Golder.co.nz

This article was downloaded by: [Mrs Mary Ann Muller]On: 19 June 2015, At: 11:15Publisher: Taylor & FrancisInforma Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House,37-41 Mortimer Street, London W1T 3JH, UK
Ozone: Science & Engineering: The Journal of the
International Ozone Association
Publication details, including instructions for authors and subscription information:
Oxidation of Emerging Contaminants during Pilot-Scale
Ozonation of Secondary Treated Municipal Effluent
Saileshkumar Singha, Rajesh Setha, Shahram Tabeab & Paul Yangb
a Civil and Environmental Engineering Department, University of Windsor, Windsor, Ontario
N9B 3P4, Canada
b Ontario Ministry of the Environment, Ontario M7A 1N3, Canada
Accepted author version posted online: 02 Feb 2015.Published online: 02 Feb 2015.
To cite this article: Saileshkumar Singh, Rajesh Seth, Shahram Tabe & Paul Yang (2015): Oxidation of Emerging Contaminants
during Pilot-Scale Ozonation of Secondary Treated Municipal Effluent, Ozone: Science & Engineering: The Journal of the
International Ozone Association, DOI:
To link to this article:
PLEASE SCROLL DOWN FOR ARTICLE
Taylor & Francis makes every effort to ensure the accuracy of all the information (the "Content") containedin the publications on our platform. However, Taylor & Francis, our agents, and our licensors make norepresentations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of theContent. Any opinions and views expressed in this publication are the opinions and views of the authors, andare not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon andshould be independently verified with primary sources of information. Taylor and Francis shall not be liable forany losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoeveror howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use ofthe Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematicreproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in anyform to anyone is expressly forbidden. T
Ozone: Science & Engineering, 37: 1–7Copyright 2015 International Ozone AssociationISSN: 0191-9512 print / 1547-6545 onlineDOI: 10.1080/01919512.2014.998755
Oxidation of Emerging Contaminants during Pilot-Scale
Ozonation of Secondary Treated Municipal Effluent
Saileshkumar Singh,Rajesh Seth,Shahram Tabe,and Paul Yang
1Civil and Environmental Engineering Department, University of Windsor, Windsor, Ontario N9B 3P4, Canada
2Ontario Ministry of the Environment, Ontario M7A 1N3, Canada
The transformation of 41 target emerging contaminants in
precautionary principle dictates that their discharge to the
secondary treated municipal wastewater effluent in Canada
environment should be limited to the extent possible.
was examined at pilot-scale, at transferred ozone doses of
Studies conducted in various parts of the world have
2.8 mg/L (0.46 O3/mg DOC) and 4.4 mg/L (0.72 mg O3/mgDOC). In general, transformation efficiencies of CECs either
detected the presence of several CECs in potable water
increased or were retained at the higher ozone dose. The
sources (Auriol et al. Benotti et al. Daughton and
higher ozone dose of 0.72 mg O3/mg DOC (Zspec = 0.6 mg
Ternes Huber et al. Tabe et al. Municipal
O3/mg DOC) was sufficient to transform 21 of the 31 detected
wastewater treatment plants are an important point source
CECs by over 80% as well as achieving the disinfection target
for many CECs released to such sources (Daughton and
of < 200 MPN E. coli per 100 mL.
Ternes Petrovi´c et al. It makes logical sense to
treat and remove the CECs from the municipal wastewater
Emerging Concern, Municipal Wastewater, Pharma-
effluent (MWWE) when their concentration is higher, rather
than from a water supply for potable water in which they
Secondary Treated Effluent, Wastewater Treatment
have been diluted several orders of magnitude (Oneby et al.
Tabe et al. The conventional technologies totreat MWWE are efficient in removing suspended solids,
organics, and nutrients. However, they are not effective inremoving many CECs that are present in trace quantities
Studies in the 1990s reported trace amounts of contami-
(Ternes Hence, new treatment technologies or addi-
nants in wastewater treatment plant effluent that could induce
tional treatment processes are required to remove these CECs,
estrogenic effects and were suspected to be responsible for
which are normally low molecular weight compounds in
the feminization of male fish in water bodies receiving such
the size range of about 150 to 500 Daltons (Snyder et al.
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
effluent discharges (Folmar et al. Harries et al.
