1471-2199-8-44.fm
BMC Molecular Biology
Research article
Hin-mediated DNA knotting and recombining promote replicon
dysfunction and mutation
Richard W Deibler†1,2,3, Jennifer K Mann†2,4, De Witt L Sumners4 and
Lynn Zechiedrich*1,2,4
Address: 1Interdepartmental Program in Cell and Molecular Biology, Baylor College of Medicine, Houston, Texas 77030-3411 USA, 2Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas 77030-3411 USA, 3Department of Systems Biology, Harvard Medical School, Boston, Massachusetts 02115 USA and 4Department of Mathematics, Florida State University, Tallahassee, Florida 32306-4510 USA
Email: Richard W Deibler* -
[email protected]; Jennifer K Mann -
[email protected]; De Witt L Sumners -
[email protected]; Lynn Zechiedrich -
[email protected]
* Corresponding author
Published: 25 May 2007
Received: 23 January 2007Accepted: 25 May 2007
BMC Molecular Biology 2007,
8:44
2007 Deibler et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: The genetic code imposes a dilemma for cells. The DNA must be long enough to
encode for the complexity of an organism, yet thin and flexible enough to fit within the cell. The
combination of these properties greatly favors DNA collisions, which can knot and drive
recombination of the DNA. Despite the well-accepted propensity of cellular DNA to collide and
react with itself, it has not been established what the physiological consequences are.
Results: Here we analyze the effects of recombined and knotted plasmids in
E. coli using the Hin
site-specific recombination system. We show that Hin-mediated DNA knotting and recombination
(i) promote replicon loss by blocking DNA replication; (ii) block gene transcription; and (iii) cause
genetic rearrangements at a rate three to four orders of magnitude higher than the rate for an
unknotted, unrecombined plasmid.
Conclusion: These results show that DNA reactivity leading to recombined and knotted DNA is
potentially toxic and may help drive genetic evolution.
crystalline state of 80 – 100 mg/ml ]. Understanding
Much of DNA metabolism is understood in the context of
how DNA functions requires understanding its conforma-
the linear sequence of nucleotides that compose the
tion under such compact conditions.
nucleic acid. For example, gene promoters, replication ori-gins, partitioning sequences and genes themselves are
DNA conformation is affected not only by crowding but
defined by their particular DNA sequences. However, the
also by its physical structure. Intuitively, anything long,
physical, mechanical and topological properties of DNA
thin and flexible can become self-entangled. Interestingly,
also exert significant influence over DNA metabo].
for 200 kb DNA molecules at thermal equilibrium, the
Inside cells, the long (1.6 mm for
Escherichia coli) and flex-
most energetically favorable conformation is the trefoil
ible (persistence length ≈ 50 nm) DNA must be com-
knot, 31 is 20-fold smaller than the chromo-
pacted into a very small volume, achieving a liquid
some of
E. coli. Thus, it is not surprising that when cells are
(page number not for citation purposes)
BMC Molecular Biology 2007,
8:44
lysed, a small portion ( 1%) of plasmid DNA, which is
intracellular DNA can alter its activity. This system ties
only on the order of 4 kb, is found knotted [. The
knots topologically identical to those observed
in vivo
propensity for DNA to knot is predicted to be even greater
. Although studying the effects of knots in chro-
for the longer and more folded eukaryotic chromosomes
mosomal DNA would be optimal, it is not technically fea-
ver, if we apply the figure of 1% DNA knotting
sible because there is no direct way to measure
to human chromosomes, then nearly every other diploid
chromosomal knotting. Therefore, we have examined
human cell would have a knot.
what happens when DNA strands collide to recombineand knot a 5.4 kb plasmid containing a gene required for
Although DNA knotting is clearly energetically favorable
cell survival. Plasmids appear to be a reasonable model
for DNA, several observations suggest that the intracellu-
for chromosomal metabolism. For example, supercoiling
lar environment should further exacerbate knotting.
changes in reporter plasmids ] mirror changes in the
Experiments with the bacteriophage P4 demonstrated that
supercoiling of the chromosome []. The recombined
the confinement of DNA in a small volume stimulates the
plasmid products generated by Hin are easily analyzed
knotting of DNA []. Furthermore, DNA inside the cell is
because of their small size. A recombination event occur-
negatively supercoiled. Negative supercoiling promotes a
ring in the chromosome would be much more difficult to
number of genetic processes, including gene expression
detect. Although Hin recombines and knots at the
hix
and DNA replication, in part because it promotes opening
sites, the resulting knots can move during DNA metabo-
of the DN]. DNA supercoiling also com-
lism. On the chromosome, this knot sliding could be as
pacts the DNA and brings distant strands into close prox-
far as the size of a topological dom], which
imity [uence, supercoiling promotes
would be more difficult to detect experimentally.
strand collision and DNA tangling. Indeed, computer sim-ulations have revealed that supercoiling should drive
Here we show that Hin-mediated site-specific recombina-
DNA knotting because writhe in a knot is less stressful on
tion and knotting led to dysfunction of the replicon and
the DNA than writhe in an unknotted, supercoiled mole-
blocked expression of a gene on the plasmid. This process
is highly mutagenic, and our results suggest that unlessrecombination and knotting are carefully controlled,
Collisions of DNA helices with one another are poten-
intracellular DNA can be unstable. We suggest that such
tially problematic because DNA is a self-reactive mole-
instability of the genetic material could help drive evolu-
cule. The repair of double strand breaks, single strand gaps
tionary variation.
and stalled replication forks involve recombination,which requires physical contact with a homologous DNA
molecule. Similarly, transposition, site-specific recombi-
nation and modulation of transcription (by enhancers
The experimental approach we use here to study the cellu-
and other
cis-regulatory elements) often involve DNA-
lar effect of recombining and knotting DNA is outlined in
DNA interactions. However, it has not been well estab-
Figure ave shown previously that Hin recom-
lished whether DNA strand collisions and the potential
bines and knots plasmid DNA in
E. coli that topoisomer-
resulting entanglements affect DNA metabolism in the
ase IV unti]. The Hin site-specific recombination
system models two
in vivo processes: it tangles the DNA tocreate knots identical to those formed inside the cell and
One indication that DNA knotting is deleterious to cells is
shuffles the DNA sequence to model DNA recombination
the universal prevalence of type-2 topoisomerases. These
e
hin recombinase gene is provided by the plas-
are essential enzymes that cleave both strands of a DNA
mid pKH66 (hereafter referred to as pHIN) and is
double helix, pass another duplex through this transient
expressed from the
tac promoter following induction by
gate and reseal the break. Type-2 topoisomerases are the
isopropyl-β-D-thiogalactopyranoside (IPTG). pHIN also
enzymes responsible for unknotting DNA, and, in
E. coli,
encodes for spectinomycin resistance.
