Luteinizing hormone reduces the activity of the npr2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic gmp decrease that promotes resumption of meiosis in oocytes


Contents lists available at
Developmental Biology
journal homepage:
Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclasein mouse ovarian follicles, contributing to the cyclic GMP decrease thatpromotes resumption of meiosis in oocytes
Jerid W. Robinson ,1, Meijia Zhang , Leia C. Shuhaibar , Rachael P. Norris , Andreas Geerts Frank Wunder , John J. Eppig , Lincoln R. Potter nn, Laurinda A. Jaffe n
a Department of Pharmacology, University of Minnesota, Minneapolis, MN, USAb State Key Laboratory of Agro-biotechnology, College of Biological Sciences, China Agricultural University, Beijing, People's Republic of Chinac Department of Cell Biology, University of Connecticut Health Center, Farmington, CT, USAd Bayer Pharma AG, Pharma Research Center, Wuppertal, Germanye The Jackson Laboratory, Bar Harbor, ME, USAf Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN, USA
In preovulatory ovarian follicles of mice, meiotic prophase arrest in the oocyte is maintained by cyclic
Received 8 March 2012
GMP from the surrounding granulosa cells that diffuses into the oocyte through gap junctions. The
Received in revised form
cGMP is synthesized in the granulosa cells by the transmembrane guanylyl cyclase natriuretic peptide
receptor 2 (NPR2) in response to the agonist C-type natriuretic peptide (CNP). In response to luteinizing
Accepted 12 April 2012
hormone (LH), cGMP in the granulosa cells decreases, and as a consequence, oocyte cGMP decreases
Available online 21 April 2012
and meiosis resumes. Here we report that within 20 min, LH treatment results in decreased guanylyl
cyclase activity of NPR2, as determined in the presence of a maximally activating concentration of CNP.
This occurs by a process that does not reduce the amount of NPR2 protein. We also show that by a
slower process, first detected at 2 h, LH decreases the amount of CNP available to bind to the receptor.
Mouse ovarian follicle
Both of these LH actions contribute to decreasing cGMP in the follicle, thus signaling meiotic
Luteinizing hormoneGuanylyl cyclase
resumption in the oocyte.
& 2012 Elsevier Inc. All rights reserved.
with the transition from prophase to metaphase, marked by thebreakdown of the nuclear envelope about 2 h after LH exposure.
Mammalian oocytes are maintained in meiotic prophase for
However, other events of the prophase-to-metaphase transition
prolonged periods. During prophase arrest, the oocyte is located in
occur before nuclear envelope breakdown: microtubule organizing
a follicle in which it is surrounded by granulosa cells ((A)). As
centers assemble (, chromatin condenses
the follicle grows to its full size (� 400–500 mm in mice), the oocyte
, and cell cycle regulatory proteins
acquires the ability to resume meiosis, but due to inhibitory signals
undergo changes in activity and localization (
from the granulosa cells, the oocyte remains in prophase
Recent studies of the mouse ovary have shown that a key
. Then during each reproductive
inhibitory substance for maintaining prophase arrest is cGMP,
cycle, luteinizing hormone (LH) from the pituitary acts on the
which diffuses from the granulosa cells into the oocyte through
granulosa cells of the fully grown follicle to cause the oocyte to
gap junctions ). In the
mature into a fertilizable egg and be ovulated. This process begins
oocyte, cGMP inhibits the cAMP phosphodiesterase PDE3A, andthus prevents the degradation of cAMP. Elevated cAMP activatesprotein kinase A, which acts through a complex of mechanisms to
n Corresponding author. Fax: þ1 860 679 1269.
nn
inhibit the activity of the CDK1-cyclin B kinase and thus to inhibit
the prophase-to-metaphase transition (
E-mail addresses:
If cGMP in a follicle-enclosed oocyte is experimen-
tally decreased, by injection of a cGMP-specific phosphodiester-
ase, cAMP is decreased, and as a consequence meiosis resumes
Contributed equally to this work.
0012-1606/$ - see front matter & 2012 Elsevier Inc. All rights reserved.
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
is located in the mural granulosa cells, mostly within the outerseveral layers of cells, and is absent in the cumulus cells(; ). In response to LH,the permeability of the gap junctions between the granulosa cellsthroughout the follicle is reduced, such that intercellular diffusionwithin the follicle of molecules of the size of cGMP is slowed(). In parallel,cGMP levels in the follicle decrease ; ), from a basal level of � 3 mM, to
� 0.5 mM at 20 min and � 0.1 mM at 1 h after applying LH
(). CNP levels also decrease ; ), but the earliest of these measure-ments were made at 4 h after LH application, while the cGMPdecrease occurs by 20 min, so their functional significance has notbeen certain. As cGMP in the follicle decreases, cGMP in theinterconnected oocyte falls correspondingly, to a few percent ofthe basal level at 1 h. As a consequence, the inhibition of PDE3A isrelieved, cAMP decreases, and meiosis resumes
The decrease in cGMP in the follicle could be caused by a
decrease in cGMP synthesis, an increase in cGMP degradation,and/or an increase in cGMP efflux. Here we report that onemechanism by which LH signaling reduces cGMP is by reducingthe activity of the guanylyl cyclase NPR2.
Materials and methods
Mice and hormones
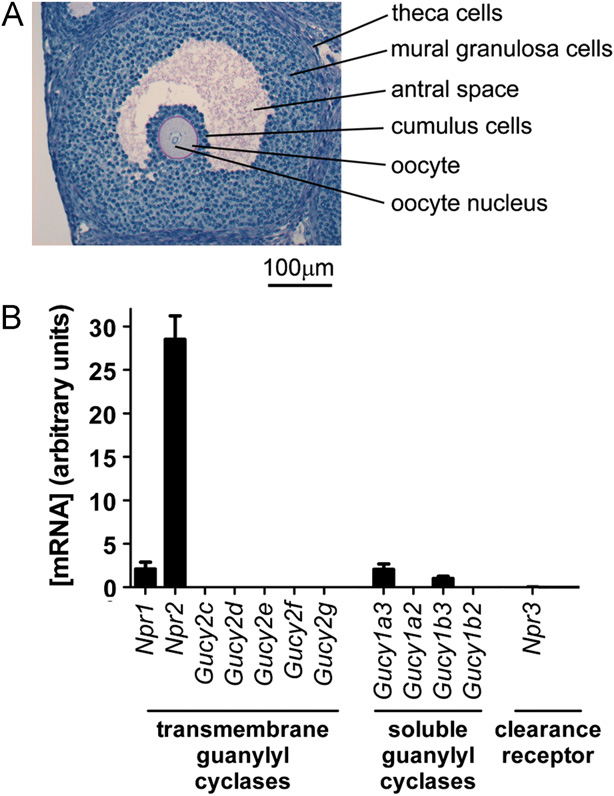
Fig. 1. In mural granulosa cells, Npr2 mRNA is present at a higher concentrationthan mRNAs for other guanylyl cyclases. (A) Histological section of a mouse ovary,showing an antral follicle, and indicating the mural granulosa cells collected for
Ovaries were obtained from prepubertal B6SJLF1 mice (23–25
analysis, as well as other cell types and structures in and around the follicle. (B)
day old) from The Jackson Laboratory (Bar Harbor, ME); proce-
Relative concentrations of each guanylyl cyclase mRNA in isolated mural granu-
dures were approved by the animal care committees of the
losa cells. Results for the natriuretic peptide clearance receptor, Npr3, are also
University of Connecticut Health Center, China Agricultural Uni-
shown. Where no bars are visible, concentrations of mRNAs were o0.1% of Npr2.