Since then, several other trace contaminants of human and
The scientific community has been aware of the disinfec-
ecological health concern have been detected in waters and
tion property of ozone since the start of the 20th century.
wastewaters (Shon et al. Snyder et al. Ternes
Recent studies that demonstrated the potential of ozone to
et al. These contaminants are grouped commonly
oxidize and transform many CECs have renewed interest in
as contaminants of emerging concern (CECs), and include
the technology for wastewater treatment and reuse applica-
pharmaceutically active compounds (PhACs), personal care
tions (Bahr et al. Hansen et al. Snyder et al.
products (PCPs), fire retardants, and nanomaterials. Although
Ternes et al. Further benefits of ozonation of MWWE
evidence of actual observed environmental effects from expo-
include an increase in its dissolved oxygen levels, decreases
sure to CECs is limited (Bloetscher and Plummer
in its chemical oxygen demand, and improvement in aes-thetic characteristics due to reduction in turbidity and color.
Received 2/11/2014; Accepted 11/27/2014
In addition, due to the significant advances in the ozone man-
Address correspondence to Dr. Rajesh Seth, Civil and
ufacturing technology in the last couple of decades and the
Environmental Engineering Department, University of Windsor,
experience gained from studies on ozone treatment of water
401 Sunset Avenue, Windsor, Ontario, N9B 3P4, Canada. E-mail:
and wastewater, costs for ozone treatment have declined to
Ozonation of Secondary Treated Municipal Effluent
July–August 2015
become competitive with UV for disinfection (Drury et al.
biological treatment (activated sludge process). The plant
Oneby et al. and ozonation is now a matured
adds alum after grit removal to enhance coagulation and
technology for such applications (Leong et al.
phosphorus removal. It discharges the effluent with or with-
Although studies have demonstrated the potential for
out disinfection to Little River, which leads into the Detroit
ozonation to transform effectively many CECs present in
River. The plant meets the specified disinfection requirement
MWWE, such studies are still limited both in number and
of < 200 MPN E. coli/100 mL during the months of April–
list of CECs examined. Studies have further shown that the
October with UV disinfection. In this study, the municipal
effectiveness of such transformations by ozone is strongly
wastewater effluent (MWWE) before disinfection was the
dependent on the properties of CEC and the matrix, par-
feed water (influent) to the pilot unit.
ticularly the nature and concentration of dissolved organic
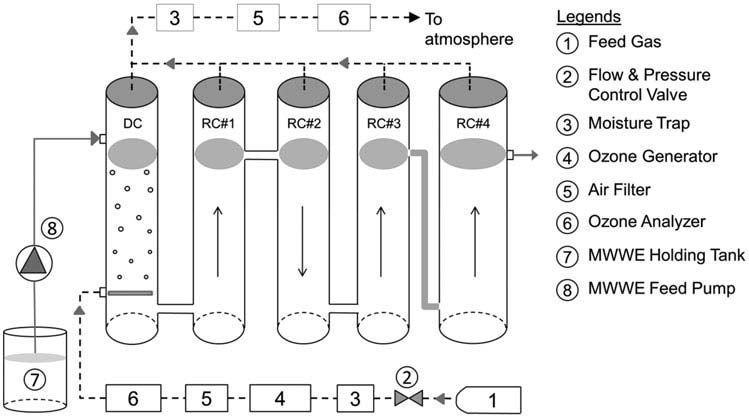
shows the schematics of the pilot unit. It includes
carbon (Huber et al. Uslu et al. In addition,
an ozone contactor, ozone generator (Lab2B, Triogen,
the list of CECs in the water environment continues to grow.
Glasgow, UK), and ozone monitor (Model 454, Teledyne,
Hence, there is a continued need to study the ozone and
San Diego, USA). A combination of dry air and pure oxy-
ozone-based advanced oxidation processes (AOPs) for dif-
gen (Praxair Canada) at a rate of 4 L/min was the feed
ferent wastewater matrices to better understand and apply
gas to generate ozone. The ozone contactor was comprised
them for wastewater treatment. The objective of this study
of a dissolution chamber (DC) and four reaction chambers
was to examine the effect of ozonation of secondary-treated
(RC#1 to RC#4). The material of contactor columns was clear
MWWE in Ontario, Canada on transformation of selected
PVC. The fittings were either ozone-resistant stainless steel
CECs as well as disinfection. The 41 selected CECs include
or Teflon. MWWE was collected in a 300-L feed tank from
antiphlogistics, antibiotics, lipid regulators, estrogen replace-
where it was transferred to the top of the DC by a peri-
ment agents, and reproductive hormones. The disinfection
staltic pump. A coarse bubble glass diffuser dispersed the air
target for MWWE in Ontario is < 200 MPN E. coli per
enriched with ozone at the bottom of the dissolution cham-
100 mL (Ontario MOE To the best knowledge of
ber. The water flowed countercurrent to the rising gas bubbles.
