMA) and Milliman have estimated that new therapies, including Sovaldi and Olysio, will increase Medicare Part D federal spending
including Sovaldi® and Olysio®, will increase Medicare Part D
by $2.9 billion to $5.8 billion in 2015.21 Viekira Pak™, another breakthrough hepatitis C
federal spending by $2.9 billion to $5.8 billion in 2015.
treatment, was added to Express Scripts formularies for commercially insured plan sponsors and, as of January 1, 2015, is the exclusive option for those plans' patients with genotype 1 hepatitis C, regardless of symptoms or disease progression.
meDicAre yeAr in review
However, the specifications from which CMS draws evidence for current Star Ratings use
Following all of this industry dialogue, in February's 2016 Draft Call Letter, CMS announced
treatment guidelines issued in 2003. The Pharmacy Quality Alliance (PQA) decided in January
it would retire the Part D Diabetes Treatment measure for CY 2017 Star Ratings. This
2015 to retire the Star Ratings measure related to appropriate treatment of hypertension
measure will still be included in the 2016 Star Ratings, based on plan sponsors' 2014
in patients with diabetes, and this retired measure reflected pre-JNC 8 recommendations.
data. However, going forward the Diabetes Treatment measure will not impact Star Ratings performance. Medicare plans should seek to understand the impact of this change within
Data from an exclusive Express Scripts study looked at pharmacy claims for nearly 2.4
their broader Star Ratings strategy and clinical approach.
million Medicare patients in 2013. The data suggested that the JNC 8 guideline changes are consistent with how physicians are already treating older patients with hypertension. The percentage of Medicare beneficiaries who filled a prescription for an ACE inhibitor or
soluTions For meDicAre's chAllenges
an ARB declined with age, while the use of calcium channel blockers and beta blockers increased (see chart below). Part D plans now have research to support the prescribing
To address plans' Star Ratings challenges, Express Scripts offers a range of clinical
trends seen and, as such, should be encouraged to continue monitoring prescribing pattern
solutions to boost Part C and Part D performance. Plans wanting to improve clinical Star
changes that fall in line with updates to treatment guidelines.
measures benefit from our highly specialized solutions that align programs for pharmacists, physicians, patients and case managers, and help drive optimal performance against plans' pharmacy measures and medical measures. Because client needs are unique, our menu of Stars Solutions is deep – including ScreenRx®, which uses predictive modeling and tailored
percenT of MeDIcare BenefIcIarIes usIng hIgh BlooD
interventions to drive adherence; RationalMed®, which uses proprietary clinical analysis of
prescription, medical and laboratory data to identify safety risks; and solutions that target diabetes-treatment initiatives and support prescribing-physician best practices.
by age grouP
The clinically specialized Therapeutic Resource Centers (TRCs) of the Express Scripts
Pharmacy and Accredo Specialty Pharmacy deliver superior treatment outcomes and cost-
effectiveness by supporting patients through the challenges of complex and costly diseases. Our specialist pharmacists and nurses each receive clinically specialized training in one
disease state, and the work they do focuses almost exclusively on that clinical condition.
This specialized expertise and commitment enables an optimal patient experience and ensures the highest performance in pharmacy safety, improved medication adherence and
closing gaps in care. With their highly specialized knowledge of the complex disease states
and complicated treatment protocols they manage, these TRC pharmacists and nurses often have more experience in rare conditions than some of the physicians who prescribe
the treatments.
In addition, the adherence benefits for Medicare members who choose the Express Scripts
Pharmacy for home delivery of prescription medications are well documented. A growing
body of evidence suggests that the choice of where Medicare beneficiaries fill their
ACE Inhibitor or ARB
medications is vitally linked to adherence success.
meDicAre yeAr in review
In 2014, Express Scripts conducted the first known, well-controlled, head-to-head comparison of prescriptions dispensed at retail pharmacies and at home delivery pharmacies. The peer-reviewed study, which controlled for socioeconomic status and excluded patients using home delivery automatic refill programs, examined 29 million de-identified claims corresponding to nearly 1 million Medicare beneficiaries taking medication for diabetes, high blood pressure or high cholesterol over a two-year period. The study found that beneficiaries who receive medication for diabetes, high blood pressure and high cholesterol via home delivery pharmacy are between 25% and 29% more likely to be adherent to prescription drug therapy than patients with these conditions who fill their prescriptions at a retail pharmacy.24 Programs like MyRxChoices® are designed to educate members on the benefits of home delivery pharmacy and thus maximize the adherence performance benefits to plans.
Medicare beneficiaries who receive
medication for diabetes, high blood
pressure and high cholesterol via
home delivery pharmacy are between
25% and 29% more likely to adhere
to prescription drug therapy than
patients with these conditions who fill
their prescriptions at a retail pharmacy.24
meDicAre TherApy cl Ass review
a look aT MeDIcare overall
coMponenTs of MeDIcare TrenD
Drug TrenD for 2014
e further examined Medicare trend in 2014 by Medicare plan type: Medicare
pMpy spenD
unIT cosT
Advantage Part D (MAPD), Prescription Drug Plan (PDP) and Employer Group
W Waiver Plan (EGWP). Traditional spend for MAPDs increased 7.0%, with a per-
member-per-year (PMPY) spend of $1,751.11, stemming from a 6.1% increase in unit cost
combined with a 0.9% increase in utilization. The specialty PMPY spend for MAPDs in 2014
January-December 2014 compared to same period in 2013
increased to $542.05, a 43.3% increase over 2013.
EGWPs, which comprise plan sponsors that continue to offer benefits to their retirees, tend
to have broader formularies, lower copayments and fewer member restrictions. In 2014,
pMpy spenD
unIT cosT
EGWP plans had the highest PMPY spend ($2,800.49) for traditional drugs among the three
Medicare plan types, but they also had a slightly lower increase in utilization for traditional
drugs than both MAPD and PDP plans. For specialty drugs, EGWPs had a 33.9% increase
to $738.25 PMPY spend.