The results show the mean 7s.e.m. for 3 RNA preparations.
versity, and The Jackson Laboratory. For granulosa cell collection,cumulus-oocyte complex collection, CNP ELISA assays, and histo-logical analysis, the mice were injected with 5 I.U. equine
The generation of the cGMP that maintains meiotic arrest
chorionic gonadotropin (eCG) 40–48 h before use, to stimulate
requires the function in the granulosa cells of the transmembrane
follicle growth and LH receptor expression. Mice for antral follicle
guanylyl cyclase natriuretic peptide receptor 2 (NPR2, also known
isolation were not injected with eCG; instead the follicles were
as guanylyl cyclase-B) and its extracellular agonist C-type
exposed to 10 ng/ml follicle stimulating hormone (FSH) in vitro.
natriuretic peptide (CNP, also known as natriuretic peptide C,
Ovine LH, human LH, ovine FSH, and eCG, purified from
NPPC) (). In ovaries of mice carrying mutations
biological sources, were obtained from A.F. Parlow (National
in Npr2 or Nppc genes, meiosis resumes precociously (
Hormone and Peptide Program, Torrance, CA). Human recombi-
Although there is also evidence for expression of other
nant LH was obtained from EMD Serono Research Institute, Inc.
guanylyl cyclases in granulosa cells ) and
(Rockland, MA). Human chorionic gonadotropin (hCG) was pur-
some evidence that these may contribute to the maintenance of
chased from Sigma-Aldrich (St. Louis, MO). Ovine LH was used
meiotic arrest (¨
for studies of isolated follicles (10 mg/ml). Because of their
and the response of the follicle to LH
slower rate of degradation ), human
), CNP-dependent activation of NPR2 is
LH or hCG was used for injection into mice (10 mg or 5 I.U.,
fundamental for generating the inhibitory levels of cGMP.
CNP is synthesized by the outer (mural) granulosa cells, and
binds to NPR2 throughout the follicle to stimulate cGMP produc-
Measurement of relative amounts of guanylyl cyclase mRNAs in
tion (; The connection of
the cumulus cells to the mural granulosa cells is essential formaintaining meiotic arrest, since when this connection is broken,
Mural granulosa cells were collected by puncturing antral
leaving the cumulus-oocyte complex free in the antral space,
follicles of isolated ovaries with 30 gauge needles. RNA was
meiosis resumes ). This supports
extracted using TRIzol reagent (Invitrogen Corporation, Carlsbad,
the concept that although Npr2 mRNA is most concentrated in the
CA). DNAse I digestion was performed to remove residual geno-
cumulus cells ), cGMP generated by NPR2 in the
mic DNA, and mRNAs were reverse transcribed using random
mural layers also provides a critical part of the inhibitory cGMP to
Quantitative TaqMan analysis was performed using the
Despite this knowledge of how CNP, NPR2, and cGMP function
Applied Biosystems PRISM 7900 sequence detection system, to
to maintain meiotic arrest, less is known about how signaling by
determine the relative concentration of each guanylyl cyclase
LH reverses the arrest. LH acts on a G-protein-linked receptor
mRNA in granulosa cells. Primer sequences are listed in
(LHCGR) ), which in rats and mice,
in the supplementary material. Differences in primer efficiency
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
were determined by measuring the cycle threshold (Ct) values for
Samples were prepared by a method modified from
each primer pair using 30 ng of genomic DNA. Only small
. Two ovaries in 70 ml of 1.0 M acetic acid were heated
differences were detected, and these were corrected for by use
at 95 1C for 10 min, then lysed with a probe sonicator. 350 ml of
of the following formula:
MeOH was added to solubilize lipids, and the tube was centri-
Ct (corrected)¼Ct (measured)þCt (mean of all probes, genomic
fuged at 30,000 � g at 4 1C for 15 min. The supernatant (‘‘ovary
DNA)� Ct (genomic DNA)
extract'') contained � 250 mg of protein. �50% of the CNP was
Normalization was performed using the housekeeping gene Rpl32
recovered in this extract (determined by adding a known amount
as a control. The resulting expression is given in arbitrary units.
of CNP to the ovaries before extraction). For each sample, 10, 5,and 2 mg of the extract protein were lyophilized and assayed,
2.3. Measurement of guanylyl cyclase activity in a crude membrane
following the manufacturer's instructions. Data were analyzed
fraction of follicles
using Prism software. Statistical significance of the data wastested using one-way ANOVA with a Dunnett multiple compar-
For each experiment, antral follicles from 4 mice were isolated
isons post-test. The concentration of CNP in the ovary, if CNP was
and cultured for 24–30 h in the presence of FSH to stimulate
uniformly distributed, was estimated based on a volume per
follicle growth and LH receptor expression
ovary of �4 ml ( �4 mg wet weight).
The follicles were divided into 2 equal groups, and halfwere exposed to LH for the indicated time. The 40–50 follicles in
Histological analysis of nuclear envelope breakdown kinetics
each group were washed in PBS and lysed in phosphataseinhibitor buffer in a 100 ml glass homogeni-
Serial sections of mouse ovaries were prepared as previously
zer. To obtain a crude membrane fraction, the homogenate
described (Follicles with a diameter
(200 ml volume) was centrifuged at 10,000 � g for 20 min; the
of Z350 mm in at least one dimension, as measured in the
pellet ( � 1 ml volume) was resuspended in 50 ml of phosphatase
section containing the nucleolus or chromosomes, were analyzed
inhibitor buffer and sonicated briefly. Protein content was deter-
for the presence of an intact nucleus (see (A)).
mined by solubilizing a 4 ml aliquot in 1% SDS and performing aBCA assay (Pierce, Thermo Fisher Scientific, Rockford, IL). The
Results and discussion
crude membrane fraction contained � 1 mg of protein per follicle.
The samples were frozen in liquid N2 and stored at �80 1C.
In mural granulosa cells, mRNA encoding NPR2 is present at a higher
Guanylyl cyclase assays were conducted for each pair of
concentration than mRNAs encoding other guanylyl cyclases
follicle samples prepared as described above (one sample thathad been treated with LH, one control sample without LH), using
Although NPR2 is known to be present in mural granulosa cells
methods as previously described ).
and functionally important for maintaining meiotic arrest, there is
Assays were performed at 37 1C using 1–2 mg of follicle protein
also evidence that NPR1 and soluble guanylyl cyclase subunits
per assay tube, in the presence or absence of 1 mM CNP (or ANP),
could contribute to the control of meiotic arrest and progression
which are maximally activating concentrations for their respec-
(see Introduction). Because previous studies did not determine
tive receptors (; ).
the relative expression levels of mRNA for NPR2 and other
0.5 mM isobutylmethylxanthine (IBMX) was included to inhibit
guanylyl cyclases in mouse granulosa cells, and because not all
cGMP phosphodiesterase activity. CNP (or ANP) dependent gua-
of the guanylyl cyclases were investigated, we quantitatively
nylyl cyclase activity refers to the activity measured in the
compared the amounts of mRNA in mural granulosa cells for
presence of the natriuretic peptide minus the activity measured
each of the mouse guanylyl cyclase genes (
in the absence of the natriuretic peptide. Statistical significance of
The mouse genome contains 7 transmembrane and 4 soluble
the data was tested using two-way repeated measures ANOVA
guanylyl cyclase genes ). We detected mRNA
with a Bonferroni post-test; control and LH-treated samples that
encoding two transmembrane guanylyl cyclases, NPR1 and
had been prepared and assayed together were analyzed as pairs.