the authors of this article, this study was first of its kind in
Ozonated wastewater from the DC entered the first reaction
chamber (RC#1) from bottom and flowed upwards. Similarly,the flow entered the column RC#2 from top and the columnsRC#3 and RC#4 from bottom. The column RC#4 provided
MATERIALS AND METHODS
additional contact time to ensure that effluent from the pilot
unit did not contain any residual ozone. When operated incontinuous flow mode at a flow rate of 4 L/min, the hydraulic
A pilot plant was established at the Little River Pollution
retention time (HRT) in each of the DC and RC#1 - 3 was
Control Plant (LRPCP), Windsor, Ontario, Canada for ozone
1.7 min, and was 10 min in RC#4.
treatment of secondary-treated MWWE. LRPCP has a treat-
Dissolved organic carbon (DOC) and total organic car-
ment capacity of 73,000 m3/day. The treatment process
bon (TOC) was analyzed as per Standard Method 5310
consists of preliminary and primary treatment, followed by
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
FIGURE 1.
Schematic of the pilot unit.
S. Singh et al.
July–August 2015
(Clesceri et al. with a Shimadzu TOC-VCSH analyzer,
Transformation of CECs at TOD of 4.4 mg/L
Kyoto, Japan. The ozone monitor measured ozone concen-
(0.72 mg O3/mg DOC)
trations in the ozone-enriched feed gas and the vent gas.
presents the data related to the concentration of
These concentrations were used to calculate transferred ozone
the detected CECs, and their transformation on reaction with
dose (TOD) and ozone transfer efficiency. The ozone trans-
ozone at TOD of 4.4 mg/L (0.72 mg O3/mg DOC).
fer efficiencies were ∼50%. The concentration of residual
Significant variation in concentrations of CECs in MWWE
ozone in the aqueous phase was measured as per Method
was observed during the two experimental runs, which is quite
4500-O3 of Standard Methods (Clesceri et al. The
common in literature as well (Tabe et al. Ternes
UV254 absorbance was measured with a Varian Cary 50 Scan
although the concentration of ibuprofen during one of the
spectrophotometer (Palo Alto, CA, USA). The characteristics
experimental runs was unusually high which needs to be fur-
of MWWE before and after ozonation were determined in
ther investigated. The results show that ozonation is effective
the Environmental Engineering Laboratories at the University
in transforming the majority of the detected CECs.
of Windsor. Samples packed in ice were shipped overnight
The transformations of all drugs in the antiphlogistics
to Mass Spectroscopy Laboratory of Ontario Ministry of the
group were above 90%, except for ketoprofen for which the
Environment (Toronto, Canada) for the analyses of the target
range was 79–86%. Earlier studies have also reported above
CECs using LC/MS-MS.
90% reduction of acetaminophen, diclofenac, indomethacin,
The DOC of MWWE was similar during the two exper-
and naproxen at ozone doses up to 0.5 mg O3/mg DOC (Bahr
imental runs and measured at 6.1 mg/L and accounted for
et al. Reungoat et al. Reungoat et al. Rosal
greater than 95% of the TOC. MWWE pH was in the range
et al. For ibuprofen, studies have reported less than
6.9–7.1, temperature 18–21 ◦C, and alkalinity 95–180 mg/L
50% removal at ozone doses up to 0.5 mg O3/mg DOC and
as CaCO3. The ozone dose in the first experimental run was
above 90% transformation at an ozone dose of 0.8 mg O3/mg
8.8 mg/L (TOD = 4.4 mg/L or 0.72 mg O3/mg DOC). Ozone
DOC or higher (Bahr et al. Snyder et al. Ternes
doses in the second experimental run were 8.8 mg/L (TOD =
et al. Wert et al. The literature is variable on
4.4 mg/L or 0.72 mg O3/mg DOC) and 5.6 mg/L (TOD =
ketoprofen. At TOD/DOC ratio of 0.6–0.8, ketoprofen trans-
2.8 mg/L or 0.46 mg O3/mg DOC). The specific ozone con-
formation reported by Bahr et al. is 82%, then by Kim
sumption (Zspec), i.e., the ratio of ozone consumption to the
and Tanaka it ranges from 31 to 71%.