E. coli cells harbor-
the responsibility falls solely on topoisomerase IV
ing pHIN also contained either pBR322 (pBR), which
The loss of topoisomerase IV activity has additional affects
lacks recombination sites and serves as a negative control,
in cells that include hyper-negative supercoiling and the
or one of two pBR22-derived plasmids pTGSE4 (pREC) or
inability to segregate newly replicated DN
pRJ862 (pKNOT) that carry sites recognized by the Hin
Therefore, the effects of knots needed to be evaluated sep-
recombinase. All three plasmids contain the
bla gene,
arately from supercoils and catenanes.
which encodes β-lactamase and provides resistance toampicillin. We used the
bla gene as a reporter to assess the
Here we use the previously characterized Hin site-specific
effects of recombining and knotting the DNA.
recombination and DNA knotting system [, tounderstand how the physical constraints placed upon
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
Figure 1 cal effects of Hin-mediated recombination/knotting
Physiological effects of Hin-mediated recombination/knotting. (A) Assay for the effect of knotting on the function of a
gene. The ovals represent E. coli cells. The Hin expression vector, pHIN, and plasmid substrates pBR, pREC and pKNOT con-
taining the bla gene (encoding β-lactamase) are depicted. Wild-type recombination sites are depicted as black arrows. The
mutant hix site is shown as a grey arrow. (B) Effect of DNA knotting on ampicillin sensitivity of E. coli strain W3110 containing
pHIN and either pBR, pREC or pKNOT. Single colonies were streaked from left to right across LB-agar that contained an amp-
icillin gradient and constant IPTG (1 mM) and spectinomycin (50 μg/ml) for Hin overexpression and maintenance. The experi-
ment was repeated five times in either strain C600 or W3110, and was carried out either from high to low or from low to high
ampicillin concentration with identical results. (C) Ampicillin sensitivity (MIC ) was quantified using the plate dilution method.
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
Hin binds two 26-bp recognition sites and makes double-
Hin-mediated recombination and knotting of a plasmid
stranded breaks at the center of these sites leaving a two-
alter function of a reporter gene
bp overhang within each break ext, Hin rotates
We first assessed the effect of Hin-mediated DNA recom-
the DNA strands in a right-hand direction as dictated by
bination and knotting on resistance to ampicillin con-
the required DNA negative superco. If both sites
ferred by the bla gene on pBR, pREC and pKNOT. LB-agar
are wild-type hix (or gix) sites (black arrows Figure
contained a gradient of ampicillin], a constant con-
in pREC, the two-bp overhangs are complimentary and
centration of spectinomycin to maintain the Hin expres-
religation may occur after a 180° rotation. However, if the
sion vector and IPTG to induce expression of Hin. Wild-
Hin substrate has one wild-type and one mutant site (grey
type E. coli K12 strains, C600 or W3110, containing pHIN
arrow Figure NOT, the overhangs are not
and either pBR, pREC or pKNOT were streaked across the
complimentary and a 360° rotation (or some multiple of
LB-agar. Whereas the strains containing pBR and pREC
360°) is necessary for religation to occur. Thus, Hin
were able to grow on the highest ampicillin concentra-
recombines pREC and knots pKNOT. Although we ini-
tions, growth of the strain carrying pKNOT was limited
tially anticipated that pREC would serve to differentiate
We determined whether this effect was spe-
between effects caused by recombination and those
cific to the gene encoded by the plasmid being targeted,
caused by DNA knotting, we (data not shown) and others
pKNOT, or caused a general increased susceptibility to
observed that, in vivo, Hin will occasionally mediate
antimicrobial agents. Knotting (and recombination) had
processive recombination events to knot plasmids con-
no effect on resistance encoded on a separate plasmid or
taining wild-type recombination sites at a steady-state
on the chromosome: strains harboring the three plasmids
level of 2 – 3% (Tab) [. pKNOT is extensively knot-
were all growth inhibited at identical concentrations of
ted by Hin because the mismatch between the sites
spectinomycin (resistance encoded by pHIN) or nor-
requires processive recombination [,] for religa-
floxacin (targets encoded by the chromosome) (data not
tion. Hin expression increases the steady-state knotting of
shown). These results indicate that the sensitivity of E. coli
pKNOT approximately 5- to 10-fold over endogenous lev-
to ampicillin is affected negatively when a knotted plas-
els in the presence of topoisomerase IV function and 35-
mid encodes its resistance. Knots impair the function of
to 45-fold when topoisomerase IV function is blocked
the replicon on which they form rather than cause a gen-
]. Twist knots with three (31), five (52) and seven
eral effect on the cell.
(72) negative nodes as well as composite knots with six(31#31), eight (31#52) and nine (31#31#31) nodes are
We determined minimal inhibitory concentration
readily generated when Hin knots pKNOT [. Higher
(MIC50) values (the ampicillin concentration that inhibits
noded knots are seen in decreasing abundance. Only twist
50% of bacterial growth) to quantify the Hin-mediated
knots with three (31) and four (41) nodes are seen when
sensitivity to ampicillin. Strains harboring pKNOT were
Hin knots a plasmid with two wild-type recognition sites
killed at a lower ampicillin concentration (1.4 mg/ml)
Previous studies examined the effect of Hin and
than pBR (4.7 mg/ml) or pREC (2.3 mg/m
other site-specific recombinases on gene expression
The intermediate sensitivity of the pREC-containing strain
]. However, a key distinction between those studies
to ampicillin compared to the pBR- and pKNOT-contain-
and the experiments performed here is that in those exper-
ing strains could be caused either by the intermediate level
iments the recombinase binding sites were placed
of DNA knotting that occurs in pREC or by Hin-mediated
between the promoter and the gene whereas here the
recombination of pREC.
reporter gene is distant to the site of recombination. Thus,here we examine the global effects on the DNA molecule
Hin recombination and knotting alter β-lactamase levels
rather than the local effects on promoter function.
To dissect the molecular mechanism by which Hin affectsthe function of the bla gene on pREC and pKNOT, we per-formed immunoblots to assay β-lactamase levels. Strainswere grown in liquid medium containing IPTG and spec-tinomycin until mid-logarithmic phase (OD600 = 0.3).
Table 1: Hin-mediated knotting.
in vivo + NORb
35% [21], 45% [25]
ano Hin inductionbnorfloxacin (to block unknotting by topoisomerase IV)
(page number not for citation purposes)


BMC Molecular Biology 2007, 8:44
Equal amounts of whole cell extracts were submitted to
shown). Therefore, the reduction in protein levels is spe-
SDS-PAGE and either stained with Coomassie blue or
cific to genes encoded on the plasmid rather than a gen-
subjected to Western blotting with anti-β-lactamase antis-
eral inhibition of gene expression. Subsequently, AcrA
er. The Coomassie blue stained gels indicated
levels were used to standardize loading.
that equal amounts of protein had been loaded (data notshown). The pHIN-containing C600 strain with pKNOT
If it is knotting that caused the increased susceptibility to
produced four- and three-fold less β-lactamase than the
ampicillin, then, because topoisomerase IV resolves knots
pBR- or pREC-containing strain, respectively, in either rich
in E. coli, inhibiting topoisomerase IV should increase the
(Lal M9 medium (Figure
amount of knots and cause an additional reduction of β-
reduction in β-lactamase levels correlates well with the
lactamase production from pKNOT. To test whether knot-
reduction in ampicillin resistance (compare Figure
ting increased ampicillin susceptibility, we utilized a tem-
wi There was no effect of pKNOT on levels of
perature-sensitive topoisomerase IV mutant, parCts.
the chromosomally encoded topoisomerase IV subunits,
Although cell growth and viability are reduced at the non-
ParC or ParE, or on levels of AcrA (Fand data not
permissive temperatures for parCts, cell division contin-
fect on β-lactamase protein levels
Hin-mediated effect on β-lactamase protein levels. Cultures of C600 (left) and parCts (right) were grown in rich (LB) or
minimal (M9) medium. Results here are from a typical experiment performed at 42°C. Immunoblotting was performed on total
cellular lysates. Blots were probed with anti-AcrA, anti-β-lactamase, anti-ParC or anti-ParE antibodies. Shown below the blot
from cells grown in LB is the mean of four independent experiments (except for parCts pREC, which was performed three
times) and standard deviations. The values below the M9 experiment show the quantification of that blot, but the M9 experi-
ment was repeated with the same results.
