Www3.gehealthcare.com.au
Prevention and the
of breast cancer
A report commissioned by GE Healthcare, authored by
Bengt Jönsson and Nils Wilking
Br grow cure
begins cases
Blood skin
nodes Among although
familiar screening malignant
organs seven


About the authors
Professor Bengt Jönsson
Economists Association.
Nils Wilking MD PhD
Bengt Jönsson is Professor Emeritus (Health Economics), department of economics, Stockholm School of Economics, and Director of the
Associate Professor Nils Wilking MD PhD has worked in clinical
Center for Health Economics. He is currently member of the European
oncology for 30 years after graduating from the Karolinska Institute
Academy of Cancer Sciences, and of the EU Expert Panel on effective
in Stockholm, Sweden. He has worked for many years in the field of
ways of investing in health.
surgical oncology, but has since the late 1980s mainly worked within medical oncology with a focus on breast and GI cancer.
He has been a member of the Karolinska University Hospital Board, and the Scientific Advisory Board, National Board of Health and
He headed the Breast and GI Cancer Unit at the Karolinska Hospital
Welfare, Sweden. He was also Chair of the Expert Group to the
during 1992-1998. During this period, he also set up and headed the
Committee on Funding and Organization of Health Services and
clinical trial unit at the Department of Oncology.
Medical Care in Sweden (HSU 2000), and a member of the National Social Insurance Board from 1992 to 1994, and of SBU (The Swedish
In 1998, he joined Eli Lilly as a senior research physician. In 2001, he
Council on Technology Assessment in Health Care), Scientific Advisory
moved to BMS where he held a European position in their oncology
Board 1988-2004.
team. Since 2003, he has worked in a research context linked to the Karolinska Institute. Since 2010, he also serves as Senior Strategic
Professor Jönsson is a member of the editorial boards of several
Advisor for the Southern Health Care Region in Sweden.
journals, including the Journal of Cancer Policy, European Journal of Health Economics, and International Journal of Technology
His main focus has been on research in relation to health service
Assessment in Health Care. He has also been a temporary adviser to
delivery. This work, in collaboration with Professor Bengt Jönsson
the WHO and a consultant to OECD and UNIDO. Professor Jönsson's
at the Stockholm School of Economics, has resulted in a number of
extensive publications in the field of health economics include over
reports with a focus on patient's access to cancer therapy. These
200 papers, reports, and book chapters. Presently he is past president
reports include information on more than 55 countries.
and member of the board of the SHEA, the Swedish Health Economics Association and past president of iHEA, the international Health
As of 2013 Nils Wilking is the head of the department of oncology at Skånes University Hospital, Lund/ Malmö Sweden.
Prevention and the economic burden of breast cancer
Executive Summary
Disease Adjusted Life Years (DALYs)
Outcome in breast cancer
Prevention in high risk groups
Secondary prevention, early diagnosis
Mammography screening programs
Current controversy about screening
Breast MRI and other emerging technologies
Cost effectiveness of breast cancer screening
Conclusions and policy implications
Prevention and the economic burden of breast cancer
• New evidence on chemoprevention to reduce breast cancer risk
has resulted in revised guidelines, recommending this preventive
• The disease burden of breast cancer is high; every year over 1.4
option for women at high risk.
million women worldwide get breast cancer, and approximately
459,000 women die of the disease. Breast cancer is the second most
• While screening has contributed to improvements in survival,
common cancer form overall and the most common cancer for
there is a debate about benefits, harms and cost-effectiveness of
women, constituting 25% of all female cancer.
alternative approaches to screening. While decreasing incidence in
some populations and improved outcome of treatment may have
• Accurate data on breast cancer incidence at a national level
reduced the benefits of screening, this is not evidence for dismissing
is lacking in several countries due to limited cancer registration.
this option for prevention. In populations with increasing incidence
Available data show that breast cancer incidence rates have
and high mortality/incidence rate, the potential benefits of early
steadily increased in developed countries over the last 50 years.
detection are increasing. There is still a need and opportunities for
In the last decades increased incidence rates are also being seen
appropriately designed screening programs, and for new options
in many developing countries, in particular in parts of Asia. The
for early detection. Targeting specific risk groups and improving
increased incidence of breast cancer is mainly due to increased life
the internal efficiency of the diagnostic process can improve
expectancy but also relates to lifestyle changes, such as women
cost-effectiveness. The present focus in health care systems on
having fewer children as well as hormonal interventions like post-
comparative effectiveness makes it mandatory for new effective
menopausal hormonal therapy.
methods to provide data for assessment of their value in relation to
established alternatives.
• The mortality trends are diverging, with declining mortality
in many developed countries, while mortality is increasing in
developing and newly industrialized countries. Survival rates are
also lower in these countries mainly due to the late stage of the
The sustainability of health care systems, and the need to make
disease when diagnosed, but also due to limitations in access to
priorities for investments in improved health are at the top of the
proper diagnostics and treatment of both early and advanced
health policy agenda (1). Breast cancer is an important and interesting
breast cancer.
example of the issues involved. The disease is well defined, and over the last four decades a number of new technologies for prevention,
• The economic burden of breast cancer is considerable in terms
early detection and treatment have been introduced, that have
of both direct and indirect costs. The direct health care costs
significantly improved the outcome. But the implementation varies
attributable to breast cancer vary greatly between countries,
between health care systems, and the search for evidence on best
reflecting differences in total health care spending. Hospitalizations
practices is still far from completed. The epidemiology of the disease
dominate the direct costs, but costs of pharmaceuticals are also
is also changing, and the most important change is the growing
high and increasing. The indirect costs of breast cancer are larger
incidence of breast cancer in developing countries. It is also in the
than the direct treatment costs since many breast cancer cases
developing countries that we see the shortest survival due to late
occur in women below 65 years.
diagnosis of the disease. There are also variations in survival within developed countries related to socioeconomic status. In the US, despite
• While progress in treatment has contributed to improvements
improvements in survival across poverty levels for all known stages
in survival, the cost of treatment, particularly for advanced breast
of disease, relative survival remains lower among women residing in
cancer, has also increased significantly. The potential benefit of
poor areas compared with affluent areas. This poor outcome probably
primary and secondary prevention thus remains high, both in
relates to a more advanced stage of disease at diagnosis. In 2008,
health and economic terms, and this applies to both developed and
51.4% of poor women had undergone a screening mammogram in the
developing countries.
past 2 years compared with 72.8% of more affluent women (2).
Prevention and the economic burden of breast cancer
Investing in health is not only about sustainability of the health care
the ageing of populations is a major factor behind the increasing
systems, equity in health and access to health care. Breast cancer is
an example where investments in health create benefits outside the health care system as breast cancer affects many women of working
age. The opportunities to reduce the number of working days lost
are an important additional benefit from investment in improving
outcomes in breast cancer.
This paper reviews the economic burden of breast cancer, with
particular focus on opportunities to reduce the burden through
prevention and early detection. The focus on prevention and early
detection is important for several reasons. During the last decade
focus has been on the costs and survival benefits from the introduction
of new drugs for treatment of breast cancer, and to some extent on
the refinement of breast surgery, aimed at improving quality of life.
During the same period we have seen a growing controversy about
the benefits and harms of mammography for early detection in
developed countries with decreasing incidence of breast cancer. But
at the same time we see increasing incidence in developing and newly
industrialized countries, where cases are detected at a late stage.