January-December 2014 compared to same period in 2013
Medicare traditional drug spend increased 8.6%, to $2,403.49, for PDP plans, driven largely by a 7.6% increase in unit cost. In addition, PDP plans had a higher specialty spend
increase (60.0%) than the two other types of Medicare plans.
pMpy spenD
unIT cosT
Overall, we saw the impact of benefit design driving significant differences in MAPD,
PDP and EGWP trend. For example, we saw PDP members' increased adherence in two
measures (D12-blood pressure medication adherence and D13-cholesterol medication
$3,276.38
adherence), which was reflected in higher PMPY spend and utilization for these commonly
January-December 2014 compared to same period in 2013
used medications than MAPDs experienced. Again, richer plan designs are associated with PDPs, whereas EGWPs are driving higher PMPY spend. However, we will be closely watching
this trend in 2015 and beyond, because PDP and EGWP plan designs are now changing to
include more tightly managed benefit designs and closed formulary options.
pMpy spenD
unIT cosT
$3,538.74
January-December 2014 compared to same period in 2013
meDicAre TherApy cl Ass review
MeDIcare: TraDITIonal Therapy classes anD InsIghTs
coMponenTs of TrenD for The Top 10 MeDIcare TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
High Blood Cholesterol
High Blood Pressure/Heart Disease
Heartburn/Ulcer Disease
Urinary Disorders
$2,262.41
ogether, the top three Medicare traditional therapy classes ranked by per-
Diabetes saw a higher PMPY
member-per-year (PMPY) spend – diabetes, high blood cholesterol and pain/
spend ($358.93) than any other
inflammation – contributed 33.6% of total PMPY spend for all traditional
medications used by Medicare beneficiaries in 2014. Total traditional trend was 6.4%,
traditional therapy class among
mainly the result of increased unit costs.
meDicAre TherApy cl Ass review
• Diabetes saw a higher PMPY spend ($358.93) than any other traditional therapy class
among Medicare beneficiaries. Trend for diabetes medications was 26.4%, driven by a small increase in utilization (4.9%) and a significant increase in unit cost (21.5%). Highly utilized medications with double-digit increases in unit-cost trend, including Lantus® (insulin glargine [rDNA origin]) and Januvia® (sitagliptin), are driving spend in this therapy class. Invokana® (canagliflozin), the first in a relatively new class of diabetes treatments known as sodium-glucose co-transporter 2 (SGLT2) inhibitors, experienced a substantial increase in utilization after gaining exposure in the market. Two other SGLT2 inhibitors, Farxiga™ (dapagliflozin) and Jardiance® (empagliflozin), were approved in 2014. Competition among them is likely to have an impact on trend in 2015.
• Total PMPY spend for medications used to treat pain and inflammation increased 9.1%,
despite a slight drop in utilization. Some of the most commonly used medications in this therapy class – including Celebrex® (celecoxib), the only available COX-2 inhibitor – had double-digit increases in unit-cost trend. For Celebrex, the increase was likely related to the manufacturer's profit-protection efforts, stemming from the drug's patent expiration in June 2014.
• In 2014, the trends for both high blood pressure/heart disease medications and
depression medications were negative. Declines in unit costs attributed to the genericization of these classes were responsible. In 2014, the generic fill rate (GFR) for the high blood pressure/heart disease class increased to 96.2%, while the GFR for
In 2014, the generic fill rate (GFR)
depression treatments increased to 97.4%.
for the high blood pressure/heart
• The double-digit increase in unit cost for traditional anticoagulant medications
disease class increased to 96.2%,
was primarily driven by unit-cost increases for Xarelto® (rivaroxaban) and Eliquis® (apixaban). Both drugs also experienced significant increases in utilization, likely as
while the GFR for depression
a result of patients switching from less-convenient warfarin and specialty injectable anticoagulants. Overall trend for the class was 60.2%.
treatments increased to 97.4%.
meDicAre TherApy cl Ass review
Top 10 MeDIcare TraDITIonal Drugs
hree of the top 10 most-expensive traditional drugs when ranked by Medicare
December 2013. Lantus was the most-expensive traditional therapy drug, with a 2014
per-member-per-year (PMPY) spend – Lantus, Januvia and Levemir – are
PMPY spend of $77.17. Total trend for Lantus was 23.3%. Nexium was the second-most-
T indicated to treat diabetes. Together, these three drugs contributed 6.7% of expensive drug when ranked by PMPY spend. Despite the patent expiration for Nexium
all Medicare traditional drug spend in 2014. Individuals age 65 and older are almost twice
in 2014, generics for the brand were not approved until early 2015, due to regulatory
as likely to have diagnosed diabetes as younger individuals.25 Brand drugs dominated the
problems. Utilization declined in six of the top 10 drugs, and two others saw moderate
top 10 drugs, with the sole generic, duloxetine, ranking in ninth place. Total trend was
increases. Large double-digit increases in utilization and cost were observed for duloxetine
highest for duloxetine, as its utilization increased steadily throughout its first full year
of availability, after the patent protection of the branded version, Cymbalta®, expired in
Top 10 MeDIcare TraDITIonal Therapy Drugs
ranKed by 2014 PMPy sPend
% of ToTal
Drug naMe
pMpy spenD TraDITIonal spenD
unIT cosT
Lantus® (insulin glargine injection) Diabetes
Nexium® (esomeprazole
Heartburn/Ulcer Disease
Crestor® (rosuvastatin)
High Blood Cholesterol
Abilify® (aripiprazole)
Advair Diskus® (fluticasone/
Spiriva® (tiotropium)
Namenda XR® (memantine extended Mental/Neurological Disorders
Januvia® (sitagliptin)
Levemir® (insulin detemir injection) Diabetes
meDicAre TherApy cl Ass review
coMparIson of MeDIcare anD coMMercIal TrenD:
TraDITIonal Therapy classes
MeDIcare TrenD versus coMMercIal TrenD for The Top 10 MeDIcare TraDITIonal Therapy classes
ranKed by MedICare trend
Urinary Disorders
High Blood Cholesterol
Heartburn/Ulcer Disease
High Blood Pressure/Heart Disease
verall, the traditional therapy class trend for Medicare clients and for
greater for Medicare clients than for commercial clients because the prevalence of disease
commercially insured clients was identical (6.4%). In addition, the trend for
is higher in older individuals. Conversely, the increase in spend for asthma medications
O each of the 10 drugs in the class moved in the same direction for both groups
observed among Medicare beneficiaries (as opposed to the decline in spend seen for the
of clients, with the exception of medications used to treat urinary disorders and asthma.
commercially insured) is likely related to the utilization of some treatments classified as
The Medicare trend was moderately lower than the commercial trend for two classes –
asthma medications to treat chronic obstructive pulmonary disease (COPD), which usually
pain/inflammation and mental/neurological disorders – and was only marginally lower for
affects older populations.
