631-635
631-635 10/9/07 13:09 Page 631
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 20: 631-635, 2007
Effective microorganism fermentation extract (EM-X)
attenuates airway hyperreactivity and inflammation
through selective inhibition of the TH2 response
independently of antioxidant activity
JEONG-SU DO, HYO-JUNG SEO, JIN-KI HWANG, JUN-HEE KIM and SANG-YUN NAM
Department of Biological Science, Jeonju University, Jeonju, Korea
Received May 7, 2007; Accepted July 16, 2007
Abstract. The effective microorganism fermentation extract
and its prevalence and severity have risen dramatically in
(EM-X) is an antioxidant cocktail derived from the fermen-
recent decades (1,2). Allergic asthma is a complex inflam-
tation of plant material with effective microorganisms, and
matory disease of the airways characterized by reversible
its clinical application is being increasingly scrutinized. In
airflow obstruction (3) and is accompanied by chronic
the current study, the antiasthmatic effect of EM-X was
inflammation of the bronchial mucosa and a denudation of
investigated using a mouse model. Inhalation of EM-X during
the epithelial lining of the bronchi and bronchioli (4).
OVA challenge resulted in a significant reduction in airway
Murine models of pulmonary inflammation and airway
hyperreactivity (AHR) and airway recruitment of leukocytes
hyperresponsiveness have demonstrated that CD4+ type 2
including eosinophils. However, the level of 8-isoprostane in
helper T (TH2) cells play a pivotal role in allergic asthma.
bronchoalveolar lavage fluid (BALF), a marker of oxidative
Being activated with allergen, they secrete cytokines which are
stress in asthmatic patients, was unaltered by EM-X inhalation.
involved in the production of IgE and the activation and
Instead, ELISA data showed that levels of IL-4, IL-5 and
airway recruitment of inflammatory leukocytes (5). This
IL-13 in BALF or lung tissues were significantly lower in
knowledge has come from extensive investigation showing
EM-X-inhaling mice than in the control mice, but not the
that depletion of CD4+ T cells and neutralization of TH2
IFN-γ level. A considerably lower amount of Ag-specific IgE
cytokines abolishes allergic asthma in mouse models (6-9).
and IgG1 was detected in the serum of EM-X-inhaling mice
Activated eosinophils are another major source of multiple
than in the serum of the controls, whereas their IgG2a
TH2 cytokines which are known mediators of asthma (10-
secretion was similar. In addition, Ag-specific
ex vivo IL-4,
13). Of those, IL-4 is crucial for TH2 development and
IL-5 and IL-13 production of draining lymph node cells was
induction of IgE synthesis (14,15), and IL-5 regulates
markedly diminished by EM-X inhalation, but not IFN-γ.
eosinophil activation, recruitment and maturation in the bone
These data clearly show that inhaled EM-X suppresses type 2
marrow (16-18). IL-13 has very similar biological activities
helper T (TH2), but not type 1 helper T (TH1), response. In
to IL-4 (19), although their action in T cell proliferation and
conclusion, inhalation of EM-X attenuates AHR and airway
differentiation is distinctly displayed, that is, directly by IL-4
inflammation which results from selective inhibition of the
(20) or indirectly by IL-13 (21).
TH2 response to allergen, but independently of antioxidant
Oxidative stress, an imbalance between reactive oxygen
activity. Our data also suggest that EM-X may be effectively
species and the body's defense system, has also been described
applied for control of allergic asthma.
as a pathological mediator in allergic asthma. Oxidativestress associated with a large variety of reactive oxygen
species plays an important role in airway inflammationthrough epithelial cell damage and the loss of cellular
Asthma is a major health problem worldwide. There is
integrity (22,23). Although profound insights have been made
currently an epidemic of this disease in the western world,
into the pathology of asthma so far, the exact mechanismsinducing and regulating the disease still remain elusive.