There is thus the need to review the case for development of cost-
a Data taken from GLOBOCAN (31).
b ASR = age standardised rate per 100,000 population, using the WHO World Standard Population (32).
c MR:IR= ratio of mortality rate to incidence rate for the region specified in the mortality column.
effective methods for prevention and early detection of breast cancer.
Table 1 Estimated number of cases and age-standardized rates* for
incidence and mortality of female breast cancer by world region in
2008. Source (3).
Epidemiological data is the basis for determining the burden of a
*An age-standardized rate (ASR) is a summary measure of the rate that a population
would have if it had a standard age structure.
Breast cancer is the most common cancer form in women; with
Table 1 shows the incidence and mortality for breast cancer in different
an estimated 1.4 million new cases worldwide each year, breast
regions of the world in 2008. The total number of cases, or crude
cancer constitutes about 25% of all cancer cases in women and is
incidence, and mortality, are the best measures for assessing the
the second most common cancer form overall (3). Breast cancer can
actual health and economic burden of breast cancer in a country or
also occur in men, but this is very uncommon. Incidence rates of
region. The age-adjusted incidence and mortality is of interest as it
breast cancer are significantly higher in developed countries than in
shows the influence of risk factors other than age in the development
developing countries (72 and 29 per 100,000 respectively, see table 1);
of breast cancer. The age span of women affected by breast cancer is
the difference in incidence rates between developed and developing
broad. Although uncommon, breast cancer may affect women already
countries is probably due to a combination of demographic, hereditary, in their 20s and 30s, but close to 90% of all cases are diagnosed from environmental and lifestyle risk factors. Incidence rates are rapidly
the age of 40 and onwards .Countries with younger populations have
increasing in many newly industrialized countries due to changing
lower crude than age-adjusted incidence rates.
lifestyles reflecting those patterns in developed countries where we already see high incidence rates. Risk factors that may contribute to
The ratio of mortality divided by incidence is an indicator of how
breast cancer incidence include: low parity, late first pregnancy, early
successful a country is in early detection and treatment of breast
start of menstruation as well as exposure to hormonal treatment, oral
cancer; the lower the ratio in Table 1 the better. If all cases were cured,
contraception, obesity and alcohol consumption. As explained below,
Prevention and the economic burden of breast cancer
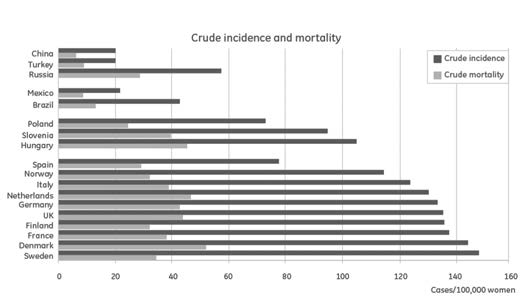
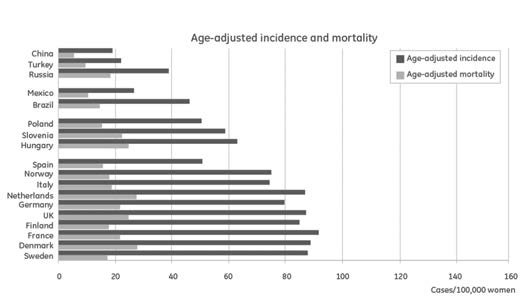
i.e. women diagnosed with breast cancer would die from something
Figures 1a and 1b show incidence and mortality, both crude and age-
else, the ratio should be zero, and if all die from breast cancer, the ratio
adjusted, for a selection of countries. As can be seen when comparing
is 1.0. The ratio of mortality to incidence is 0.24 in more developed
figures, in Mexico, Brazil, and Turkey, the crude incidence is lower than
regions, and 0.40 in less developed regions. The differences are large
the age-adjusted incidence since these countries have relatively young
with a ratio of 0.20 in countries such as Sweden for example, and a
populations. In these countries the burden of breast cancer is expected
ratio of 0.60 in parts of Africa and Melanesia. This difference is mainly
to increase rapidly with increasing life expectancy and life style
explained by differences in the stage of the tumour at diagnosis, which
changes. For example, the total number of reported cases in Mexico
reflects the presence of screening programs as well as education and
in 1999 was 10,000 compared to 7,000 in Sweden in 2007, although
public awareness. Well organized breast cancer care is also a key
Mexico has a population 10 times as large as the Swedish population.
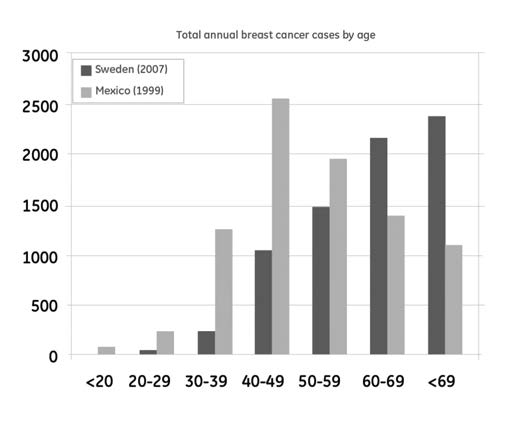
Figure 2 (below), illustrates the age distribution across the total number
North America has an age adjusted incidence and mortality of 83.5
of breast cancer cases in Mexico and Sweden respectively. In Mexico,
and 16.7 respectively which gives a mortality incidence ratio of 0.20,
the average age at diagnosis of breast cancer is approximately 50
similar to the countries in Western Europe. A closer look at statistics
years while the average age at diagnosis in Sweden is 60 years due to
from the US, reveals large differences between segments of the
the younger population on average in Mexico compared to Sweden [5,
population. There are ethnic differences with a higher rate of breast
6]. In both countries, the majority of women are under 65 years when
cancer and an inferior outcome in African American women compared
they are first affected by breast cancer, which contributes to the large
to white. On the other hand the incidence in Asian American women
health and economic burden of the disease.
and in Hispanic/ Latina women is lower than that among white and African American women (2).
Figure 2a Breast cancer cases per age group in Mexico and Sweden.
Source (4).
Figure 1a and 1b. Source (4).
Prevention and the economic burden of breast cancer
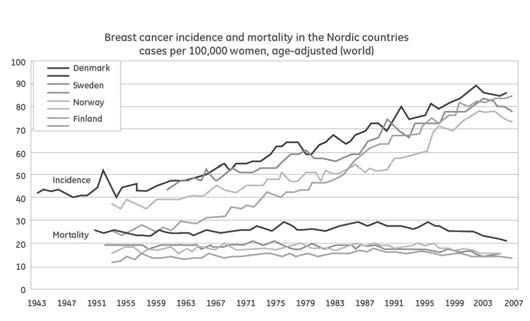
A barrier to the estimation of global breast cancer incidence is the limited data availability in many countries. Incidence figures in many countries are based on data from small geographic areas that are pooled and extrapolated to represent national data. This is not only the case in most developing countries; the majority of the European countries do not have a 100% national coverage of cancer registration. The Nordic countries are exceptions and Denmark was the first of the countries to establish a national cancer registry in 1942, and other Nordic countries followed with registration on a national level in the 1950s. (10, 11).
Figure 3 presents the breast cancer incidence and mortality trends over the last 60 years from the Nordic registries (12).