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
ues to occur and produces enough viable offspring that we
pBR or pREC relative to pHIN (Figure e was even
were able to obtain sufficient growth (OD600 = 0.3) to per-
less pKNOT DNA isolated from the parCts strain grown at
form immunoblots. When either of the non-permissive
42°CA, right side). When knotted, the copy
temperatures, 37°C or 42°C, for the parCts allele was
number of pKNOT was reduced from 20 – 40 copies/cell
used, the results were the same. pKNOT in the parCts
,] to lower than pHIN levels in parCts cells (Figure
strain produced 8.5-fold less β-lactamase than pREC and
β-lactamase levels were determined from immunob-
17-fold less than pBR when cells were grown in LB or M9
lotting and densitometry of total cell lysates following
medium at the non-permissive temperatur,
SDS-PAGE. Although the DNA knots caused a reproduci-
right panels). Therefore, inhibiting the enzyme that unties
ble reduction of pKNOT copy number, the magnitude of
the knots exacerbates the reduction in β-lactamase pro-
this effect (two- to six-fold) was never as large as the effect
duction. As with the MIC50 data above, it is unclear
on β-lactamase levels (eight- to twenty-fold). In addition,
whether the β-lactamase differences between pBR and
pREC copy number was unchanged although there was a
pREC are caused by Hin binding, recombining or knotting
less than two-fold decrease in β-lactamase production
pREC. There is less β-lactamase produced in the parCts
from pREC in parCts cells at 42°C. Therefore, decreased β-
harboring pKNOT than C600 containing pKNOT. How-
lactamase levels seen for pREC must not be at the level of
ever, the plasmids carried in the two strains have different
replicon copy number. The difference between the effect
superhelical densities. In the parCts strain, DNA is more
on β-lactamase levels and the effect on plasmid copy
negatively supercoiled at the non-permissive temperature
number suggests that DNA knots mediate their effects
e increased negative supercoiling should, if any-
through a combination of promoting replicon loss and
thing, slightly stimulate β-lactamase production. How-
blocking gene transcription.
ever, the knots counter this increase in β-lactamaseproduction. Thus, the inhibitory effects of DNA knotting
It was possible that some cells had lost all their plasmid
may be greater than measured because some effects are
DNA to become plasmid free and other cells retained nor-
potentially being masked by the increase in negative
mal plasmid levels or that all cells generally had reduced
plasmid levels. To distinguish between these possibilities,we grew cells harboring pHIN and either pBR, pREC or
Molecular analysis of Hin-mediated effects
pKNOT in the presence of IPTG and spectinomycin, under
It has been observed in vitro that DNA knots can diminish
conditions identical to those used to evaluate plasmid lev-
transcript]. Thus, the effect on β-lactamase produc-
els and β-lactamase production, except ampicillin was not
tion and ampicillin resistance we observed could be
included. Cell culture dilutions were spread on LB-agar
explained by an inhibition of bla transcription. However,
and incubated overnight at 30°C. The following day, the
it could also result from knots interfering with DNA repli-
colonies were replica plated onto LB-agar ± ampicillin,
cation, which would reduce the number of copies of the
grown overnight at 30°C and counted. The frequency of
bla gene and, consequently, the amount of β-lactamase
plasmid-free cells was the same among all the three strains
generated. An effect of DNA knotting on replication in
and was similar to what others have observed for loss of
vitro or in vivo has not been documented previously. Addi-
pBR in E. coli grown in a rich medium (LB) over the time
tionally, knots could reduce bla expression by causing
period comparable to the one used here ( 3 h) [. Thus,
pKNOT to break by weakening the tensile strength of
in these experiments, the Hin-mediated effect does not
DNA. Precedence of knots weakening and breaking poly-
lead to complete loss of pKNOT. However, it is possible
mers has been predicted by molecular dynamics simula-
that given enough time complete plasmid loss might
tions of polyethyleneown using optical
tweezers on actin filaments trated withsoft macroscopic strings [ ]. It
DNA catenanes are produced as intermediates of replica-
is possible that Hin mediates its effects through a combi-
tion and they accumulate in temperature-sensitive topoi-
nation of blocking transcription, interfering with replica-
somerase IV mutants at the non-permissive temperature
tion and breaking pKNOT.
,]. When DNA replication is disrupted, replicationcatenanes do not accumulate and data not shown).
To determine whether plasmid stability and copy number
We examined the levels of catenanes in parCts carrying
are affected by Hin activity we quantified the levels of
pHIN and either pBR, pREC or pKNOT. Plasmid DNA was
pBR, pREC or pKNOT DNA. Cultures were grown to mid-
isolated from parCts strains grown for 40 min. at 42°C as
logarithmic phase and divided in half. Plasmid DNA lev-
before, nicked to remove supercoiling and analyzed by
els were measured from one half and β-lactamase levels
high-resolution agarose gel electrophoresis ]. Cate-
from the other half. DNA levels were determined by den-
nated pBR and pREC products were clearly visible, but
sitometric analysis of agarose gels. Following Hin induc-
DNA catenanes were greatly reduced for pKNOT (Figure
tion, cells contained roughly two-fold less pKNOT than
e catenanes were seen under conditions where
(page number not for citation purposes)


BMC Molecular Biology 2007, 8:44
Hin-mediated effect
on plasmid replication
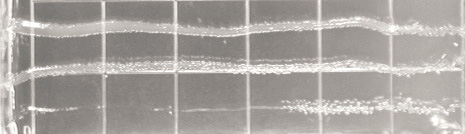
Hin-mediated effect on plasmid replication. (A) Plasmid DNA was isolated from strains C600 or parCts containing the
plasmids indicated. The DNA was linearized with HindIII, which cuts all the plasmids once, including pHIN. Shown is a repre-
sentative ethidium bromide-stained agarose gel from an experiment performed at 42°C. Given below the gel image are the
mean plasmid level values of three independent experiments with standard deviations. (B) Plasmid DNA was isolated 50 min.
after IPTG induction of Hin, nicked, displayed by high-resolution gel electrophoresis and visualized by Southern blotting. Shown
is an autoradiogram. All lanes contain plasmid DNA from the pHIN-harboring parCts cells with pBR, pREC or pKNOT. The
positions of nicked dimer (
), linear dimer (
) catenanes (e.g.,
) are indicated.