The effective microorganism fermentation extract (EM-X)
is a refreshment drink, and it is produced by fermentation ofunpolished rice, papaya, and seaweed with effective micro-
Correspondence to: Professor Sang-Yun Nam, Department of
organisms including photosynthetic bacteria, lactic acid
Biological Science, Jeonu University, 3-1200 Hyoja-dong, Jeonu
bacteria and yeast (24). EM-X inhibits
in vitro growth and
reduces the regenerative potential of cancer cells (25). EM-X
E-mail:
[email protected]
also
in vivo protects the liver and kidney from oxidativestress-dependent damage (26) and shows anti-inflammatory
Key words: effective microorganism fermentation extract, airway
(27) and neuroprotective effects for retinal (28) and dopa-
hyperreactivity, inflammation, TH2 response, cytokine
minergic neurons (29). Although the underlying mechanisms
631-635 10/9/07 13:09 Page 632
DO
et al: EM-X ATTENUATES AHR AND AIRWAY INFLAMMATION
are unclear, accumulated evidence shows that the antioxidant
Collection of serum. After mice were anesthetized, blood was
properties displayed by flavonoids, saponins, ubiquinones,
extracted by cardiac puncture. Blood was allowed to clot at
lycopene and vitamin E are most probably associated with
room temperature for 30 min, and the serum recovered by
the activities of EM-X (26,27,30). Despite a recently
centrifugation (10,000 rpm, 5 min at 4˚C) was stored at 70˚C
increasing number of reports on the clinical use of EM-X, its
antiasthmatic action has never been described. Here, wereport conclusive evidence showing the potential of EM-X
Preparation of peribronchial lymph node (LN) cells. The LN
for clinical use to prevent or control asthma and other allergic
cells were derived from the paratracheal and parabronchial
diseases by selective suppression of the TH2 response but
regions. Freshly isolated LN cells were made into single-cell
independently of antioxidant activity.
suspensions, and contaminated erythrocytes were lysed byhypotonic shock with sterile distilled water. Cells were
Materials and methods
stimulated with OVA (200 μg/ml) at 1x106 cells/ml in IMDMmedium (Gibco Laboratories, Grand Island, NY) supplemented
Animals. Female BALB/c 5- to 6-week-old mice were
with 10% heat-inactivated FBS (Gibco), 100 U/ml penicillin,
supplied from Samtaco (Osan, Korea), the Korean branch of
100 μg/ml streptomycin, 0.25 μg/ml amphotericin B (Sigma)
Taconics (Germantown, NY). The mice were maintained in
and 5x10-5 M 2-mercaptoethanol (2ME).
an environmentally controlled rearing system and used forexperiments when 7- to 8-weeks old. Mice were age-matched
Enzyme-linked immunosorbent assay (ELISA). The levels
for each experiment, and all experiments in this study were
of the cytokines in the culture supernatants and Ig in the
performed in accordance with Jeonju University Institutional
serum were determined in appropriate dilutions by sandwich
Animal Care and Use Committee guidelines.
Induction of allergic airway inflammation. All mice were
IgG2a (BD Pharmingen) following the manufacturer's
sensitized and challenged with OVA (chicken egg albumin,
grade V; Sigma, St. Louis, MO). Systemic sensitization wasperformed by two i.p. injections of 50 μg OVA absorbed to
8-isoprostane assay. The levels of 8-isoprostane in BALF
1 mg alum (aluminum ammonium sulfate, Sigma) in 0.3 ml
were determined by competitive ELISA (Cayman Chemical,
PBS (phosphate-buffer saline, pH. 7.4) on days 0 and 7. On
Ann Arbor, MI) according to the the manufacturer's
days 15, 16 and 18, mice were anesthetized by i.p. injection
of Avertin (2.5% wt/vol in PBS) and intranasally challengedwith 2% OVA in PBS (50 μl/mouse).
Statistical analysis. Statistical analysis and graphicalpresentation were conducted using SigmaPlot 6.0 (SPSS Inc.,
EM-X and treatment. EM-X was kindly supplied by EM
Chicago, IL). Values were provided as the means ± SE, and
Korea (Jeonju, Korea). Mice inhaled aerosol containing 16-
group means were compared with the Student's t-test in
fold diluted EM-X in PBS with a nebulizer (Schuco 2000,
which p<0.05 was considered significant.
Allied Health Care Products, St. Louis, MO) from day 14 for5 consecutive days.
