Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the epic clinical trial and observational study,


Contemporary Clinical Trials 30 (2009) 256–268
Contents lists available at
Contemporary Clinical Trials
Early anti-pseudomonal acquisition in young patients with cystic fibrosis:Rationale and design of the EPIC clinical trial and observational study
Miriam M. Treggiari Margaret Rosenfeld Nicole Mayer-Hamblett , George Retsch-Bogart Ronald L. Gibson Judy Williams Julia Emerson Richard A . Kronmal Bonnie W. Ramsey
a Department of Anesthesiology and Pain Medicine, University of Washington School of Medicine, Seattle, WA, USAb Cystic Fibrosis Therapeutics Development Network, Cystic Fibrosis Foundation, Seattle, WA, USAc Department of Pediatrics, University of Washington, Children's Hospital and Medical Regional Center, Seattle, WA, USAd Department of Pediatrics, University of North Carolina, Chapel Hill, NC, USAe Department of Biostatistics, School of Public Health and Community Medicine, University of Washington, Seattle, WA, USA
Background: The primary cause of morbidity and mortality in patients with cystic fibrosis (CF)
Received 7 August 2008
is progressive obstructive pulmonary disease due to chronic endobronchial infection,
Accepted 6 January 2009
particularly with Pseudomonas aeruginosa (Pa). Risk factors for and clinical impact of early Painfection in young CF patients are less well understood.
Purpose: The present studies are designed to evaluate risk factors and outcomes associated with
early Pa acquisition, and the benefits and harms of four anti-pseudomonal treatment regimens
Inhaled tobramycin
in young CF patients initiated after the first Pa positive respiratory culture.
Methods: The Early Pseudomonas Infection Control (EPIC) program consists of two studies, a
Pseudomonas aeruginosa
randomized multicenter trial in CF patients ages 1–12 years at first isolation of Pa from a respiratory
culture, and a longitudinal cohort study enrolling Pa-negative patients. Using a factorial design, trial
participants are assigned for 18 months to either anti-pseudomonal treatment on a scheduledquarterly basis (cycled therapy) or based on recovery of Pa from quarterly respiratory cultures(culture-based therapy). The study drugs include inhaled tobramycin (300 mg BID) for 28 days,combined with either oral ciprofloxacin (15–20 mg/kg BID) or oral placebo for 14 days. The primaryendpoints of the trial are the time to pulmonary exacerbation requiring IV antibiotics orhospitalization for respiratory symptoms, and the proportion of patients with new Pa-positiverespiratory cultures during the study. The broad goals of the observational study are to describe therisk factors and outcomes associated with early acquisition of Pa. 306 patients were randomized inthe clinical trial and 1787 were enrolled in the cohort study.
Conclusions: These companion studies will provide valuable epidemiological and microbiologicalinformation on early CF lung disease and Pa acquisition, and safety and clinical efficacy data on anti-pseudomonal treatment strategies for early Pa infections in the airways of young children with CF.
2009 Elsevier Inc. All rights reserved.
☆ Financial support: The research for this article was supported in part by the Cystic Fibrosis Foundation grants number EPIC0K0 and OBSERV04K0, the National
Heart Lung and Blood Institute (NHLBI) and National Institute for Digestive Disorders and Kidney (NIDDK) grant number U01-HL080310, and the National Centerfor Research Resources (NCRR) grant number ULI-RR2501401. Study drugs and devices were supplied free of charges by Novartis Pharmaceutical Corp. (inhaledtobramycin) and Bayer Healthcare AG (oral ciprofloxacin and oral placebo), compressors and nebulizers were provided by PARI Respiratory Equipment Inc.
☆☆ ClinicalTrial.gov numbers: NCT00676169; NCT00097773.
⁎ Corresponding author. Department of Anesthesiology and Pain Medicine, Box 359724, Harborview Anesthesiology Research Center, Harborview Medical
Center — University of Washington, 325 Ninth Avenue — Seattle, WA 98104, USA. Tel.: +1 206 744 3059; fax: +1 206 744 8090.
E-mail address: (M.M. Treggiari).
1 Participating clinical sites and investigators are listed in Appendix A.
1551-7144/$ – see front matter 2009 Elsevier Inc. All rights reserved.
doi:
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
which enrolled young Pa-negative patients and clinical trialparticipants. The goal of the observational study is to
1.1. Study rationale
determine improved strategies for prevention and treatmentof early Pa infection.
Cystic fibrosis (CF), an autosomal recessive disease lacking
a curative therapy, has a current median survival of over
1.2. Study overview
36 years, and affects approximately 25,000–30,000 indivi-duals in the United States and 70,000 people worldwide .
In order to assess the clinical and microbiologic efficacy of
The primary cause of morbidity and mortality in patients with
early anti-pseudomonal therapy, and more thoroughly
CF is progressive obstructive pulmonary disease associated
address issues of safety and antimicrobial resistance, the
with chronic Pseudomonas aeruginosa (Pa) endobronchial
Early Pseudomonas Infection Control multi-center clinical trial
bacterial infection and an intense neutrophil-dominated host
(EPIC-CT) and an observational study (EPIC-OBS) target
inflammatory response . Pa, a ubiquitous environmental
children with CF younger than 12 years of age.
bacterium, is the most important pathogen in CF lung disease.
The EPIC-OBS serves both as a freestanding epidemiologic
Pa infection can begin very early in life, and the prevalence of
study of risk factors associated with early Pa airway infection,
Pa in respiratory cultures increases with age, from 10–30% at
and as an adjunct to the EPIC-CT by providing pre-study data
ages 0–5 years to 80% at ages ≥18 years . Unlike established
on risk factors potentially affecting response to the trial
Pa infection, features of early Pa infection, including suscept-
regimens and post-enrollment follow-up for the clinical trial
ibility to antibiotics, non-mucoid phenotype, and low bacterial
participants for up to five years ).
density, appear to provide a "window of opportunity" during
The EPIC-CT was designed to allow the randomized
which time anti-pseudomonal therapy may be effective in
controlled evaluation of early intervention with inhaled and
eradicating Pa Over time, the distinct microenviron-
oral anti-pseudomonal therapy in young patients with CF at
ment in the CF airways allows selection of Pa uniquely adapted
first isolation of Pa from respiratory cultures. Participants
for chronic, persistent infection. These organisms are mucoid,
meeting the eligibility criteria were offered the opportunity to
form biofilms, become increasingly antibiotic-resistant, are
be enrolled in the clinical trial and initiate or continue
present at high density, and are virtually impossible to
simultaneous participation in the observational study. The
eradicate. Chronic Pa infection is clearly associated with
clinical trial assigned children to two different antimicrobial
poorer clinical outcomes among patients with CF The
treatment strategies: (1) Cycled antibiotic therapy, i.e.,
risk factors for and clinical impact of early Pa infection are
treatment provided in quarterly cycles regardless of findings
even less understood, yet are of great import to clinicians
from respiratory cultures obtained quarterly, and (2) anti-
caring for young patients with CF. Preliminary data suggest a
biotic therapy based upon cultures, i.e., treatment based on
favorable effect of aggressive treatment at first isolation of Pa
recovery of Pa from respiratory cultures obtained at sched-
from respiratory cultures, but data from large randomized
uled quarterly intervals throughout the 18-month study
trials are lacking .
Thus, we designed a randomized trial in children with CF
To summarize, the overall objectives of the clinical trial are
to investigate the benefits and harms of aggressive, early anti-
to compare the clinical and microbiological efficacy and safety
pseudomonal interventions with the goal of delaying or
of cycled therapy versus culture-based therapy, initiated at
preventing chronic Pa infection and its clinical consequences.
the time of early Pa infection of the respiratory tract and given
Companion to this clinical trial is the first large, multicenter,
over an 18-month study period. Because a true placebo group
longitudinal observational study of early lung disease in CF,
receiving no anti-pseudomonal antibiotic therapy was not
Fig. 1. EPIC study diagram and overlap between the clinical trial and the observational study.
