Goodhealth-manchester.nhs.uk2
ACMD Advisory Council on the Misuse of Drugs
Chair: Professor Les Iversen
Secretary: Rachel Fowler
3rd Floor (SW), Seacole Building
2 Marsham Street
Tel: 020 7035 0555
Norman Baker MP, Minister of State for Crime Prevention Home Office 2 Marsham Street London SW1P 4DF
28 November 2013
Dear Minister, In May 2013, the ACMD advised that the synthetic benzofuran series of chemicals: 1-(benzofuran-5-yl)-propan-2-amine (5-APB) and 1(benzofuran-6-yl)-propan-2-amine) (6-APB) and some closely related analogues, be subject to a temporary class drug order. The Home Secretary and the Minister for Crime Prevention at the time, accepted this advice and a temporary class drug order came into force on 10 June 2013. These substances were marketed as a legal form of Ecstasy prior to this and there have been reported fatalities associated with these in the UK. The ACMD has followed its initial assessment with further consideration of the evidence available on 5- and 6-APB and related compounds in the context of the Misuse of Drugs Act (1971). I enclose this advice and generic definition with this letter. 5- and 6-APB are phenethylamine-type materials. The Misuse of Drugs Act (1971) controls certain substances of the phenethylamine family as class A substances, however, differences in their chemical structure mean that 5- and 6-APB and related compounds fall outside of these generic controls. The generic definition proposed in this report would bring these substances under the control of the Misuse of Drugs Act (1971). The ACMD therefore recommends that the compounds listed in the generic at Annex B, are controlled under the Misuse of Drugs Act (1971) as class B substances; and they should be scheduled under Schedule I of the Misuse of Drugs Regulations (2001) as the ACMD are not aware of any medicinal use.
Yours sincerely,
Professor Les Iversen
Cc: Home Secretary, Rt Hon. Theresa May MP
ACMD Advisory Council on the Misuse of Drugs
Benzofurans: A review of the evidence
Contents
1. Introduction
2. Background
3. Prevalence and Chemistry
4. Pharmacology
5. Medical harms
6. Social harms
7. Related Compounds
8. Recommendation
Annex A - Structures of MDA, 5- and 6-APB and
related materials, including ‘5-IT'
Annex B – Generic Definition
Annex C – Examples of compounds covered by
the proposed generic
References
1. Introduction
1.1. In early June 2012, the ACMD became aware of a suspected 6-APB
("Benzofury")-related death at the Scottish RockNess Music Festival, although this turned out not to be related to 6-APB, ACMD was prompted to review this series of chemicals. 5- and 6-APB were first mentioned in user forums in 2010 [http://www.erowid.or/chemicals/6-APB]. This report reviews the evidence of misuse and harm in relation to the benzofuran-type substances. The evidence concerning these substances and closely related analogues (see ―related compounds‖ and Annex A) was considered by the ACMD at meetings of the Novel Psychoactive Substances Committee and by the full Council in 2012 and 2013. A Temporary Class Drug Order (TCDO) for these substances and some closely related analogues came into force on 10 June 2013.1
2. Background
2.1. Two materials, 5- and 6-APB: 1-(benzofuran-5-yl)-propan-2-amine and 1-
(benzofuran-6-yl)-propan-2-amine) and their
N-methyl derivatives were sold as ―Benzofury‟ (the colloquial name presumably refers to the materials being benzofurans); (Jebadurai
et al., 2013). They are phenethylamine-type materials, related to methylenedioxyphenethylamines such
methylenedioxyamphetamine (MDA). 5- and 6-APB are structural isomers of one another. Benzofuran analogues of MDA were originally synthesised in the 1990s by David Nichols at Purdue University, who was examining the structure-activity relationships of ecstasy-type materials (Monte,
et al., 1993).
2.2. Chemically, 5- and 6-APB are two steps away from MDA (in the methylenedioxy
ring, one oxygen is replaced by a carbon and a double bond is present). As a result of these differences, they fall outside the generic controls on phenethylamine derivatives within the Misuse of Drugs Act, 1971.
2.3. Prior to the laying of a temporary class drug order the materials were being
marketed as a legal form of Ecstasy (for example: [www.officialbenzofury.com]). They were on sale as powder or as tablets, although the latter are always referred to as ―pellets‖, to avoid any overt suggestion that they are for human use. Packaging usually included statements that the materials are research chemicals and not for human consumption. ―Pellets‖ claimed to contain a 120 mg dose were being sold by this and other web sites at about £10 per pellet, with reductions for multi-unit purchases. Powder was being sold for about £35 per gram. The web sites, such as [www.officialbenzofury.com], no longer offer 5- or 6-APB but describe the ethyl analogue 5-EAPB: (1-(benzofuran-5-yl)-
N-ethylpropan-2-amine) as a legal alternative to 5- or 6-APB.