depression and anticoagulants. The magnitude of trend for the diabetes therapy class was
meDicAre TherApy cl Ass review
MeDIcare: specIalTy Therapy classes anD InsIghTs
coMponenTs of TrenD for The Top 10 MeDIcare specIalTy Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Multiple Sclerosis
Inflammatory Conditions
Pulmonary Arterial Hypertension
Miscellaneous Specialty Conditions
Immune Deficiency
Blood Cell Deficiency
mong Medicare beneficiaries, overall trend for specialty medications was
Ranked by per-member-per-year
45.9% in 2014, composed of an 11.6% increase in utilization and a 34.3%
(PMPY) spend, the top three therapy
increase in unit cost. Ranked by PMPY spend, the top three therapy classes –
oncology, multiple sclerosis and hepatitis C – together contributed 57.8% of total specialty
classes together contributed 57.8%
PMPY spend. All but one of the top 10 therapy classes – anticoagulants – had significant increases in total spend in 2014. However, the key drivers of trend were by far the new
of total specialty PMPY spend.
drugs to treat hepatitis C. Therapies for blood cell deficiency and immune deficiency – the ninth and 10th ranked specialty classes, respectively – were unique to the top 10 list for Medicare beneficiaries when compared to the commercially insured and Medicaid populations, primarily because medications in the classes are used to treat conditions that more commonly affect older adults.
meDicAre TherApy cl Ass review
• The increase in PMPY spend for cancer treatments continued to top that of other specialty
medications. In 2014, PMPY spend increased 37.2%. Trend was driven by a substantial 24.6% increase in utilization and a 12.6% increase in unit cost. This utilization increase is likely the result of several factors, including the expansion of indications for several drugs; the continued development of newer, more targeted therapies; and an increase in the survival rates of patients living with cancer but continuing medication therapy.
Oncology trend was driven by a
Nine new oncology agents were approved in 2014: Beleodaq™ (belinostat), Blincyto™
substantial 24.6% increase in utilization
(blinatumomab), Cyramza® (ramucirumab), Keytruda® (pembrolizumab), Lynparza™ (olaparib), Opdivo® (nivolumab), Sylvant™ (siltuximab), Zydelig® (idelalisib) and
and a 12.6% increase in unit cost.
Zykadia™ (ceritinib).
• Total trend for multiple sclerosis (MS) medications was 27.9%, due to increases in both
utilization and unit cost. Tecfidera® (dimethyl fumarate), released in April 2013, continued to capture market share, likely because the oral administration it offers patients with MS is more convenient than that offered by injectables. Generics to Copaxone® (glatiramer), the market leader in this class, are expected to hit the market in September 2015, but the brand manufacturer is hoping to continue the shift of existing Copaxone users to a new, longer-acting formulation of the drug that has patent protection until 2030.
• Spend for inflammatory conditions increased 30.7%. Utilization increased substantially,
but the main factor was the 17.8% increase in unit cost. One of the key treatments in this class is Xeljanz® (tofacitinib), the only oral disease modifying anti-rheumatic drug available. At the time of U.S. Food and Drug Administration (FDA) approval in 2012, its place in therapy was unclear due to questions concerning its safety profile. Now that longer-term safety and effectiveness data are available, Xeljanz has begun to capture Medicare market share from some more-established injectable treatments.
• Trend for medications used to treat blood cell deficiencies, some a temporary result
of taking powerful chemotherapy agents, increased 9.6% in 2014, the unit-cost trend dampened somewhat by a 1.7% decrease in utilization. Many of the price tags for these branded medications increased in advance of biosimilars to drugs like Neupogen® (filgrastim), which could receive FDA approval in mid-2015, and Procrit® (epoetin alfa), which may be approved in the next two years.
meDicAre TherApy cl Ass review
Top 10 MeDIcare specIalTy Drugs
or Medicare plans, the top 10 specialty drugs accounted for 44.4% of per-
11.7% of all Medicare specialty drug spend in 2014. Harvoni was only just launched in
member-per-year (PMPY) spend for all specialty drugs in 2014. PMPY spend
October 2014, and in that short time contributed 2.5% of specialty spend. Olysio had the
F ranged from a low of $17.66 for Avonex, an established multiple sclerosis largest increase in total PMPY spend (26,028.7%), partly driven by increased utilization
treatment, to a high of $66.22 for Sovaldi, one of three new hepatitis C treatments, all of
after the market availability of the drug reached a full calendar year. Aside from the
which are in the top 10 list. Three cancer medications ranked in the top 10: Revlimid (the
hepatitis C medications, the largest increase in utilization and total spend was observed
second-most-expensive specialty medication), Gleevec (the sixth) and Zytiga (the seventh).
for Zytiga, an oncology medication indicated for the treatment of metastatic prostate
Even though they were launched in late 2013, Sovaldi and Olysio together contributed
cancer. The incidence of prostate cancer rises substantially with age.26
Top 10 MeDIcare specIalTy Therapy Drugs
ranKed by 2014 PMPy sPend
% of ToTal
Drug naMe
pMpy spenD
unIT cosT
Sovaldi® (sofosbuvir)
Copaxone® (glatiramer)
Multiple Sclerosis
Enbrel® (etanercept)
Inflammatory Conditions
Humira® (adalimumab)
Inflammatory Conditions
Gleevec® (imatinib)
Zytiga® (abiraterone)
Olysio® (simeprevir)
Avonex® (interferon beta 1-a)
Multiple Sclerosis
meDicAre TherApy cl Ass review
coMparIson of MeDIcare anD coMMercIal TrenD:
specIalTy Therapy classes
MeDIcare TrenD versus coMMercIal TrenD for The Top 10 MeDIcare specIalTy Therapy classes
ranKed by MedICare trend
Miscellaneous Specialty Conditions
Inflammatory Conditions
Multiple Sclerosis
Pulmonary Arterial Hypertension
Immune Deficiency
Blood Cell Deficiency
verall, the trend for specialty therapy classes experienced by Medicare clients
Although the number of Medicare beneficiaries with HIV has risen dramatically,27 new
(45.9%) was significantly higher than that experienced by commercially
infections are still more likely to be diagnosed in individuals under, rather than over, the
O insured clients (30.9%), related to differences in disease prevalence and drug
age of 55.28 Newer anticoagulants classified as traditional drugs because of their ease of
utilization for older patients versus younger ones. In each therapy class, specialty drug trend
administration captured additional market share among Medicare beneficiaries, who are
for Medicare and commercially insured clients moved in the same direction. HIV treatments
much older, on average, than their commercially insured counterparts and therefore more
and anticoagulants were the only therapy classes in the top 10 for which the trend for
likely to use anticoagulant therapy.