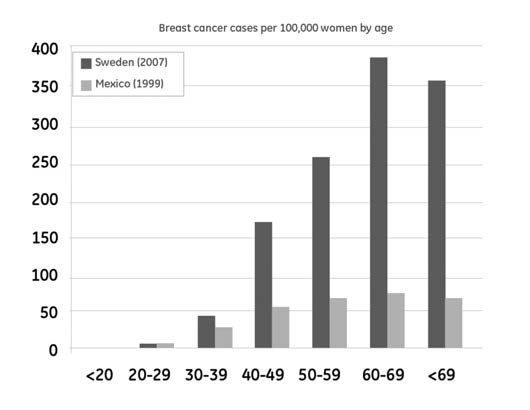
Figure 2b shows the huge difference in the incidence of breast cancer
at different ages in Sweden and Mexico. Source (4).
China currently has the lowest age-adjusted incidence of the study countries, but one may expect that the fundamental changes in reproductive patterns in China brought about by the implementation in the 1970s of the one-child policy, as well as current lifestyle changes in China caused by rapid economic growth, will potentially lead to dramatically increased rates of breast cancer in Chinese women. Such trends can already be seen in the middle-aged population in urban areas of China, where a 20–30% increase in breast cancer incidence has been documented over the past decade, although part of the increase may also be due to earlier diagnosis and better diagnostic
Figure 3 Breast cancer incidence and mortality rates in the Nordic
methods, such as the introduction of mammography (7).
countries since the 1950s. Source (4).
A review of breast cancer incidence and mortality in 9 countries in
The main picture is one of increasing incidence, and since mid 1990s a
the Middle East (8) showed that incidence rates are comparatively
declining mortality. Incidence rates seem to have been stable or fallen
low, with Lebanon as an exception, which partly can be explained by
slightly during the last part of the period. Still, in countries like Sweden
underreporting. Incidence rates are increasing, and rates also vary
there has been an increased incidence over the last 5 years (see figure
within countries between different parts of the population. While
4) (overleaf). This increase is at present difficult to explain, especially
progress is made in terms of early detection, many cancers are
since the use of post-menopausal hormone therapy has declined
diagnosed at a late stage.
significantly over the last decade.
The incidence of breast cancer is low in India, but rising. Due to low awareness of the disease, and absence of screening programs, the majority of breast cancers are diagnosed in a rather late stage (9).
Prevention and the economic burden of breast cancer
US and Canada show a similar pattern to the Nordic countries, with reduced incidence and mortality since the early 2000s. For Asian countries, including Japan, incidence is increasing rapidly. (76).
Disability- adjusted life years (DALYs)
Disability-adjusted life years (DALYs) is a measurement for the overall
burden of disease that combines years of potential life lost due to
premature mortality and years of productive life lost due to disability,
with the intention to quantify the gap between current health status
and an ideal health situation (13).
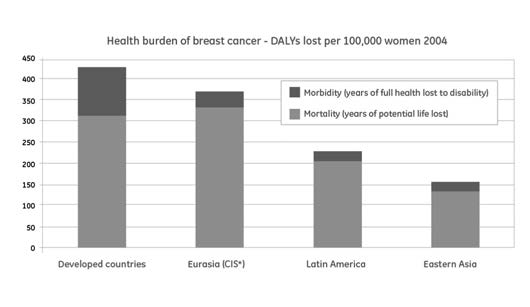
Figure 5 (below), shows the estimated disease burden of breast cancer in DALYs per 100,000 women, separated into years of life lost and years lost due to disability, in the relevant WHO MDG (Millennium Development Goals) Regions.
Figure 5 Number of DALYs lost per 100,000 women, 2004. Distributed
after loss due to mortality and morbidity respectively. Source (13).
The first conclusion is that mortality is the main contributor to the burden of breast cancer measured by DALY. But when more cases are detected, and survival increases, the impact of quality of life during and after treatment is of increasing importance.
Although the burden per 100,000 women is highest in developed countries, where incidence rates are high, it is important to recognize
Figure 4 Recent development of incidence and mortality of breast
that the disease burden per breast cancer case is higher in developing
cancer in Sweden. Number of cases per 100,000 women 1997-2011.
countries due to higher mortality rates in breast cancer and the
younger age of women at diagnosis.
Prevention and the economic burden of breast cancer
DALYs estimates must be interpreted with some caution. The incidence
COST ITEM
TOTAL MILLION SEK
PER CENT OF TOTAL
and mortality data that serve as input to the DALYs estimates are
for some countries based on estimates and extrapolation and the
Direct cost
calculation method in itself is based on a range of assumptions in order
to make this kind of assessment possible. However, the DALY estimates
are important both for comparisons between diseases, countries and
over time. Table 2 shows an update from 2008 of DALYs lost distributed
on more or less developed regions. About two thirds of DALYs lost occur
in developing countries, mainly due to reduced survival.
Breat cancer (2008)
Estimated disability-adjusted life years (DALYs), years of life lost (YLLs) and years lived with disability (YLDs)
Table 3 Cost of breast cancer distributed by the different cost items.
Source (14). 1 SEK=0.15 USD=0.11 Euro.
This Swedish study estimated that direct health care costs account for less than 10 per cent of the total social costs. Indirect costs for loss of
Table 2 Breast cancer (2008) Estimated disability-adjusted life years
production are twice the direct costs. Screening accounts for about
(DALYs), years of life lost (YLLs) and years lived with disability (YLDs).
two percent of the total social costs and drugs only about one percent.
However, since 2002 the costs for drugs have increased, and in 2011 expenditures on trastuzumab (Herceptin) (for the treatment of HER2
positive breast cancer; approximately 20% of the total breast cancer
population) alone was 326 million Swedish Krona (SEK); four times
Breast cancer is not only a large health burden but also a significant
higher than the total cost of drugs in 2002 (83 million SEK).
economic burden to society. The economic burden of breast cancer is not well documented and comprehensive estimations of the cost of
Intangible costs are valued at 600,000 SEK per Quality Adjusted Life
breast cancer are limited. A Swedish cost-of-illness study estimated
Years (QALY) lost, which is less than what is accepted for cancer drugs
that while the direct costs (the costs directly linked to treatment,
in Sweden (1 million SEK) (77), but a figure representing a more relevant
detection, prevention, or care), of breast cancer are significant, the
value for intra-marginal changes. It is slightly higher than what has
indirect costs of the disease (the cost of lost productivity due to the
been accepted in National Institute for Clinical Excellence (NICE)
patients' disability and illness and premature mortality), are more
decisions about recommending cancer drugs for the NHS in England.
than twice as large as the direct costs (14). This Swedish study also estimated a monetary value of intangible costs due to loss of quality of
The estimates above represent the cost of breast cancer from a
life and reduced survival, thus providing a link to the measure of health
social perspective. It is common that estimates of the costs of breast
burden as DALY lost. A comparison of the size of the different cost
cancer only take into account part of the social costs. In a study of the
components is shown in table 3 below.
cost of breast cancer in the Nordic countries, direct treatment costs were estimated at 538 million Euro or just over 20 million Euro per million population, and the public expenditures for sickness and early retirement payments at 216 million Euro (Denmark excluded due to lack of data) (15). The direct cost estimates also excluded part of the costs for ambulatory care, and public expenditures for sickness and
Prevention and the economic burden of breast cancer
early retirement account for only about one third of the social costs due to lost productivity. Indirect costs due to premature mortality were ignored as well.
