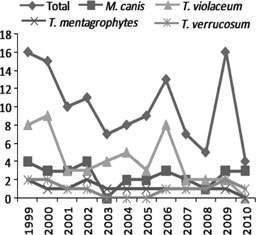
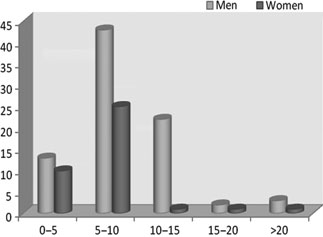
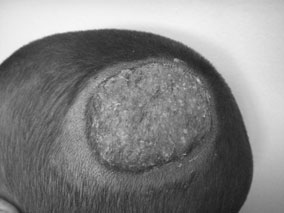
azal.az
Annex № 1 to the Order № February 15- "Rules of Carriage of Passengers, Baggage and Cargo" "Azerbaijan Airlines" Closed Joint Stock Company Table of contents 1. General provisions Terms, Definitions and abbreviations Compliance with laws and requirements of state authorities Electronic Ticket