Measurement of airway hyperreactivity (AHR). AHR was
EM-X inhalation attenuates AHR in a mouse model of
measured 24 h after the last OVA challenge (day 19), by
asthma. To examine the effect of EM-X in the development
recording respiratory pressure curves in response to inhaled
of allergen-induced AHR and lung inflammation, we
nebulized methacholine (acetyl-ß-methylcholine chloride;
sensitized and challenged mice with OVA. As shown in Fig. 1,
Sigma) using whole-body plethysmography (All-Medicus,
control mice developed significant AHR, however, the Penh
Seoul, Korea) as previously described (31). AHR was
values in EM-X-inhaling mice, sensitized and challenged in
expressed in enhanced pause (Penh), an index of airway
the same manner, were significantly lower than in the control
obstruction. Penh values were obtained for 5 min and
mice when they were elicited with increasing concentrations
averaged. Mice were exposed to a series of methacholine
of methacholine. Significant differences in Penh value were
aerosols (0, 6.25, 12.5, 25 and 50 mg/ml).
shown at 25 and 50 mg/ml methacholine (p<0.05, n=12).
These results show that EM-X exerts an inhibitory effect on
Analysis of BALF (bronchoalveolar lavage fluid). BALF
AHR in asthmatic mice.
was obtained immediately after bleeding of the mice bylavage of the airways through a tracheal cannula with PBS.
EM-X inhalation suppresses lung inflammation. To validate
For differential BALF cell counts, cytospin preparations
that EM-X also effectively reduces lung inflammation, the
were made (1,000 rpm, 5 min) using a cytocentrifuge
airway recruitment of leukocytes was analyzed. The total
(Shandon Southern Products, Runcorn, Cheshire, UK) and
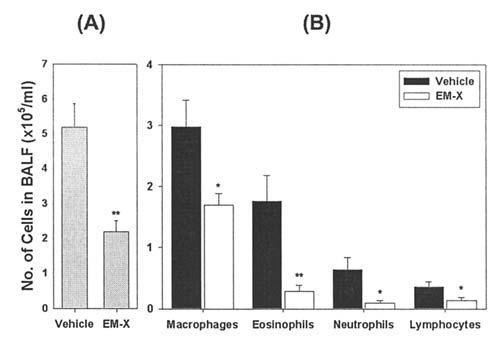
number of leukocytes in BALF was severely decreased in
stained with Diff-Quick (Baxter Healthcare, Miami, FL). For
EM-X-inhaling mice (2.2±0.3x105 cells/ml), when compared
each cytospin preparation, a minimum of 500 cells was
to those in BALF of the control mice (5.2±0.3x105 cells/ml;
counted and differentiated into macrophages, eosinophils,
p<0.01, n=10) (Fig. 2A), and the absolute numbers of
neutrophils and lymphocytes by morphology and staining
leukocyte subpopulations were also significantly reduced
characteristics.
631-635 10/9/07 13:09 Page 633
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 20: 631-635, 2007
Figure 3. Effect of EM-X inhalation on the level of 8-isoprostane in BALF.
BALF was recovered from non-immunized and asthmatic mice as describedin Fig. 2, and levels of 8-isoprostane were determined by competitive
Figure 1. Effect of EM-X inhalation on airway hyperreactivity in a mouse
ELISA. Data are shown as the cumulative means ± SE of 10 individual mice
model of asthma. Mice were sensitized with OVA at days 0 and 7, and then
from 3 independent experiments. **p<0.01 vs the control (PBS/Vehicle).
intranasally challenged with OVA on days 15, 16 and 18. Control miceunderwent the same procedure but received PBS instead of OVA.
Aerosolized EM-X was administered from day 14 for 5 consecutive days.
One day after last challenge, Penh was determined in response to increasingdoses of methacholine. Data are shown as the cumulative means ± SE of 12individual mice from 4 independent experiments. *p<0.05 vs each control(OVA/Vehicle).
Figure 4. Effect of EM-X inhalation on the cytokine levels in BALF. BALFand lung homogenate were recovered 1 day after last challenge, and levelsof IL-4 (A), IL-5 (B), IL-13 (C) or IFN-γ (D) were determined by ELISA.
Data are shown as the cumulative means ± SE of 10 individual mice from 3independent experiments. *p<0.05 and **p<0.01 vs vehicle control.