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
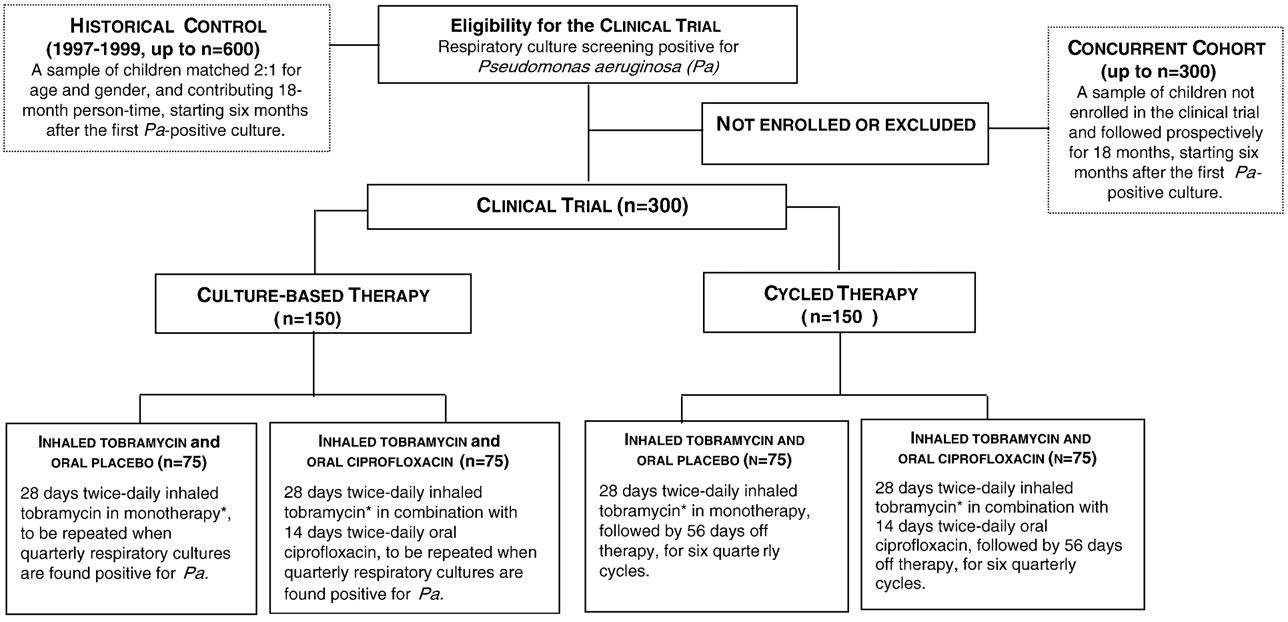
Fig. 2. Randomization assignment of participants enrolling in the clinical trial component of EPIC. ⁎Patients with culture positive for Pa at the end of 28 days ofinhaled therapy will receive a second 28-day treatment course for the first cycle only.
feasible at the time of study initiation in 2004, clinical trial
associated with isolation of Staphylococcus aureus (S. aureus)
participants will be also compared with two external controls,
from respiratory cultures, and with emergence of methicillin-
a concurrent cohort (derived from the EPIC Observational
resistant S. aureus (MRSA) will also be evaluated. DNA
study) that will allow the evaluation of the generalizability of
samples extracted from whole blood are being collected and
the clinical trial results, and a historical group that will allow
banked from participants and their parents of origin for a
the evaluation of low versus high intensity exposure to
separate planned evaluation of genetic factors that may be
antipseudomonal antibiotics. This historical group, derived
associated with CF pathogenesis, disease progression, and
from an existing patient registry and covering a period
clinical outcomes. Finally, Pa isolates and serum samples from
ranging from 1997–1999, had significantly less exposure to
observational participants are banked for future studies to
inhaled tobramycin prior to the commercial product's (TOBI)
enhance the understanding of microbiological aspects of early
FDA approval in 1998. Children in the historical group will be
CF lung disease.
matched 2:1 for age and gender to each EPIC-CT participant.
For subjects who enroll in the EPIC-CT, the observational
study provides pre-enrollment data to allow examination of
2. The observational study component
the association between risk factors prior to trial enrollment,particularly Pa serology and Pa phenotype (mucoid versus
2.1. Objectives and aims of EPIC-OBS
non-mucoid), and response to trial regimens. Moreover, theobservational study provides follow-up data on trial end-
The primary aim of EPIC-OBS is the identification of risk
points after completion of trial participation to allow
factors associated with early age at first isolation of Pa from
assessment of the long-term effects of trial regimens
respiratory cultures and with the early emergence of mucoid
(The observational study will also allow characteriza-
and antibiotic-resistant strains of Pa, in particular, modifiable
tion of patients who declined enrollment in the clinical trial to
exposures, such as environmental tobacco smoke, breastfeed-
estimate the presence of potential selection bias and to
ing and daycare. The EPIC-OBS also aims to describe the
evaluate the effect of non-protocol based antimicrobial
longitudinal changes in clinical endpoints (e.g., lung function,
approaches on the clinical, microbiology, and serology end-
growth, exacerbation frequency) associated with initial
points of interest in the clinical trial.
acquisition of Pa, as well as changes in clinical endpointsassociated with emergence of mucoid Pa and antibiotic-
2.2. Study design of EPIC-OBS
resistant Pa. Among subjects not enrolled in the clinical trial,the effect of type and length of anti-pseudomonal therapy on
The EPIC-OBS is an ongoing prospective, observational
clinical endpoints, subsequent Pa serology, Pa antimicrobial
cohort study. The size of the original cohort was 1787
resistance, Pa genotype, and emergence of other pathogens
participants at 59 CF centers. CF patients ≤12 years of age
will be examined. The longitudinal relationships between
at the time of enrollment were eligible if they had no prior
anti-pseudomonal serology, isolation of Pa from respiratory
isolation of Pa from respiratory cultures, or if prior history of
cultures, and evolving clinical signs and symptoms during
isolation of Pa from respiratory cultures, at least a two-year
early CF lung disease will be described. Clinical outcomes
period with Pa negative cultures (documented by at least 1
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
culture annually) or were concurrently enrolled in the EPIC-
on statistics from the 2000 CFF National Patient Registry
CT. The study was approved by the Institutional Review Board
and questionnaire data from prospective sites participating in
at each participating site, and all participants or their
the EPIC studies. Combining the information from both
surrogates provided written informed consent.
sources indicated that at approximately 60 sites, a sample of
The EPIC-OBS involves prospective data and specimen
about 2000 total Pa negative patients age 12 and younger was
collection (Clinical care and monitoring is not affected
available. The sample size justification was based on the
by study participation. The Cystic Fibrosis Foundation (CFF)
primary EPIC-OBS aim, i.e., to define risk factors for early age
maintains a National Patient Registry containing a wide array of
at first isolation of Pa. We hypothesized that environmental
demographic, clinical, and actuarial data on all patients seen at
tobacco smoke exposure and other modifiable exposures
CF care centers. Registry data entry is encounter-based and
would have an effect on age at first isolation of Pa at least as
submitted via a secure web site ). The EPIC-
great as that of other known risk factors evaluated in prior
OBS study is made possible by the existence of this resource:
studies, such as aerosol use, mother's education, and delta
data collection occurs at each encounter and via an annual
F508 homozygous status . The prevalence of smoking
family survey utilizing an augmented version of the CFF
among parents of children with CF ranges from 15 to 40%
Registry containing the standard Registry data collection
nationally. The minimum detectable hazard ratios, varying
forms as well as additional, study-specific forms. The EPIC-
the underlying prevalence of the risk factor from 0.2 to 0.5,
OBS annual family survey is a parent/family questionnaire on
and as a function of study power, ranged from 1.32 to 1.25 for
potential risk factors for Pa acquisition, such as environmental
a power of 0.8. These detectable hazard ratios are within the
tobacco smoke and daycare attendance. Study sites are also
range of risk estimates reported in prior studies. All calcula-
queried annually regarding issues such as infection control
tions were based on the log-rank statistic, assumed a two-
practices and use of standardized monitoring and treatment
sided .05 significance level and the occurrence of 650 events
regimens in young CF patients.
(conversions to Pa positive status during the study period)
EPIC-OBS participants have a serum sample collected
and uniform accrual of the study participants over the two
annually for evaluation of Pa serology and for banking. After
year enrollment period.
the first isolation of Pa from respiratory culture at the local
provides the projected number of patients
site laboratory, an annual respiratory specimen is sent to the
converting to Pa-positive during the study period. The
Coordinating Center Core Microbiology Laboratory for semi-
following assumptions were made to estimate duration of
quantitative culture, evaluation of Pa mucoidy and banking. If
enrollment in EPIC-OBS at the participating sites: 1. An
available, initial Pa isolates from the local site laboratory are
enrollment rate of 70% of eligible patients; 2. 15% annual rate
also shipped to the Core Microbiology Laboratory for banking.
of conversion to Pa-positive; 3. 40% of Pa positive patients
A single whole blood sample is collected from participants
would enroll in EPIC-CT; and 4. 10% of enrolled subjects would
and a whole blood sample or buccal swab is collected from
drop-out or be lost to follow-up by the end of the study. Based
parents of origin for DNA extraction and banking.
on these assumptions, enrollment into the EPIC-OBS wasneeded for the first two years of the study to achieve a sample
2.3. Sample size and power for EPIC-OBS
of approximately 1400 Pa negative patients, of whom 650participants were expected to convert to Pa positive before
The sample size, number of sites for both the EPIC-OBS and
completion of the observation period, from which approxi-
EPIC-CT, and duration of enrollment were determined based
mately 300 were expected to enroll in the EPIC-CT.