initial DOC, at TODs of 4.4 and 2.8 mg/L was calculated to
In the fluoroquinolones subgroup of antibiotics, transfor-
be 0.6 and 0.43 mg O3/mg DOC, respectively.
mation of ciprofloxacin and norfloxacin was in the range of71 to >99%. Rosal et al. and Kim and Tanaka have reported 67 to >99% transformation of these drugs at
ozone doses between 0.6 and 0.9 mg O3/mg C. The trans-
If the concentration of a CEC was below its detection limit
formation of enrofloxacin as quantified from one of the two
(DL) in ozone-treated MWWE, its transformation efficiency
experiments was only 23% as compared to >99% transfor-
was calculated only if its concentration in MWWE before
mation at TOD/ DOC ratio of ∼ 0.6 reported by Dodd et al.
ozone treatment was equal to or greater than five times the
DL. In addition, if a CEC had concentration below its DL in
In macrolides subgroup, the transformation efficiency of
the ozonated effluent, a value equal to half of its DL was used
the antibiotic lincomycin was close to 99%. This is consis-
to calculate its transformation efficiency.
tent with the finding by Rosal et al. and Kim andTanaka However, the transformation of erythromycinand roxithromycin at 50% and 3.5% was lower as compared
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
RESULTS AND DISCUSSION
with > 90% transformation previously reported for the twocompounds at similar ozone doses (Reungoat et al.
The results from three experiments from two experi-
Reungoat et al. Rosal et al. Schaar et al.
mental runs are presented. Out of the total 41 CECs ana-
The removal of chlortetracycline and oxytetracycline of
lyzed, the following eight CECs were not detected in both
the tetracyclines group of antibiotics was between 9 and
the experiments: carbadox, chloramphenicol, diethylstilbe-
38%. Mean transformation of doxycycline, meclocycline, and
strol, lasalocid A, monensin sodium, sulfachloropyridazine,
tetracycline was in the range of 75 to 98%. Dodd et al.
sulfadimethoxine, and warfarin. Results from other experi-
have reported >99% reduction of tetracycline from
ments (not reported) showed that the Ontario Ministry of
wastewater at a TOD/TOC ratio of around 0.3. No data were
the Environment's disinfection target of < 200 MPN E.
found in the literature on transformation of the remaining
coli/100 mL for MWWE was consistently met at TOD of
compounds in the group on ozonation of MWWE.
4.4 mg/L but not always at TOD of 2.8 mg/L. The MWWE
Ozonation reduced the concentration of sulfamethazine
E.coli concentrations in these experiments ranged between
and sulfamethoxazole drugs of the sulfonamide group of
2,500– 21,500 MPN/100 mL, which were reduced to between
antibiotics by over 99% and their concentration decreased
5–11 and 20–463 MPN/100 mL at TODs of ∼ 4.4 mg/L and
to below detection limit or close to the detection limit after
2.8 mg/L, respectively.
ozone treatment. These results are consistent with the findings
Ozonation of Secondary Treated Municipal Effluent
July–August 2015
Transformation of CECs at TOD of 0.72 mg O3/mg DOC
Ozonated Effluent
Antibiotic – Fluoroquinolones
Antibiotic – Macrolides
Antibiotic – Tetracyclines
Antibiotic – Sulfonamides
Antibiotic – Miscellaneous
Estrogen replacement agents
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
Bisphenol-A (BPA)
Ovulation Inhibitors
Reproductive Hormones
17-β-Estradiol (E2)
Legend: DL: Detection Limit Exp; Experiment ND; Not detected.
S. Singh et al.
July–August 2015
of other studies (Hollender et al. Huber et al.
Transformation of CECs at TOD of 2.8 mg/L
Reungoat et al. Snyder et al.
(0.46 O3/mg DOC) and TOD of 4.4 mg/L (0.72 mg
The transformation of antidepressant and lipid regulator
drugs such as carbamazepine, bezafibrate, clofibric acid, and
During the second experimental run, the MWWE was
gemfibrozil was above 90%. Bahr et al. have reported
treated with two different ozone doses. The concentrations
17 to 21% transformation of bezafibrate and clofibric acid at
of target CECs in MWWE are presented in (Exp-2).
ozone dose of 0.4 mg O3/mg DOC. They observed 74 to 98%
presents summary of the observed transformation
reduction at a higher ozone dose of 0.8 mg O3/mg DOC.
efficiencies of the detected CECs at the two ozone doses.