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
either bla was fully functioning (pREC) or impaired
ance (Table Ampicillin at 4.8 mg/ml (3.4-fold higher
(pKNOT), it does not seem likely that catenanes block
than the MIC50 for pKNOT) was high enough to block all
replication and transcription (or cause mutagenesis). This
growth and select for hyper-resistant mutants in the C600
experiment also provided an indirect method to examine
strain containing pHIN and pKNOT. At this concentration
the effect of knotted DNA on DNA replication. Because
of ampicillin, using the MSS maximum likelihood
DNA replication is the only source of the catenanes, the
method OT strain yields 3.4 × 10-6 muta-
observation that the level of DNA catenanes is reduced
tions/cell/generation. At an ampicillin concentration of
provides additional support that DNA replication is
16.1 mg/ml (3.4-fold higher than the MIC50), C600 con-
impaired in pKNOT.
taining pHIN and pBR yields 4.8 × 10-10 mutations/cell/generation. At an ampicillin concentration of 7.9 mg/ml
Hin-mediated recombination/knotting is mutagenic
(3.4-fold higher than the MIC50), C600 containing pHIN
We propose two models to explain how Hin blocks the
and pREC had a mutation rate of 4.7 × 10-7 mutations/
function of the bla gene (Fig. These possibilities are
cell/generation. Thus, Hin-mediated recombination and
not mutually exclusive. The first is the "roadblock" model:
knotting increased the mutation rate three to four orders
Hin, bound to or cleaving DNA, or the knots themselves
of magnitude compared to the spontaneous mutation rate
form an impasse to RNA and/or DNA polymerases. The
of cells with pBR and Hin expression (Table
second is the "breakage" model. Although it is not clearhow knots localize in DNA, it has been suggested from
To determine the molecular basis for the hyper-resistance
numerical studies that knots may spontaneously pull into
to ampicillin, plasmid DNA was isolated from mutant col-
a tight conforA within the cell is con-
onies and analyzed (FigurThere were two notable
stantly being subjected to a number of pulling forces gen-
and unanticipated features of these rearrangements. First,
erated by transcription, replication and segregation. A
the isolated plasmid DNA was much larger than the
force (15 pN) comparable to that generated by a single
parental pKNOT. This result was surprising because any
replication or transcription comp], has been
number of deletions or substitution mutations could dis-
shown to tighten a DNA trefoil to a diameter less than 25
rupt Hin recombination and these types of changes would
nm]. Either the roadblock or the breakage model
either result in a smaller plasmid or no change in plasmid
predicts that DNA knots would be mutagenic through rep-
size. However, these latter types of alterations were not
lication fork arrest or through a DNA double-strand break.
apparent. Second, we found that not only was pKNOT
In addition, either could induce the SOS response, which
altered, but pHIN was also changed in the hyper-resistant
could lead to a genome-wide increase in mutation fre-
mutants. Gross genetic rearrangements of the plasmid
were visible by restriction endonuclease digestion of eachsample (data not shown). These results suggest that
While measuring the ampicillin resistance of pBR-, pREC-
recombination between pHIN and pKNOT is responsible
and pKNOT-containing cells, we observed that, following
for the rearrangements and the phenotype of hyper-resist-
overnight growth, C600 or parCts strains harboring
ance to ampicillin. Although Hin does not directly recom-
pKNOT formed large, robust colonies in the zone of drug-
bine or knot pHIN, it is likely that recombination between
mediated clearing (Figure trast, no colonies
pHIN and pKNOT results in a fused plasmid that is either
were observed in the cleared zones around filter discs con-
refractory to additional knotting/recombination or
taining any concentration of ampicillin in lawns of C600
expresses β-lactamase at a sufficient level to confer hyper-
or parCts strains containing pBR or pREC (Figur
resistance to ampicillin. Without causing ampicillin
data not shown). We found that the effect was specific to
hyper-resistance, pKNOT-pKNOT fusions would not be
β-lactam (ampicillin or cefotaxime) resistance, as no col-
selected. In an attempt to analyze the role of homologous
onies were found in the zones of drug clearance for pBR-,
recombination in this plasmid rearrangement, we tried,
pREC- or pKNOT-containing C600 or parCts when nor-
but were unable to transform mutant strains lacking recA
floxacin was used (data not shown). These results suggest
or recD with pHIN.
that increased mutation rate is occurring specifically forpKNOT and not the genome as a whole.
The plasmid changes and ampicillin hyper-resistance wereheritable. Plasmid DNA was isolated from the colonies
One hundred percent of the colonies that grew in the
that arose in the zones of clearance and transformed into
drug-cleared zone had a higher resistance to ampicillin
C600 cells harboring pHIN. The plasmids conferred a
than the original pKNOT-containing C600 cells and 69%
higher level of ampicillin resistance than pKNOT as deter-
had higher resistance than pBR-containing cells (Figure
mined by Kirby-Bauer assay (data not shown). We found
ing the MIC50 values of the original pBR, pREC and
that in four of five transformants tested, the mutant plas-
pKNOT strains (Figurermed fluctuation
mid-transformed cells retained hyper-resistance to ampi-
assays to determine the mutation rate to ampicillin resist-
cillin and there were no visible colonies in the new zones
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
Intracellular DNA is supercoiled, compacted and highlyconcentrated. Consequently, DNA will collide frequently
with itself, and the result of these collisions increases the
potential for DNA recombining and knotting. We have
analyzed what can happen when the collisions lead to
recombining and knotting. The results are that both repli-cation and transcription are blocked and genetic rear-rangements are increased.
Mechanism of the Hin-mediated effect
ls for the Hin-mediated effect
DNA knots, and not recombination, are likely the pre-
Potential models for the Hin-mediated effect. Plasmid
dominant cause of Hin-dependent replication and tran-
pKNOT is recombined by Hin to knot the DNA (a single line
scription blocks and mutagenesis because the effect for
represents the central axis of the double helix). In the road-
pKNOT is more severe than for pREC. The effects were not
block model, the knot (or possibly Hin bound to or cleaving
caused by inherent differences in the three plasmids.
DNA) is impassable and stalls polymerase. Alternatively, in
Ampicillin MIC50 values of C600 strains harboring pHIN
the breakage model, knots may break DNA as a result of
and either pBR, pREC or pKNOT grown in the absence of
forces on the plasmid.