Variability in the methodology used and costs included in different studies should be kept in mind when assessing estimates from different countries of the total cost of breast cancer. In France, the total healthcare cost for breast cancer was calculated to be €1,456 million in 2004, of which 55% was for hospital care and 45% was for primary care. Surgery represented approximately 34% of the total hospital care, drug administration and drugs 37%, and radiotherapy 13%. Total indirect costs in France due to potential lost production capacity were estimated to be €1,652 million (16). In Germany the total direct cost of female breast cancer in 2008 was estimated to be €1,956 million. In addition, there is 59,000 years of lost production annually; 17,000 due to disability, 19,000 due to invalidity and 23,000 due to premature mortality (17). In Finland, the cost of breast cancer including direct costs and transfer payments, i.e. healthcare costs, sick day payments and invalidity pension, were estimated to be €65 million in 2004 (16).
Figure 6 gives an overview of the cost estimated from a identified sample of studies.
Figure 6b Average indirect costs per breast cancer case (16-19).
Data was recalculated to the average cost per new breast cancer case to make it possible to relate data from different countries; however explicit comparisons between countries must be done with caution since the studies have used different methods. The indirect cost per breast cancer case in Germany presented in the figure was calculated by multiplying the years of lost productivity due to breast cancer in Germany with the average gross salary in Germany.
Most of these cost analyses were based on cost data that are now 5- 7 years old. Since then, some relevant changes have taken place in the treatment of breast cancer, specifically when it comes to the range of drugs available to patients. Drugs were estimated to constitute approximately 10% of direct healthcare costs in breast cancer care in the Swedish study based on data from 2002 (14). The drug share of the total cost of care in cancer has increased in recent years with the introduction of new, targeted biological therapies for the treatment of breast cancer (15). In metastatic breast cancer, according to available data, drug costs constitute a significantly larger share of total healthcare costs in Sweden. They represent 35-40% of total costs (20, 21) and 25% of total costs in the UK (22). The treatment of advanced
Figure 6a Average direct costs per breast cancer case (16-19).
Prevention and the economic burden of breast cancer
stages of breast cancer is generally more expensive than treatment in
There is no direct link between per capita expenditures in healthcare
earlier stages (19- 23).
(see figure 7 above) and the resources or health care services available
to the individual patient. Therefore, one must also take into account
Studies of the cost of breast cancer from an incidence perspective
how efficiently available resources are utilized in the healthcare system
generally only include estimates of the direct health care expenditures.
and the different relative costs of, for example, healthcare personnel
A review of studies of the cost of treating breast cancer in the US,
in different countries. For countries with low health care spending in
reveal that the costs are high during the first year after diagnosis
an international context, it is important to make a distinction between
and in the last phases of the disease (24). Estimates of lifetime per-
resources that are available locally at relatively low costs, and those
patient costs of breast cancer ranged from $US20,000 to $US100,000.
that need to be imported at international prices.
Estimates of life time costs are important as a source of data for modeling the cost-effectiveness of different interventions, from
Some of the therapies used in the treatment of breast cancer, such as
screening and initial treatment to strategies for management of late
radiation and diagnostic equipment, require sophisticated technology
stages of the disease. It is thus not surprising that cost estimates vary,
for which the cost of establishing and maintaining these medical
but a consistent result is that treatment costs for later stages (stage III
facilities is high. The WHO recommends that in limited-resource
and IV), are higher than for treatment of breast cancer in stage I and II.
countries, medical facilities should initially be concentrated in relatively few places to optimize the use of resources. On the other hand, in
Economic evaluations of trastuzumab (Herceptin®) in Sweden, indicate
countries with social and economic inequalities, high-tech medical
typical life time costs for both adjuvant treatment and treatment of
facilities may often be based in areas of the country where wealth is
recurrent disease are, 50-60,000 Euros, over an estimated survival
concentrated, resulting in a sharp contrast in access to treatments that
time of 12 versus 2 years respectively. Cost for adjuvant treatment is
the wealthier and poorer populations can achieve, which can be further
reduced due to lower costs from fewer recurrences (25-26).
compounded by the remoteness of the more often poorer rural regions(28).
Even though data on the economic burden of breast cancer is only available from a selection of countries, the available cost analysis
Access to innovative medicines may also be a problem in countries
presented above illustrates how the cost per patient differs significantly with low income and health care expenditures, since prices are between countries. This is, to a large extent, a consequence of the total
determined by ability to pay in high income countries with well
healthcare resources available in a country, and difference in the care
established health insurance systems. Patients may thus have to wait
provided (e.g. access to high cost cancer drugs).
for access to these medicines until the patent has expired and low price generics become available. However, it should be recognized that there is also a challenge to make optimal use of old medicines, since resources are needed for diagnosis and follow-up to make the treatments effective in clinical practice.
Outcome in breast cancer
The long-term prognosis for breast cancer patients has improved significantly; 10-year survival rates are now 80% in those countries with the best outcomes compared to just over fifty per cent 50 years ago. But we can observe very large differences in survival rates between countries. Such comparisons must be interpreted with caution, since studies often refer to different time periods and patient
Figure 7 Per capita expenditure on health for selected countries.
populations. Table 4 below shows latest data from GLOBOCAN 2008(3)
Prevention and the economic burden of breast cancer
Estimates of 5-year relative survival for female breast cancer in selected countries.a
5-year relative survival (%)
(95% confidence intervalb)
Unites States of America 2005-2007
Republic of Korea
a Data taken from Refs. [47,68,70,74-78].
b Confidence intervals are not intended for comparative purposes, but rather to indicate the precision of the estimate.
c Country estimates were only provided to integer precision
n.a. = 95% confidence interval not available
Table 4 Five year survival rates for female breast cancer in different
countries 2008. Source (3).
Survival rates are higher for developed than developing countries and regions. This is mainly due to the observed differences in survival linked to different stages of the disease at diagnosis. Much of the variation in breast cancer survival between countries is thus likely to be caused be disparities in early detection programs and access to appropriate diagnostics and treatment services. Figure 8 below illustrates the continuous improvement in 5 year survival over time, but also the persistent great difference in long term survival for breast cancer detected at different stages.
The 5-year observed survival rate refers to the percentage of patients who live atleast 5 years after being diagnosed with cancer (Figure 8). Many of these patients live much longer than 5 years after diagnosis. A relative survival rate compares the observed survival with what would be expected for people without the cancer. This helps to correct for the
Figure 8 Survival by disease stage in Norway. Source: Kreftregisteret
deaths caused by something besides cancer, and as an alternative way
Institutt for populasjonsbasert kreftforskning" (Cancer statistics,
to describe the effect of cancer on survival.
Institute for population based cancer research), Norway, 2009.
Prevention and the economic burden of breast cancer
Latest five-year relative survival figures from the US shows 100% in
The specific life-style risk factors of breast cancer that are susceptible
stage I, 93% in stage II, 72% in stage III and 22% in stage IV. (78).
to primary prevention measures include: breast feeding, obesity after menopause, diet, alcohol, physical activity, oral contraception close to
Early detection and treatment are thus the major strategies for
menopause, and post-menopausal hormonal treatment (33). The more
improved survival after diagnosis. We will later discuss strategies for
extensive treatment guidelines from some countries discuss breast
prevention and early detection. The importance of early treatment can
cancer risk factors, but in the majority of guidelines, risk factors are
be illustrated with the introduction of traztuzumab for HER 2 positive
only mentioned briefly or not at all (34).
breast cancer. The drug was first introduced for metastatic disease, and in that stage, gave an improved survival corresponding to 0.60–1.00 QALY per treated woman, compared to treatment without trastuzumab, while when used for adjuvant (early) treatment, gave a survival benefit of 0.97–1.22 QALY per treated woman in an estimate for Sweden (29), and 0.56 QALY versus 1.70 QALY in an estimate for the US (30).