Hollender et al. have also reported average 88% and
The result shows that the number of CECs detected in the
66% removal of bezafibrate and clofibric acid at ozone doses
unozonated MWWE and but not in the ozonated MWWE
of 0.6 to 0.67 mg O3/mg DOC. Snyder et al. and
increased from three at the TOD of 2.8 mg/L to seven at the
Dickenson et al. have reported > 93% transformation
TOD of 4.4 mg/L. In general, the transformation of CECs
of gemfibrozil at an ozone dose of around 0.3 mg O3/mg C.
was higher at the higher ozone dose. The transformation of
Concentrations observed of most of the potential EDCs
BPA, carbamazepine, diclofenac, indomethacin, lincomycin,
(estrogen replacement agents, ovulation inhibitors, repro-
sulfamethoxazole, trimethoprim, and was more than 80% at
ductive hormones) were low. Transformations of 19-
both ozone doses. There was a noticeable increase in the
norethisterone, estrone (E1), estriol (E3), and bisphenol-A
transformation of naproxen, ciprofloxacin, bezafibrate, and
(BPA) were in the range of 93–99%, which is consistent
clofibric acid from 27–67% at TOD of 2.8 mg/L (0.46 O3/mg
with values reported in literature (Huber et al. Nakada
DOC) to 79– >99% at TOD of 4.4 mg/L (0.72 O3/mg
et al. Ternes et al. Wert et al. The trans-
DOC). Transformations of enrofloxacin, roxithromycin,
formation of E2 was in the range of 14 to 63%. Equilin
oxytetracycline, and chlortetracycline were below 30% at both
and 17-α-estradiol were detected in concentrations less than
the ozone dose.
10 ng/L in one sampling event only and their transformationafter ozonation was 44% and 33%.
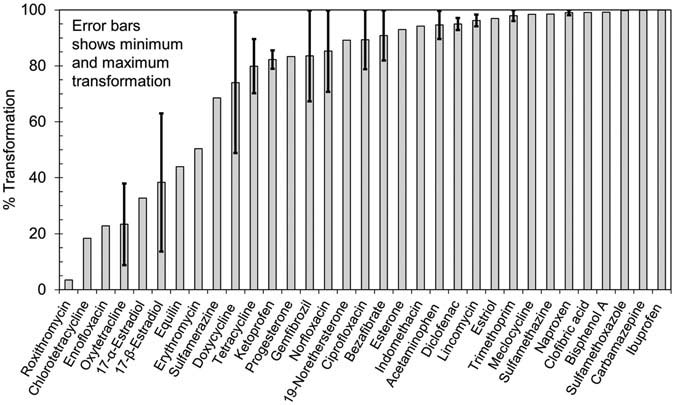
shows the transformation observed of CECs
at TOD of 4.4 mg/L. For 21 of 31 CECs for whichtransformation efficiency could be calculated, average trans-
This study reports results from pilot-scale ozone treat-
formation efficiency exceeded 80%. The average trans-
ment of secondary-treated municipal wastewater effluent in
formation of another six CECs including 17-α-estradiol,
Canada for 41 target CECs at TODs of 2.8 mg/L (0.46 O
17-β-estradiol, erythromycin, sulfamerazine, doxycycline,
DOC) and 4.4 mg/L (0.72 mg O
and tetracycline, was between 30–80%. For the remaining
3/mg DOC). In general, the
transformation efficiencies (TEs) of CECs either increased or
four (roxithromycin, chlortetracycline, enrofloxacin, and
maintained at the higher ozone dose. TEs of > 80% were
oxytetracycline; all antibiotics), the average transformation
observed at both ozone doses for seven CECs (bisphenol
was less than 30%.
A, carbamazepine, diclofenac, indomethacin, lincomycin,
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
FIGURE 2.
Transformation of CECs at TOD of 4.4 mg/L (0.72 mg O3/mg DOC).