IPTG were identical (data not shown). In addition, themagnitude of the pKNOT-mediated effects was increased
of clearance. Because of this, it appears that either no fur-
by compromising the activity of the enzyme, topoisomer-
ther DNA rearrangements are occurring or, if they are,
ase IV, responsible for unknotting DNA. However, in
these additional rearrangements do not confer ampicillin
addition to unknotting, topoisomerase IV carries out two
hyper-resistance. The transformant (1/5) that behaved
other cellular tasks: decatenation (reviewed in []) and
similarly to pKNOT-containing strains indeed harbored
DNA supercoil re. Removal of the decate-
pKNOT. Thus, the fused mutant plasmid appears to have
nation activity of topoisomerase IV did not account for
resolved back into pHIN and pKNOT. To compensate for
the increased pKNOT-mediated effects because far more
reduced production of β-lactamase, the mutant plasmids
catenated replication intermediates were seen in parCts
could contain either a mutated bla gene that produces an
cells containing either pBR or pREC than in those that
enzyme more efficient at metabolizing ampicillin, or the
contain pKNOT (Figuree DNA supercoiling shift
mutations could allow for increased production of the
resulting from the inhibition of topoisomerase IV is not
enzyme. Using immunoblot analysis as described above,
enough to stimulate either the transcription of the super-
we found that all the cells carrying the rearranged plas-
coiling-dependent leu-500 prom or the λ inte-
mids that were examined had increased β-lactamase pro-
grase recombination system [in vivo, suggesting that
duction relative to pKNOT (Fig
the increase in negative supercoiling resulting from inhib-iting topoisomerase IV activity is unlikely to affect Hin
To determine whether the larger molecular weight plas-
recombination. It is possible that mechanistic differences
mid that had replaced pKNOT and pHIN contained DNA
in recombination on a substrate with two wild-type sites
originally present in both pHIN and pKNOT, we trans-
(pREC) compared to a substrate with one wild-type and
formed C600 with total plasmid DNA from the ampicillin
one mutant site (pKNOT) could account for the Hin-
hyper-resistant isolates. Plasmid DNA from four of the
mediated effects. For example, in a purified system, DNA
ampicillin resistant colonies was used in independent
cleavage by Hin is stimulated by a single mutant recombi-
transformations. For each transformation, half of the
nation ally, in vivo, DNA cleavage of
transformed cells was spread on LB-agar containing amp-
pKNOT by pHIN has been detected].
icillin (100 μg/ml, sufficient to select for the parentalpKNOT), and half was spread on LB-agar containing spec-
Plasmids replicate completely in less than six seconds and
tinomycin (50 μg/ml, to select for pHIN). We found that
do so asynchronously. Moreover, they transcribe con-
64/64 spectinomycin resistant transformants were also
stantly. Thus, a slight increase of a lethal DNA form could
resistant to ampicillin and 28/32 ampicillin resistant
have large consequences. Although topoisomerase IV rap-
transformants were also resistant to spectinomycin. These
idly unties knots, perhaps knot-induced problems, such as
results are consistent with a fusion between pHIN and
stalled replication forks, or stalled or blocked transcrip-
pKNOT being responsible for the ampicillin hyper-resist-
tion, persist longer than the knots themselves. Indeed
ance phenotype observed in the majority of mutants.
because topoisomerase IV can resolve DNA knots as theyare formed, then, as the copy number of the plasmid goesdown, there should come a point at which topoisomerase
(page number not for citation purposes)



BMC Molecular Biology 2007, 8:44
MT7 MT19 MT1 MT4 MT5 MT9 MT10 MT1 MT12 MT21 MT8 MT13 MT2 MT3 MT6 MT27 MT15 MT16 MT17 MT18 MT20 MT22 MT23 MT24 MT25 MT26
nicked pREC/pKNOT
sc pREC/pKNOTsc pBR
Plasmid pBR pREC pKNOT MT9 MT10 MT11 MT12 MT13 MT14
0.11 0.59 0.65 0.34 0.48 0.43 0.41 Relative
Hin-mediated mutag
Hin-mediated mutagenesis. (A) Ampicillin resistant colonies growing in the zone of clearance around a filter containing 4 mg ampicillin. (B) Quantitation of ampicillin resistance of individual colonies. (C) Ethidium bromide-stained gel of plasmid DNA iso-lated from mutant colonies growing within the zone of clearance and separated by agarose gel electrophoresis. Lane a is a supercoiled molecular weight standard. Lanes b, c and d contain plasmid DNA from the parental strains harboring pHIN and either pBR, pREC or pKNOT, respectively. Lanes e-j contain plasmid DNA isolated from mutant pKNOT colonies. (D) Total cell lysates of mutants grown in 1 mM IPTG were separated by SDS-PAGE and submitted to immunoblotting. Immunoblots were probed with anti-AcrA antibodies (for a loading control) or anti-β-lactamase antibodies. Shown below the blot are signal intensities in arbitrary units. AcrA and anti-β-lactamase levels for C600 strains containing pHIN and either pBR, pREC or pKNOT are shown for comparison.
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
Table 2: Hin-mediated mutation rates
Selective AMP
Mutation rate per cell division
Mutation rate normalized to pBR
IV can resolve all the knots produced by the Hin system.
helped drive selection and genetic change. In addition,
The result would not be a complete loss of plasmid, but
cellular stress causes a number of recombinases and trans-
instead a steady-state level lower than that found with
posases to be acps this activation
unknotted DNA, which is what we observed (Figur
creates a transient "hypermutable state" that allows cells
It is difficult to envision a process analogous to topoi-
to develop a mechanism to overcome the stress. Such an
somerase IV unknotting that would reverse the effects of
event would be similar to that suggested to occur during
Hin-mediated site-specific recombination. Thus, if recom-
adaptive mutagenesis when E. coli are starved for lactose
bination were leading to the loss of plasmids, it would
-istent with this idea, cells harboring trans-
seem that the unchecked altered plasmid would be lost
posons such as Tn10, which can recombine and knot
completely from a population of cells in the absence of
DNA, will out-compete cells lacking Tn10 that are other-
selection, which was not observed.
wise isogenic, which suggests that the transposon confersa greater evolutionary fitnes
The DNA knot- or recombination-created blockage couldimpinge upon either the initiation or elongation of gene
transcription or DNA replication. Gene promoters and
Our results suggest that recombined and knotted forms of
replication origins are small relative to plasmids. Unless
DNA are problematic for the cell. Thus, it is the DNA con-
DNA knots preferentially form in or are localized to pro-
formation, rather than the primary sequence, that causes
moters or origins, or are hotspots for recombination, then
malfunctions. Effects of transient changes in conforma-
it is expected that the polymerase roadblocks would occur
tion may then persist through induced mutations in the
at arbitrary positions on the DNA. Thus, such blockages
primary sequence. Unexpectedly, the DNA molecule
would likely be outside of where transcription or DNA
undergoing site-specific recombination/knotting can
"attack" a bystander DNA, and thus both DNA moleculesmay be altered.
It has been demonstrated that when topoisomerase IVactivity is reduced by mutation, priA, which encodes the
PriA protein that plays an important role in restarting
Strains and Plasmids
blocked replication forks, becomes an essential gene
E. coli strains C600, ParC1215 (parCts) and W3110 were
]. It is possible that the stalling of replication forks
described previomid pKH66 (pHIN)
at knots is the cause of this need for PriA and would
contains the S. typhimurium hin gene under control of the
explain why the presence of gyrase, which can remove
tac promoter and expresses Hin upon addition of isopro-
positive supercoils, but not knots, is insufficient to keep
pyl-1-thio-β-galactoside (IPTE4 (pREC)
replication moving in these cells.