Since the cost of early treatment is about the same as the cost of treatment of metastatic disease, (50-60 000 USD), but there is improved effectiveness with early treatment, the cost-effectiveness of early treatment is better than later treatment. But it should also be noted that the estimated survival increases are limited to about one year, which reinforces the point that to have a significant effect on the burden of disease, further improvement in survival from primary and secondary prevention is necessary. New drugs show promise for further improvement in outcome of treatment, but they also come at a significant increase in costs (31-32).
The quality of life of patients during and after disease and treatment is an important outcome of breast cancer care and is considered an
Prevention in high-risk groups
essential outcome measure in cancer clinical trials. There is still very
limited information on quality of life of breast cancer patients from
It is estimated that 20–30% of breast cancers are related to genetic
clinical trials as well as from clinical practice. Quality of life is more
factors that in combination with lifestyle factors can trigger the
affected in younger women with breast cancer and in women with
development of the disease. Around 4-7% of breast cancer cases are
recurrent and metastatic disease (4).
directly attributable to certain genetic mutations, most commonly in the BRCA1 and BRCA2 genes, which predispose women to a 60-80%
life-time risk of developing breast cancer, often already at a young
Primary prevention
age. For women with a high genetic predisposition for breast cancer, preventive measures can be taken including; more frequent screening,
Primary prevention measures aim to reduce the risk factors for a
and at a younger age, or chemoprevention with endocrine therapy.
specific disease and/or the individual perceptibility for such risk factors.
These drugs however, may have limited impact since BRCA1 carriers
Primary prevention of breast cancer is more difficult to achieve than
are frequently endocrine unresponsive. The most established strategy
for some other cancer forms, for example lung cancer, since most of
is preventive surgery including removal of the breasts, although the
the breast cancer risk factors are currently not amenable to primary
evidence base for this strategy is limited (35).
prevention interventions. However it should be noted that a healthy lifestyle reduces the risk for breast cancer as well as many other
Tamoxifen and raloxifen have been available for use as preventive
agents in the US for many years (36-39). A recent review, confirms
Prevention and the economic burden of breast cancer
that the prophylactic use of tamoxifen and raloxifen reduces the
Secondary prevention/early diagnosis
incidence of invasive breast cancer. Subgroup analyses and decision models suggest that high-risk women, particularly those who have
The aim of secondary prevention is to reduce the severity of disease
had a hysterectomy, derive the most benefit with the least harm, the
(risk of recurrent and/or metastatic disease) and the risk of dying from
researchers report (40).
it. As discussed earlier, outcomes are significantly better if the breast cancer is detected before it has spread outside of the breast.
Both drugs have recently been granted market authorization for this indication from EMA in Europe. Women in England with a family
However, early-stage breast cancer is not symptomatic in all patients.
history of cancer will be able to get the drugs tamoxifen and raloxifen
The main objective of early detection or secondary prevention through
on the National Health Service as a protection against breast cancer,
screening is to detect early stage cancers when they can be treated
under new guidelines from the National Institute of Health and Care
most effectively. Early detection has been shown to be important
Excellence (NICE)(41). It is estimated that about 3% of the female
due to the strong association between stage at diagnosis (or tumor
population would be indicated for prevention. However, experience
size) and survival (44). For most types of breast cancer the likelihood
from the US market tells that rather few women use this treatment,
of lymph node invasion and worsening tumor grade increases as
mainly due to the side effects involved.
tumor size increases (45), leading to poorer long-term survival. Early detection is only valuable if it leads to timely diagnostic follow-up and
The American Society of Clinical Oncology (ASCO) has recently issued
effective treatment. The principal secondary prevention measure in
updated guidelines for chemoprevention (42). In women at increased
breast cancer is population-based mammography, combined with
risk of breast cancer age ≥ 35 years, tamoxifen (20 mg per day for 5
ultrasound examination in dense breasts in some countries, which has
years) should be discussed as an option to reduce the risk of estrogen
been shown to improve outcomes as it leads to a larger share of breast
receptor (ER)–positive breast cancer. In postmenopausal women,
cancers being diagnosed at an early stage in the screened population.
raloxifen (60 mg per day for 5 years) and exemestane (25 mg per day
Regular self-examination of the breast has also been put forward as
for 5 years) should also be discussed as options for breast cancer
a measure for early detection of breast cancer. However, there is no
risk reduction. Those at increased breast cancer risk are defined as
evidence that self-examination has any effect on earlier diagnosis.
individuals with a 5-year projected absolute risk of breast cancer ≥
Nevertheless, in many countries with limited coverage of breast cancer
1.66% (based on the National Cancer Institute Breast Cancer Risk
screening, the majority of breast cancer is detected when women
Assessment Tool or an equivalent measure) or women diagnosed with
seek care after having noticed a breast lump, therefore initiatives to
lobular carcinoma in situ. Use of other selective ER modulators or other
increase the awareness of breast cancer are extremely important so
aromatase inhibitors to lower breast cancer risk is not recommended
that women are conscious that breast lumps and other changes to the
outside of a clinical trial. Health care providers are encouraged to
breasts can be a sign of cancer and do not postpone seeking care until
discuss the option of chemoprevention among women at increased
the symptoms have reached a critical stage. It is also interesting to
breast cancer risk. The discussion should include the specific risks and
note that a recent retrospective ‘failure analysis' of BC mortality in over
benefits associated with each chemo preventive agent.
7000 women found that >70% of deaths from breast cancer occurred
However, an ongoing problem is that it is difficult to detect the high-
in women who did not receive regular screening mammograms (46).
risk woman, who may benefit most from treatment. New research
A critical factor in relation to this is access to well organized breast
indicates that it may be possible to identify the gene patterns that
cancer care, including diagnostic work-up, surgical and non-surgical
predict which women are likely to have a positive response to
treatment with Selective Estrogen Receptor Modulators (43).
Mammography screening programs
Since the drugs are generic, costs and cost-effectiveness are not an issue, but the resources for identification of risk groups and follow up
Although the outcome of breast cancer screening has been debated,
may be a matter for consideration.
the increased level of evidence available from the countries that implemented screening programs in the 1980s has resulted in breast
Prevention and the economic burden of breast cancer
cancer screening now being recommended by both the WHO, (in countries where resources are available to ensure effective and reliable screening of at least 70% of the target age group), and the Council of the European Union (EU) (47-49). Screening programs have been implemented for a long time in many countries, while other countries have recently implemented screening programs on the national level or are in the process of doing so. Extensive guidelines for quality assurance of screening programs have been developed for example on the EU level (47). Figure 9 gives an overview of the coverage of screening in a selection of countries. The target population differs between countries but in most, screening is targeted to 50-69 year old women.
In Mexico, mammography is recommended but there is no national population-based screening program and the overall adherence rate to
Figure 9 Breast cancer screening coverage in selected countries. (4).
mammography controls is low; a recent survey in Mexico City indicates that many women feel uncomfortable or worried about having
Population-based screening is a complex logistical process, from the
mammography (50-51).
initial invitation of the target population to further referral of patients with a screen result that requires follow-up. The WHO's statement on
A recent survey among 1,000 Hong Kong Chinese women aged 18-69
mammography is that it is an expensive test that requires great care
years reported that almost 60% of the women had never heard of
and expertise both to perform and in the interpretation of results,
mammography screening (52). These studies indicate that increased
and that therefore population-based screening is not viable in all
communication efforts are needed to promote breast examination in
countries. Although there is insufficient evidence, good clinical breast
groups with low adherence.
examinations by specially trained health workers could have an important role when resources are limited (45).