Ozonation of Secondary Treated Municipal Effluent
July–August 2015
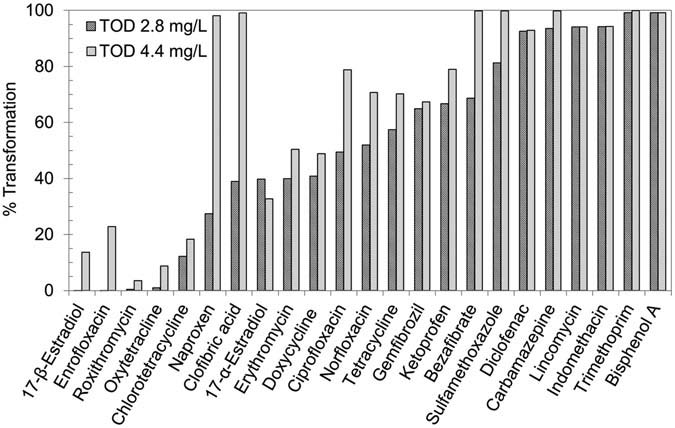
FIGURE 3.
Transformation of CECs at TOD of 2.8 and 4.4 mg/L.
sulfamethoxazole, and trimethoprim), while transformations
of Pharmaceutical Compounds and Pathogens – The Berlin Study.
of four CECs (enrofloxacin, roxithromycin, oxytetracycline,
Strasbourg: IOA.
Bahr, C., J. Schumacher, M. Ernst, F. Luck, B. Heinzmann, and M. Jekel.
and chlortetracycline) remained below 30%. The higher ozone
2007. "SUVA as Control Parameter for the Effective Ozonation of
dose of 0.72 mg O3/mg DOC (Zspec = 0.6 mg O3/mg
Organic Pollutants in Secondary Effluent." Water Science and Technology
DOC) was sufficient to transform 21 of the 31 detected
55(12): 267–274.
CECs by over 80% as well as to achieve the disinfection tar-
Benotti, M.J., R.A. Trenholm, B.J. Vanderford, J.C. Holady, B.D.
get of < 200 MPN E. coli per 100 mL. The findings are
Stanford, and S.A. Snyder. 2009. "Pharmaceuticals and EndocrineDisrupting Compounds in US Drinking Water." Environmental Science
in general consistent with studies conducted elsewhere in
& Technology 43(3): 597–603.
the world and demonstrate the effectiveness of ozonation in
Bloetscher, F., and J.D. Plummer. 2011. "Environmental Review and Case
simultaneously achieving disinfection and transformation of
Study: Evaluating the Significance of Certain Pharmaceuticals and
many CECs in secondary-treated municipal wastewaters in
Emerging Pathogens in Raw Water Supplies." Environmental Practice
Canada at transferred ozone dosages of around 0.7 mg O
13(3): 198–215.
Clesceri, L.S., A.E. Greenberg, and A.D. Eaton, editors. 1998. Standard
Methods for the Examination of Water and Wastewater, 20th Edition.
Washington, DC: American Public Health Association/American WaterWorks Association/Water Environment Federation.
Daughton, C.G., and T.A. Ternes. 1999. "Pharmaceuticals and Personal Care
Products in the Environment: Agents of Subtle Change?" Environmental
The authors acknowledge the assistance of Chris Manzon
Health Perspectives 107: 907–938.
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
and staff at Little River Pollution Control Plant, Bill
Dickenson, E.R.V., J.E. Drewes, D.L. Sedlak, E.C. Wert, and S.A. Snyder.
2009. "Applying Surrogates and Indicators to Assess Removal Efficiency
Middleton, and Matt St. Louis at the University of Windsor.
of Trace Organic Chemicals during Chemical Oxidation of Wastewaters."Environmental Science & Technology 43(16): 6242–6247.
Dodd, M.C., M.O. Buffle, and U. von Gunten. 2006. "Oxidation of
Antibacterial Molecules by Aqueous Ozone: Moiety-Specific ReactionKinetics and Application to Ozone-Based Wastewater Treatment."
The authors also acknowledge financial assistance from the
Environmental Science & Technology 40(6): 1969–1977.
Drury, D.D., S.A. Snyder, and E.C. Wert. 2006. "Using Ozone Disinfection
Ontario Ministry of the Environment.
for EDC Removal." Proceedings of the Water Environment Federation2006(12): 1249–1258.
Folmar, L.C., N.D. Denslow, V. Rao, M. Chow, D.A. Crain, J. Enblom,
J. Marcino, and L.J. Guillette, Jr. 1996. "Vitellogenin Inductionand Reduced Serum Testosterone Concentrations in Feral Male Carp
Auriol, M., Y. Filali-Meknassi, R.D. Tyagi, C.D. Adams, and R.Y. Surampalli.