Medicare beneficiaries was lower than that for their commercially insured counterparts.
meDicAiD yeAr in review
MeDIcaID year In revIew
uring 2014, multiple factors affected trend for health plans that manage pharmacy benefits for Medicaid beneficiaries, including: the expansion of Medicaid coverage
D and subsequent increase in Medicaid beneficiaries that followed implementation
of the 2010 Affordable Care Act (ACA); changes to the Medicaid program by state legislatures, and clinical advances and subsequent treatment guideline updates.
Expansion of Medicaid CoverageIn support of its goal of decreasing the number of uninsured Americans and making health insurance more affordable, the ACA gave states the option of receiving federal funds to offset the costs of raising the Medicaid threshold to households with incomes less than 138% of the federal poverty level. The legislatures in 25 states, along with the government of the District of Columbia, chose to raise eligibility thresholds in 2014. In addition, the ACA extended Medicaid coverage to childless adults, who previously had not been eligible for the program.29
8.7 million adults and children
Consequently, we saw the number of Medicaid beneficiaries over age 19 grow from 40.2% in
were added to the Medicaid
2013 to 44.2% in 2014. Also contributing to growth in the Medicaid population was what has been called "the woodwork effect." As some individuals were applying for health insurance
program in 2014, bringing the
coverage through the federal exchange or one of the 25 state exchanges, they discovered that they were eligible for Medicaid coverage and thus were "drawn out of the woodwork." As a
number of Medicaid beneficiaries
result, 8.7 million adults and children were added to the Medicaid program in 2014, bringing
nationwide to 67.9 million.
the number of Medicaid beneficiaries nationwide to 67.9 million.30
With their numbers of Medicaid beneficiaries increased dramatically but unevenly, states took varying approaches to managing the Medicaid pharmacy benefit. Many that chose Medicaid expansion had previously used some form of managed care to control the operational and medical costs of managing enrollee benefits. A few states implemented managed care tools for their expansion population, using a different care model than they had previously utilized. This resulted in various levels of trend management success across states.
State LegislationIn 2014, some states enacted legislation that changed the requirements for the management of prescription drug benefits, thereby limiting the strategies available to Medicaid plan sponsors to control the cost and utilization of the pharmacy benefit.
meDicAiD yeAr in review
As part of Florida's statewide expansion of managed care Medicaid, that state now requires
Because Sovaldi was released in late 2013, after most state Medicaid agencies had calculated
all participating health plans to use a state-mandated formulary for the first year of a
their capitation rates for managed care organizations for 2014, those rates did not take into
contract. The state of Washington took a different approach, enacting regulations that
account the cost of these new, expensive treatment regimens. Neither had most state Medicaid
require health plans to maintain an "open" formulary status for patients who continue to
agencies predicted the impact that paying for these therapies would have on their budgets.
fill prescriptions for previously prescribed atypical antipsychotics, antidepressants and
This concern was raised by state Medicaid agencies in 2014, as they tried to create treatment
medications for attention deficit hyperactivity disorder (ADHD).
guidelines while facing high demand for these products and large financial outlays.
Meanwhile, New York made changes to its "Prescriber Prevails" program; implemented
The concerns of state agencies were warranted. Hepatitis C medications had a trend of 317.2%
in 2012, the program allows a prescriber's professional judgment to prevail in the
for 2014 – a higher trend than that for any other therapy class. In addition, the per-member-
prior-authorization process, thereby allowing some patients to bypass the prior authorization
per-year (PMPY) spend for this therapy class, at $55.02, was higher than the spend for all
process usually required for some drugs. Originally focused on medically necessary
but three of the top 10 therapy classes across both traditional and specialty drugs – even
atypical antipsychotics, the New York program was expanded in 2013 to apply to other drug
though the prevalence of use for hepatitis C medications was dramatically lower than that for
categories, including transplant medications, antidepressants, specialty inflammatory
other medications in the top 10 classes. This dramatic increase in spend is reminiscent of the
condition therapies and anti-seizure medications. In 2014, New York continued to add
staggering increases in price for HIV/AIDS medications that were seen in the early 2000s, when
medications to this list, which affects the ability of Medicaid payers to manage costs.
the new subclass of antiretroviral therapy became the standard of care for HIV/AIDS.
Clinical AdvancesThe largest impact on trend for Medicaid plans in 2014
The largest impact on trend for Medicaid plans in 2014 stemmed
stemmed from U.S. Food and Drug Administration (FDA) approval of three expensive new oral hepatitis C treatments:
from FDA approval of three expensive new oral hepatitis C
Sovaldi® (sofosbuvir), Olysio® (simeprevir) and Harvoni®
treatments: Sovaldi® (sofosbuvir), Olysio® (simeprevir) and
(ledipasvir/sofosbuvir). Treatment with these specialty
hepatitis C patients
drugs is one of the most expensive therapeutic regimens available, and the cost for a course of therapy can reach as high as $150,000. With more than 750,000 patients
TrenD mAnAgemenT sTrATegies
with chronic hepatitis C receiving state-funded healthcare through either Medicaid or the prison system, the burden of paying for the cost of these hepatitis C treatment regimens falls
Tighter Specialty Drug Management and Accredo
disproportionately on state budgets, as opposed to commercial insurance plan budgets.
While Express Scripts and our clients continue to advocate for more sustainable and fair drug pricing, there are important strategies that Medicaid plans can implement
Based on the pricing strategy offered by drug manufacturers when Sovaldi first launched,
to ensure the most appropriate use of these medications. One recommended strategy
Express Scripts projected that states would spend a total of more than $55.2 billion if
is tighter management for high-priced specialty medications to help ensure that
they provided all these hepatitis C patients with one of the newest therapy regimens that
the right patients receive the right treatments. This tighter management can
included Sovaldi. Express Scripts estimated that five states would be faced with the highest
occur through updated prior authorization and treatment guidelines, including
spend: California, Texas, Florida, New York and Illinois.
the use of exclusive specialty drug distribution channels like Accredo, the Express Scripts specialty pharmacy.