Based on identified data, China, Russia, Mexico, and Denmark have the lowest coverage of breast cancer screening.
The situation in the world regarding access to mammography varies.
In Russia, where mammography screening is managed at the regional level, coverage and adherence varies greatly between regions (53-55).
Prevention and the economic burden of breast cancer
One of the goals of Mexican healthcare for the period 2007-2012 is to triple the coverage of mammography screening in women 45-64 years old from the reported coverage of 22% in 2006 (56).
In China, the anti-cancer association launched a pilot project called "breast cancer screening for one million women" in 2005, with the objective to offer regular screenings to one million women aged 35-70 years (53). However the project suffered from technical problems and a lack of funding, which meant that the screening could be offered at a reduced price but not free of charge, and by 2006, only 120,000 women had been screened. In 2008, a follow-up project was initiated with the aim to provide screening to women in rural areas and this project has obtained government funding. The ambition is to screen more than half a million women in the next few years. Still this is only a small proportion of the 300 million women that would belong to the target group for mammography screening in China. Some local government schemes have started to offer cancer screening, such as the Beijing government that offers free breast examination for women within a certain age range. One issue with screening in China is that breast cancer is most common in premenopausal women and it is more difficult to detect cancer with mammography in younger women as breast tissue is more dense (57-58).
No national or regional breast screening program exists in India. Mammography is available in all major cities in both private and public hospitals for those who are willing to pay for it. Use of mammography for screening is not considered to be cost-effective, partly because of
Lately a similar debate has started in the US after the U.S. Preventive
the lack of high quality treatment facilities (9).
Services Task Force recommendation in 2009 that there was no evidence that women aged 40-49 benefit from routine screening. While
Also in countries with a high overall coverage, it has been shown
mammography undoubtedly detects early stages of breast cancer,
that two groups in particular are underrepresented in breast cancer
and reduces mortality in breast cancer (61), there is a renewed debate
screening programs; women from lower socio-economic levels and first on the balance between benefits and harms, with consequences
generation immigrants.
for the way this technology is applied in an optimal manner. The assessment of pros and cons is complicated by the fact that it is
Current controversies about screening
difficult to provide evidence about the outcome in clinical practice. It should be noted that this problem is also increasingly evident for new
The value of breast cancer screening has been considerably debated,
treatments in cancer, where outcome measures used in clinical trials,
for example in Denmark (59-60), partly with the argument that
such as progression free survival, may not easily be directly translated
population-based screening leads to over-diagnosis of cancer in situ
into improvements in quality of life and overall survival in clinical
that would not have developed into breast cancer, thus incurring
unnecessary treatment costs as well as risks and worries for affected women.
Recent important evidence comes from a study of the Norwegian breast-cancer screening program that was started in 1996 and expanded geographically during the subsequent 9 years (62).
Prevention and the economic burden of breast cancer
Women between the ages of 50 and 69 years were offered screening
A similar review from the UK estimates that for 10,000 UK women
mammography every 2 years. The study compared the incidence-
invited to screening from age 50 for 20 years, about 681 cancers will be
based rates of death from breast cancer in four groups: two groups
found of which 129 will represent over-diagnosis, and 43 deaths from
of women who from 1996 through 2005 were living in countries with
breast cancer will be prevented. In round terms, therefore, for each
screening (screening group) or without screening (non-screening
breast cancer death prevented, about three over-diagnosed cases will
group); and two historical-comparison groups that from 1986 through
be identified and treated. Of the 307,000 women aged 50–52 who are
1995 mirrored the current groups. The rate of death was reduced
invited to screening each year, just 1% would have an over-diagnosed
by 7.2 deaths per 100,000 person-years in the screening group as
cancer during the next 20 years (66).
compared with the historical screening group (rate ratio, 0.72; 95% confidence interval [CI],0.63 to 0.81) and by 4.8 deaths per 100,000
These studies conclude that screening for breast cancer has a positive
person-years in the non-screening group as compared with the
effect on mortality, confirming the results from clinical trials, but that
historical non screening group (rate ratio, 0.82; 95% CI, 0.71 to 0.93;
the effect is smaller than the effect from improvement of treatment
P<0.001 for both comparisons), for a relative reduction in mortality
and other factors. It will thus be even more important to optimize the
of 10% in the screening group (P = 0.13). Thus, the difference in the
screening programs. The development of cost-effective screening
reduction in mortality between the current and historical groups
methods and strategies is particularly important for developing
that could be attributed to screening alone was 2.4 deaths per
countries, where the potential health benefits are highest, but the
100,000 person-years, or a third of the total reduction of 7.2 deaths.
available resources the lowest (63).
The availability of screening mammography was associated with a reduction in the rate of death from breast cancer, but the screening
But also for countries with well-developed screening models,
itself accounted for only about a third of the total reduction.
improvements in the technology may change the balance of benefits, harms and costs. A particularly important issue is the improvement
A recently published paper studied changes in mortality after the
in the diagnostic workup to reduce the number of benign biopsies.
introduction of screening guidelines for breast and prostate cancers
Early detection of tumors through regular screening mammography
in the US and UK (67). They used differences in the timing of guideline
biennial as per the United States Preventative Services Task Force
adoption, which ages are recommended for screening, and which
recommendations, (USPSTF), has been shown to reduce breast cancer
cancers are detectable by screening to identify the effect of guidelines.
but many women turn out to have false positive results. False positive
Their quadruple-differencing strategy finds a moderately sized
mammograms have large economic consequences due to the cascade
mortality benefit from mammography and PSA screening guidelines
of tests that follow including diagnostic mammography, ultrasound,
among recommended age groups and little change in mortality rates
and biopsy. With an estimated 18 million screening mammograms
among age groups not recommended to receive screening. The result
conducted in the US annually, a false positive rate of 10% amounts to
can be compared with an earlier US study, which compared screening
almost $1 billion in unnecessary spending (68). Although the number of
and adjuvant therapy. The proportion of the total reduction in the rate
diagnostic mammograms and ultrasounds prompted by false-positive
of death from breast cancer attributed to screening varied in the seven
screens each year contributes significantly to the economic burden of
models from 28 to 65 percent (median, 46 percent), with adjuvant
breast cancer, the most significant cost associated with false-positive
treatment contributing the rest (64).
mammography screening is the large number of breast biopsies performed, nearly 70% of which result in benign diagnoses (69).
In an attempt to work out the balance between benefits and harm, the EUROSCREEN working group (65) published a "balance sheet" for
Simulations performed by a research group at the Fred Hutchinson
mammography screening. For every 1000 women screened biennially
cancer centre in Washington (70), indicate that the consequences of
from age 50–51 until age 68–69 and followed up to age 79, an
introducing a new diagnostic in order to reduce the number of positive
estimated seven to nine lives are saved, four cases are over-diagnosed,
biopsies or as an alternative to diagnostic mammograms and/or
170 women have at least one recall followed by non-invasive
ultrasound, are not as clear-cut as may be expected. It comes down to
assessment with a negative result and 30 women have at least one
a valuation of the different consequences of a false positive and a false
recall followed by invasive procedures yielding a negative result.
negative result. But it shows the importance of simulation models as a
Prevention and the economic burden of breast cancer
tool to help to find cost-effective diagnostic and treatment pathways.