1 Following agreement at the ACMD Council meeting on 16 May 2013
3. Prevalence and Chemistry
3.1. 1-(Benzofuran-5-yl)propan-2-amine (5-APB) was patented by Briner
et al.,
(2000), and has been sold as a designer drug since 2010. Anecdotal user reports suggest that it has empathogenic and stimulant effects and that 5-APB is stronger than 6-APB. A positional analysis of the side chain of both compounds purchased from Internet sites has been published by Stanczuk
et al., (2013).
3.2. In 2011, the UK‘s Forensic Early Warning System (FEWS) identified 5-APB and
6-APB as materials as likely to be of interest and funded the development of certified reference standards to support forensic laboratories in their work. When these standards were received by forensic laboratories, it was found that standard analytical techniques could not distinguish between the two materials, so laboratory reports normally refer to materials being identified as ―5- or 6-APB‖. Vendors are therefore unlikely to be confident about which form they are selling.
3.3. There is analytical evidence of use of these compounds in the UK. For example,
5-APB was found in analyses of pooled urine from urinals in both London and the North West of England in July 2012 (Wood
et al., 2013 and Archer
et al., 2013).
3.4. However, reports from police seizures from head shops around the country, and
from the Serious Organised Crime Agency (SOCA) in 2011 and 2012 indicated that many products which claimed to contain ―benzofury‖ in fact contained other substances.2 These included piperazines (Class C), cathinone derivatives (Class B),
diphenylprolinol (D2PM), and caffeine, rather than 5- or 6-APB. Samples which did contain 5- and 6-APB were found in many different formats, sometimes in combination with other substances, including methiopropamine and caffeine.
3.5. Scotland Police reported that 5 & 6-APB are in use across the North of Scotland,
with anecdotal evidence that they are more prevalent in more remote areas (DEWS Report to ACMD of October 2013). Avon and Somerset Police reported that 5/6-APB was being used at their open prison to avoid the drug testing regime and is popular with inmates along with ―Spice‖ products.
3.6. Anecdotal evidence from drug workers also state that empty Benzofury product
packets are routinely found when doing outreach work in public places. Prior to legislation earlier this year, Benzofury products were seen in many ―legal high‖ shops and they were quoted as one of their most popular products.
3.7. A SOCA quarterly report on seizure results taken from forensic drug
examinations (26 September 2013) reported the following number of samples taken:
1. Q1 of 2013 (January-March)
2. Q2 of 2013 (April to June)
13 samples (3 specifically 5/6- APB)
12 samples (1 specifically 5- APB)
2 SOCA is now known as the National Crime Agency
3.8. Evidence on the prevalence of the benzofury compounds is mixed and there was
no evidence in recent self-report user survey of significant use (Global Drug Survey 2013). Evidence from local drug services shows that there are no presentations for treatment stating this as their main drug of choice, however, it is frequently mentioned as a drug that is used to ―top up‖ their primary drug of choice.
4. Pharmacology
4.1. Iversen, L.
et al. (2013), reported that 5- and 6-APB are potent triple monoamine
transport inhibitors for dopamine (DAT), norepinephrine (NET) and serotonin (SERT
) in vitro, with highest potencies for DAT and NET. Their potencies on the monoamine transporters are similar to those of 3,4-methylenedioxy-methamphetamine (―Ecstasy‖). Dawson
et al (2013) confirmed the effects of 5-APB as an inhibitor of DAT and SERT
in vitro, and voltammetric studies showed that the drug also slowed DA reuptake, and at higher concentrations promoted DA release in rat brain nucleus accumbens slices.
4.2. Iversen
et al (2013) also reported that 5- and 6-APB have high affinities for h5-HT
and hαC receptors. Furthermore,
in vitro functional assays revealed that both compounds were potent full agonists at the 5-HT2B receptor. Activation of 5-HT2B receptors by 5-APB, leading to contraction of rat stomach fundus
in vitro, was also reported by Dawson et al (2013). Setola
et al (2003) found that MDMA and MDA selectively interacted with h5-HT2B receptors and induced fenfluramine-like proliferative actions on human cardiac valve tissue
in vitro. The finding that MDMA, MDA, 5- and 6-ABP activate 5-HT2B receptors suggests that there is a potential risk of cardiac toxicity associated with their long-term use, as has been reported for other 5-HT2B receptor agonists, such as fenfluramine (Rothman
et al, 2000; Dawson & Moffatt, 2012).