We have seen state Medicaid agencies implement various guidelines, such as making viral
campaign advocating for fairer drug pricing, Express Scripts announced in December a new
load testing a requirement for continued authorization of medication; split-fill dispensing,
agreement with AbbVie, makers of the new hepatitis C medication, Viekira Pak™ (ombitasvir/
allowing only 14-day dispensing; evaluation of illegal drug use; and retreatment limits.
paritaprevir/ritonavir co-packaged with dasabuvir). The unprecedented arrangement expands
We encourage health plans to review and consider the approaches taken in the marketplace
both payer affordability and patient access. The cost to cure is now low enough that plan
as they create their own guidelines.
sponsors can afford to treat all hepatitis C patients with genotype 1, not just the sickest.
Encouraging the use of exclusive specialty drug distribution channels like Accredo – rather than retail pharmacies – can dramatically improve medication adherence. For example,
Encouraging the use of exclusive specialty drug distribution
among patients with cancer, our specialized care improved medication adherence by 16 percentage points; among patients with multiple sclerosis, adherence improved
channels like Accredo – rather than retail pharmacies – can
32 percentage points.
dramatically improve medication adherence. For example, among
patients with cancer, our specialized care improved medication
Medication adherence is critically important for patients with conditions such as hepatitis C – from the standpoint of both the high costs of the newer therapies being used to treat the
adherence by 16 percentage points; among patients with multiple
condition and concerns about clinical effectiveness and possible future complications due to
sclerosis, adherence improved 32 percentage points.
nonadherence. A recent analysis underlines the importance of Accredo's programs for patients with hepatitis C. It found that hepatitis C patients who do not experience the unique clinical model available through Accredo are nearly twice as likely to fail their therapy.
Clinical SolutionsAn additional strategy that health plans can implement is Express Scripts clinical solutions
Smart Formulary Management
that align pharmacists, physicians, patients and case managers to help drive optimal
Another strategy we recommend is smart formulary management. Today, most therapy classes
performance against both pharmacy and medical measures. The clinically specialized
offer more drug choices than ever. Yet many prescription drugs that cost more deliver no
Therapeutic Resource Centers (TRCs) of the Express Scripts Pharmacy and Accredo
additional health benefit. Smart formulary management is vital to offering a sustainable
Specialty Pharmacies deliver superior treatment outcomes and cost-effectiveness by
pharmacy benefit that preserves patient access and choice while helping payers obtain fair
supporting patients through the challenges of complex and costly diseases. Our specialist
and affordable pricing. Express Scripts modeled this type of smart formulary management
pharmacists and nurses each receive clinically specialized training in one disease state,
by excluding a handful of "me-too" products from the Express Scripts National Preferred
and the work they do focuses almost exclusively on that clinical condition. This specialized
Formulary. This step created the necessary leverage to negotiate more effectively with
expertise and commitment enables an optimal patient experience and ensures the highest
manufacturers and ultimately achieve lower drug prices for the clients and patients we serve.
performance in pharmacy safety, improved medication adherence and closing gaps in care.
To ensure needed access, in the rare instance when a patient has a medical need for an off-
With their highly specialized knowledge of the complex disease states and complicated
formulary drug, we have created a pathway for the excluded drug to be covered for the patient.
treatment protocols they manage, these TRC pharmacists and nurses often have more experience in rare conditions than some of the physicians who prescribe the treatments.
Smart formulary management can also include preference for one product over another when the products are clinically equivalent. Throughout 2014, Express Scripts was largely critical of
Because each Medicaid health plan's needs are unique, our menu of available programs
a recent trend of drug manufacturers bringing to market products that, although innovative,
is designed to meet varying needs – from ScreenRx® for predictive modeling and tailored
were priced at levels that were so high that they were unsustainable for payers. For example,
interventions to drive adherence; to RationalMed® using proprietary clinical analysis of
the $84,000 treatment cost for the hepatitis C drug, Sovaldi (sofosbuvir), threatened to
prescription, medical and lab data to identify safety risks; to targeted diabetes treatment
bankrupt both private and public plan sponsors around the United States. After a year-long
initiatives supporting physician prescribing best practices.
meDicAiD TherApy cl Ass review
a look aT MeDIcaID overall
ages 0 to 19, 2014
Drug TrenD for 2014
pMpy spenD
unIT cosT
verall trend for Medicaid plans was 10.2% in 2014, due primarily to a 10.7%
increase in unit cost. Utilization was marginally negative (-0.5%), reflecting
the influx of new, adult beneficiaries less likely to use medications for chronic
conditions. Because the number of prescriptions remained similar from 2013 to 2014, but the
January-December 2014 compared to same period in 2013
number of beneficiaries across which to divide the costs grew, a decrease in utilization was reflected. Overall per-member-per-year (PMPY) spend was $882.43 (vs. $800.65 in 2013).
ages 20 to 34, 2014
Traditional trend was 2.8% – far below that observed in either the commercially insured or
Medicare populations – reflecting a 3.2% increase in unit cost, offset somewhat by a 0.5%
pMpy spenD
unIT cosT
decrease in utilization. Spend for traditional medications contributed 72.2% of total PMPY
spend in 2014 – a lower share than the 77.7% observed in 2013. The decrease in share of
spend for traditional medications was due to the impact of the new, expensive specialty
medications, Sovaldi, Olysio and Harvoni among them, which contributed significantly to
January-December 2014 compared to same period in 2013
a 35.8% increase in PMPY spend for specialty drugs in 2014 – three times that in 2013.
PMPY spend and trend varied across age groups. For beneficiaries age 20 to 34, total trend was -2.5% – the drop due largely to the increase in the number of younger adult
ages 35 to 64, 2014
beneficiaries in this age range. PMPY spend for these beneficiaries was $828.78. As in
2013, PMPY spend for beneficiaries age 35 to 64, at $2,222.82, was higher than that for all
pMpy spenD
unIT cosT
other age groups. The 4.1% trend for this age group was driven entirely by an increase in
unit cost for specialty medications. The highest trend, 12.4%, was among beneficiaries age
65 and older, again driven by increased unit costs for specialty medications.
January-December 2014 compared to same period in 2013
coMponenTs of MeDIcaID TrenD
ages 65 and older, 2014
pMpy spenD
unIT cosT
pMpy spenD
unIT cosT
January-December 2014 compared to same period in 2013
January-December 2014 compared to same period in 2013
meDicAiD TherApy cl Ass review
MeDIcaID: TraDITIonal Therapy classes anD InsIghTs
coMponenTs of TrenD for The Top 10 MeDIcaID TraDITIonal Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Attention Disorders
Chemical Dependence
High Blood Pressure/Heart Disease
anked by PMPY spend, the top 10 traditional therapy classes accounted for
Ranked by per-member-per-year
70.6% of Medicaid traditional spend. For the second year in a row, medications
(PMPY) spend, the top 10 traditional
used to treat diabetes were the most-expensive traditional therapy class,
contributing 16.6% of overall Medicaid traditional drug spend. Six of the top 10
therapy classes accounted for
traditional therapy classes had negative total trend, with the largest decline seen in spend for antidepressants.