NPR2, and two soluble guanylyl cyclase subunits, GUCY1A3
The analysis was performed using Prism software (GraphPad
(soluble guanylyl cyclase alpha 1) and GUCY1B3 (soluble guanylyl
Software, Inc., La Jolla, CA).
cyclase beta 1). Among these, Npr2 mRNA was expressed at a highlevel, Z14 times higher than any of the other guanylyl cyclases.
Measurement of cGMP in cumulus cells
We also tested for mRNA encoding NPR3, which has sequencesimilarity to the extracellular domains of NPR1 and NPR2, but
Cumulus-oocyte complexes were isolated at various times
lacks the guanylyl cyclase domain and activity (
after hCG injection, and cultured as previously described (
NPR3 is a clearance receptor for natriuretic peptides. Little or no
), with or without 30 nM CNP for 1 h. Cumulus cells
Npr3 mRNA was detected.
were then separated for measurement of cGMP using an ELISA
Although concentrations of mRNAs are not directly propor-
method as previously described ). Statistical
tional to the amounts of the proteins they encode, these mea-
significance of the data was tested using one-way ANOVA with a
surements further support the conclusion that NPR2 is the
Dunnett multiple comparisons post-test.
primary guanylyl cyclase that produces cGMP in the follicle.
Measurement of CNP in ovaries
LH signaling reduces NPR2 activity in the follicle
CNP in ovaries was assayed by an ELISA method (FEK-012-03,
One way that LH activation of its receptors in the mural
Phoenix Pharmaceuticals Inc., Burlingame, CA) with a primary
granulosa cells could decrease cGMP levels within the follicle is
antibody made against the 22 amino acid form of CNP. This
by reducing NPR2 activity. Two aspects of this question were
antibody also recognizes the 53 amino acid form of CNP, and
considered: (1) whether LH signaling decreases NPR2 activity in
presumably the precursor forms, which include the same 22
the follicle as a whole, of which most of the volume is mural
amino acids at their C-termini (;
granulosa cells, and (2) whether LH signaling decreases NPR2
activity in the cumulus cells. A decrease in NPR2 activity in either
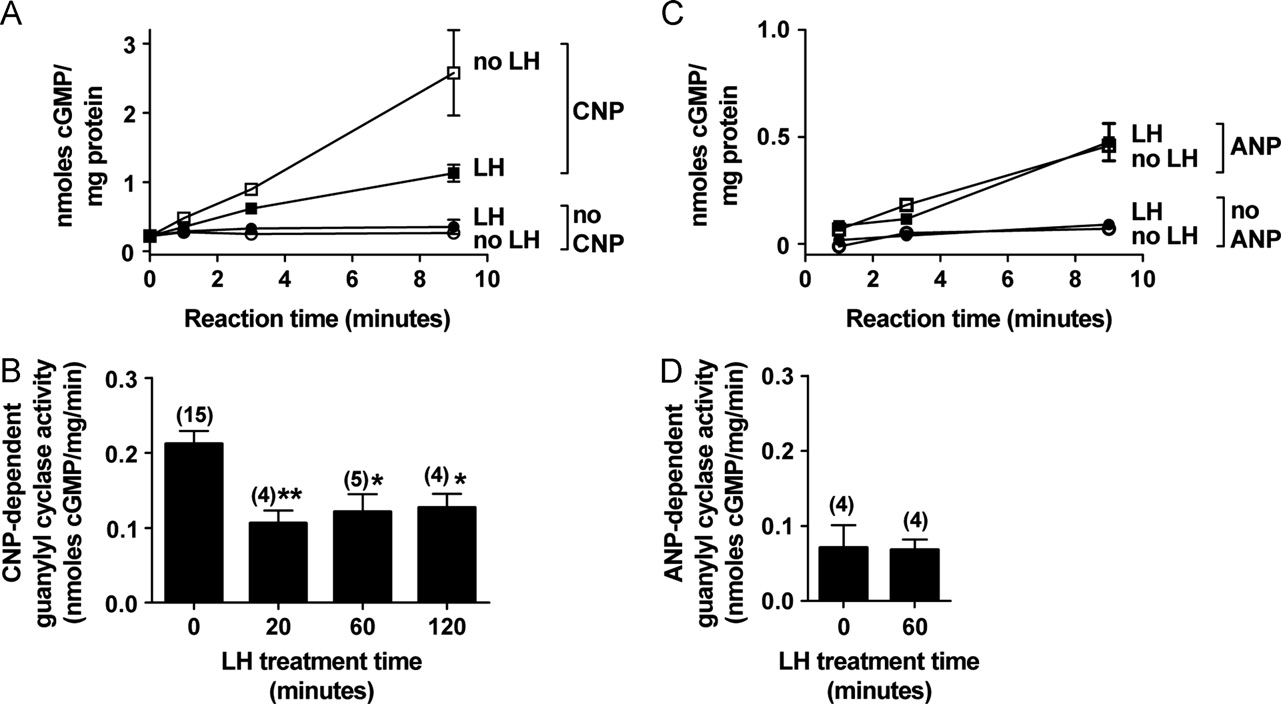
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
or both of these regions could contribute to the measured
activity was 0.0770.03 nmole cGMP/mg protein/minute, or 33% of
decrease in cGMP in the oocyte. This section describes our studies
the CNP-dependent activity (n¼4). However, the ANP-dependent
of a crude membrane fraction from whole follicles, and a sub-
activity was unchanged by LH (C) and (D)). Some of the ANP-
sequent section describes our studies of cumulus cells.
dependent guanylyl cyclase activity that we measured might be due
Guanylyl cyclase activity was measured using the particulate
to NPR1 expressed in membranes from theca cells and blood vessels
fraction obtained by centrifuging a homogenate of follicles. When
that were not completely removed from the follicle by microdissec-
this crude membrane fraction was incubated without CNP,
tion A)). The lack of effect of LH on ANP-dependent cGMP
guanylyl cyclase activity was too low to measure accurately, but
accumulation serves as a control to indicate that the LH-induced
addition of 1 mM CNP increased the activity to 0.2170.02 nmole
decrease in CNP-dependent cGMP accumulation is not due to an LH
effect on phosphodiesterase activity that could have been present in
and (B)). After a 20 min exposure of follicles to LH,
the crude membrane fraction despite the presence of IBMX.