(Cyprinus carpio) Captured Near a Major Metropolitan Sewage Treatment
2006. "Endocrine Disrupting Compounds Removal from Wastewater, a
Plant." Environmental Health Perspectives 104(10): 1096–1101.
New Challenge." Process Biochemistry 41(3): 525–539.
Hansen, K.M.S., H.R. Andersen, and A. Ledin. 2010. "Ozonation of
Bahr, C., M. Ernst, T. Reemtsma, B. Heinzmann, F. Luck, and M. Jekel.
Estrogenic Chemicals in Biologically Treated Sewage." Water Science
2005. Pilot Scale Ozonation of Treated Municipal Effluents for Removal
and Technology 62(3): 649–657.
S. Singh et al.
July–August 2015
Harries, J.E., D.A. Sheahan, S. Jobling, P. Matthiessen, M. Neall, J.P.
in a Full Scale Reclamation Plant Using Ozonation and Activated Carbon
Sumpter, T. Tylor, and N. Zaman. 1997. "Estrogenic Activity in Five
Filtration." Water Research 44(2): 625–637.
United Kingdom Rivers Detected by Measurement of Vtellogenesis in
Rosal, R., A. Rodríguez, J.A. Perdigón-Melón, A. Petre, E. García-Calvo,
Caged Male Trout." Environmental Toxicology and Chemistry 16(3):
M.J. Gómez, A. Agüera, and A.R. Fernández-Alba. 2010. "Occurrence of
Emerging Pollutants in Urban Wastewater and Their Removal Through
Hollender, J., S.G. Zimmermann, S. Koepke, M. Krauss, C.S. McArdell,
Biological Treatment Followed by Ozonation." Water Research 44(2):
C. Ort, H. Singer, U. von Gunten, and H. Siegrist. 2009. "Elimination
of Organic Micropollutants in a Municipal Wastewater Treatment Plant
Schaar, H., M. Clara, O. Gans, and N. Kreuzinger. 2010. "Micropollutant
Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration."
Removal During Biological Wastewater Treatment and a Subsequent
Environmental Science & Technology 43(20): 7862–7869.
Ozonation Step." Environmental Pollution 158(5): 1399–1404.
Huber, M.M., S. Canonica, G.Y. Park, and U. von Gunten. 2003.
Shon, H.K., S. Vigneswaran, and S.A. Snyder. 2006. "Effluent Organic Matter
"Oxidation of Pharmaceuticals During Ozonation and Advanced
(EfOM) in Wastewater: Constituents, Effects, and Treatment." Critical
Oxidation Processes." Environmental Science & Technology 37(5):
Reviews in Environmental Science and Technology 36(4): 327–374.
Snyder, S.A., E.C. Wert, D.J. Rexing, R.E. Zegers, and D.D. Drury. 2006.
Huber, M.M., A. Göbel, A. Joss, N. Hermann, D. Löffler, C.S. McArdell, A.
"Ozone Oxidation of Endocrine Disruptors and Pharmaceuticals in
Ried, H. Siegrist, T.A. Ternes, and U. von Gunten. 2005. "Oxidation of
Surface Water and Wastewater." Ozone-Science & Engineering 28(6):
Pharmaceuticals During Ozonation of Municipal Wastewater Effluents: A
Pilot Study." Environmental Science & Technology 39(11): 4290–4299.
Kim, I., and H. Tanaka. 2011. "Energy Consumption for PPCPs Removal by
"Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in
O3 and O3/UV." Ozone-Science & Engineering 33(2): 150–157.
Water: Implications for the Water Industry." Environmental Engineering
Leong, L.Y.C., J. Kuo, C.C. Tang, and Water Environment Research
Science 20(5): 449–469.
Foundation. 2008. Disinfection of Wastewater Effluent — Comparison of
Tabe, S., T. Jamal, R. Seth, C. Yue, P. Yang, X. Zhao, and L. Schweitzer. 2009.
Alternative Technologies. London: IWA Publishing.
"PPCPs and EDCs—Occurrence in the Detroit River and Their Removal
Nakada, N., H. Shinohara, A. Murata, K. Kiri, S. Managaki, N. Sato, and H.
by Ozonation." Water Research Foundation 1–207.