Table 1EPIC-OBS data and sample collection schedule.
Quarterly encounter
Hospitalization or IV antibiotic
EPIC-OBS enrollment form
Registry clinical encounter form
EPIC-OBS clinical encounter form a
Registry year-end survey
EPIC-OBS year-end survey b
Registry hospitalization/IV history
Results of respiratory culture (local site lab) c
Results of outpatient complete blood count d
Serum for serology and banking
Respiratory specimen sent to Core Microbiology Lab e
a The EPIC-OBS clinical encounter form collects information about the following characteristics: use of oral and inhaled antibiotics since the previous visit,
presence of crackles or wheezes on chest auscultation, cough frequency, cold symptoms, physical activity level, and activity limitations due to respiratorysymptoms.
b The EPIC-OBS year-end survey collects information about the following exposures: influenza and pneumococcal vaccines; environmental tobacco smoke;
wood-burning stoves; hot tubs; swimming pools; attendance at social events with individuals who have CF; playing with other children who have CF; otherhousehold members with CF; daycare attendance; Synagis® prophylaxis; breast-feeding; mother's education; and annual household income.
c Culture results from routine clinical cultures performed at the local site laboratory are recorded in the Registry clinical encounter forms.
d Result of outpatient complete blood counts are recorded on the EPIC-OBS clinical encounter form.
e After the first isolation of Pa from a respiratory culture at the local site laboratory, annual respiratory specimens (OP swab or sputum) are shipped to and
processed at the Cystic Fibrosis Foundation Therapeutics Development (TDN) Core Microbiology Laboratory. If possible, initial Pa isolates from the local sitelaboratory will also be shipped to the TDN Core Microbiology Laboratory.
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
3.2. Choice of interventions in the EPIC-CT
Estimated annual number of eligible patients among 60 U.S. CF centers in2000 based on the CFF National Patient Registry.
In young patients in whom Pa is isolated for the first time,
the goal of treatment is maintaining sustained eradication
rate of first Pa
rather than controlling a chronic infection. The main con-
siderations that were taken into account in the treatment
selection process were the following: (1) a group receiving no
active anti-pseudomonal antibiotics was not deemed feasible
in consideration of current clinical practice, (2) 28-day
therapy seemed appropriate based on previous literature
concerning cycled inhaled tobramycin , and (3) a
quarterly cycle would allow sufficient time off antibiotics tolimit exposure to antimicrobials.
Enrollment rates exceeded expectations, and the observa-
The proposed regimens were discussed among a panel of
tional study actually enrolled 1787 rather than 1400
experts from the CF community convened by the study
principal investigators and the CFF to develop the optimalrange of therapeutic approaches that would ensure adequate
3. The clinical trial component
microbial coverage for all subjects and preserve clinicalequipoise in the CF community.
3.1. Objectives of EPIC-CT
A systematic review of the literature was prepared for the
panel to assist in selection of the most effective and safest
The primary objectives of the clinical trial are to
anti-pseudomonal agents for administration in this young age
investigate if an intensive quarterly anti-pseudomonal strat-
group. Aminoglycosides are the drug class with the largest
egy (cycled therapy) reduces pulmonary exacerbations and
amount of information available about sputum–antibiotic
the isolation of Pa from respiratory cultures, compared with a
interactions in patients with CF. It was the general agreement
strategy of anti-pseudomonal administration solely based
that tobramycin for inhalation was a logical prototypical agent
upon recovery of positive respiratory cultures collected
for trials of early intervention in CF, as its safety and efficacy in
quarterly (culture-based therapy). The specific primary aim
patients six years and older is the most thoroughly docu-
is twofold and includes a clinical and a microbiological
mented of all inhaled antibiotics. It is the only FDA approved
endpoint. The clinical endpoint is the time to first pulmonary
inhaled antibiotic for the treatment of Pa infection in patients
exacerbation (requiring intravenous antibiotics or
with CF. There have been several studies in children less than
hospital admission during the 18 month study period. The
six years old demonstrating at least transient Pa eradication
microbiological endpoint is the proportion of Pa-positive
from upper and/or lower airways with inhaled tobramycin
respiratory cultures among quarterly cultures obtained after
randomization. Secondary independent clinical efficacy end-
The recommended dosage of preservative-free inhaled
points include: (1) time to pulmonary exacerbation not
tobramycin for adults and children ≥6 years of age is 300 mg.
requiring intravenous antibiotic usage or hospitalization, (2)
This dose was demonstrated to attain sputum levels adequate to
frequencies of pulmonary exacerbations, hospitalizations, anduse of concomitant oral, inhaled, and intravenous antibiotics,
Table 3Definition of pulmonary exacerbation — minimal criteria for treatment with
(3) anthropometric measures (linear growth, weight gain),
(4) pulmonary function tests including FVC, FEF25%–75%, andFEV
The presence of a pulmonary exacerbation is established by the following:
1 (patients 4 years of age and older, able to reproducibly
One of the major criteria alone or two of the minor signs/symptoms and
perform spirometry), and (5) total hospitalization days.
fulfillment of symptom duration.
Secondary microbiological endpoints include the micro-
Major criteria: (one finding alone establishes the presence of a pulmonary
biologic profile of Pa isolates from respiratory cultures as
indicated by: (1) changes in antibiotic susceptibility patterns
(1) Decrease in FEV1 of ≥10% from best baseline within past 6 months,
(minimal inhibitory concentrations of 12 antibiotics), (2)
unresponsive to albuterol (in participants able to reproducibly performspirometry)
colony morphology, and (3) the presence of mucoid isolates
(2) Oxygen saturation b90% on room air or ≥5% decline from previous
from baseline to the end of the study. The emergence of
intrinsically aminoglycoside- and ciprofloxacin-resistant non-
(3) New lobar infiltrate(s) or atelectasi(e)s on chest radiograph
pseudomonal organisms is also evaluated (e.g., B. cepacia, A.
(4) Hemoptysis (more than streaks on more than one occasion in past week)Minor signs/symptoms: (two minor signs/symptoms are required with duration
xylosoxidans, and S. maltophilia).
criteria in the absence of major criteria)
Study participants are also followed with respect to the
(1) Increased work of breathing or respiratory rate
serologic response against selected Pa surface and secretory
(2) New or increased adventitial sounds on lung exam
antigens and changes in inflammatory markers (white blood
(3) Weight loss ≥5% of body weight or decrease across 1 major percentile in
count with differential and C reactive protein).
weight percentile for age in past 6 months
(4) Increased cough
The comparison of the safety profiles between the two
(5) Decreased exercise tolerance or level of activity
groups include the emergence of organ toxicities detected by
(6) Increased chest congestion or change in sputum
serial evaluation of articular/skeletal symptoms, renal func-
Signs/symptom duration: (required with two minor signs/symptoms in absence
tion, hearing acuity, liver function, hematological profile, and
of major criteria)
(1) Duration of sign/symptoms ≥5 days or significant symptom severity
adverse events.
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
overcome potential sputum antagonism . Inhaled tobramycin
groups could receive an additional 28-day course of inhaled
has also been shown to be safe and to achieve therapeutic
tobramycin at the end of the first treatment cycle if their
concentrations in the lower airway of patients b6 years of age
respiratory cultures sampled after three weeks of the first
. Dose adjustments for age or weight are not required.
anti-pseudomonal cycle continued to be positive for Pa. They
Studies in patients chronically colonized with Pa support
did not receive a second course of ciprofloxacin or placebo.
the use of inhaled tobramycin twice a day for cycles of 28 days
Following this initial antibiotic regimen, participants
receiving drug, followed by 1 month not receiving drug. In
randomized to the cycled therapy group (n = 153) received
colonized patients, data suggested that the effect of a 28-day
therapy administrated in quarterly cycles for five additional
cycle persists at least 56 days in over half of the patients,
quarters, while participants randomized to the culture-based
indicating that a 28-day cycle followed by 56 days off therapy
therapy group (n = 153) received a course of therapy only when
may be appropriate . This discontinuous dosing has the
quarterly respiratory cultures, from either the central or the
theoretical advantages of minimizing emergence of resistance
local laboratory, were found positive for Pa for the same study
and of reducing drug exposure. Therefore, quarterly therapy
duration. The same anti-pseudomonal antibiotics, inhaled
was selected for this young population.
tobramycin twice-daily for 28 days and oral ciprofloxacin or
The consensus panel supported a combination of an
placebo twice-daily for 14 days, were used in both groups. The
inhaled antibiotic with an oral fluoroquinolone such as
inhaled tobramycin therapy was not blinded. The ciprofloxacin
ciprofloxacin for initial eradication. There are several pre-
was administered as a pill to older children taking 250 mg or
sumed advantages to this approach. First, oral fluoroquino-
higher dose and placebo tablets were provided by Bayer.