CNP-dependent guanylyl cyclase activity fell to 50% of the activitymeasured in the membrane fraction from follicles without LHexposure, and remained depressed for 2 h after applying LH
The LH-induced decrease in NPR2 activity in the follicle occurs
(A) and (B)).
without a corresponding decrease in NPR2 protein
The decrease in follicle cGMP that will result from a 50% decrease
in NPR2 activity depends on the cGMP affinity of the phosphodies-
Previous studies have shown that other biological factors that
terases present in the granulosa cells ). If the affinity is higher
rapidly decrease NPR2 activity in cultured cells do so in a manner
(lower Km), the cGMP concentration will fall to a lower level. Much
that is independent of NPR2 protein levels (;
of the cGMP phosphodiesterase activity in the follicle is sensitive to
). To test if LH decreased the amount of
sildenafil and tadalafil, indicating an important PDE5 component
NPR2 protein in follicles, we first tried Western blotting, and
Based on Km values for PDE5, a 50% reduction
immunoprecipitation followed by Western blotting. However, with
in NPR2 activity could potentially account for the decrease in follicle
the available antibodies, it was not possible to detect endogenous
cGMP from 3 mM before LH treatment to � 0.5 mM after 20 min
levels of the protein using these methods. So instead, we measured
guanylyl cyclase activity in follicle membrane fractions after treat-
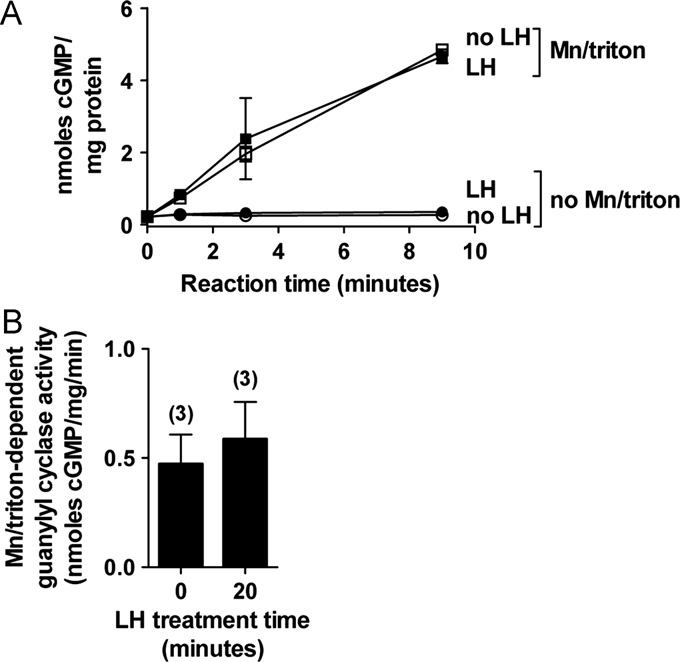
Because a small amount of Npr1 mRNA is also expressed in
ment with 1% Triton X-100 and 5 mM MnCl2, a condition known to
granulosa cells we also evaluated the effect of LH on
maximally activate NPR1 and NPR2 in the absence of natriuretic
NPR1 activity, by measuring guanylyl cyclase activity in the pre-
peptide and to be indicative of guanylyl cyclase protein levels
sence of 1 mM atrial natriuretic peptide (ANP). Studies of human
(; ). Guanylyl cyclase
NPR1 and NPR2 have shown that 1 mM ANP activates NPR1, but has
activity measured in the presence of Triton X-100 and MnCl2 is
almost no effect on NPR2 ). In the crude
independent of modification of the NPR2 protein by phosphoryla-
membrane fraction from follicles, ANP-dependent guanylyl cyclase
Fig. 2. LH signaling reduces NPR2 activity in the follicle. (A) Guanylyl cyclase activity of crude membrane fractions prepared from follicles treated with or without LH for20 min was measured with or without 1 mM CNP. Values indicate the mean7range of duplicate measurements for each condition, using one follicle preparation madeafter LH treatment, and another preparation made in parallel but without LH treatment. Where not visible, the error bars are contained within the symbol. (B) Combineddata from 15 experiments like that in A, showing CNP-dependent guanylyl cyclase activity of crude membrane fractions from follicles treated with LH for the indicatedtimes. CNP-dependent activity values were determined by subtracting the basal values measured in the absence of CNP. Activities are expressed as the mean 7s.e.m. for nfollicle preparations. Activities for follicles treated with LH for 20, 60, or 120 min differed significantly from the activity for follicles without LH treatment (np o0.05;nn, po0.01). (C) and (D) Guanylyl cyclase activity of crude membrane fractions prepared from follicles treated with or without LH for 60 min was measured with orwithout 1 mM ANP. Data are presented as described for A and B. LH did not decrease the ANP-dependent guanylyl cyclase activity.
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
measured their cGMP content. hCG is often used instead of LH,since both hormones act on the same receptor. 30 nM CNP wasused because this is approximately the minimum concentrationneeded to prevent spontaneous meiotic resumption in isolatedcumulus-oocyte complexes (). Under theseexperimental conditions, measurements of a change in cellularcGMP content in response to LH receptor stimulation couldindicate a change in guanylyl cyclase activity, or a change incGMP phosphodiesterase activity, or a change in cGMP efflux.
However, by measuring the effect of LH receptor stimulation oncGMP content in the presence and absence of CNP, we were ableto distinguish between these possibilities.
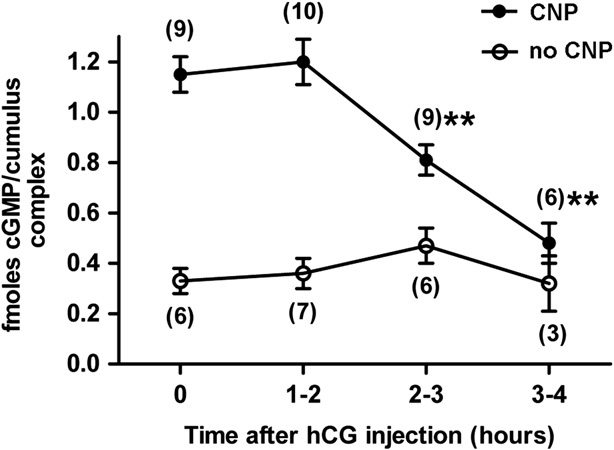
Without injection of the mice with hCG, addition of 30 nM CNP
to cumulus-oocyte complexes elevated the cGMP content of thecumulus cells by 4.1 70.9 times (n¼6). When cumulus-oocytecomplexes were isolated from mice at 1 h after hormone injec-tion, and then incubated in the presence of CNP for an additionalhour, the cGMP content of the cumulus cells was the same as thatin cumulus cells from mice without hormone injection However, when the cumulus-oocyte complexes were isolated at2 h after hormone injection, and incubated with CNP until 3 h,cGMP had decreased to 70% of values obtained without hormonetreatment ). With isolation of the complexes at 3 h,followed by a CNP incubation and measurement at 4 h, cGMP
Fig. 3. The LH-induced decrease in NPR2 activity occurs without a corresponding
had decreased to 42%
decrease in NPR2 protein. (A) Guanylyl cyclase activity of crude membrane
In the absence of CNP, the cGMP content of the cumulus cells
fractions prepared from follicles treated with or without LH for 20 min wasmeasured with or without 1% Triton X-100 and 5 mM MnCl2, to maximally
was low, as expected for this in vitro condition in which NPR2
activate guanylyl cyclase. Values indicate the mean 7range of duplicate measure-
would not be activated. Under this condition, the cGMP content
ments for each condition, using one follicle preparation made after LH treatment,
was not decreased by LH receptor stimulation ), indicating
and another preparation made in parallel but without LH treatment. (B) Combined
that LH receptor signaling does not increase cGMP phosphodies-
data from 3 experiments like that in A, showing Mn/triton-dependent guanylylcyclase activity of crude membrane fractions from follicles treated with or without
terase activity in these cells, or cause an increase in cGMP efflux.