5. Medical harms
5.1. A toxicology report from Preston Coroners described a death where a young
man consumed 6-APB, which was later confirmed in his urine, together with the anti-depressant mirtazapine. The paramedics unsuccessfully attempted resuscitation and were surprised at the elevated body temperature of the deceased 20 minutes after the resuscitation had finished. Such stimulants are known to cause elevated temperature (hyperpyrexia).
5.2. A case reported involving a 21-year old man who presented to an Emergency
Department with acute agitation and paranoia following the self-reported use of 6-APB purchased from the Internet and cannabis [Chan WL, 2013]. Subsequent screening of his urine confirmed the use of 6-APB (urine concentration 2000 ng/mL); also detected were metabolites of the synthetic cannabinoid receptor agonist JWH-122, the tetrahydrocannabinol metabolite 11-nor-9-carboxy-delta-9-tetrahydrocannabinol, 6-(2-methylaminopropane)benzofuran (6-MAPB) (30 ng/mL), amphetamine (90 ng/mL), chloroquine (5 ng/mL), ketamine metabolites (3 ng/mL), and ephedrine (800 ng/mL). Since there were a number of other
substances detected, it is possible that some or all of these other substances may have contributed to the clinical features seen in this individual.
5.3. The National Poisons Information Service TOXBASE reported 65 telephone
enquiries from health professional relating to 5- or 6-APB in the period from March 2009 to March 2013. In the same period there were 741 accesses to TOXBASE online for information on these compounds.
5.4. The Scottish Borders Alcohol and Drugs Partnership reported a death in 2012
where a young male had consumed ―Benzofury‖. 2-APB (a structural isomer), methiopropamine and cocaine were listed in the pathology report as the cause of death (2-APB is normally only seen as an impurity in samples of 5- and 6-APB). From the same party, 7 other individuals were admitted to hospital but were later discharged. Symptoms were tachycardia, increased blood pressure and hyperpyrexia.
5.5. Scottish Fatalities Unit (North) reported two deaths in 2012: one involving 6-APB,
5-IT and ecstasy; the other involving 6-APB and 5-IT only.
5.6. The Home Office‘s ‗drug early warning system‘ (DEWS) confirmed one
Benzofury-related death, reported in September 2013. The final pathology result was 2-aminopropyl-benzofuran, methiopropamine and cocaine toxicity. The DEWS also reported one client completed survey indicating Benzofury use in the last 12 months.
5.7. Detectable levels of 5/6-APB were found in one patient who had allegedly
consumed the controlled substance methoxetamine and presented with acute toxicity to an A&E department (Wood
et al., 2012).
5.8. The ‗national programme of substance associated deaths‘ (npSAD) reported that
Benzofury was identified in 10 deaths on the npSAD mortality database (report of 23/09/13- since March 2012).
6. Social Harms
6.1. Prevalence of 5/6 APB is very low in surveys with young adults conducted in
nightclubs and festivals (personal correspondence with Professor Fiona Measham). They do not seem to be a drug of choice; they are not particularly prevalent and there is no evidence that they are associated with criminal behaviour, either violent or acquisitive crime. The limited evidence available to date indicates that the Benzofuran compounds have the potential to carry similar risks of personal and social harm as Ecstasy.
7. Related Compounds
7.1. 5- and 6-APB are closely related to 3,4-methylenedioxyamphetamine (MDA) –
(see structures at Annex A). When recommending the TCDO, the ACMD recommended that a variety of substances related to 5– and 6–APB available for sale also be controlled. All carry the potential harms associated with 5- and 6-APB and ecstasy (MDMA).
7.2. Closely related are 1-(2,3-dihydro-1-benzofuran-5-yl)propan-2-amine (5-APDB)
and 1-(2,3-dihydro-1-benzofuran-6-yl)propan-2-amine (6-APDB), which are the dihydro-derivatives of 5- and 6-APB, synthesised by Monte
et al (2003), in which the double bond in the five-membered ring is saturated. They have been offered for sale as ecstasy-like ―legal highs‖.
7.3. Indanylaminopropane (IAP) or 5-(2-aminopropyl-2,3-dihydro-1H-indene (5-APDI)
is a further material in this sequence, where both oxygen atoms of MDA have been replaced by methylene groups. This compound has also been offered as a ―legal high‖ and although reported to have much weaker effects than MDA when used on its own, it is claimed to mimic the effects of Ecstasy when used in combination with a stimulant such as amphetamine.