lones are distributed systemically providing access to the
Younger children received ciprofloxacin suspension or taste
sinuses and upper airway, a potential reservoir for re-
masked placebo suspension provided by Bayer.
infection . Second, they are bactericidal agents which
Overall, the study follows a factorial design and partici-
have a broad spectrum of antimicrobial activity with excellent
pants were allocated to the treatment regimens as displayed
in vitro activity against Pa strains from CF patients and
in . Once patients were assigned to a treatment group,
documented in vitro synergism with tobramycin . Third,
patients and treating physicians had to adhere to the assigned
they are well tolerated and easily administered. Fourth, the
regimen for the 18-month study period. Except for the initial
risk of emergence of resistance can be minimized by short-
two study visits, patients were seen on a quarterly basis in
term administration . The pharmacokinetic profile of oral
conjunction with their routine clinic visits. Irrespective of
and intravenous ciprofloxacin was examined in 150 pediatric
randomization assignment, participants in each of these
patients ages 0.3–17 years, including 28 children with CF. On
groups were allowed to receive necessary antibiotic therapy
average, the most frequently used dose of ciprofloxacin in
for treatment of a pulmonary exacerbation in addition to their
children is twice daily 15–20 mg/kg/dose up to a maximum
assigned treatment regimen. An operational definition of
of 750 mg/dose, for a two-week course .
pulmonary exacerbation was developed for the purpose of thestudy ). Participants presenting in a stable condition
3.3. Study design
at the time of randomization were assigned to one of thestudy regimens immediately. Study participants presenting
This clinical trial is a multicenter, randomized study of
with new onset of a pulmonary exacerbation requiring IV
young children with CF. Fifty seven clinical centers throughout
antibiotics or hospital admission were treated at the discre-
the US participated and the enrollment goal of 300 partici-
tion of the investigator and then randomized at the following
pants was met in 2.5 years ). Participating sites are listed
quarter if they continued to meet the study eligibility criteria.
in Appendix A. All participating centers obtained IRB approvalfrom their respective institutions. After obtaining informed
consent/assent, 306 participants have been equally rando-mized to one of two early anti-pseudomonal treatment
Inhaled tobramycin was provided in an open label fashion,
algorithms (cycled or culture-based therapy groups). The
while oral ciprofloxacin was provided in a double-blinded
duration of study participation for each subject is 18 months,
fashion. To minimize potential bias due to the lack of blinding,
during which time each participant receives up to six
we developed an objective and rigorous operational definition
treatment cycles. In combination with inhaled tobramycin
of pulmonary exacerbation (and we verified all the
(Novartis Pharmaceutical Corp), patients were randomized to
hospitalization records to ensure that the reason of hospitaliza-
receive either oral ciprofloxacin or oral placebo (Bayer
tion was a pulmonary exacerbation. A patient diary was
Healthcare AG).
maintained as a corroborating mechanism to ensure the active
Randomization was carried out by permuted blocks, and
reporting of any and all respiratory symptoms. A review
performed using a computer generated sequence. The
committee is devoted to the quality control of this endpoint
randomization blocks did not account for clinical site since
in a blinded fashion. Further, all of the secondary endpoints are
we assumed that the potential clustering effect of clinical site
objective measures. Additional measures to minimize bias
would be mitigated by the large number of sites (i.e., large
included an extensive ongoing training of both physicians and
number of clusters of small size).
research coordinators by quarterly newsletters, teleconferences
At study enrollment, participants received an initial course
and annual study meetings. Educational tools for families and
of anti-pseudomonal antibiotic therapy consisting of 28 days
primary care physicians who might also be involved in the care
of inhaled tobramycin with 14 days of oral ciprofloxacin or
of the study participants have also been provided. All protocol
placebo. To promote initial eradication of Pa at the beginning
deviations and violations have been evaluated on a case-by-
of the study, participants randomized to any of the study
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
Table 4EPIC-CT study visit schedule.
Medical history review b
Interim medical history b
Concomitant medication review
Complete physical exam c
Oropharyngeal or sputum culture f
CBC with differential
C-reactive protein
Chest X-ray (PA and lateral) g
Adverse event monitoring
Additional plasma aliquot
a If needed, for pulmonary exacerbation (PE) or follow up of adverse events (AE), including musculo-skeletal, articular or neurological symptoms.
b Neurological side effects based on medical history; if abnormalities are reported, patient is referred to the facility enrolling study subjects.
c Complete physical exam, including standard articular/skeletal muscle exam.
d Height/length and weight must be measured by same equipment throughout study period. Participants initiating study with length measurements must
continue with length throughout study.
e Spirometry in participants ≥4 years of age.
f MICs at Week 0, Week 22, Week 46, and Week 70.
g Chest X-ray must not be prior to 6 months prior to inclusion in the study.
h Annual audiology at the sites is recommended. If abnormal results are found, a confirmatory audiology will be repeated four weeks later.
3.5. Choice of study population
or other methyl-xanthines within 30 days of the time ofenrollment, administration of more than one course of
Male and female subjects ≥1 year and ≤12 years of age with
intravenous anti-pseudomonal antibiotics (defined as at least
a diagnosis of CF with a documented new onset of orophar-
10 days of therapy) in the 2 years prior to baseline or more than
yngeal, sputum or lower respiratory tract culture positive for Pa
one course (at least 28 continuous days of therapy) of inhaled
within six months prior to study entry were eligible for
anti-pseudomonal antibiotics within two years prior to study
participation in this study. For study purposes, first isolation
entry, and chronic macrolides use (more than 3-month
of Pa was defined as the first lifetime documented respiratory
duration) within 3 months of baseline; presence of a condition
culture positive for Pa or as a positive Pa culture after at least
or abnormality that in the opinion of the Investigator would
two-year absence of Pa growth (minimum of one documented
compromise the safety of the patient or the quality of the data.
negative Pa culture per year). For participants ages 12 to
Intravenous or inhaled anti-pseudomonal antibiotics needed to
15 months, at least one Pa positive respiratory culture since
be completed more than 30 days prior to baseline.
birth was required. Children below the age of 1 year were notconsidered for enrollment because oral ciprofloxacin could not
3.6. Study subject screening and follow up strategies
be administered for safety concerns regarding potentialarthropathy Other eligibility criteria included: Diag-
Potential study participants were screened at the participat-
nosis of CF clinically stable with no evidence of any
ing sites whether or not they were previously enrolled in the
significant respiratory symptoms at screening that would
observational study. Patients meeting the criteria for the EPIC-
require administration of intravenous antipseudomonal anti-
CT were enrolled and randomization assignment was made
biotics, oxygen supplementation and/or hospitalization; signed
centrally via an interactive voice response system.
informed consent by parent or legal guardian.
shows the content of the baseline encounter and subsequent
Patients were excluded from the study if they had a history
quarterly study visits for the 18 month study duration. Patients
of aminoglycoside hypersensitivity or adverse reaction to
had microbiology samples from oropharyngeal swab cultures at
inhaled aminoglycoside, history of hypersensitivity or adverse
every quarterly visit. Anthropometric measures and nutritional
event associated with ciprofloxacin or other fluoroquinolones,
assessment included length (children ≤18 months) or height,
abnormal renal function (serum creatinine N1.5 times the
and weight. Spirometry data were collected in subjects 4 years
upper limit of normal for age), clinically documented chronic
of age or older, according to the guidelines stipulated in the
hearing loss, serum transaminase levels at the screening visit
1994 American Thoracic Society Guidelines with modified
N2 times the upper limit of normal range, administration of any
criteria for children
investigational drug within 30 days prior to enrollment, chronic
EPIC-CT participants, who were not previously enrolled in
administration of loop diuretics, administration of theophylline
the observational study, had the option to enroll in EPIC-OBS
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
prior to completion of the clinical trial in order to collect long-
consisted of parent recall at quarterly visits and review of parent
term clinical and safety follow-up data.
diary. The diary collected data on daily drug consumption, any
Due to potential for aminoglycoside ototoxicity, audio-
changes in patient health or new onset of symptoms, medica-
metry utilizing age appropriate testing with tympa-
tion use, and encounters with other health-care providers. The
nometry to detect the presence of fluid in the middle ear was
diary was maintained to ensure, among other things, an active
performed at study enrollment, at the end of the first year and
mechanism to capture the possible occurrence of new
at study completion. Abnormal hearing was defined as an
respiratory symptoms that could qualify as a study defined
auditory threshold ≥25 dB at any frequency (500–8000 Hz)
in either ear. If abnormal results were found during the studyperiod, confirmatory audiometry was repeated four weeks
3.8. Microbiology methods and specimen collection
later. Children with abnormal audiologic findings had atympanometry performed, and if both were abnormal, they
Oropharyngeal specimens were obtained at all study
were referred to the study investigator or medically-qualified
visits. Specimens were collected with a cotton-tipped swab
sub-investigator for an ear examination. Audiometry was not
from the posterior oropharyngeal wall and tonsillar pillars.