Figure 2. Effect of EM-X inhalation on airway inflammation in a mousemodel of asthma. Mice were immunized and EM-X was administered as
EM-X inhalation diminishes type 2 cytokine and Ig levels
described in Fig. 1. After examination of AHR, the trachea was cannulated
in vivo. The above results allow us to hypothesize that EM-X
and the lungs were lavaged. Total number of viable leukocytes (A) in
inhalation leads to immune modification suppressing the
recovered BALF was enumerated, and differential cell counts (B) wereperformed on Diff-Quik-stained cytocentrifuge preparations. Data are
allergic response. To clarify this hypothesis, we evaluated
shown as the cumulative means ± SE of 10 individual mice from 3
in vivo levels of relevant cytokines. The levels of IL-5, IL-13
independent experiments. *p<0.05 and **p<0.01 vs vehicle control.
and IFN-γ in BALF of the controls were not significantlydifferent from those of EM-X-inhaling mice. However, thelevel of IL-4 in EM-X-inhaling mice was only half(221.2±68.4 pg/ml) of that in the control mice (471.7±97.4 pg/
EM-X inhalation exerts an antiasthmatic effect independently
ml; p<0.05, n=10). While the levels of IL-13 and IFN-γ in
of antioxidant activity. EM-X is well known as an anti-
the lung tissues were comparable between the two groups,
oxidant cocktail, and the antiasthmatic activity of EM-X may
IL-4 (108.0±33.4 pg/ml) and IL-5 (126.4±15.4 pg/ml) levels
be attributed to its antioxidant action. To address this issue,
in EM-X-inhaling mice were significantly lower than those of
we analyzed the level of 8-isoprostane in BALF, a marker of
the control mice (IL-4, 304.6±56.0, p<0.01; IL-5, 282.1±59.4 pg/
oxidative stress in asthmatic patients (32). The level of 8-
ml, p<0.05, n=10) (Fig. 4).
isoprostane in BALF was considerably increased by OVA
To assess the efficacy of EM-X on the Ag-specific humoral
challenge (from 6.2±0.9 to 57.7±8.1 pg/ml; p<0.01, n=10).
immune response, OVA-specific Ig levels in serum were
However, EM-X inhalation resulted in no significant
measured. As shown in Fig. 5, markedly lower levels of OVA-
difference (45.8±5.1 pg/ml) (Fig. 3), suggesting that the anti-
specific IgE and IgG1 were observed in the serum of EM-X-
asthmatic effect of EM-X is uncoupled with its antioxidant
inhaling mice (Fig. 5A and B), whereas their IgG2a secretion
was similar (Fig. 5C).
631-635 10/9/07 13:09 Page 634
DO et al: EM-X ATTENUATES AHR AND AIRWAY INFLAMMATION
Figure 5. Effect of EM-X inhalation on Ag-specific antibody secretion in vivo. After examination of airway lavage, blood was taken by cardiac puncture andserum was recovered. Ag-specific serum IgE (A), IgG1 (B) and IgG2a (C) levels were evaluated by ELISA. Data are shown as the cumulative means ± SE of6 individual mice from 3 independent experiments. *p<0.05 and **p<0.01 vs each control.
application has been validated by recent reports showing anin vivo protective effect on the liver and kidney (26) as wellas anti-inflammatory (27) and neuroprotective effects(28,29). Recently, clinical application for the treatment ofcancer and several intractable diseases is being increasinglyscrutinized (33). However, published data addressing theantiasthmatic effect of EM-X are still unavailable. In thecurrent study, using a mouse model, we investigated whetherin vivo EM-X treatment reduces allergic asthma.
Our data clearly showed that EM-X attenuates the allergic
response upon analysis of AHR and leukocyte recruitmentinto airways (Figs. 1 and 2). Inasmuch as the antioxidativepotential has been well documented as a major action of
Figure 6. Effect of EM-X inhalation on Ag-specific cytokine production of
EM-X, antioxidation was implicated as a mechanism under-
lymph node cells. One day after last challenge, peribronchial LN cells were
lying the antiasthmatic activity of EM-X. As a target molecule
prepared and stimulated with OVA for 3 days, and their ex vivo cytokine
to test the antioxidative outcome related with asthmatic
production was examined by ELISA. Data are shown as the cumulativemeans ± SE of 12 individual mice from 4 independent experiments. *p<0.05
responses, 8-isoprostane appeared the most likely candidate.
and **p<0.01 vs each control.