] is a pBR322-derived plasmid containing the Ginrecombination (gix) sites and enhancer from bacteri-
Implications for cellular physiology and evolution
ophage Mu. Gin, Hin and their respective recombination
Given (i) the abundance of recombinases, transposases
sites are interchange62 (pKNOT) contains
and topoisomerases found in both prokaryotic and
hix recombination sites and the enhancer binding site for
eukaryotic organisms, (ii) the lack of sequence specificity
the Hin recombinase from S. typhimurium ]. One hix
by these enzymes, (iii) the confined space for the chromo-
site contains a single base pair change, which forces a sec-
somes and (iv) the propensity of DNA to react and entan-
ond round of recombination to tie knots by preventing
gle with itself, DNA rearrangements that lead to cellular
religation after only one roun. To create the strains
transformation or death, or that contribute to the muta-
used throughout this work, we used a CaCl2 method to
tions that shape evolution seem likely to occur. In other
transform wild-type E. coli with plasmid DNA (typically
words, an intrinsic lack of DNA stability might have
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
Norman), anti-ParC or anti-ParE (kind gifts of the late
Gradient pl and Kirby-Bauer [ disc diffusion
N.R. Cozzarelli, University of California, Berkeley), incu-
assays were used to measure antibiotic resistance. Satu-
bated in SuperSignal West chemiluminescent reagent
rated overnight cultures containing the strains were
(Pierce, Rockford, IL) and visualized with a charge cou-
diluted 30- to 100-fold in LB containing 1 mM IPTG and
pled display camera.
50 μg/ml spectinomycin. The freshly diluted cultures weregrown at 37°C until they reached OD600 = 0.3. For the
Plasmid loss assay
Kirby-Bauer disc diffusion assays, cells were spread on LB-
Cells were grown as for Western blot analysis. Plasmid
agar containing 1 mM IPTG and 50 μg/ml spectinomycin.
DNA was isolated by the alkaline lysis method , line-
The plates were allowed to dry for 20 min. and discs con-
arized with HindIII (which cuts pKNOT and pHIN once)
taining 10 μl of different ampicillin concentrations (0 –
and separated by electrophoresis on 1% agarose (TAE)
500 mg/ml) were placed onto the agar. The plates were
gels. Plasmid levels were quantified by densitometric
then incubated overnight at 37°C. The diameter of the
scanning (NucleoVision software, NucleoTech Corp., San
cleared zone around each disc was measured. For the gra-
Mateo, CA) of images of ethidium bromide-stained gels.
dient plate assay, cells were spread on square plates con-
Assuming pHIN levels do not change among the strains,
taining a gradient from 0 to 17.5 mg/ml ampicillin, and
plasmid bands were first normalized within each lane to
then incubated overnight at 37°C. The plate dilution
the pHIN vector. These standardized band values are
method was used to determine the ampicillin MIC50 val-
shown relative to the value for pBR within each strain
ues. The three E. coli strains harboring pHIN and either
background. To determine whether entire plasmid popu-
pBR, pREC or pKNOT were grown overnight in LB
lations were lost from cells, various dilutions of cells
medium containing 100 μg/ml ampicillin, 50 μg/ml spec-
grown in LB medium were spread onto LB-agar and rep-
tinomycin and no IPTG. These cultures were diluted 500-
lica plated on agar ± 100 μg/ml ampicillin. Colonies were
fold in LB medium containing 50 μg/ml spectinomycin
counted following overnight incubation at 30°C (C600
and 1 mM IPTG, but no ampicillin. The freshly diluted
and parCts) or 37°C (C600).
cultures were grown with shaking to mid-logarithmicphase (OD600 = 0.3 – 0.4) at 37°C. Appropriate dilutions
DNA catenane analysis
(to final cell counts of approximately 100 and 1000 per
DNA catenanes were analyzed as done pr].
plate) were spread onto LB-agar alone and LB-agar con-
parCts cells containing pHIN and either pBR, pREC or
taining ampicillin concentrations from 1.3 to 4.8 mg/ml,
pKNOT were grown at 30°C to mid-logarithmic phase
50 μg/ml spectinomycin, but no IPTG. Colonies were
(OD600 = 0.3 – 0.4). IPTG was added to a final concentra-
counted following overnight incubation at 37°C. For each
tion of 1 mM to induce Hin expression. After 10 min.,
of the three strains, regression analysis was performed to
cells were shifted to 42°C to inactivate the mutant topoi-
determine the best-fit curve through the data points
somerase IV. Forty minutes later, plasmid DNA was iso-
(2670: n = 10, 2671: n = 9 and 2672: n = 8) in the plot of
, nicked with DNase I to remove supercoiling
survival as a function of ampicillin concentration. From
] and displayed by high-resolution gel electrophoresis
this best-fit curve, the ampicillin MIC50 values were
]. The DNA was then transferred to a Zeta Probe nylon
membrane (Bio-Rad Laboratories, Hercules, CA) andprobed with [α-32P]-dCTP (GE Healthcare, Little Chal-
Antibodies and immunoblotting
font, UK) labeled pBR322 (made by random priming,
Isogenic C600 and ParC1215 (parCts) strains were grown
Amersham Megaprime™ DNA labeling systems, GE
overnight in LB medium without IPTG. Cells were diluted
Healthcare, Little Chalfont, UK), which hybridizes all
1/100 into LB medium and grown with shaking ± 1 mM
three plasmids.
IPTG and 50 μg/ml spectinomycin to mid-logarithmicphase (OD600 = 0.3 – 0.4) at 37°C or 42°C. Duplicate sets
Isolation of ampicillin resistant colonies and fluctuation
of whole cell extracts were made by resuspending equal
amounts of pelleted cells in loading buffer (125 mM Tris-
Ampicillin resistant colonies that grew inside the zone of
HCl, pH 6.8; 1.4 M β-mercaptoethanol; 20% glycerol; 2%
clearance (FigurA) were streaked onto LB-agar plates
SDS; 0.1% Bromophenol blue), boiling for 3 min. and
containing 1 mM IPTG, 50 μg/ml spectinomycin and 1
subjecting to 10% SDS-PAGE. One set was stained with
mg/ml ampicillin and incubated overnight at 30°C. These
Coomassie blue to ensure equal protein amounts were
conditions were used to prevent the accumulation of
loaded. The other set was blotted to a nitrocellulose Pro-
revertants to ampicillin sensitivity. To determine the
tran membrane. The blots were probed with (1:10,000
mutation rate, E. coli harboring pHIN and either pBR,
dilution for all) antisera to β-lactamase (a kind gift of T.
pREC or pKNOT were grown overnight in LB medium
Palzkill, Baylor College of Medicine, Houston), anti-AcrA
containing 100 μg/ml ampicillin, 50 μg/ml spectinomy-
(a kind gift of H. I. Zgurskaya, University of Oklahoma,
cin and no IPTG. The overnight cultures were diluted
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
6,000-fold into LB medium ( 105 cells/ml) containing no
ampicillin, 50 μg/ml spectinomycin and 1mM IPTG and
Cozzarelli NR, Cost GJ, Nollmann M, Viard T, Stray JE:
divided into ten 1.2-ml aliquots. These aliquots were
Nat Rev Mol Cell Biol 2006, 7(8):580-588.