LH for 20 min (mean 7s.e.m.). LH did not decrease the Mn/triton-dependent
Thus the LH receptor-induced decrease in cumulus cell cGMP
guanylyl cyclase activity.
seen in the presence of CNP can be attributed to a decrease incGMP production. These findings indicate that LH signalingdecreases NPR2 activity in the cumulus cells, but only after 2–
Detergent-dependent guanylyl cyclase activity was the same
3 h, versus 20 min in the mural cells. This delay is likely to be a
in samples from follicles with or without LH treatment for 20 min
consequence of the localization of the LH receptors in separate
(Since NPR1 and NPR2 are the only detectable membrane
cells (the mural granulosa). As will be discussed below, this
guanylyl cyclases in granulosa cells, and since NPR1 is a relatively
intercellular signaling is most likely mediated by the release of
minor component, these detergent measurements indicate that at
EGF-like growth factors from the mural granulosa cells.
20 min, LH does not decrease the amount of NPR2 protein. A
The decrease in cGMP production in the cumulus cells could
possible cause of the rapid LH-induced decrease in NPR2 activity
result from a decrease in the amount of NPR2 protein, or from a
is dephosphorylation, which can result from elevation of intra-cellular Ca2 þ and/or activation of protein kinase C ;
In the cumulus cells, CNP-dependent cGMP production decreases inresponse to LH receptor stimulation, but more slowly than in themural granulosa cells
Because of the direct connection between the cumulus cells and
the oocyte, and because of the higher level of Npr2 mRNA in thecumulus cells compared with the mural cells itwas of particular interest to investigate whether LH signals that areinitiated in the mural granulosa cells regulate NPR2 activity in thecumulus cells. As described above, LH receptors are not present inthe cumulus cells, so such regulation would have to involvesignaling between different regions of the follicle.
Due to the small amount of protein that could be obtained, we
could not analyze guanylyl cyclase activity in a cumulus cell
Fig. 4. In the cumulus cells, CNP-dependent cGMP production decreases in
membrane fraction as we did for the more abundant material
response to LH receptor stimulation, but more slowly than in the mural granulosa
from whole follicles. Instead, we isolated cumulus-oocyte com-
cells. Cumulus-oocyte complexes were collected at various times after hCG
plexes from ovaries at various times after injection of mice with
injection into mice and incubated for one hour with or without 30 nM CNP. Thecumulus cells were separated and cGMP content was measured. Values indicate
human chorionic gonadotropin (hCG) to stimulate the LH recep-
the mean 7s.e.m. for the number of experiments shown in parentheses. Values for
tor, incubated the complexes in the presence or absence of 30 nM
2–3 and 3–4 h post hCG were significantly different from the no hCG value
CNP for an additional hour, then isolated the cumulus cells and
(nnp o0.01).
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
modification of the NPR2 protein such as dephosphorylation. In
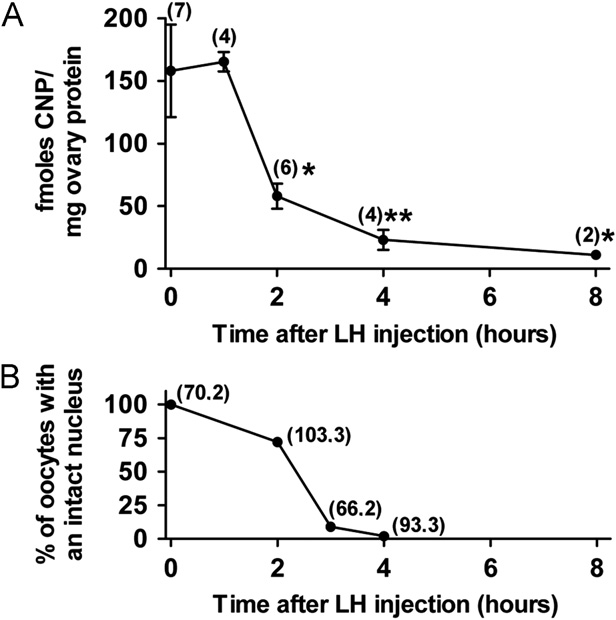
37% (A)). The decrease in CNP at 2 h corresponds to the time
support of the first possibility, the amount of Npr2 mRNA in the
at which nuclear envelope breakdown in the oocyte is beginning,
cumulus cells decreases to �50% of the basal level at 3 h after LH
as determined from histological sections of ovaries of similarly
receptor stimulation (However, it is unknown
treated mice ((B)). Thus, the CNP decrease occurs well after
how rapidly NPR2 protein would decrease as a consequence.
cGMP decreases in the follicle (detected at 20 min after LH,
Protein turnover rates for NPR2 have not been investigated, but
), but early enough to potentially contribute to
turnover of the closely related protein NPR1 in cultured cells is a
stimulating nuclear envelope breakdown. After nuclear envelope
slow process, with a half-time of Z8 h
breakdown, CNP continued to decrease, reaching 15% at 4 h afterLH, and 7% at 8 h
The amount of CNP in the ovary decreases in response to LH,
A likely cause of the CNP decrease is that Nppc mRNA
preceding nuclear envelope breakdown
decreases to about half of the basal level by 2 h after LH receptorstimulation Thus LH signaling might
Another factor that could contribute to the LH-induced reduction
reduce Nppc mRNA synthesis or increase its degradation. Because
in cGMP levels in the follicle is a decrease in CNP. CNP decreases
the turnover of CNP is very rapid, with a half-life of about 3 min in
have been reported previously for rat, mouse, and human
plasma a decrease in Nppc mRNA could rapidly
, but the earliest of
decrease the amount of CNP. Other possible factors that could
these measurements were made at 4 h after LH receptor stimula-
contribute to the decrease in CNP are an increase in the natriure-
tion, so it was unclear if the CNP decrease occurred early enough to
tic peptide clearance receptor NPR3, and an increase in the
contribute to causing nuclear envelope breakdown and the events
activity of proteases that degrade CNP
that precede it, vs later events leading to ovulation. To investigatethe time course of the decrease in CNP, we injected mice with LH,and at various times afterwards, collected their ovaries for analysis
The amount of CNP in the ovary increases as follicles develop to the
of CNP content using an ELISA based on an antibody that should
preovulatory stage
recognize all forms of CNP and its precursor NPPC.