70.6% of Medicaid traditional spend.
meDicAiD TherApy cl Ass review
highlighTs
• PMPY spend for diabetes in 2014 was higher than that for any other traditional therapy
class. Total trend for diabetes medications was 18.4%, resulting mainly from a significant increase in unit cost (17.0%). Brand inflation for insulin therapies contributed heavily to the unit-cost increase. For example, the unit cost for Lantus® (insulin glargine [rDNA origin]) increased 32.8% – likely in anticipation of biosimilar insulin glargine expected in 2017 and probable competition from ultra-long acting insulins that are in the pipeline for approval in the next few years. Costs for other insulins, like Humalog® (insulin lispro [rDNA origin]), also went up.
• Despite a 3.2% decrease in utilization, trend for pain/inflammation treatments was 9.1%
in 2014. The class, which includes a variety of treatments for pain – including not only opioids like hydrocodone, but also GABA receptor agonists like gabapentin and NSAIDs like naproxen – is almost exclusively generic; the generic fill rate (GFR) for the class was 97.5% in 2014. However, market dynamics in 2014 led to double-digit increases in unit costs for two of the most commonly used treatments among Medicaid beneficiaries, lidocaine and oxycodone/acetaminophen.
• Total trend for medications used to treat attention disorders was a modest 1.0%, mostly
as a result of a 4.1% decrease in utilization. This decrease may be partly related to increased scrutiny of medications for attention deficit hyperactivity disorder (ADHD) that are used by Medicaid beneficiaries.31 The decrease in utilization of therapies for attention deficit – which are prescribed most often for children – may also be attributable to age shifts in the Medicaid population following implementation of the ACA.
• Although utilization of depression medications increased 5.7% in 2014, the 22.1%
Brand inflation for insulin therapies
decrease in unit cost led the class to the largest negative total trend (-16.4%) among the top 10 traditional therapy classes. The biggest reason for the decline in the unit
contributed heavily to the 17.0%
cost was an increase in the GFR from 94.0% in 2013 to 98.6% in 2014. One of the
increase in unit cost for diabetes
last remaining brand serotonin norepinephrine reuptake inhibitors (SNRIs), Cymbalta® (duloxetine), lost patent protection at the end of 2013, with the result that all of the top
10 most commonly used antidepressants in 2014 were generics.
meDicAiD TherApy cl Ass review
Top 10 MeDIcaID TraDITIonal Drugs
ine of the top 10 traditional drugs used by Medicaid beneficiaries in 2014
As in 2013, the Medicaid traditional
were brands, which contributed 25.8% of per-member-per-year (PMPY)
N spend for all traditional therapy drugs. The only generic medication in the therapy drug with the highest PMPY
top 10, methylphenidate, is used to treat attention deficit hyperactivity disorder (ADHD).
spend was the antipsychotic Abilify.
As in 2013, the Medicaid traditional therapy drug with the highest PMPY spend was the antipsychotic Abilify. Total trend was 6.0% for Abilify, as the decrease in utilization was
Total trend was 6.0% for Abilify.
offset by an increase in unit cost; the increase in unit cost likely occurred in anticipation of the expiration of the drug's patent in April 2015. The highest trend, for Symbicort (53.2%) was driven mostly by a 42.7% increase in utilization.
Top 10 MeDIcaID TraDITIonal Therapy proDucTs
ranKed by 2014 PMPy sPend
% of ToTal
Drug naMe
pMpy spenD TraDITIonal spenD
unIT cosT
Abilify® (aripiprazole)
Lantus® (insulin glargine injection) Diabetes
Humalog® (insulin lispro injection)
OneTouch Ultra® Test Strips
Chemical Dependence
Symbicort® (budesonide/
methylphenidate extended release
Attention Disorders
Ventolin® HFA (albuterol)
Spiriva® (tiotropium)
Chronic Obstructive Pulmonary
Advair Diskus® (fluticasone/
meDicAiD TherApy cl Ass review
MeDIcaID: specIalTy Therapy classes anD InsIghTs
coMponenTs of TrenD for The Top 10 MeDIcaID specIalTy Therapy classes
ranKed by 2014 PMPy sPend
pMpy spenD
unIT cosT
Inflammatory Conditions
Multiple Sclerosis
Miscellaneous Specialty Conditions
Growth Deficiency
Pulmonary Arterial Hypertension
verall trend for specialty medications used by Medicaid beneficiaries was 35.8% in 2014, fueled by the significant increase in unit cost related to the
O launch of three new hepatitis C treatments – Sovaldi® (sofosbuvir), Olysio®
(simeprevir) and Harvoni® (ledipasvir/sofosbuvir). Hepatitis C medications contributed 65.0% of the total increase in specialty spend. Accordingly, hepatitis C medications were the most-expensive Medicaid specialty therapy class when ranked by PMPY spend.
Hepatitis C medications contributed
The top three therapy classes – hepatitis C, HIV and inflammatory conditions – together contributed 54.7% of total specialty PMPY spend. Two of the top 10 therapy classes –
65.0% of the total increase in
growth deficiency and anticoagulants – had negative total trend in 2014. Only pulmonary
specialty spend.
arterial hypertension and anticoagulants had a decrease in unit cost from 2013 to 2014.
meDicAiD TherApy cl Ass review
highlighTs
• Total trend for HIV medications was 11.0%, almost exclusively from an increase in unit
cost. Although the wave of patent expirations in this class continues, generic availability may not reduce the cost of HIV therapy because a constantly changing pipeline is needed to address mutations in virus strains that cause resistance to drugs. Additionally, older HIV drugs that may have generic equivalents must be taken several times a day in combination with other drugs, and newer treatments that combine multiple medications into one-dose form offer more convenient dosing regimens. For example, Stribild®(elvitegravir/cobicistat/emtricitabine/tenofovir) is taken only once a day; and although Tivicay® (dolutegravir), another relatively recent FDA approval, must be taken with other drugs, dosage is only twice a day. Both brand medications gained significant market share in 2014, but both come with much higher price tags than generics.