grown with shaking to mid-logarithmic phase (OD600 =
Bohrmann B, Haider M, Kellenberger E:
0.3 – 0.4) at 37°C to obtain parallel, independent cul-
tures. The number of ampicillin resistant mutants that
originated in each culture was determined by spreading 2
× 70 μl (pBR- and pREC-containing strains) or 2 × 200 μl
(pKNOT-containing strain) of undiluted culture onto LB-
Annu Rev Biophys Biomol Struct 2004,
agar containing various ampicillin concentrations, 50 μg/
ml spectinomycin, but no IPTG. 16.1 mg/ml ampicillin
Yan J, Magnasco MO, Marko JF: Nature
was used for the strain harboring pBR; 7.9 mg/ml ampicil-
lin was used for the strain harboring pREC; and 4.8 mg/
Shishido K, Komiyama N, Ikawa S: Increased production of a
knotted form of plasmid pBR322 DNA in Escherichia col
ml ampicillin was used for the strain harboring pKNOT.
J Mol Biol 1987, 195(1):215-218.
Each of these ampicillin concentrations is 3.4-fold higher
Martin-Parras L, Lucas I, Martinez-Robles ML, Hernandez P, Krimer
than the corresponding strain's ampicillin MIC
DB, Hyrien O, Schvartzman JB:
total number of cells was determined by spreading dilu-
Nucleic Acids Res 1998,
tions of each culture on LB-agar. Colonies were counted
Deibler RW: The biological implications of DNA knots and the in vivo activ-
after incubation overnight at 37°C. The probable number
ity of topoisomerase IV Houston: Baylor College of Medicine; PhD the-
of mutations per culture (m) was calculated from the dis-
tribution of hyper-resistant mutants in the independent
Shishido K, Ishii S, Komiyama N: The presence of the region on
pBR322 that encodes resistance to tetracycline is responsi-
cultures using the MSS maximum likelihood method.
ble for high levels of plasmid DNA knotting in Escherichia co
Then the mutation rate (μ) was calculated as μ = m/2N
Nucleic Acids Res 1989,
t is the total number of cells per cultur].
Ishii S, Murakami T, Shishido K: Gyrase inhibitors increase the
content of knotted DNA species of plasmid pBR322 in
Escherichia coli J Bacteriol 1991, 173(17):5551-5553.
Sikorav JL, Jannink G:
RWD carried out antibiotic resistance measurements,
immunoblotting, plasmid loss assays, DNA catenane
Biophys J 1994, 66(3 Pt 1):827-837.
analysis and isolation of ampicillin resistant colonies,
Arsuaga J, Vazquez M, Trigueros S, Sumners D W, Roca J:
conceived of and participated in the design of the study
Proc Natl Acad Sci USA
and drafted the manuscript. JKM carried out antibiotic
Baker TA, Sekimizu K, Funnell BE, Kornberg A: Extensive unwind-
resistance measurements, plasmid loss assays, isolation of
ing of the plasmid template during staged enzymatic initia-
ampicillin resistant colonies and fluctuation analysis, per-
tion of DNA replication from the origin of the Escherichia col
formed the statistical analysis, participated in the design
Cell 1986, 45(1):53-64.
Hatfield GW, Benham CJ: DNA topology-mediated control of
of the study and helped to draft the manuscript. DWS par-
global gene expression in Escherichi Annu Rev Genet 2002,
ticipated in the design and coordination of the study, ana-
lyzed experimental results and helped to draft the
Liu Y, Bondarenko V, Ninfa A, Studitsky VM: Proc Natl Acad Sci
manuscript. LZ conceived of and participated in the
USA 2001, 98:14883-14888.
design and coordination of the study, analyzed experi-
Steck TR, Franco RJ, Wang JY, Drlica K: Topoisomerase muta-
tions affect the relative abundance of many Escherichia coli
mental results and helped to draft the manuscript. All
Mol Microbiol 1993, 10(3):473-481.
authors read and approved the final manuscript.
Vologodskii AV, Cozzarelli NR: J Mol Biol 1993, 232(4):1130-1140.
Vologodskii AV, Levene SD, Klenin KV, Frank-Kamenetskii MD, Coz-
We thank Dr. Stacy Merickel and Dr. Reid Johnson for Hin reagents pKH66
J Mol Biol 1992, 227(4):1224-1243.
and pRJ862 and for sharing unpublished results. We are grateful to Dr. Tim-
Podtelezhnikov AA, Cozzarelli NR, Vologodskii AV:
othy Palzkill and Dr. Helena I. Zgurskaya for providing antibodies. We are
Proc Natl Acad Sci USA 1999,
grateful to Dr. Mary-Jane Lombardo for comments on the manuscript.
Funding for this work was provided by the National Science Foundation
Vologodskii AV, Marko JF:
MCB-0090880, the National Institutes of Health RO1 AI054830, a Bur-
Biophys J 1997, 73(1):123-132.
Deibler RW, Rahmati S, Zechiedrich EL: Topoisomerase IV,
roughs Wellcome Fund New Investigator Award in the Toxicological Sci-
alone, unknots DNA in E. coliGenes Dev 2001, 15:748-761.
ences and the Curtis Hankamer Research Award to LZ. RWD and JKM
Adams DE, Shekhtman EM, Zechiedrich EL, Schmid MB, Cozzarelli
were supported by pre-doctoral fellowships from the Program in Mathe-
matics and Molecular Biology at Florida State University with funding from
the National Science Foundation and the Burroughs Wellcome Fund Inter-
Cell 1992, 71(2):277-288.
Zechiedrich EL, Cozzarelli NR: Roles of topoisomerase IV and
faces Program.
DNA gyrase in DNA unlinking during replication in
Escherichia coli Genes Dev 1995, 9(22):2859-2869.
(page number not for citation purposes)
BMC Molecular Biology 2007, 8:44
Zechiedrich EL, Khodursky AB, Bachellier S, Schneider R, Chen D, Lil-
Davenport RJ, Wuite GJ, Landick R, Bustamante C: Single-molecule
ley DM, Cozzarelli NR: Roles of topoisomerases in maintaining
study of transcriptional pausing and arrest by E. co
steady-state DNA supercoiling in Escherichia coli J Biol Chem
Bao XR, Lee HJ, Quake SR:
Merickel SK, Johnson RC: Topological analysis of Hin-catalysed
Phys Rev Lett 2003, 91(26 Pt 1):265506.
DNA recombination in vivo and in vitro Mol Microbiol 2004,
Beaber JW, Hochhut B, Waldor MK:
Heichman KA, Moskowitz IPG, Johnson RC:
Nature 2004, 427(6969):72-74.
Friedberg EC, Walker GC, Siede W: DNA repair and mutagenesis edn.
Washington: ASM Press; 1995.
Genes Dev 1991, 5(9):1622-1634.