Without LH injection, there were �150 fmoles of CNP per mg of
We also examined the effect of equine chorionic gonadotropin
ovary extract protein ), corresponding to an overall con-
(eCG, also called PMSG) on CNP levels. Unlike human chorionic
centration of � 10 nM. However, the immunoreactive material
gonadotropin (hCG), which binds to the LH receptor, eCG binds to
detected by the ELISA contains both extracellular peptide and
the follicle stimulating hormone receptor, which stimulates antral
intracellular precursor protein, and only the peptide that has been
follicle growth and LH receptor expression. eCG is often used
secreted into the extracellular space can activate NPR2. Thus the
experimentally to cause follicles to grow and to progress to the
concentration of peptide that could function to regulate NPR2 is
preovulatory stage; it was used for this purpose for the CNP
measurements described above.
No change from the pre-LH level of CNP was seen at 1 h after
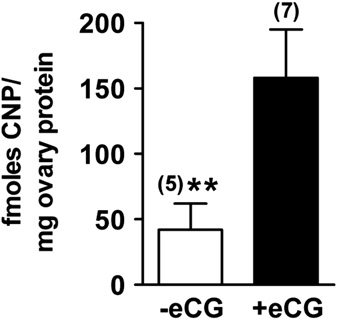
We found that eCG injection of the mice, 44 h before collecting
injection of LH, but by 2 h, the amount of CNP had decreased to
the ovaries, increased their CNP content ). The increase in CNPin response to eCG is consistent with findings that mRNA encodingthe CNP precursor (NPPC) increases in mouse ovaries in response toeCG ), and that CNP and cGMP increasebetween the days of diestrus and proestrus, in rats and hamsters(). Thus ourfindings add to the accumulating evidence that during folliclegrowth to the preovulatory stage, CNP and cGMP content of theovary increases. At the preantral stage, cyclic nucleotide regulationis not needed to maintain meiotic arrest ). Then with follicle growth, as the oocyte accumulates moreCDK1 and other factors that result in meiotic competence (
Fig. 5. The amount of CNP in the ovary decreases in response to LH, precedingnuclear envelope breakdown. (A) Time course of the decrease in the CNP content
Fig. 6. The amount of CNP in the ovary increases in response to activation of
of ovary extracts, following LH injection into mice. Values indicate the mean 7
follicle stimulating hormone receptors. The mice to be used for the CNP
s.e.m. for the number of mice shown in parentheses. Values for 2, 4, and 8 h LH
measurements shown in (A) were injected 44 h previously with eCG to
treatments were significantly different from the no LH value (np o0.05;
stimulate follicle growth. In response to eCG, the amount of CNP per mg of ovary
nnpo0.01). (B) Time course of nuclear envelope breakdown, following LH injection
protein increased � 4 times. The þ eCG data shown in are the same as the no
into mice. Values indicate the percentage of fully grown follicles ( Z350 mm in
LH (0 h) data shown in (A), so the statistical significance of these data was
diameter) in which the oocyte contained a prophase-arrested nucleus; the number
tested together. Values for ovaries from mice with or without eCG treatment were
of follicles and the number of mice counted are indicated.
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
, CNP is synthesized in order to prevent premature
elevation and protein kinase C activation can lead to dephosphor-
ylation and inactivation of NPR2 (). Calciumelevation could also increase the activity of the PDE1 family of cGMPphosphodiesterases ).
Pathways by which LH signaling in the mural granulosa cells causes
Since the mRNA encoding the precursor protein of CNP is
cGMP to decrease in the oocyte
expressed in the same cells as the LH receptor exposure to LH could result in a reduction in the amount of CNP
The connections between LH-induced activation of G-proteins
by signaling within the same cells. Likewise, since the LH receptor
in the outer layers of the mural granulosa cells and the ensuing
is expressed in the outer several layers of the mural granulosa
events in the follicle that lead to the decrease in cGMP and
cells the LH-induced decrease in NPR2
resumption of meiosis in the oocyte are only partially understood
activity in these cells could result from signaling within the same
cell. However, the LH-induced decrease in NPR2 activity in the
These connections include not only the path-
cumulus cells, or in the inner layers of the mural cell epithelium,
ways leading to a decrease in granulosa cell guanylyl cyclase
both of which regions lack LH receptors (
activity, but also pathways that reduce gap junction permeability
), must involve signaling between cells.
through MAP kinase-dependent phosphorylation of connexin 43
Based on evidence that the cGMP decrease in the follicle is
partially dependent on EGF receptor signaling
diffusion of cGMP into the oocyte. Both the decrease in gap
junction permeability and the decrease in granulosa cell guanylyl
growth factors released from the outer layers of mural granulosa
cyclase activity contribute to the decrease in oocyte cGMP.
cells in response to LH are mediators of the paracrine signals, and
At the level of the mural granulosa cells, LH receptor signaling
could also contribute to autocrine signaling in the outer layers of the
activates Gs and adenylyl cyclase
mural granulosa cells. EGF receptor signaling is essential for LH-
thus elevating cAMP. LH receptor signaling also activates Gi, Gq, and
induced nuclear envelope breakdown (), and EGF
phospholipase Cb ; ¨
receptor activation, as indicated by increased phosphorylation of the
thus elevating calcium (via IP3) in granulosa
receptor protein, occurs as early as 30 min after LH treatment
cells in culture ; ). However,
). However, it remains unknown how LH
activation of protein kinase C by the diacylglycerol that is generated
receptor signaling triggers the synthesis and/or release of the EGF-
by phospholipase C has, to our knowledge, not been detected so far
like growth factors epiregulin and amphiregulin. RNA encoding
. Further studies of these signaling events
precursors of these growth factors increases by 2 h after LH receptor
using intact follicles will be informative, because both calcium
stimulation but in addition, LH signaling mightactivate the proteases that release epiregulin and amphiregulin frompre-existing precursors
EGF receptor signaling is required for much of the increase in
MAP kinase activity in response to LH (andthus contributes to phosphorylation of connexin 43 and theresulting decrease in gap junction permeability (; ). EGF receptor signaling also activatesphospholipase Cg ), and could thuselevate calcium and protein kinase C activity, amplifying the LHreceptor signaling that may occur through through phospholipaseCb. As discussed above, these signaling events could decreaseNPR2 activity, and possibly increase PDE1 activity, thus loweringcGMP in the granulosa cells and oocyte.
By 20 min after applying LH to ovarian follicles, the guanylyl
cyclase activity of NPR2 elicited by a saturating concentration ofCNP is decreased by half. This correlates with a similarly rapiddecrease in follicle cGMP. There is then a slower decrease in NPR2responsiveness to CNP in the cumulus cells, first seen at 2–3 h. By2 h, LH signaling also induces a decrease in the amount of CNP inthe ovary. Together, these 3 factors that decrease guanylyl cyclaseactivity contribute to the decrease in cGMP in the follicle. Becausethe mural granulosa cells, cumulus cells, and oocyte are con-nected by gap junctions to form a syncitium with respect tocGMP, cGMP in the oocyte equilibrates with that in the surround-ing somatic cell compartment, and the resulting decrease inoocyte cGMP promotes meiotic resumption.
Fig. 7. Signaling pathways connecting LH binding to its receptors in the outerlayers of the mural granulosa cells to resumption of meiosis in a mammalian
We thank Tracy Uliasz, Amber Selko, and Marilyn O'Brien for
oocyte. The green box indicates the findings of this study in the context of otheraspects of the signaling network.
technical assistance, William Ratzan, Lisa Mehlmann, Jim Watras,
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
and Deborah Dickey for their generous help, Viacheslav Nikolaev,
Oogenesis: The Universal Process. John Wiley & Sons Ltd., Chichester, UK,
Mark Terasaki, Michaela Kuhn, Dieter M ¨uller, Jolanta Gutkowska,
pp. 181–197.