collected on all participants because some sites did not have
Participants were encouraged to cough prior to collection of
the capability to perform audiometric testing in this age
the OP specimen. With the exception of the specimen
group and some children were unable to comply with the
obtained at the end of the first treatment cycle (which was
testing procedure or had uninterpretable results due to
processed at the site laboratory), all specimens were
pneumatic equalizing tube placement.
processed at the core microbiology laboratory at Children's
Blood samples were obtained for assessment of clinical
Hospital and Regional Medical Center in Seattle, WA.
status and included blood chemistry (creatinine, blood urea
Oropharyngeal swab specimens were sent on wet ice by
nitrogen, and liver function tests including hepatic transami-
overnight express shipment to the core microbiology labora-
nases [AST and ALT] and γGT, and C reactive protein), and a
tory, and needed to be received and cultured within 2
complete blood count (hemoglobin, hematocrit, red blood
calendar days of collection. Bacterial culture techniques
cell count, white blood cell count, and white blood cell
were performed according to core microbiology laboratory
differential count). After baseline, blood samples were
standard procedures using a semi-quantitative bacterial
obtained twice a year and at the end of the study. Blood
culture technique All organisms were identified
sampling also included serum banking for Pa serology assays.
using standard techniques, including standard biochemical
For this purpose, the blood sample was centrifuged, the serum
testing and PCR techniques . All Pa isolates were assessed
extracted, and the specimen stored at −70 °C.
for mucoid phenotype. Minimal inhibitory concentrations
Chronic use of azithromycin was not permitted. All
(MIC) for Pa of 12 antibiotics were determined using a semi-
participants were encouraged to remain on the same medica-
automated microbroth dilution (Sensititre, AccuMed, Wes-
tions throughout the entire study period, as medically feasible.
tlake, OH), according to standard National Committee on
Study participants maintained a diary while on the study and
Clinical Laboratory Standards methods. MICs for any Pa
the information recorded in the patient diary was abstracted at
isolate were performed at baseline and every six months.
every clinic visit. The diary collected data among others on
We conducted quality control procedures to ensure that the
treatment adherence and changes in concomitant medications.
sample shipment and processing methods would yield accurateresults. For this purpose we spiked 50 samples with inoculums
3.7. Drug distribution and adherence to treatment regimen
of known micro-organism species (Pa, Stenotrophomonasmaltophilia, Achromobacter xylosoxidans, and "no organism")
Within seven days of the study visit participants were
and density (103 to 105 CFU) that were sent blindly to the core
contacted to report the microbiology results and to review the
microbiology laboratory. The specificity for Pa identification
treatment plan. If participants required study medication based
was excellent (100%), as there were no instances of Pa mis-
on group assignment, the drug was prescribed at this time. Study
identification. There was a single instance of the laboratory
drugs (tobramycin solution for inhalation, ciprofloxacin suspen-
finding no isolates from a Pa positive sample, yielding a
sion or tablets, or a matched oral placebo) were distributed by a
sensitivity for Pa isolation of 97%. To further evaluate measure-
central pharmacy and mailed to the study participants' domicile
ment error due to sampling, manipulation, and transport, data
within 48 h of prescription. At the first treatment cycle patients
were collected from the clinical site microbiology laboratory to
were also provided with a nebulizer (PARI-Proneb® Ultra
evaluate concordance between results for those participants
compressors and PARI LC Plus® reusable nebulizers, PARI
who had two simultaneous or sequential oropharyngeal
Respiratory Equipment Inc., Midlothian, VA). Participants were
cultures collected at the same study visit.
contacted within 48 h of prescription of the study drugs to verifydrug receipt, and were instructed to initiate treatment upon
3.9. Sample size and power of EPIC-CT
receipt of the study drugs. Subsequently, participants werecontacted within 14 days of the clinic visit to identify occurrence
By design, the primary study endpoint for which the study is
of adverse events including a musculoskeletal assessment
powered is the clinical efficacy endpoint, time to first exacer-
survey, and to discuss study medication adherence. All partici-
bation requiring intravenous antibiotics and/or hospitalization,
pants were again contacted within 6 weeks of the clinic visit to
which will be compared between treatment groups using a
evaluate possible changes in health status.
hazard ratio as an estimate of the relative risk. The primary
Further monitoring for patient adherence to prescribed
analysis will compare the more aggressive treatment group,
treatment regimen (cycled and culture-based treatment arms)
cycled therapy, to the less aggressive treatment group, culture-
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
based therapy to evaluate the relative reduction in pulmonary
tial interactions between the tobramycin and ciprofloxacin
exacerbations achieved by the more aggressive therapy. To
regimens. These analyses will involve comparing smaller
determine the statistical power and sample size for this clinical
subgroups of participants in each of the four relevant
efficacy endpoint, we first obtained expected rates of exacer-
subgroups (approximately 75 participants per group).
bation in this patient population.
A modified intent-to-treat (ITT) population was defined as all
To determine a reasonable relative risk size for which to
randomized participants who received at least one dose of study
power this clinical efficacy endpoint, we estimated the annual
exacerbation event rate using data on over 40,000 person-yearsrepresented in the CFF National Patient Registry during 1985–
3.11. Statistical analysis plan
2000 All patients born in 1985 or later and who werebetween the ages of 1 and 12 were classified into three mutually
By design, the primary study endpoint for which the study
exclusive groups at each calendar year: (i) patients with no
is powered is the clinical efficacy endpoint, time to first
positive Pa culture since birth; (ii) patients who had the first
protocol-defined pulmonary exacerbation requiring intrave-
positive culture during that year, and (iii) patients with at least
nous antibiotics and/or hospitalization. The censored failure
one positive culture in previous years. Based on the percentages
time will be taken as the diagnosis date of the pulmonary
of patients experiencing at least one exacerbation related event
exacerbation, or the end of follow-up if no exacerbation
during a calendar year, we estimated that the annual incidence
meeting these criteria has occurred. End of follow-up is
rate of exacerbations was 0.17 in group (i), 0.36 in group (ii),
defined as the date of the last study visit completed by the
and 0.37 in group (iii). These rates appeared to be consistent
participant. Time will be measured in number of days post
across age categories within each group. We assumed that an
Day 0, the first day study therapy was started. The null
aggressive early antibiotic therapy could potentially reduce the
hypothesis is that there is no difference between the cycled
risk of exacerbation by as much as 50% when comparing group
therapy and culture-based therapy groups in terms of the
(ii) to group (i). Based on the annual exacerbation incidence
time to first protocol-defined exacerbation requiring intrave-
rates estimated in the registry, an 18-month study with 300
nous antibiotics and/or hospitalization.
patients had 80% power to detect a relative risk of approxi-
A Kaplan–Meier plot will be used to graphically display
mately 0.6 or lower, equivalent to detecting a 40% or greater
estimates of the survivor function in terms of the proportion of
reduction in risk of pulmonary exacerbations. The primary
participants who were exacerbation free over time for both the
microbiologic analysis of the proportion of Pa positive cultures
cycled and culture-based groups. Relative risk due to treatment
in the 18-month study period will also compare the cycled
will be estimated using a Cox proportional hazards regression
therapy arm to the culture-based therapy arm. For this
model, with covariate adjustment for baseline age group (1–3,
objective, a sample size of 300 subjects provides 80% power
N3–6, and N6 years). The significance of the treatment group
to detect an odds ratio 0.6 or smaller for Pa-positive respiratory
variable will be tested by the likelihood ratio test at a two-sided
cultures between the two treatment strategies.