7.4. Related materials of interest include 5- and 6-(2-aminopropyl)indole (5- and 6-
API), which have structures similar to 5- and 6-APB, except that the oxygen atom in the heterocyclic ring is replaced by a nitrogen atom. 5-API is also known as 5-IT and was sold as a ―legal high‖. It is a positional isomer of alpha-methyltryptamine. Shulgin (1997) reported that an oral dose of 20 milligrams of 5-IT ―is a long-lived stimulant producing increased heart-rate, anorexia, diuresis, and slight hyperthermia for about twelve hours‖. Concern about this material at European level has resulted in the preparation of an EMCDDA-Europol joint report: [www.emcdda.eu/publications/joint-reports/5-IT].
7.5. The
N-methyl analogue of 5-APB was offered for sale as ―5-MAPB‖
[www.buyresearch chemicals.net; www. Buckledbonzi.com]. Structurally this material is closer to Ecstasy than the APBs.
7.6. The
N-ethyl analogue of 5-APB, 5-EAPB, (1-(benzofuran-5-yl)-N-ethylpropan—
2-amine) is currently offered for sale as a legal alternative to other benzofurans, controlled under the TCDO. 5-EAPB has been associated with a death at the recent ‗Brownstock‘ festival, although the full toxicology results were not available at the time of reporting.
8. Recommendation
8.1. The ACMD consider that, in the case of the compounds listed below and those
covered by the generic scope in Annex B, they are drugs that are being, or are likely to be, misused, and that misuse is having, or is capable of having, harmful effects. The ACMD recommends that they are controlled under the Misuse of Drugs Act, 1971, as Class B substances, and as they have no legitimate medical uses, as Schedule 1 substances under the Misuse of Drugs Regulations, 2001.
5- and 6-APB: (1-(benzofuran-5-yl)-propan-2-amine and 1-(benzofuran-6-yl)-
propan-2-amine) and their
N-methyl derivatives.
5- and 6-APDB: (1-(2,3-dihydro-1-benzofuran-5-yl)-propan-2-amine and 1-
5- and 6-IT: (2-(1H-indol-5-yl)-1-methylethylamine and 2-(1H-indol-6-yl)-1-
methylethylamine).
5-EAPB : (1-(benzofuran-5-yl)-N-ethylpropan-2-amine)
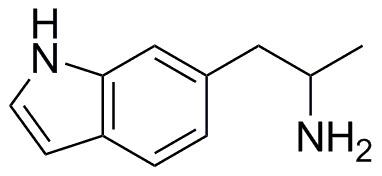
Annex A - Structures of MDA, 5- and 6-APB and related materials, including ‘5-
IT'
3,4-Methylenedioxyamphetamine (MDA); Class A, Schedule 1
5-(2-Aminopropyl)benzofuran (5-APB) 6-(2-Aminopropyl)benzofuran (6-APB)
5-(2-Aminopropyl)-2,3-dihydrobenzofuran 6-(2-Aminopropyl)-2,3-dihydrobenzofuran (5-APDB) (6-APDB)
5-(2-Aminopropyl) -2,3-dihydro-1H-indene (5-APDI) or Indanylaminopropane (IAP)
5-(2-Aminopropyl)indole (5-API or ‗5-IT‘) 6-(2-Aminopropyl)indole (6-API)
Annex B – Generic Definition
The following paragraph to be added to Schedule 1 Part 2 (Class B dugs)
Any compound (not being a compound for the time being specified in paragraph
1(ba) of Part 1 of this Schedule) structurally derived from 1-benzofuran, 2,3-dihydro-
1-benzofuran, 1H-indole, indoline, 1H-indene, or indane by substitution in the 6-
membered ring with a 2-ethylamino substituent whether or not further substituted in
the ring system to any extent with alkyl, alkoxy, halide or haloalkyl substituents and
whether or not substituted in the ethylamino side-chain with one or more alkyl
substituents.