Jankowski, M., Reis, A.M., Mukaddam-Daher, S., Dam, T.-V., Farookhi, R., Gut-
Matthew Movsesian, Claire Lugnier, Marina Freudzon, and Melina
kowska, J., 1997. C-type natriuretic peptide and the guanylyl cyclase receptors
Schuh for stimulating discussions, and Joseph Burleson for statis-
in the rat ovary are modulated by the estrous cycle. Biol. Reprod. 56,
tical advice. This work was supported by grants R01 HD014939 to
L.A.J. and U01 HD21970 to J.J.E., a grant from the National Basic
Kawamura, K., Cheng, Y., Kawamura, N., Takae, S., Okada, A., Kawagoe, Y., Mulders,
S., Terada, Y., Hsueh, A.J.W., 2011. Pre-ovulatory LH/hCG surge decreases
Research Program of China (2012CB944401) to M.Z., a grant from
C-type natriuretic peptide secretion by ovarian granulosa cells to promote
the University of Minnesota Graduate School (21,922) to L.R.P.,
meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 26, 3094–3101.
and NIH training grant T32AR050938 to J.W.R.
Killock, D.J., Ivetic, A., 2010. The cytoplasmic domains of TNFa-converting enzyme
(TACE/ADAM17) and L-selectin are regulated differently by p38 MAPK andPKC to promote ectodomain shedding. Biochem. J. 428, 293–304.
K ¨uhn, B., Gudermann, T., 1999. The luteinizing hormone receptor activates
Gi2. Biochemistry 38,
Appendix A. supplementary material
Mehlmann, L.M., Saeki, Y., Tanaka, S., Brennan, T.J., Evsikov, A.V., Pendola, F.L.,
Knowles, B.B., Eppig, J.J., Jaffe, L.A., 2004. The G
Supplementary data associated with this article can be found
maintains meiotic arrest in mammalian oocytes. Science 306, 1947–1950.
in the online version at
Mock, E.J., Niswender, G.D., 1983. Differences in the rates of internalization of 125I-
labeled human chorionic gonadotropin, luteinizing hormone, and epidermalgrowth factor by ovine luteal cells. Endocrinology 113, 259–264.
Norris, R.P., Freudzon, M., Mehlmann, L.M., Cowan, A.E., Simon, A.M., Paul, D.L.,
Lampe, P.D., Jaffe, L.A., 2008. Luteinizing hormone causes MAPK-dependent
phosphorylation and closure of Cx43 gap junctions in mouse ovarian follicles:one of two paths to meiotic resumption. Development 135, 3229–3238.
Norris, R.P., Ratzan, W.J., Freudzon, M., Mehlmann, L.M., Krall, J., Movsesian, M.A.,
Abbey, S.E., Potter, L.R., 2003. Lysophosphatidic acid inhibits C-type natriuretic
Wang, H., Ke, H., Nikolaev, V.O., Jaffe, L.A., 2009. Cyclic GMP from the
peptide activation of guanylyl cyclase-B. Endocrinology 144, 240–246.
surrounding somatic cells regulates cyclic AMP and meiosis in the mouse
Abbey-Hosch, S.E., Cody, A.N., Potter, L.R., 2004. Sphingosine-1-phosphate inhibits
oocyte. Development 136, 1869–1878.
C-type natriuretic peptide activation of guanylyl cyclase B (GC-B/NPR-B).
Norris, R.P., Freudzon, M., Nikolaev, V.O., Jaffe, L.A., 2010. Epidermal growth factor
Hypertension 43, 1103–1109.
receptor kinase activity is required for gap junction closure and for part of the
Abbey-Hosch, S.E., Smirnov, D., Potter, L.R., 2005. Differential regulation of NPR-B/
decrease in ovarian follicle cGMP in response to LH. Reproduction 140,
GC-B by protein kinase C and calcium. Biochem. Pharmacol. 70, 686–694.
Amsterdam, A., Koch, Y., Lieberman, M.E., Lindner, H.R., 1975. Distribution of
Panigone, S., Hsieh, M., Fu, M., Persani, L., Conti, M., 2008. Luteinizing hormone
binding sites for human chorionic gonadotropin in the preovulatory follicle of
signaling in preovulatory follicles involves early activation of the epidermal
the rat. J. Cell. Biol. 67, 894–900.
growth factor receptor pathway. Mol. Endocrinol. 22, 924–936.
Blobel, C.P., Carpenter, G., Freeman, M., 2009. The role of protease activity in ErbB
Park, J.Y., Su, Y.Q., Ariga, M., Law, E., Jin, S.L., Conti, M., 2004. EGF-like growth
biology. Exp. Cell. Res. 315, 671–682.
factors as mediators of LH action in the ovulatory follicle. Science 303,
Chattopadhyay, A., Vecchi, M., Ji, Q.-S., Mernaugh, R., Carpenter, G., 1999. The role
of individual SH2 domains in mediating association of phospholipase C-g1
Potter, L.R., 2011a. Guanylyl cyclase structure, function and regulation. Cell.
with the activated EGF receptor. J. Biol. Chem. 274, 26091–26097.
Signalling 23, 1921–1926.
Chesnel, F., Eppig, J.J., 1995. Synthesis and accumulation of p34cdc2 and cyclin B in
Potter, L.R., 2011b. Regulation and therapeutic targeting of peptide-activated
mouse oocytes during acquisition of competence to resume meiosis. Mol.
receptor guanylyl cyclases. Pharmacol. Ther. 130, 71–82.
Reprod. Dev. 40, 503–508.
Potter, L.R., 2011c. Natriuretic peptide metabolism, clearance and degradation.
Conti, M., Hsieh, M., Zamah, A.M., Oh, J.S., 2012. Novel signaling mechanisms in the
FEBS J. 278, 1808–1817.
ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 356,
Potter, L.R., Hunter, T., 1998. Identification and characterization of the major
phosphorylation sites of the B-type natriuretic peptide receptor. J. Biol. Chem.
Davis, J.S., Weakland, L.L., West, L.A., Farese, R.V., 1986. Lutenizing hormone
273, 15533–15539.
stimulates the formation of inositol trisphosphate and cyclic AMP in rat
Potter, L.R., Hunter, T., 1999. A constitutively ‘‘phosphorylated'' guanylyl cyclase-
granulosa cells. Biochem. J. 238, 597–604.
linked atrial natriuretic peptide receptor mutant is resistant to desensitization.
Dickey, D.M., Flora, D.R., Bryan, P.M., Xu, X., Chen, Y., Potter, L.R., 2007. Differential
Mol. Biol. Cell. 10, 1811–1820.
regulation of membrane guanylyl cyclases in congestive heart failure:
Potter, L.R., Yoder, A.R., Flora, D.R., Antos, L.K., Dickey, D.M., 2009. Natriuretic
natriuretic peptide receptor (NPR)-B, not NPR-A, is the predominant natriure-
peptides: their structures, receptors, physiologic functions and therapeutic
tic peptide receptor in the failing heart. Endocrinology 148, 3518–3522.
applications. Handb. Exp. Pharmacol. 191, 341–366.