0.05 level of significance. The relative risk due to treatment fromthis model and corresponding 95% confidence interval will be
3.10. Factorial aspects of sample size and power
the primary measure of treatment effect. Secondary analyseswill evaluate the interaction between the inhaled and oral
The design of this study makes it possible to perform
treatment groups within the context of this model, and similar
separate evaluations of (1) the effect of two different
models will be used to investigate differences between
tobramycin treatment strategies and (2) the effect of oral
treatment groups with respect to an important secondary
ciprofloxacin versus oral placebo, each based on comparing
endpoint, time to pulmonary exacerbation requiring any
two groups of 150 study participants. The first evaluation
antibiotics (inhaled, oral, or IV) and/or hospitalization. The
would compare the cycled versus the culture-based group,
following covariates will be considered for adjustment in
and the second evaluation would compare the group assigned
exploratory models for these endpoints: gender, enrollment
to receive oral ciprofloxacin (regardless of tobramycin regi-
season, Pa status at enrollment, and/or geographic region.
men) with the group assigned to receive oral placebo. The
The microbiology endpoint is the proportion of Pa-positive
secondary analysis will explore the main effect of ciproflox-
respiratory cultures among the seven respiratory cultures taken
acin by comparing all participants randomized to oral
during the 18 months of the follow-up. The respiratory culture
ciprofloxacin to all participants randomized to placebo.
results obtained from oropharyngeal cultures or expectorated
Since the sample size in each of these groups is the same as
sputum cultures obtained at weeks 3, 10, 22, 34, 46, 58, and 70
for the primary comparison, the study will have the same
will be used in this analysis. The response will be binary
power for this analysis as the primary analysis. Assuming lack
(positive or negative culture). In the rare event that both an
of interaction between cycled therapy and ciprofloxacin, the
oropharyngeal culture and an expectorated sputum culture are
factorial structure of the study will allow performing two
available at a given visit and produce discordant results, a
independent comparisons with adequate power, by spending
positive result will be used in the analysis for this visit. The ITT
only one degree of freedom on each comparison. The study
population will be used for this longitudinal analysis and
would have reduced power for finding an effect if there is a
missing data will not be included. A generalized estimating
negative interaction between cycled therapy and ciproflox-
equation (GEE) model using a logit link will be used to model
acin which is thought to be unlikely. However, we could also
this data with an independence working correlation matrix. The
gain power if there was a positive synergistic effect between
significance of the treatment group variable will be tested by
the cycled therapy and ciprofloxacin. Importantly, further
the Wald test using a two-sided 0.05 level of significance. The
exploratory analyses will be performed to investigate poten-
treatment associated odds ratio from this model and

M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
corresponding 95% confidence interval will be the primary
Each adverse event is entered into the case report form and
measure of treatment effect. The estimated treatment effect will
evaluated with respect to intensity, seriousness, causality
be interpreted as the marginal odds ratio of Pa-positive
(relationship to treatment) and actions taken. A summary of
respiratory cultures during the 18 months.
potential trends or unexpected events is provided to the Data
Sensitivity analyses will be performed in order to evaluate the
Safety Monitoring Board for further review and evaluation.
robustness of the primary microbiologic results to missingculture data, which by virtue of study design should be minimal.
3.13. Data and Safety Monitoring Board (DSMB)
Specifically, we will perform three additional analyses for theprimary microbiologic endpoint: (1) an analysis which imputes
Safety is monitored on an ongoing basis throughout the trial
missing respiratory culture data using the Last Observation
by a Data and Safety Monitoring Board (DSMB) appointed by the
Carried Forward (LOCF) method, (2) an analysis which assumes
National Heart Lung and Blood Institute (NHLBI). The DSMB
all missing culture data is negative for Pa, and (3) an analysis
convenes at fixed times during the study in open and closed
which assumes all missing culture data is positive for Pa. A
meetings at approximately 6 month intervals, including an
further analysis will investigate the sensitivity of the results to the
annual in-person meeting. At each meeting, the DSMB reviews
augmentation of core laboratory results with available results
safety data, enrollment data, protocol violations, and overall
from individual site microbiology laboratories. In some instances,
study progress. Descriptions of serious adverse events are
participants were double-swabbed and one swab was sent to the
communicated to the DSMB Chair within 24 h of their occurrence.
core lab and one to the site lab. In instances for which the site lab
There are no pre-specified stopping rules for efficacy or
result was positive for Pa and the core lab was negative, the final
futility. If the data from this study are unable to support the
result will be treated as positive. Additional secondary endpoints
superiority of the cycled based treatment regimen to the culture
including longitudinal changes in anthropometric measures and
based therapy in terms of both clinical and microbiologic
spirometry will be modeled using the GEE framework to test for
efficacy, this would be an important result that could influence
differences in the 18-month change in each endpoint. Adverse
clinical care. In particular, there would be no supportive data to
events will be descriptively summarized by MEDRA system
suggest that newly colonized CF patients be aggressively treated
organ class and preferred term. Colony morphology will be
regardless of their culture positivity. If the two regimens are
summarized by the eradication and incidence patterns during
equal in terms of efficacy, the secondary endpoints regarding
the follow up period as compared to baseline for each of the
safety and microbiologic resistance become even more impor-
following bacterial organisms: P. aeruginosa, A. xylosoxidan, B.
tant for determining which regimen is superior and these must
cepacia, S. aureus and S. maltophilia. This analysis will primarily
be evaluated for the entire duration of the study. The DSMB
be descriptive, and the proportion of patients who have
follows the safety (quarterly) and efficacy (semi-annually)
eradicated or newly acquired the organism by the end of the
endpoints on a regular basis and could stop the study at any
study will be summarized with corresponding 95% confidence
time if they felt it was ethically necessary.
3.14. Study enrollment
3.12. Adverse event monitoring
During the 2.5 years enrollment period, from December
In the case of a serious adverse event (as defined by the FDA
20, 2004 to February 7, 2008, the actual accrual was excellent
21 CFR 312.32) the site investigator notifies the Coordinating
due to excellent participation by all sites, closely overlapping
Center within 24 h of learning of the event.
the projected figures (Clinical site performance is
Fig. 3. EPIC clinical trial enrollment by month. Solid line indicates actual enrollment, dotted line indicates projected enrollment.
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
monitored on an ongoing basis and, if deficiencies are
dosing, safety, and efficacy in this young population. A site survey
identified, these are addressed in a timely fashion.
was also distributed to confirm that the proposed strategies wereacceptable in the context of the local practices and in equipoise
with current treatments in use at each participating institution.
This process ensured that the proposed strategies would be well
The major objective of EPIC-CT is to help define a safe,
accepted by the CF community and facilitated successful
effective and systematic approach to the treatment of first
enrollment throughout the study period.
isolation of Pa from young CF patients. These young patients
In an effort to minimize the burden of a long term trial on
potentially have the most to gain from aggressive early
participants and their families, we scheduled the clinical trial
intervention, but also the most to lose in terms of cumulative
study visits to coincide with routine clinic visits and study
drug toxicities and acquisition of resistant pathogens. If the
drugs were shipped directly to the participant's residence.
study demonstrates both a microbiologic effect and clinical
Because of frequent microbiology sampling, the study also
efficacy of aggressive therapy without significant adverse
avoided the use of invasive sampling procedures and ensured
events or high rates of acquisition of resistant organisms, then
that sampling occurred in conjunction with procedures
there would be a strong rationale for aggressive early interven-
performed (blood draws and oropharyngeal swabs) for
tion. If the two treatment approaches are not different then the
routine care. Although Pa monitoring via oropharyngeal
use of aggressive cycled antibiotic therapy must be reassessed.
cultures is suboptimal, more invasive approaches were not
The major objectives of the EPIC-OBS study are 1) to
feasible in a study of this size and in the age range of the study
provide a closely-monitored cohort of young children prior to
participants. Results of study oropharyngeal cultures were
potential EPIC-CT entry, 2) to provide long-term follow-up of
provided to the sites to allow continued clinical care.
the treatment regimens for the subset of randomized
This study did not examine patient/family reported
patients, and 3) to establish a large, multi-center, well-
outcomes due to the lack of availability of validated instru-
characterized cohort of young children with CF to obtain more
ments for participants of ages 6 years or younger . Future
generalizable data on the risk factors for and impact of Pa
studies should consider collecting age-appropriate quality of
acquisition and infection. The ultimate goal is for this cohort
life measures to evaluate the effect of long term interventions
to be closely monitored for at least 10 years with both study-
on treatment burden and health related quality of life.
specific data collection and ongoing use of the CF Patient
The emphasis placed on engaging the clinical sites in the
Registry. We anticipate that this longitudinal study will be
early phase of study development and designing the study to
informative on both risks factors for and clinical outcomes of
meet the needs of both the sites and the participant's families
initial, chronic, and mucoid Pa infection, and will explore the
likely contributed to the excellent enrollment rate that
impact of polymicrobial infections (interaction of S. aureus
exceeded projections by 6 months. In addition, the attrition
and Pa) in children with CF. The banked serum will provide a
rate has remained at less than 10% easily meeting expectations.
rich resource for the analyses of serologic markers of early and
In conclusion, this study will provide valuable clinical and
chronic Pa infection, and other potential biomarkers of early
microbiologic efficacy and safety data regarding the optimal
CF lung disease. The DNA bank from whole blood of EPIC-OBS
use of antipseudomonal therapy in young children with CF,
participants will provide a unique resource for the evaluation
and the long-term follow-up of this unique cohort of children
of genetic modifiers of early CF disease.
will supply important data on the effect of Pa infection on
This initiative represents the largest cohort and therapeu-
subsequent health status, and the linked serum and DNA
tic study ever conducted in children with CF and provides a
banks will contribute valuable information on surrogate
model of how to conduct a phase 3 trial in a population of
markers and genetic modifiers of early CF lung disease.