These substances include silicone oil and polyacrylamide gel.
Surgical biopsies are still performed at many institutions, but can
German investigators reported at the 2013 Breast Cancer Symposium
in most instances be replaced by far less expensive percutaneous
in San Francisco that an abridged magnetic resonance imaging
(MRI) protocol can accurately detect cancers among women whose mammographic screenings were negative. MRI, therefore, may reveal
There may be significant opportunities to reduce costs and improve
the type of tumor that mammography typically misses, and can do so
outcomes also with existing methods for breast cancer screening. A
in a time-efficient fashion, thus making MRI feasible for breast cancer
Nordic study revealed differences in cost per patient screened between
screening (73).
countries; from 34 Euro in Sweden, to 127 in Finland, and with Norway and Denmark in between (15).
Digital breast tomosynthesis (DBT) (74) is a new breast imaging technology that uses tomography and 3-D reconstruction to improve
Breast MRI and other emerging technologies
lesion visibility. The U.S. Food and Drug Administration recently approved DBT equipment, and research on its effectiveness continues
Magnetic resonance imaging (MRI) has been shown to detect cancers
around the world. In 2012, the Automated Breast Ultrasound System
not visible on mammograms. The chief strength of breast MRI is its
(ABUS) was approved by the FDA as an adjunct to mammography
very high negative predictive value. A negative MRI can rule out the
for breast cancer screening in asymptomatic women for whom
presence of cancer to a high degree of certainty, making it an excellent
screening mammography findings are normal or benign (BI-RADS
tool for screening in patients at high genetic risk or radiographically
Assessment Category 1 or 2), with dense breast parenchyma (BI-RADS
dense breasts, and for pre-treatment staging where the extent of
Composition/Density 3 or 4), and have not had previous clinical breast
disease is difficult to determine on mammography and ultrasound.
However, breast MRI has long been regarded to have disadvantages. For example, although it is 27–36% more sensitive, it has been
New technological options are welcome, but need to be carefully
claimed to be less specific than mammography (72). As a result, MRI
evaluated in clinical practice, including an assessment of cost-
studies may have more false positives (up to 30%), which may have
effectiveness in the screening situation. At present these new
undesirable financial and psychological costs. It is also a relatively
modalities seems to be very valuable tools in the work-up of women
expensive procedure, and one which requires the intravenous injection
selected through mammographic screening in order to identify false
of gadolinium, which has been implicated in a rare reaction called
positive cases, as well as mapping the extent of disease in true positive
nephrogenic system fibrosis. Another limitation is access to MRI
scanners / available capacity for screening linked to the investment required (cost of the MRI equipment).
Cost-effectiveness of breast cancer screening
Proposed indications for using MRI for screening include:
Cost-effectiveness analyses compare the costs and health effects (outcomes) of an intervention to determine the extent to which it
• Strong family history of breast cancer
can be regarded as providing value for money. This can be used to
• Patients with BRCA-1 or BRCA-2 oncogene mutations
help inform decision makers who have to determine where and how
• Evaluation of women with breast implants
to best allocate resources. The cost-effectiveness of breast cancer
• History of previous lumpectomy or breast biopsy surgeries
screening varies by country and depends on many factors e.g. disease
• Axillary metastasis with an unknown primary tumor
epidemiology, health care system, costs and compliance rate. The
• Very dense or scarred breast tissue
majority of studies have been conducted in developed countries and cannot be directly translated to low-resource countries. Many of the
In addition, breast MRI may be helpful for screening in women who
cost-effectiveness analyses in breast cancer screening have focused
have had breast augmentation procedures involving intramammary
on comparing different strategies for screening in high-income
injections of various foreign substances that may mask the
countries, e.g. age range, screening test, frequency of screening
appearances of breast cancer on mammography and/or ultrasound.
Prevention and the economic burden of breast cancer
Assuming that mammography screening reduces mortality in breast
discussed, than left to speculation. One advantage of this is that
cancer by 20%, 43 deaths are avoided per 10,000 screened using
systematic studies usually lead to improvements in the data and
figures from the UK screening program (76). The same source gives
improved understanding of the drivers of cost-effectiveness, which
the estimate of 17 years of life gained per death avoided, which gives
can make better decisions and thus better outcomes. The increasing
0.073 LYG per screened woman. The costs of screening programs
number of options for early detection of breast cancer makes it also
vary between countries, and how the costs are calculated. Ideally, a
necessary to use cost-effectiveness to define the detection programs
cost-effectiveness calculation should include all costs for screening and that give most value for money. diagnosis and treatment.
Many cost-effectiveness studies of mammography have been
Using data from the Nordic study, costs vary from 34 Euro to 127
performed, but since the determinates of both relative effectiveness
Euro per woman screened (15). We will use 100 Euro as the base case
and costs changes over time, new studies need to be performed.
estimate (15). Let us further assume that over a 20-year period, there
The development of cost-effective strategies for early detection for
are seven screening occasions. This will give a cost per life year gained
countries with low and medium income and health care spending
of 10,000 Euro. This estimate is consistent with, but in the lower range,
levels should be a priority for reducing the burden of breast cancer
of earlier studies of cost-effectiveness which have arrived at estimates
between 1000 and 30,000 Euro per LYG (75-76). This indicates that
screening is a cost-effective method for improving outcomes in breast
Conclusions and policy implications
cancer compared to no screening.
• Accurate data on breast cancer incidence and mortality on the
The variation in results between studies is not surprising, taking into
national level is lacking in several countries due to limited cancer
account the uncertainties about the clinical outcome of screening.
registration. Such data are important for documentation, assessment
There is also limited data to adjust life years gained to quality adjusted
and communication of the burden of the disease. There is a need for an
life years gained, taking into account both the fact that all life years
initiative for collection of such data in a way that makes international
gained are not of full quality, and impact of diagnosis and treatment
comparisons possible, as well as comparisons over time to assess
on quality of life. In addition, it is both conceptually and empirically
progress and impact of policy development.
difficult to estimate the incremental costs of screening. How much of treatment costs should be included in the cost of screening. Without
• Available data show that breast cancer incidence rates have
a screening program, cancer may have been detected later, with a
steadily increased in developed countries over the last 50 years.
different stream of costs. Changes in survival also have an impact on
In the last decades increased incidence rates are also being seen in
costs for treatment of other diseases, and it is debated if changes in
many developing countries, in particular in parts of Asia. The increased
costs for treatment of other diseases should be included or not. The
incidence of breast cancer is mainly due to increased life expectancy
inclusion of changes in indirect costs due to reduced morbidity in
but also relates to lifestyle changes, such as women having fewer
women who are of working age, and the inclusion of costs in added
children as well as hormonal interventions like post-menopausal
years of life, will also affect the estimates of cost-effectiveness. Finally,
hormonal therapy.
cost-effectiveness is determined by the design of the screening program, and will differ between patient characteristics, such as age.
• Data on the economic burden of breast cancer in terms of
direct and indirect costs are sparse. Such data are important as
Performing cost-effectiveness studies of screening programs are
complements to data on health burden, and for decisions about
thus a complicated task and the results will carry a great degree
resource allocation for prevention and treatment. They are also
of uncertainty. However, decisions about the implementation and
necessary for the performance of comparative studies between
design of screening programs will be influenced by such estimates.
countries and between regions (populations) within countries. There
It is therefore better that such studies are performed in a systematic
is a need to learn from such comparisons for the development of best
way, with data limits recognized and uncertainties around estimates
practices for prevention, early detection and treatment.