Takada. 2007. "Removal of Selected Pharmaceuticals and Personal Care
Ternes, T.A. 1998. "Occurrence of Drugs in German Sewage Treatment Plants
Products (PPCPs) and Endocrine-Disrupting Chemicals (EDCs) During
and Rivers." Water Research 32(11): 3245–3260.
Sand Filtration and Ozonation at a Municipal Sewage Treatment Plant."
Ternes, T.A., A. Joss, and H. Siegrist. 2004. "Scrutinizing Pharmaceuticals
Water Research 41(19): 4373–4382.
and Personal Care Products in Wastewater Treatment." Environmental
Oneby, M.A., C.O. Bromley, J.H. Borchardt, and D.S. Harrison. 2010.
Science & Technology 38(20): 392a–399a.
"Ozone Treatment of Secondary Effluent at U.S. Municipal Wastewater
Ternes, T.A., J. Stüber, N. Herrmann, D. McDowell, A. Ried, M. Kampmann,
Treatment Plants." Ozone-Science & Engineering 32(1): 43–55.
and B. Teiser. 2003. "Ozonation: A Tool for Removal of Pharmaceuticals,
Ontario Ministry of the Environment (MOE). 2008. Design Guidelines for
Contrast Media and Musk Fragrances from Wastewater?" Water Research
Sewage Works. Ontario, Canada: Ontario Ministry of the Environment.
37(8): 1976–1982.
Petrovi´c, M., S. Gonzalez, and D. Barceló. 2003. "Analysis and Removal of
Uslu, M.O., R. Seth, N. Biswas, S. Jasim, and S. Tabe, S. 2015. "Reaction
Emerging Contaminants in Wastewater and Drinking Water." Trac-Trends
Kinetics of Ozone with Selected Pharmaceutical Products and Their
in Analytical Chemistry 22(10): 685–696.
Removal Potential from Municipal Wastewater Effluents in the Great
Reungoat, J., B.I. Escher, M. Macova, F.X. Argaud, W. Gernjak, and J.
Lakes Basin." Ozone Science & Engineering 37(1): 36–44.
Keller. 2012. "Ozonation and Biological Activated Carbon Filtration of
Wert, E.C., F.L. Rosario-Ortiz, and S.A. Snyder. 2009. "Effect of
Wastewater Treatment Plant Effluents." Water Research 46(3): 863–872.
Ozone Exposure on the Oxidation of Trace Organic Contaminants in
Reungoat, J., M. Macova, B. I. Escher, S. Carswell, J.F. Mueller, and J. Keller.
Wastewater." Water Research 43(4): 1005–1014.
2010. "Removal of Micropollutants and Reduction of Biological Activity
Downloaded by [Mrs Mary Ann Muller] at 11:15 19 June 2015
Ozonation of Secondary Treated Municipal Effluent
July–August 2015
Source: http://www.golder.co.nz/modules.php?name=Publication&op=showlivepdf&sp_id=389&lang_id=3
The Future Mortality of High Mortality Countries: A Model Incorporating Expert Arguments Garbero, A., Pamuk, E., Garenne, M., Masquelier, B. and Pelletier, F. IIASA Interim ReportOctober 2013 Garbero, A., Pamuk, E., Garenne, M., Masquelier, B. and Pelletier, F. (2013) The Future Mortality of High Mortality Countries: A Model Incorporating Expert Arguments. IIASA Interim Report . IIASA, Laxenburg, Austria, IR-13-017
Personalisierte Medizin Wie ist es möglich, dass zwei Menschen mit der gleichen Krankheit unterschiedlich auf die Behandlung mit demselben Medikament reagieren? Die Antwort liegt in den Genen. 1. Weniger Nebenwirkungen dank Pharmakogenomik Vergleicht man das Erbgut zweier Menschen, zum Beispiel das Erbgut einer Schülerin und ihres Banknachbarn, so wird man feststellen, dass sich die beiden Genome an etwa 30 bis 60 Millionen Basenpaaren, den «Buchstaben» des Erbguts, unterscheiden (einzige Ausnahme: der Banknachbar ist zugleich der eineiige Zwilling). Das entspricht etwa 1 bis 2 Prozent des gesamten Erbguts. Noch vor fünf Jahren meinten Wissenschafter, dass sich zwei Menschen nur etwa zu 0,1 Prozent genetisch voneinander unterscheiden.