young children. The project was made possible under theauspices of the Cystic Fibrosis Foundation and the National
Heart, Lung and Blood Institute who joined forces to create acooperative program, along with the support of industry who
The research for this article was supported in part by the
donated study drugs and supplies. The studies made use of
Cystic Fibrosis Foundation grants number EPIC0K0 and
existing resources both during the planning and implementa-
OBSERV04K0, the National Heart Lung and Blood Institute
tion phase as provided by the linkage to the National CFF
(NHLBI) and National Institute for Digestive Disorders and Kidney
Registry. Similar to the design of the Women's Health Initiative
(NIDDK) grant number U01-HL080310, and the National Center
that combined a cohort study and a clinical trial, the
for Research Resources (NCRR) grant number ULI-RR2501401.
observational cohort study not only serves as a free-standing
Study drugs and devices were supplied free of charges by
epidemiologic study of early CF lung disease, but also provides
Novartis Pharmaceutical Corp. (inhaled tobramycin) and Bayer
a pool of potential clinical trial participants and provides long
Healthcare AG (oral ciprofloxacin and oral placebo), compressors
term follow-up data on clinical trial participants. These
and nebulizers were provided by PARI Respiratory Equipment Inc.
features should provide an ideal setting for the interpretation
The Sponsors had no role in designing the study, in the
of the clinical trial findings and their generalizability. A large
data collection, or in the writing of the manuscript. They have
number of sites were recruited to ensure timely enrollment
no access to the study data.
and provide a broad geographic distribution and to ensure theinclusion of a representative sample of the population.
Appendix A. — Epic investigators
In the trial planning stage, a consensus process among experts
in the CF field was used to ensure that the study treatment
Program Office: National Institute of Health: Susan Banks-
strategies were optimal in terms of administration schedule,
Schlegel, PhD, National Heart, Lung and Blood Institute, Project
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
Director; Gail Weinman, MD, National Heart, Lung and Blood
College, Westchester Medical Center, Valhalla, NY, Allen
Institute, Executive Secretary; Cystic Fibrosis Foundation Ther-
Dozor, MD and Nikhil Amin, MD; Northern California Kaiser
apeutics: Robert J. Beall, Ph.D., Preston W. Campbell III, MD, Bruce
CF Center, Kaiser Permenente Medical Center, Oakland, CA,
C. Marshall, MD.
Greg Shay, MD and Albin Leong, MD; Oregon Health Sciences
Data and Safety Monitoring Board: Lynne Quittell, MD (Chair)
University, Portland, OR, Michael Wall, MD; Penn State Milton
Columbia University; William Clarke, PhD, University of Iowa;
S. Hershey Medical Center, Hershey, PA, Gavin Graff, MD;
Mary Jane Kennedy, PharmD, Kosair Charities Pediatric Clinical
Rainbow Babies & Childrens Hospital, Cleveland, OH, Michael
Research Unit, Louisville; Robert Nelson, MD, PhD, University of
W. Konstan, MD; Rhode Island Hospital, Providence, RI, Mary
Pennsylvania; Kenneth N. Oliver, MD, MPH, National Institute of
Ann Passero (EPIC-OBS only); Riley Hospital, Indiana Uni-
Allergy and Infectious Disease; Ronald Rubenstein, MD, PhD,
versity, Indianapolis, IN, Michelle Howenstine, MD; Schneider
Children's Hospital of Philadelphia; O. Dale Williams, PhD,
Children's Hospital, New Hyde Park, NY, Joan DeCelie-
University of Alabama at Birmingham; Gail Weinman, MD,
Germana, MD; Spectrum Health Hospitals – DeVos Children's
National Heart, Lung and Blood Institute; Susan Banks-Schlegel,
Butterworth Hospital, Grand Rapids, MI, Susan Millard, MD; St.
PhD, National Heart, Lung and Blood Institute; Jungman Joo, PhD,
Christopher's Hospital for Children, Philadelphia, PA, Laurie
National Heart, Lung and Blood Institute.
Varlotta, MD; Stanford University, Packard Children's Hosp.,
Coordinating Center: Study monitors: Amanda Bailey,
Palo Alto, CA, Richard Moss, MD; SUNY Upstate Medical
Molly Andrina, Amy Feldman, Robin Hill, Tamara Potter,
University, Upstate Medical Center, Syracuse, NY, Ran D. Anbar,
Deborah Chambers, Shirley Desmon; Data Management:
MD; Texas Children's Hospital, Houston, TX, Peter Hiatt, MD;
Barbara Mathewson, MS, David Escobar, MPH; Biostatistical
Tulane University School of Medicine, New Orleans, LA, Scott
Unit: Umer Khan, MS, Kelli Joubran, MS; Microbiology Core
H. Davis, MD; University of Alabama at Birmingham, Birming-
Lab: Jane Burns, MD, Jenny Stapp, Anne Marie Buccatt, Griffith
ham, AL, Hector H. Gutierrez, MD; University of California, San
Adam; Medical Monitor: Christopher Goss, MD, MSc.
Francisco, San Francisco, CA, Dennis Nielson, MD; University of
Clinical Centers: Albany Medical College, Albany, NY, Paul
Florida College of Medicine, Gainesville, FL, Terry Spencer, MD;
Comber, MD, PhD; All Children's Hospital CF Center, St.
University of Iowa, Iowa City, IA, Richard C. Ahrens, MD;
Petersburg, FL, Magdalen Gondor, MD; Cardinal Glennon
University of Kentucky, Lexington, KY, Jamshed F. Kanga, MD;
Children's Hospital — St. Louis University, St. Louis, MO,
University of Mass Memorial Health Care, Worcester, MA,
Blakeslee E. Noyes, MD; Nationwide Children's Hospital,
Brian P. O'Sullivan, MD; University of Michigan, Ann Arbor, MI,
Columbus, OH, Karen McCoy, MD; Children's Hospital &
Samya Nasr, MD; University of Mississippi Medical Center,
Regional Medical Center, Seattle, WA, Ronald L. Gibson, MD,
Jackson, MS, Alicia DePaula, MD and Fadel Ruiz, MD;
PhD (EPIC-CT), Margaret Rosenfeld, MD, MPH (EPIC-OBS);
University of Nebraska, Omaha, NE, John L. Colombo, MD;
Denver Children's Hospital, Denver, CO, Frank Accurso, MD
University of New Mexico, Albuquerque, NM, L. Francine
and Jeffrey Wagener, MD; Children's Hospital Medical Center
Caffey, MD; University of North Carolina, Chapel Hill, Chapel
of Akron, Akron, OH, Greg Omlor, MD; Children's Hospital of
Hill, NC, George Retsch-Bogart, MD; University of Rochester,
Michigan, Detroit, MI, Debbie Toder, MD; Children's Hospital
Rochester, NY, Clement L. Ren, MD; University of Utah, Salt
of Pittsburgh, Pittsburgh, PA, David Orenstein, MD; Children's
Lake City, UT, Barbara A. Chatfield, MD; University of Virginia,
Hospital of Wisconsin, Milwaukee, WI, William M. Gershan,
Charlottesville, VA, Deborah K. Froh, MD; University of
MD; Children's Hospital, Boston, Boston, MA, Terry Spencer,
Wisconsin Hospital and Clinics, Madison, WI, Michael Rock,
MD, Thomas Martin, MD, and David Waltz, MD; Children's
MD; Vanderbilt University Medical Center, Nashville, TN,
Hospitals & Clinics, Minneapolis, MN, John McNamara, MD;
Elizabeth Perkett, MD and Christopher Harris, MD; Vermont
Children's Medical Center of Dayton, Dayton, OH, Robert J.
Children's Hospital at Fletcher Allen Health Care, Burlington,
Fink, MD; Children's Memorial Hospital, Chicago, IL, Adrienne
VT, Thomas Lahiri, MD; Washington University School of
Prestridge, MD; Children's Mercy Hospital, Kansas City, MO,
Medicine, St. Louis Children's Hospital, St. Louis, MO, Thomas
Philip Black, MD; Cook Children's Medical Center, Ft. Worth,
Ferkol, MD; Women & Children's Hosp of Buffalo, Children's
TX, Maynard Dyson, MD; Dartmouth-Hitchcock Medical
Hospital of Buffalo, Buffalo, NY, Daniel Sheehan, MD; Chil-
Center, New Hampshire CF Center, Lebanon, NH, H. Worth
dren's Hospital of Los Angeles, USC Medical School, Los
Parker, MD and Dennis Stokes, MD; duPont Hospital for
Angeles, CA, Marlyn Woo, MD.