It is a marker of oxidative stress in asthmatic patients (32),and its concentrations are elevated during acute asthma anddecrease with recovery (34). However, analysis of the levelof 8-isoprostane in BALF revealed no difference between the
EM-X inhalation suppresses Ag-specific type 2 cytokine
EM-X-inhaling and control groups (Fig. 3). This result
production by peribronchial lymph node cells. Data in Fig. 4
suggests that the antiasthmatic activity of EM-X is uncoupled
clearly show that EM-X selectively suppresses the TH2
with antioxidative activity.
response. To further evaluate this effectiveness, we assayed
On the other hand, inhalation of EM-X resulted in a
ex vivo Ag-specific cytokine production of draining lymph
marked decrease in the TH2 response. EM-X inhalation
node cells. The numbers of peribronchial LN cells were not
decreased the in vivo secretion of IL-4 and IL-5 (Fig. 4) and
greatly different (data not shown), demonstrating that EM-X
OVA-specific IgE and IgG1 (Fig. 5) as well as ex vivo IL-4,
does not act as a booster for Ag-specific proliferation of
IL-5 and IL-13 production of OVA-stimulated LN cells
lymphocytes. However, their capacity to produce ex vivo
(Fig. 6). One possible explanation for the failure to show a
TH2 cytokines such as IL-4 (765.8±145.4 vs. 360.4±55.4 pg/
significant decrease in all TH2 cytokine levels in BALF is
ml, p<0.05), IL-5 (1563.4±239.3 vs. 761.1±178.8 pg/ml,
that protein quantity is variably detected depending on
p<0.05) and IL-13 (1238.5±132.7 vs. 591.5±68.3 pg/ml,
consumption by responding cells or decay under the local
p<0.01) was diminished by half when EM-X was inhaled,
environment. Clinical and experimental investigations have
whereas IFN-γ production was increased without significance
confirmed that allergen-specific CD4+ TH2-type cells (35,36)
and the cytokines IL-4 (14,15), IL-5 (16-18) and IL-13 (19)play central roles in initiating and sustaining an asthmatic
response by regulating the recruitment and/or activation ofairway mast cells and eosinophils. Our data in the present
Effective microorganism-X is a refreshment drink developed
study strongly affirms that EM-X inhalation attenuates
in Japan (24), and the potential of EM-X for clinical
allergic manifestations through selective inhibition of the
631-635 10/9/07 13:09 Page 635
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 20: 631-635, 2007
TH2 response and also suggest a therapeutic potential of
16. Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG
EM-X against other allergic diseases.
and Vadas MA: Recombinant human interleukin 5 is a selectiveactivator of human eosinophil function. J Exp Med 167: 219-224,
Despite intensive studies on pathogenesis as well as
therapy, asthma remains an increasingly prevalent disease of
17. Clutterbuck EJ, Hirst EM and Sanderson CJ: Human inter-
the industrialized nations (1,2). Yet, current asthma therapies
leukin-5 (IL-5) regulates the production of eosinophils in humanbone marrow cultures: comparison and interaction with IL-1,
are not cures, and only pharmacological control of asthmatic
IL-3, IL-6, and GMCSF. Blood 73: 1504-1512, 1989.
symptoms is being achieved in most asthmatics with anti-
18. Foster PS, Hogan SP, Ramsay AJ, Matthaei KI and Young IG:
inflammatory agents (37) and/or long-acting bronchodilators
Interleukin 5 deficiency abolishes eosinophilia, airway hyper-reactivity, and lung damage in a mouse asthma model. J Exp
(1,38). Therefore, there is the obvious need for a new therapy
Med 183: 195-201, 1996.
for severe asthma, and the use of EM-X as a TH2-suppressing
19. Huang SK, Xiao HQ, Kleine-Tebbe J, et al: IL-13 expression at
reagent for immunotherapy in allergic diseases is likely to
the sites of allergen challenge in patients with asthma. JImmunol 155: 2688-2694, 1995.
have great potential.
20. Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H
The active mechanism of EM-X is only beginning to be
and Kohler G: Disruption of the murine IL-4 gene blocks Th2
understood. Several lines of evidence have shown that the
cytokine responses. Nature 362: 245-248, 1993.