Dickey, D.M., Burnett, J.C., Potter, L.R., 2008. Novel bifunctional natriuretic
Racowsky, C., Baldwin, K.V., 1989. In vitro and in vivo studies reveal that hamster
peptides as potential therapeutics. J. Biol. Chem. 283, 35003–35009.
oocyte meiotic arrest is maintained only transiently by follicular fluid, but
Eppig, J.J., Wigglesworth, K., Pendola, F., Hirao, Y., 1997. Murine oocytes suppress
persistently by membrana/cumulus granulosa cell contact. Dev. Biol. 13,
expression of luteinizing hormone receptor messenger ribonucleic acid by
granulosa cells. Biol. Reprod. 56, 976–984.
Rajagopalan-Gupta, R.M., Lamm, M.L.G., Mukherjee, S., Rasenick, M.M., Hunzicker-
Erickson, G.F., Sorensen, R.A., 1974. In vitro maturation of mouse oocytes isolated
from late, middle, and pre-antral Graafian follicles. J. Exp. Zool. 190, 123–127.
Dunn, M., 1998. Luteinizing hormone/choriogonadotropin receptor-mediated
Flora, D.R., Potter, L.R., 2010. Prolonged atrial natriuretic peptide exposure
activation of heterotrimeric guanine nucleotide binding proteins in ovarian
stimulates guanylyl cyclase-a degradation. Endocrinology 151, 2769–2776.
follicular membranes. Endocrinology 139, 4547–4555.
Flores, J.A., Aguirre, C., Sharma, O.P., Veldhuis, J.D., 1998. Luteinizing hormone (LH)
Robinson, J.W., Potter, L.R., 2011. ATP potentiates competitive inhibition of
stimulates both intracellular calcium ion ([Ca2 þ ]
guanylyl cyclase A and B by the staurosporine anoalog, Go6976: reciprocal
i) mobilization and trans-
membrane cation influx in single ovarian (granulosa) cells: recruitment as a
regulation of ATP and GTP binding. J. Biol. Chem. 286, 33841–33844.
cellular mechanism of LH-[Ca2þ ]
Salvador, L.M., Maizels, E., Hales, D.B., Miyamoto, E., Yamamoto, H., Hunzicker-
dose response. Endocrinology 139,
Dunn, M., 2002. Acute signaling by the LH receptor is independent of protein
Francis, S.H., Blount, M.A., Corbin, J.D., 2011. Mammalian cyclic nucleotide
kinase C activation. Endocrinology 143, 2986–2994.
phosphodiesterases: molecular mechanisms and physiological functions. Phy-
Schuh, M., Ellenberg, J., 2007. Self-organization of MTOC's replaces centrosome
siol. Rev. 91, 651–690.
function during acentrosomal spindle assembly in live mouse oocytes. Cell
Hsieh, M., Thao, K., Conti, M., 2011. Genetic dissection of epidermal growth factor
130, 484–498.
receptor signaling during luteinizing hormone-induced oocyte maturation.
Sela-Abramovich, S., Chorev, E., Galiani, D., Dekel, N., 2005. Mitogen-activated
PLoS ONE 6, e21574.
protein kinase mediates luteinizing hormone-induced breakdown of commu-
Hubbard, C.J., 1986. Cyclic AMP changes in the component cells of Graafian
nication and oocyte maturation in rat ovarian follicles. Endocrinology 146,
follicles: possible influences on maturation in the follicle-enclosed oocytes of
hamsters. Dev. Biol. 118, 343–351.
Sela-Abramovich, S., Galiani, D., Nevo, N., Dekel, N., 2008. Inhibition of rat oocyte
Hubbard, C.J., Greenwald, G.S., 1982. Cyclic nucleotides, DNA, and steroid levels in
maturation and ovulation by nitric oxide: mechanism of action. Biol. Reprod.
ovarian follicles and corpora lutea of the cyclic hamster. Biol. Reprod. 26,
78, 1111–1118.
Solc, P., Schultz, R.M., Motlik, J., 2010. Prophase I arrest and progression to
Hunt, P.J., Richards, A.M., Espiner, E.A., Nicholls, M.G., Yandle, T.G., 1994. Bioactiv-
metaphase I in mouse oocytes: comparison of resumption of meiosis and
ity and metabolism of C-type natriuretic peptide in normal man. J. Clin.
recovery from G2-arrest in somatic cells. Mol. Human Reprod 16, 654–664.
Endocrinol. Metab. 78, 1428–1435.
Sriraman, V., Rudd, M.D., Lohmann, S.M., Mulders, S.M., Richards, J.S., 2006. Cyclic
Jaffe, L.A., Norris, R.P., 2010. Initiation of the meiotic prophase-to-metaphase
guanosine 50-monophosphate-dependent protein kinase II is induced by
transition in mammalian oocytes. In: Verlhac, M.-H., Villeneuve, A. (Eds.),
luteinizing hormone and progesterone receptor-dependent mechanisms in
J.W. Robinson et al. / Developmental Biology 366 (2012) 308–316
granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol.
Zhang, M., Su, Y.-Q., Sugiura, K., Xia, G., Eppig, J.J., 2010. Granulosa cell ligand NPPC
Endocrinol. 20, 348–361.
and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330,
T ¨ornell, J., Carlsson, B., Billig, H., 1990. Atrial natriuretic peptide inhibits sponta-
neous rat oocyte maturation. Endocrinology 126, 1504–1508.
Zhang, M., Su, Y.-Q., Sugiura, K., Wigglesworth, K., Xia, G., Eppig, J.J., 2011. Estradiol
Vaccari, S., Weeks, J.L., Hsieh, M., Menniti, F.S., Conti, M., 2009. Cyclic GMP
promotes and maintains cumulus cell expression of natriuretic peptide
signaling is involved in the LH-dependent meiotic maturation of mouse
receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology
oocytes. Biol. Reprod. 81, 595–604.
152, 4377–4385.
Wu, C., Wu, F., Pan, J., Morser, J., Wu, Q., 2003. Furin-mediated processing of pro-C-
type natriuretic peptide. J. Biol. Chem. 278, 25847–25852.
Source: http://cell.uchc.edu/pdf/jaffe/robinson_2012.pdf
UNIVERSITE DE OUAGADOUGOU UNITE DE FORMATION ET DE RECHERCHE EN SCIENCES HUMAINES (UFR/SH DEPARTEMENT DE SOCIOLOGIE MEMOIRE DE MAITRISE LA COMMUNAUTE FACE AUX RISQUES DE COMPLICATIONS LIEES A LA MATERNITE : DISCOURS ET PRATIQUES. CAS DE KOMSILGA ET RAKAYE
A Comparative study of the Anticonvulsant effect of Nimodipine andKetamine combination with standardanticonvulsant drug in Rodents Prasanand S1, Pushpalatha C2, Mohsin MD3, Sam Pavan Kumar G4, Gundappa Rao S5 Aim of the study: To evaluate and compare the anticonvulsant property of nimodipine andketamine combination with a standard drug like Sodium valproate in electrically and chemically