Children, A.I. duPont Inst. Med. Center, Wilmington, DE, AaronChidekel, MD; Emory University CF Center, Atlanta, GA,Michael Schecter, MD, Lawrence McKean, MD and Daniel
Caplan, MD; Johns Hopkins University, Baltimore, MD, PeterMogayzel, MD, PhD; LeBonheur Children's Medical Center,
[1] Cystic Fibrosis Foundation. Patient Registry 2004 annual data report.
Memphis, TN, Robert A. Schoumacher, MD; Maine Medical
Bethesda, Maryland: Cystic Fibrosis Foundation; 2005.
Center, Portland, ME, Anne Marie Cairns, DO; Massachusetts
[2] IACFA Newsl 1999(Issue 56):8.
[3] Kosorok MR, Wei WH, Farrell PM. The incidence of cystic fibrosis. Stat
General Hospital, Boston, MA, Allen Lapey, MD, and Henry L.
Dorkin, MD; Medical College of Georgia, Augusta, GA,
[4] Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of
Margaret F. Guill, MD; Miller Children's Hospital, Memorial
pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med2003;168:918–51.
Miller Children's Hosp, University of California, Irvine, Long
[5] Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al.
Beach, CA, Felice Adler-Shohet, MD and Jay Lieberman, MD;
Significant microbiological effect of inhaled tobramycin in young
Monmouth Medical Center, Long Branch, NJ, Robert L. Zanni,
children with cystic fibrosis. Am J Respir Crit Care Med 2003;167:841–9.
[6] Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, et al.
MD; Nemours Children's Clinic, Jacksonville, FL, David Schaef-
Early pulmonary infection, inflammation, and clinical outcomes in
fer, MD and Kathryn Blake, PharmD; New York Medical
infants with cystic fibrosis. Pediatr Pulmonol 2001;32:356–66.
M.M. Treggiari et al. / Contemporary Clinical Trials 30 (2009) 256–268
[7] Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al.
[23] Haller I. Comprehensive evaluation of ciprofloxacin–aminoglycoside
Longitudinal assessment of Pseudomonas aeruginosa in young children
combinations against Enterobacteriaceae and Pseudomonas aeruginosa
with cystic fibrosis. J Infect Dis 2001;183:444–52.
strains. Antimicrob Agents Chemother 1985;28:663–6.
[8] Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL.
[24] Ball P. Emergent resistance to ciprofloxacin amongst Pseudomonas
Inflammation, infection, and pulmonary function in infants and young
aeruginosa and Staphylococcus aureus: clinical significance and ther-
children with cystic fibrosis. Am J Respir Crit Care Med 2002;165:904–10.
apeutic approaches. J Antimicrob Chemother 1990;26:165–79 Suppl F.
[9] Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis:
[25] Bosso JA. Use of ciprofloxacin in cystic fibrosis patients. Am J Med
Pseudomonas aeruginosa infection. J Clin Epidemiol 1995;48:1041–9.
[10] Proesmans M, Balinska-Miskiewicz W, Dupont L, Bossuyt X, Verhaegen J,
[26] Burkhardt JE, Hill MA, Carlton WW, Kesterson JW. Histologic and
Hoiby N, et al. Evaluating the "Leeds criteria" for Pseudomonas aeruginosa
histochemical changes in articular cartilages of immature beagle dogs
infection in a cystic fibrosis centre. Eur Respir J 2006;27:937–43.
dosed with difloxacin, a fluoroquinolone. Vet Pathol 1990;27:162–70.
[11] Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation
[27] Chysky V, Kapila K, Hullmann R, Arcieri G, Schacht P, Echols R. Safety of
of a new definition for chronic Pseudomonas aeruginosa infection in
ciprofloxacin in children: worldwide clinical experience based on compas-
cystic fibrosis patients. J Cyst Fibros 2003;2:29–34.
sionate use. Emphasis on joint evaluation. Infection 1991;19:289–96.
[12] Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas
[28] Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus
aeruginosa colonisation in cystic fibrosis by early treatment. Lancet
statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr
[13] Taccetti G, Campana S, Festini F, Mascherini M, Doring G. Early
[29] De Boeck K, Wilschanski M, Castellani C, Taylor C, Cuppens H, Dodge J, et al.
eradication therapy against Pseudomonas aeruginosa in cystic fibrosis
Cystic fibrosis: terminology and diagnostic algorithms. Thorax
patients. Eur Respir J 2005;26:458–61.
[14] Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial
[30] Standardization of spirometry, 1994 update. American Thoracic Society.
colonization with Pseudomonas aeruginosa postpones chronic infection
Am J Respir Crit Care Med 1995;152:1107–36.
and prevents deterioration of pulmonary function in cystic fibrosis.
[31] Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An
Pediatr Pulmonol 1997;23:330–5.
official American Thoracic Society/European Respiratory Society state-
[15] Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, et al.
ment: pulmonary function testing in preschool children. Am J Respir
Comprehensive analysis of risk factors for acquisition of Pseudomonas
Crit Care Med 2007;175:1304–45.
aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol
[32] Thompson G, Wilson WR. Clinical application of visual reinforcement
audiometry. Semin Hear 1984;5:85–99.
[16] Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ.
[33] Gravel GS. Behavioral assessment of auditory function. Semin Hear
Risk factors for initial acquisition of Pseudomonas aeruginosa in children
with cystic fibrosis identified by newborn screening. Pediatr Pulmonol
[34] Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, et al.
Microbiology of sputum from patients at cystic fibrosis centers in the
[17] Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-
United States. Clin Infect Dis 1998;27:158–63.
Warren J, et al. Intermittent administration of inhaled tobramycin in
[35] Burns JL, Van Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al.
patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study
Effect of chronic intermittent administration of inhaled tobramycin on
Group. N Engl J Med 1999;340:23–30.
respiratory microbial flora in patients with cystic fibrosis. J Infect Dis
[18] Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early
Pseudomonas aeruginosa colonisation in patients with cystic fibrosis.
[36] Qin X, Emerson J, Stapp J, Stapp L, Abe P, Burns JL. Use of real-time PCR
with multiple targets to identify Pseudomonas aeruginosa and other
[19] Wiesemann HG, Steinkamp G, Ratjen F, Bauernfeind A, Przyklenk B,
nonfermenting gram-negative bacilli from patients with cystic fibrosis.
Doring G, et al. Placebo-controlled, double-blind, randomized study of
J Clin Microbiol 2003;41:4312–7.
aerosolized tobramycin for early treatment of Pseudomonas aeruginosa
[37] Cystic Fibrosis Foundation. Patient Registry 2002 annual data report.
colonization in cystic fibrosis. Pediatr Pulmonol 1998;25:88–92.
Bethesda, Maryland: Cystic Fibrosis Foundation; 2003.
[20] Rosenfeld M, Gibson R, McNamara S, Emerson J, McCoyd KS, Shell R, et al.
[38] Design of the Women's Health Initiative clinical trial and observational
Serum and lower respiratory tract drug concentrations after tobramycin
study. The Women's Health Initiative Study Group. Control Clin Trials
inhalation in young children with cystic fibrosis. J Pediatr 2001;139:572–7.
[21] Smith MJ, White LO, Bowyer H, Willis J, Hodson ME, Batten JC.
[39] Modi AC, Quittner AL. Validation of a disease-specific measure of
Pharmacokinetics and sputum penetration of ciprofloxacin in patients
health-related quality of life for children with cystic fibrosis. J Pediatr
with cystic fibrosis. Antimicrob Agents Chemother 1986;30:614–6.
[22] Klinger JD, Aronoff SC. In-vitro activity of ciprofloxacin and other antibacterial
agents against Pseudomonas aeruginosa and Pseudomonas cepacia from cysticfibrosis patients. J Antimicrob Chemother 1985;15:679–84.
Source: http://www.centre-reference-muco-nantes.fr/downloads/Reunions_medicales/octobre_2013/Anti-pseudomonal_acquisition_young_patients.pdf
Carotid endarterectomy—An evidence-based review: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology S. Chaturvedi, A. Bruno, T. Feasby, R. Holloway, O. Benavente, S. N. Cohen, R. Cote, D. Hess, J. Saver, J. D. Spence, B. Stern and J. Wilterdink Neurology 2005;65;794-801 DOI: 10.1212/01.wnl.0000176036.07558.82
Journal of Anxiety Disorders 23 (2009) 563–574 Contents lists available at Journal of Anxiety Disorders The use of virtual reality in acrophobia research and treatment Carlos M. Coelho ,Allison M. Waters , Trevor J. Hine Guy Wallis a School of Human Movement Studies, University of Queensland, Level 5, Building 26, St. Lucia, QLD 4072, Australiab Griffith Institute of Health and Medical Research and School of Psychology, Griffith University, Brisbane, Australiac Queensland Brain Institute, University of Queensland, Brisbane, Australia