21. McKenzie GJ, Emson CL, Bell SE, et al: Impaired development
clinical efficacy of EM-X is largely attributed to the
of Th2 cells in IL-13-deficient mice. Immunity 9: 423-432,
antioxidant properties displayed by flavonoids, saponins,
ubiquinones, lycopene and vitamin E (26,27,30). The
22. Rahman I, Morrison D, Donaldson K and MacNee W: Systemic
oxidative stress in asthma, COPD, and smokers. Am J Respir
identification of the active component(s) responsible for the
Crit Care Med 154: 1055-1060, 1996.
antiasthmatic activity in EM-X will indeed extend its clinical
23. Henricks PA and Nijkamp FP: Reactive oxygen species as
potential for treatment of related diseases.
mediators in asthma. Pulm Pharmacol Ther 14: 409-420, 2001.
24. Higa T and Ke B: Clinical and basic medical research on EMX.
EMRO Okinawa, 2001.
25. Chui CH, Cheng GY, Ke B, et al: Growth inhibitory potential of
effective microorganism fermentation extract (EM-X) on cancer
1. ISAAC: Worldwide variation in prevalence of symptoms of
cells. Int J Mol Med 14: 925-929, 2004.
asthma, allergic rhinoconjunctivitis, and atopic eczema; ISAAC.
26. Aruoma OI, Deiana M, Rosa A, et al: Assessment of the ability
The International Study of Asthma and Allergies in Childhood
of the antioxidant cocktail-derived from fermentation of plants
(ISAAC) Steering Committee. Lancet 351: 1225-1232, 1998.
with effective microorganisms (EM-X) to modulate oxidative
2. Umetsu DT, McIntire JJ, Akbari O, Macaubas C and
damage in the kidney and liver of rats in vivo: studies upon the
DeKruyff RH: Asthma: an epidemic of dysregulated immunity.
profile of poly- and mono-unsaturated fatty acids. Toxicol Lett
Nat Immunol 3: 715-720, 2002.
135: 209-217, 2002.
3. Maddox L and Schwartz DA: The pathophysiology of asthma.
27. Deiana M, Dessi MA, Ke B, et al: The antioxidant cocktail
Annu Rev Med 53: 477-498, 2002.
effective microorganism X (EM-X) inhibits oxidant-induced
4. Wills-Karp M: Immunologic basis of antigen-induced airway
interleukin-8 release and the peroxidation of phospholipids
hyperresponsiveness. Annu Rev Immunol 17: 255-281, 1999.
in vitro. Biochem Biophys Res Commun 296: 1148-1151, 2002.
5. Coyle AJ, Le Gros G, Bertrand C, et al: Interleukin-4 is
28. Aruoma OI, Moncaster JA, Walsh DT, et al: The antioxidant
required for the induction of lung Th2 mucosal immunity. Am J
cocktail, effective microorganism X (EM-X), protects retinal
Respir Cell Mol Biol 13: 54-59, 1995.
neurons in rats against N-methyl-D-aspartate excitotoxicity
6. Gavett SH, Chen X, Finkelman F and Wills-Karp M: Depletion
in vivo. Free Radic Res 37: 91-97, 2003.
of murine CD4+ T lymphocytes prevents antigen-induced
29. Datla KP, Bennett RD, Zbarsky V, et al: The antioxidant drink
airway hyperreactivity and pulmonary eosinophilia. Am J
effective microorganism-X (EM-X) pre-treatment attenuates
Respir Cell Mol Biol 10: 587-593, 1994.
the loss of nigrostriatal dopaminergic neurons in 6-hydroxy-
7. Hogan SP, Koskinen A and Foster PS: Interleukin-5 and
dopamine-lesion rat model of Parkinson's disease. J Pharm
eosinophils induce airway damage and bronchial hyperreactivity
Pharmacol 56: 649-654, 2004.
during allergic airway inflammation in BALB/c mice. Immunol
30. Yuan HC: EM-X as a free radical scavenger. Center for
Cell Biol 75: 284-288, 1997.