Annex C- Examples of compounds covered by the proposed generic
5- and 6-APB and closely related compounds as Class B drugs
References
Archer JRH, Dargan PI, Chan WL, Hudson S, Wood DM (2013). Variability in recreational drugs and novel psychoactive substances detected in anonymous pooled urine samples from street pissoirs (street urinals) over time: A technique to monitor trends in drugs use. Clin. Toxicol. (Phila) 2013; 51:343-344. Baron, M.; Elie, M.; Elie, L. (2011). An analysis of legal highs-do they contain what it says on the tin? Drug Testing and Analysis 3: 576-581. Briner, K.; Burkhart, J. P.; Burkholder, T. P.; et al., (2000). Preparation of aminoalkylbenzofurans as 5-HT2C agonists PCT Int. Appl. WO 2000044737 A1 20000803. Chan WL, Wood DM, Hudson S, Dargan PI.(2013) Acute Psychosis Associated with Recreational Use of Benzofuran 6-(2-Aminopropyl)Benzofuran (6-APB) and Cannabis. J. Med. Toxicol. 2013;9: 278-281 Dawson,PO. Moffott, J.D. (2012). Cardiovascular toxicity of novel psychoactive drugs: Lessons from the past. Prog.Neuro-psychopharm. Biol. Pschiatr. 39: 244-252 Dawson,P., Opacka-Juffry, J., Moffatt, J.D., Daniju, Y., Dutta, N., Ramsey, J., Davidson, C. (2014) The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Progress in Neuro-Psychopharmacology and Biological Psychiatry 48, 57–63. Florvall, L. et al., (1986). Selective monoamine oxidase inhibitors. 3. Cyclic compounds related to 4-aminophenethylamine. Preparation and neuron-selective action of some 5-(2-aminoethyl)-2,3-dihydroindoles. J. Med. Chem. 29(8), 1406-12. Iversen, L., Gibbons S, Treble R, Sedtola V, Huang X-P, Rolth BL., (2013). Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 700: 147-151 Jebadurai J, Schifano F, Deluca P.(2013). Recreational use of 1-(2-naphthyl)-2-(1-pyrrolidinyl)-1-pentanone hydrochloride (NRG-1), 6-(2-aminopropyl) benzofuran (Benzofury/ 6-APB) and NRG-2 with review of available evidence-based literature. Hum. Psychopharmacol. 28: 356-64. Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE. (1993). Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4(methylenedioxy)amphetamine. J. Med. Chem. 36:3700-6. Rothman,R.B., Baumann M.H., Savage,J.E. et al. 2000. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac vulvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102: 2836-41. Setola, V., Hufeisen,S.J., Grande-Allen, J., Veseley, I., Glennon, R.A., Blough, B., Rothman,
(MDMA.‖Ecstasy‖) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol.Pharmac. 63, 1223-1229. Shulgin, A. (1997) ―TiHKAL: Tryptamines I have known and loved‖, Berkeley, Transform Press. Stanczuk A, Morris N, Gardner EA, Kavanagh P. (2013) Identification of (2-aminopropyl)benzofuran (APB) phenyl ring positional isomers in Internet purchased products. Drug Test. Anal. 5: 270-6. Troxler, F. et al.; (1968). Synthetic indole compounds. V. Syntheses of indoles with (2-aminoethyl)-, (2-aminopropyl)-, or alkanolamine side chains on the six-membered ring. Helvetica Chim. Acta. 51: 1616-28. Wood, D.M., Davies, S., Puchnarewicz, M., Johnston, A., Dargan P.I. (2012). Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur. J. Clin. Pharmacol. 68: 853-6. Wood DM, Archer JRH, Measham F, Hudson S, Dargan PI. (2013) Detection of use of novel psychoactive substances by attendees at a music festival in the North West of England. Clin. Toxicol. (Phila) 51: 340-341. Data on benzofury and its N-methyl derivatives, reported to the ACMD by the EMCDDA‘s EDND Personal communication received by the NPS Standing Committee from Avon and Somerset Police
Source: http://www.goodhealth-manchester.nhs.uk/downloads/drugs/2013-11-28%20Benzofuran%20compounds%20report%20-%20v1.0%20version%20for%20publication.pdf
Copyright ©1999 by Jim Shull All photographs by the author. All rights reserved. Amherst Media, Inc. Buffalo, N.Y. 14226 Fax: 716-874-4508 Publisher: Craig Alesse Senior Editor/Project Manager: Richard Lynch Associate Editor: Michelle Perkins ISBN: 0-936262-70-2 Library of Congress Card Catalog Number: 98-71750 Printed in the United States of America.
European Heart Journal (2011) 32, 2499–2506 Novel therapeutic concepts Hypertension management 2011: optimalcombination therapy Peter S. Sever 1* and Franz H. Messerli 2 1International Centre for Circulatory Health, Imperial College London, 59 North Wharf Road, London W2 1LA, UK; and 2Division of Cardiology, St Luke's and Roosevelt Hospitals,Columbia University College of Physicians and Surgeons, New York, NY, USA