• A 9.2% decrease in utilization was offset by a 4.2% increase in unit cost, resulting in
a -5.0% trend for growth deficiency treatments among Medicaid beneficiaries. Growth hormone treatments are another class of medications that is primarily used by children, so a shift in the age structure of the Medicaid population following the expansion of the Medicaid program in some states could be contributing to the decrease in utilization.
• Both the cost and the utilization of cystic fibrosis (CF) treatments increased in 2014.
However, utilization of Kalydeco® (ivacaftor), the first drug to treat the underlying cause of disease in patients with a rare form of CF, dropped 6.7%. The annual price tag for Kalydeco, an oral drug, can exceed $300,000. A new drug, lumacaftor, is in development;
Utilization of Kalydeco® (ivacaftor), the
if approved, it will be taken in combination with Kalydeco to treat underlying genetic mutations that cause the most common form of CF.
first drug to treat the underlying cause
• Medicaid spend for specialty anticoagulant medications continued to decline, spurred
of disease in patients with a rare form
by a 13.2% decrease in unit cost. The 99.6% generic fill rate (GFR) and its associated lower costs far outweighed the 2014 price increases observed for the few remaining
of CF, dropped 6.7%.
brands in the class.
meDicAiD TherApy cl Ass review
The Top 10 MeDIcaID specIalTy Drugs
or Medicaid plans, the top 10 specialty drugs accounted for 44.5% of per-member-per-year (PMPY) spend for all specialty drugs in 2014. PMPY spend
F ranged from $43.13 for Sovaldi to $4.32 for generic enoxaparin. Sovaldi
captured 17.6% of PMPY spend for all specialty drugs in 2014. The PMPY spend for Sovaldi
PMPY spend ranged from $43.13 for
was also more than triple the PMPY spend for the second-most-expensive drug, Humira,
which had almost three times the utilization Sovaldi did in 2014. Three HIV medications
$4.32 for generic enoxaparin.
captured spots in the top 10: Atripla, Truvada and Viread, which ranked fourth, fifth and seventh, respectively. Aside from Sovaldi, the oral MS treatment, Tecfidera, had the highest trend (247.7%), driven by a triple-digit increase in utilization, likely spurred by the added convenience of administration for an oral medication over an injectable treatment.
Top 10 MeDIcaID specIalTy Therapy Drugs
ranKed by 2014 PMPy sPend
% of ToTal
Drug naMe
pMpy spenD
unIT cosT
Sovaldi® (sofosbuvir)
Humira® (adalimumab)
Inflammatory Conditions
Enbrel® (etanercept)
Inflammatory Conditions
Copaxone® (glatiramer)
Multiple Sclerosis
Viread® (tenofovir)
Tecfidera® (dimethyl fumarate)
Multiple Sclerosis
Avonex® (interferon beta 1-a)
Multiple Sclerosis
The Drug TrenD reporT
MeThoDology
Prescription drug use for members with drug coverage provided by Express Scripts plan
The Express Scripts Prescription Price Index (PPI) measures inflation in prescription drug
sponsors32 was analyzed for the Drug Trend Report. The plan sponsors providing the
prices by monitoring changes in consumer prices for a fixed market basket of commonly
pharmacy benefit paid at least some portion of the cost for the prescriptions dispensed to
used prescription drugs. Separate market baskets are defined for brand drugs and for
their members, providing what is known as a funded benefit.
generic drugs, and are based on the top 80% of utilized drugs.
Both traditional and specialty drugs are included. Specialty medications include injectable
Please Note: Although up to nine decimal places were allowed in making all calculations,
and noninjectable drugs that are typically used to treat chronic, complex conditions and may
in most cases the results were rounded down to one or two decimals for easier reading.
have one or more of the following qualities: frequent dosing adjustments or intensive clinical
Therefore, dollar and percentage calculations may be slightly off due to rounding.
monitoring; intensive patient training and compliance assistance; limited distribution; and specialized handling or administration. Nonprescription medications (with the exception of diabetic supplies billed under the pharmacy benefit) and prescriptions that were dispensed in hospitals, long-term care facilities and other institutional settings, or billed under the medical benefit are not included.
Trend and other measures are calculated separately for those members with commercial insurance coverage, for Medicaid recipients and for Medicare beneficiaries receiving prescription benefits through Employer Group Waiver Plans (EGWPs), managed Medicare Prescription Drug Plans (PDPs) or Medicare Advantage Prescription Drug Plans (MAPDs). Members used Express Scripts for retail and home delivery pharmacy services; they used Accredo, the Express Scripts specialty pharmacies, for specialty drug prescriptions.
Total trend measures the rate of change in plan costs, which include ingredient costs, taxes, dispensing fees and administrative fees. Rebates are not included as a component of cost. Total trend comprises utilization trend and unit cost trend. Utilization trend is defined as the rate of change in total days' supply of medication per member, across prescriptions. Unit cost trend is defined as the rate of change in costs due to inflation, discounts, drug mix and member cost share. Utilization and cost are determined on a per-member-per-year (PMPY) basis. Metrics are calculated by dividing totals by the total number of member-months, which is determined by adding the number of months of eligibility for all members in the sample.
cITaTIons
1. Dolgin E. Big pharma moves from ‘blockbusters' to ‘niche busters'. Nat Med.
11. Iyengar R, Henderson R, Visaria J, Frazee SG. Dispensing channel and medication
adherence: evidence across 3 therapy classes. Am J Manag Care. 2013;19(10):798-804
2. Braun MM, Farag El Massah S, Xu K, Cote TR. Emergence of orphan drugs in the
United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov.
12. Express Scripts Research, 2014
13. Rotenstein LS, Ran N, Shivers JP, Yarchoan M, Close KL. Opportunities and challenges
3. American Diabetes Association. Statistics about diabetes. http://www.diabetes.org/
for biosimilars: what's on the horizon in the global insulin market? Clin Diabetes.
diabetes-basics/statistics/. Sept. 10, 2014. Accessed Nov. 5, 2014.
4. Pharmaceutical Research and Manufacturers of America. Medicines in development
14. Wettermark B, Brandt L, Kieler H, Boden R. Pregabalin is increasingly prescribed
for neuropathic pain, generalized anxiety disorder and epilepsy but many patients
Feb. 11, 2014. Accessed Nov. 26, 2014.
discontinue treatment. Int J Clin Pract. 2014;68(1):104-110.