Condensed Matter Science, National Taiwan University, Taipei,
8. Henderson WR Jr, Chi EY and Maliszewski CR: Soluble IL-4
receptor inhibits airway inflammation following allergen
31. Hamelmann E, Schwarze J, Takeda K, et al: Noninvasive
challenge in a mouse model of asthma. J Immunol 164:
measurement of airway responsiveness in allergic mice using
1086-1095, 2000.
barometric plethysmography. Am J Respir Crit Care Med 156:
9. Hamelmann E, Oshiba A, Loader J, et al: Antiinterleukin-5
766-775, 1997.
antibody prevents airway hyperresponsiveness in a murine
32. Baraldi E, Ghiro L, Piovan V, et al: Increased exhaled 8-iso-
model of airway sensitization. Am J Respir Crit Care Med 155:
prostane in childhood asthma. Chest 124: 25-31, 2003.
819-825, 1997.
33. Committee IEMCE: Proceedings of The 2nd International EM
10. Broide DH, Paine MM and Firestein GS: Eosinophils express
Medical Conference. IEMC Executive Committee Secretariat,
interleukin 5 and granulocyte macrophage-colony-stimulating
Okinawa, 2003.
factor mRNA at sites of allergic inflammation in asthmatics. J
34. Wood LG, Garg ML, Simpson JL, et al: Induced sputum 8-
Clin Invest 90: 1414-1424, 1992.
isoprostane concentrations in inflammatory airway diseases. Am
11. Desreumaux P, Janin A, Dubucquoi S, et al: Synthesis of inter-
J Respir Crit Care Med 171: 426-430, 2005.
leukin-5 by activated eosinophils in patients with eosinophilic
35. Walker C, Virchow JC Jr, Bruijnzeel PL and Blaser K: T cell
heart diseases. Blood 82: 1553-1560, 1993.
subsets and their soluble products regulate eosinophilia in allergic
12. Woerly G, Lacy P, Younes AB, et al: Human eosinophils
and nonallergic asthma. J Immunol 146: 1829-1835, 1991.
express and release IL-13 following CD28-dependent
36. Robinson DS, Hamid Q, Ying S, et al: Predominant TH2-like
activation. J Leukoc Biol 72: 769-779, 2002.
bronchoalveolar T-lymphocyte population in atopic asthma. N
13. Bandeira-Melo C, Sugiyama K, Woods LJ and Weller PF:
Engl J Med 326: 298-304, 1992.
Cutting edge: eotaxin elicits rapid vesicular transport-mediated
release of preformed IL-4 from human eosinophils. J Immunol
Camargo CA Jr: Corticosteroid therapy for acute asthma.
166: 4813-4817, 2001.
Respir Med 98: 275-284, 2004.
14. Liu Z, Liu Q, Hamed H, et al: IL-2 and autocrine IL-4 drive the
38. Nimmagadda SR, Spahn JD, Nelson HS, Jenkins J, Szefler SJ
in vivo development of antigen-specific Th2 T cells elicited by
and Leung DY: Fluticasone propionate results in improved
nematode parasites. J Immunol 174: 2242-2249, 2005.
glucocorticoid receptor binding affinity and reduced oral gluco-
15. Xu WF, Ji YY, Wu YD, Lin GM, Ye M and Sun B: Roles of
corticoid requirements in severe asthma. Ann Allergy Asthma
IL-4 and other factors in the trichosanthin-induced ovalbumin-
Immunol 81: 35-40, 1998.
specific IgE response. Acta Pharmacol Sin 22: 736-740, 2001.
Source: http://www.effektive-mikroorganismen.ch/wp-content/uploads/International_Journal_of_Molecular_Medicine_2007_PDF.pdf
Chemistry Notes for class 12 Chapter 16 Chemistry in Everyday Life Medicines or Drugs Chemicals which may be used for the treatment of diseases and for reducing the suffering from pain are called medicines or drugs. The branch of science which makes use of chemicals for the treatment of disseases [therapeutic effect] is called chemotherapy. Some important classes of drugs are
Original article 1 Chronic inhibition of NO synthesis per se promotes structural intimal remodeling of the rat aorta Marcos A. Rossi and Massimo Colombini-Netto Objective We characterized, using histomorphometry and thickened (60% increase in comparison with that of transmission and scanning electron microscopy, the controls and 65% thinner as compared with L-NAME- intimal remodeling of the thoracic aorta of