Rosche WA, Foster PL:
Rochman M, Aviv M, Glaser G, Muskhelishv
Methods 2000, 20(1):4-17.
Levine C, Hiasa H, Marians KJ:
EMBO Rep 2002, 3:355-360.
Hardy CD, Cozzarelli NR: A genetic selection for supercoiling
Biochim Biophys Acta
mutants of Escherichia coli
Mol Microbiol 2005, 57(6):1636-1652.
Kato J-I, Nishimura Y, Imamura R, Niki H, Hiraga S, Susuki H: New
Postow L, Hardy CD, Arsuaga J, Cozzarelli NR: Topological
topoisomerase essential for chromosome segregation in
domain structure of the Escherichia coli Genes
Escherichia coli Cell 1990, 63(2):393-404.
Dev 2004, 18(14):1766-1779.
Zechiedrich EL, Khodursky AB, Cozzarelli NR: Topoisomerase IV,
Glasgow AC, Bruist MF, Simon MI
not gyrase, decatenates products of site-specific recombina-
J Biol Chem 1989, 264(17):10072-10082.
tion in Escherichia col Genes Dev 1997, 11(19):2580-2592.
Johnson RC, Bruist MF
Grompone G, Bidnenko V, Ehrlich SD, Michel B: PriA is essential
for viability of the Escherichia coli topoisomerase IV
Embo J 1989, 8(5):1581-1590.
parEJ Bacteriol 2004, 186(4):1197-1199.
Lee SY, Lee HJ, Lee H, Kim S, Cho EH, Lim HM:
Heller RC, Marians KJ:
Nature 2006,
J Bacteriol 1998, 180(22):5954-5960.
Tam CK, Hackett J, Morris C: Rate of inversion of the Salmonella
Posfai G, Plunkett G 3rd, Feher T, Frisch D, Keil GM, Umenhoffer K,
Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, Burland V, Har-
Infect Immun 2005, 73(9):5568-5577.
cum SW, Blattner FR: Emergent properties of reduced-genome
Eisenstadt E, Carlton BC, Brown BJ: Gene Mutation. In Methods for
Escherichia coli Science 2006, 312(5776):1044-1046.
General and Molecular Bacteriology Edited by: Gerhardt P, Murray RGE,
Haniford DB: Transpososome dynamics and regulation in Tn
Wood WA, Krieg NR. Washington, D.C.: American Society for
10 Crit Rev Biochem Mol Biol 2006, 41(6):407-424.
Microbiology; 1994:304.
Hastings PJ, Slack A, Petrosino JF, Rosenberg SM:
Portugal J, Rodriguez-Campos
Acids Res 1996, 24(24):4890-4894.
PLoS Biol 2004, 2(12):e399.
Saitta AM, Soper PD, Wasserman E, Klein ML:
Hersh MN, Ponder RG, Hastings PJ, Rosenberg SM: Adaptive muta-
Nature 1999,
tion and amplification in Escherichia coli
Res Microbiol 2004,
Arai Y, Yasuda R, Akashi K, Harada Y, Miyata H, Kinosita KJ, Itoh H
Nature 1999,
Mutat Res 2005, 569(1-2):3-11.
Pieranski P, Kasas S, Dietler G, Dubochet J, Stasiak A: Localization
Chao L, McBroom SM: Evolution of transposable elements: an
of breakage points in knotted strings. New Journal of Physics
IS10 insertion increases fitness in Escherichia coli Mol Biol Evol
2001, 3:10.
Uehara H, Kimura H, Aoyama A, Yamanobe T, Komoto T: Effects of
Chao L, Vargas C, Spear BB, Cox EC:
knot characteristics on tensile breaking of a polymeric
Nature 1983, 303(5918):633-635.
monofilament. New Journal of Physics 2007, 9:65.
Kato J-I, Nishimura Y, Yamada M, Suzuki H, Hirota Y: Gene organ-
Lee CL, Ow DS, Oh SK:
ization in the region containing a new gene involved in chro-
mosome partition in Escherichia co J Bacteriol 1988,
J Microbiol Methods 2006, 65(2):258-267.
Lee C, Kim J, Shin SG, Hwang S: Absolute and relative QPCR
Hughes KT, Gaines PC, Karlinsey JE, Vinayak R, Simon MI:
quantification of plasmid copy number in Escherichia coJ
Sequence-specific interaction of the Salmonella
Embo J 1992,
Noack D, Roth M, Geuther R, Muller G, Undisz K, Hoffmeier C,
Gaspar S: Maintenance and genetic stability of vector plas-
Crisona NJ, Kanaar R, Gonzalez TN, Zechiedrich EL, Klippel A, Coz-
mids pBR322 and pBR325 in Escherichia coli
Mol Gen Genet 1981, 184(1):121-124.
Sundin O, Varshavsky A:
J Mol Biol
Cell 1981, 25(3):659-669.
Plasterk RHA, Brinkman A, van de Putte P: DNA inversions in the
Katritch V, Olson WK, Vologodskii AV, Dubochet J, Stasiak A: Tight-
chromosome of Escherichia coli
ness of random knotting. Phys Rev E 2000, 61(5 Pt B):5545-5549.
Metzler R, Hanke A, Dommersnes PG, Kantor Y, Kardar M:
Proc Natl Acad Sci USA 1983, 80(17):5355-5358.
Phys Rev Lett 2002, 88(18):188101.
Bauer AW, Kirby WMM, Sherris JC, Turck M:
Wuite GJ, Smith SB, Young M, Keller D, Bustamante C:
Am J Clin
Pathol 1966, 45:493-496.
Nature 2000, 404(6773):103-106.
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning: a laboratory man-
Maier B, Bensimon D, Croquette V:
ual 2nd edition. Cold Spring Harbor: Cold Spring Harbor Laboratory
Proc Natl
Acad Sci USA 2000, 97(22):12002-12007.
Wang MD, Schnitzer MJ, Yin H, Landick R, Gelles J, Block SM:
J Mol Biol 1973, 74(4):739-742.
Science 1998, 282(5390):902-907.
(page number not for citation purposes)
Source: https://www.mathi.uni-heidelberg.de/~banagl/pdfdocs/sumners/HinKnottingBMCMOLB.pdf
Control of Residues in Live Animals and Animal Products. Results 2005, plan 2006. Originating from the Faroe Islands (FO), Pursuant to Council Directive 96/23/EC. PRESENTATION OF THE RESIDUE CONTROL 2005 RESIDUE CONTROL PLAN 2006 Country: Faroe Date: 30th March 2006 Commission Reference Number (Stamp): Period Covered:
CLINICAL TRIALS AND OBSERVATIONS Eradication of minimal residual disease in hairy cell leukemia Farhad Ravandi, Jeffrey L. Jorgensen, Susan M. O'Brien, Srdan Verstovsek, Charles A. Koller, Stefan Faderl, Francis J. Giles,Alessandra Ferrajoli, William G. Wierda, Shirley Odinga, Xuelin Huang, Deborah A. Thomas, Emil J. Freireich, Dan Jones,Michael J. Keating, and Hagop M. Kantarjian