5. Staton T. Payers fret about the next drug doomsday: pricey PCSK9 cholesterol meds.
15. Hungin AP, Hill C, Molloy-Bland M, Raghunath A. Systematic review: patterns of
proton pump inhibitor use and adherence in gastroesophageal reflux disease. Clin
Gastroenterol Hepatol. 2012;10(2):109-116.
May 7, 2014. Accessed Nov. 28, 2014.
16. O'Callaghan P. Adherence to stimulants in adult ADHD. Atten Defic Hyperact Disord.
6. McPherson TB, Fontane PE, Jackson KD, et al. Prevalence of compounding in
independent community pharmacy practice. J Am Pharm Assoc. 2003;46(5):568-573.
17. Mojtabai R, Olfson M. National trends in long-term use of antidepressant
7. Food and Drug Administration Modernization Act of 1997. Pub. L. No. 105-115 § 127,
medications: results from the U.S. National Health and Nutrition Examination Survey.
111 Stat. 2286, 2328. 1997. (codified at 21 U.S.C. § 353a)(2006).
J Clin Psych. 2014;75(2):169-177.
8. Boodoo JM. Compounding problems and compounding confusion: Federal regulation
18. Schrijvers LH1, Uitslager N, Schuurmans MJ, Fischer K. Barriers and motivators
of compounded drug products and the FDMAM circuit split. Am J Law Med.
of adherence to prophylactic treatment in haemophilia: a systematic review.
9. Government Accountability Office. Compounded drugs. TRICARE's payment
19. Hoadley J, Summer L, Hargrave E, Cubanski J, Neuman T. Medicare Part D in its ninth
practices should be more consistent with regulations. (GAO Publication No. 15-
year: the 2014 marketplace and key trends, 2006-2014. The Henry J. Kaiser Family
64). Washington, D.C.: U.S. Government Printing Office. http://www.gao.gov/
assets/670/666339.pdf. October 2014. Accessed March 3, 2015.
2014-marketplace-and-key-trends-2006-2014/. Aug. 18, 2014. Accessed Jan. 19,
10. Katz A. Surprise! Generic drug prices spike. Bloomberg Businessweek.
http://www.businessweek.com/printer/articles/172832-surprise-generic-drug-prices-spike. Dec. 12, 2013. Accessed Nov. 25, 2014.
20. American Association for the Study of Liver Diseases and Infection Diseases Society
28. Centers for Disease Control and Prevention. HIV/AIDS: HIV among older Americans.
of America. Recommendations for testing, managing and treating Hepatitis C.
http://www.cdc.gov/hiv/risk/age/olderamericans/. Dec. 20, 2013. Accessed
http://www.hcvguidelines.org/full-report-view. Updated Dec. 19, 2014. Accessed
Jan. 31, 2015.
Dec. 28, 2014.
29. The Henry J. Kaiser Family Foundation. Medicaid eligibility for adults as of
21. Milliman. The impact of new hepatitis C drug therapy on individual Medicare
Part D spending. http://www.pcmanet.org/images/stories/uploads/2014/
of-january-1-2014/. Oct. 1, 2013. Accessed Dec. 28, 2014.
partdpremiumstudymilliman.pdf. July 2014. Accessed Jan. 19, 2015.
22. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, et al. 2014
30. Mann C. Medicaid and CHIP enrollment grows by 8.7 million additional Americans.
Department of Health and Human Services website. http://www.hhs.gov/healthcare/
Evidence-based guidelines for the management of high blood pressure in adults.
facts/blog/2014/10/medicaid-chip-enrollment-august.html. Oct. 17, 2014. Accessed
Report from the panel members appointed to the eighth joint national committee
Dec. 28, 2014.
(JNC8). JAMA. 2014;311(5):507-520.
31. Vestal C. Medicaid ADHD treatment under scrutiny. The Pew Charitable Trust. http://
23. Centers for Medicare and Medicaid Services. Chronic conditions among Medicare
beneficiaries, Chartbook, 2012 Edition. Baltimore, MD, 2012. http://www.cms.gov/
adhd-treatment-under-scrutiny. Oct. 18, 2014. Accessed Jan. 31, 2015.
32. Plan sponsors were excluded if they were not Express Scripts clients in both
periods, if they had less than 12 months of claims data in either period, if they
24. Iyengar RN, Balagere D, Henderson R, LeFrancois A, Rabbitt R, Frazee S. Association
had retail-only benefits, if they had 100% or 0% copayment benefits, if they had
between dispensing channel and medication adherence among Medicare
eligibility shifts exceeding 20% for commercial plans (eligibility shifts exceeding
beneficiaries taking medications to treat diabetes, high blood pressure, or high blood
50% for Medicare and Medicaid plans), or if they were contractually prohibited
cholesterol. J Manag Care Pharm. 2014; 20(8):851-61.
from inclusion. Individual members might be covered, and thus included, for only a
25. Centers for Disease Control and Prevention. Diabetes public health resource: rate per
portion of the time periods of interest.
100 of civilian, noninstitutionalized population diagnosed diabetes, by age, United States, 1980-2011. http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.
htm. Sept. 16, 2014. Accessed Jan. 31, 2015.
26. Centers for Disease Control and Prevention. Prostate cancer: prostate cancer risk
by age. http://www.cdc.gov/cancer/prostate/statistics/age.htm. Sept. 16, 2014. Accessed Jan. 31, 2015.
27. Henry J. Kaiser Family Foundation. Medicare and HIV/AIDS.
https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7171_04.pdf. February 2009. Accessed Jan. 31, 2015.
2015 Express Scripts Holding Company. All Rights Reserved. 14-EME27270
Source: http://w.emacromall.com/reference/ExpressScripts_DrugTrendReport.pdf
NATURE Vol 465 20 May 2010 parasites (Toxoplasma, Leishmania and active compounds — and an earlier, partial y These reports1,2 offer tremendous opportunities trypano somes) and on replicating human cel described set8 identified in a high-throughput to develop the next generation of antimalarial lines, and found that most of the compounds screen against P. falciparum — should be a first drugs. They also sound a call for the academic
the Pituitary Foundation inFormation leaFlets Monday - Friday 9:00am – 5:00pm Email: [email protected] 0845 450 0375 Endocrine Nurse HelpLine available scheduled hours The Pituitary Foundation publishes a library 0845 450 0377 of leaflets on pituitary conditions, treatments and wel -being issues. For more information please visit our website, or call our HelpLine.