Prevention and the economic burden of breast cancer
• The economic burden of breast cancer is considerable in terms of
• New data indicate that options for primary prevention with
both direct and indirect costs. The direct health care costs attributable chemotherapy should be considered in all health care systems.
to breast cancer vary greatly between study countries, reflecting
Programs for this should be developed, and combined with evaluation
differences in total health care spending. The indirect costs of breast
to find out optimal strategies for different risk groups.
cancer are larger than the direct treatment costs since many breast cancer cases occur in women below 65 years, specifically in new
• Screening for early detection of breast cancer with the aim of
industrialized countries.
improving survival is a key component of a strategy for prevention.
Such programs must be designed to meet the specific situation in
• Improved outcome is related to earlier diagnosis, where there is
different health care systems, taking into account the need to balance
a marked correlation between the stage at diagnosis in a country and
potential benefits and harms, as well as cost-effectiveness and
overall survival rates in breast cancer. Methods for early detection incur affordability. costs and potential harms, as well as benefits, and must be designed according to evidence of the balance between costs and improved
• There is great uncertainty around estimates of reductions in
mortality and the magnitude of over-diagnosis from screening, but it
is possible to conclude that breast cancer screening provides important
• The largest survival improvements over the last decades have
benefits and should be continued. Data on cost-effectiveness of
been seen in patients diagnosed with stage II or III disease, which
alternative screening strategies are still limited, and both data and
is mainly due to early detection and to the introduction of adjuvant
methods for evaluation must be improved to provide the basis for
treatment. Currently we see a rapid introduction of new effective drugs
for treatment of breast cancer that improve survival but also increase the costs of treatment; particularly at the late stages of the disease.
• There are significant variations in breast cancer outcomes
in countries with comparable levels of resources dedicated to
healthcare. This indicates an opportunity to improve outcome through
the identification and implementation of best practices in diagnosis
and treatment.
• The lack of detailed, patient linked, data on outcome in relation to
treatment patterns and stage of diagnosis in many countries impedes
and limits analyses of how changes in clinical practice affect outcome.
• Survival is the main focus in the treatment of breast cancer,
but with increasing survival rates more women are living with
the disease, making quality of life during and after treatment
increasingly important. Data on quality of life at different stages of
the disease, and related to different treatment options are still scarce.
There is a need for collecting data on quality of life and quality of care
routinely in clinical practice, for both monitoring and assessment of
outcome.
Prevention and the economic burden of breast cancer
21. Lidgren, M., et al., Resource use and costs associated with different states of breast cancer. Int J Technol Assess Health Care, 2007. 23 (2): p. 223- 31.
1. European Commission, DG Health and Consumers. Investing in Health.
22. Thomas, R.J., et al., The total hospital and community UK costs of
Commission Staff Working Document, Social Investment Package,
managing patients with relapsed breast cancer. Br J Cancer, 2009. 100 (4):
p. 598- 600.
23. Remak, E. and L. Brazil, Cost of managing women presenting with stage
2. Carol DeSantis, Rebecca Siegel, Priti Bandi, Ahmedin Jemal, DVM, Breast
IV breast cancer in the United Kingdom. Br J Cancer, 2004. 91 (1): p. 77- 83
cancer statistics 2011. CA CANCER J CLIN 2011;61:409–418
24. Campbell JD and Ramsey SD, the costs of treating breast cancer in the
3. Danny R. Youlden, Susanna M. Cramb, Nathan A.M. Dunn, Jennifer M.
US: a synthesis of published evidence pharmacoeconomics; 2006;27(3):199-
Muller, Christopher M. Pyke , Peter D. Baade. The descriptive epidemiology of
female breast cancer: An international comparison of screening, incidence,
25. Lidgren M, Jonsson, B, Rehnberg C,Wilking, N 6 Berg J, Cost-effectiveness
survival and mortality. Cancer Epidemiology 36 (2012) 237–248
of HER 2 testing and 1-year adjuvant trastuzumab therapy for early breast
4. Wilking N, Kasteng F A review of breast cancer care in 18 countries in
cancer. Ann Oncol 2008;19, 487-95
Europe, Asia and Latin America 2009. www.comparatorreports.se.
26. Lidgren M, Wilking N, Jonsson, B and Rehnberg C, Acta Oncol 2008;47,
5. Rodriguez- Cuevas, S., et al., Breast carcinoma presents a decade
earlier in Mexican women than in women in the United States or European
27. From (2), based on WHO Statistical Information information systems
countries. Cancer, 2001. 91 (4): p. 863-8.
(WHOSIS), 2009.
6. Cancer i siffror 2009. Socialstyrelsen & Cancerfonden, 2009.
28. National Cancer Control Programmes: Policies and Managerial
7. Fan, L., et al., Breast cancer in a transitional society over 18 years: trends
Guidelines. World Health Organization, Geneva, Switzerland, 2002.
and present status in Shanghai, China. Breast Cancer Res Treat, 2009. 117
29. Lundqvist A, Wilking N, Gerdtham U-G, Persson U, Steen Carlsson
K (2013). Målriktad behandling av bröstcancer. Stockholm, Sverige,
8. Kasteng F, Wilking N, Jönsson B, Patient Access to Cancer Drugs in
Studieförbundet näringsliv och samhälle, SNS.
Nine Countries in the Middle East (www.comparatorreports.se. For data
30. Garrison, LP, Jr. och Veenstra, DL, The economic value of innovative
on Turkey see Vahit Özmen, Breast cancer in the World and Turkey, The
treatments over the product life cycle: the case of targeted trastuzumab
Journal of Breast Health).
therapy for breast cancer. Value in health : the journal of the International
9. Gaurav Agarwal and Pooja Ramarkant. Breast cancer care in India:The
Society for Pharmacoeconomics and Outcomes Research, 2009. 12(8): s.
Current scenario and Challenges for the Future of Breast Care 2008;3:21-27
10. Medical Oncology Status in Europe Survey (MOSES) Phase II. ESMO
31. Swain, S. M., et al. (2013), ‘Pertuzumab, trastuzumab, and docetaxel for
MOSES Task Force, 2006.
HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival
11. Coleman, M.P., et al., EUROCARE- 3 summary: cancer survival in Europe
results from a randomised, double-blind, placebo-controlled, phase 3 study',
at the end of the 20th century. Ann Oncol, 2003. 14 Suppl 5: p. v128-49
Lancet Oncol, 14 (6), 461-71.
12. Engholm, G., et al., NORDCAN: Cancer Incidence, Mortality and
32. Breast cancer (HER2 positive, metastatic) - pertuzumab (with
Prevalence in the Nordic Countries. Association of Nordic Cancer Registries,
trastuzumab and docetaxel) [ID523] NICE Guidance (in development, Sept.
Danish Cancer Society [Online at:www.ancr.nu. Accessed August 2009],
2009(Version 3.4).
33. Mayo, I., et al., Epidemiology of breast cancer. Lancet Oncol. 2001;2:133-
13. The global burden of disease: 2004 update. World Health Organization,
34. See (2) for references
14. Lidgren, M., N. Wilking, and B. Jonsson, Cost of breast cancer in Sweden
35. Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ‘t Veer L, Garber
in 2002. Eur J Health Econ, 2007.
JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk
15. Cost of cancer in the Nordic Countries. A comparative study of health
in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. Journal
care costs and public income loss compensation payments related to
of clinical oncology : official journal of the American Society of Clinical
cancer in the Nordic countries in 2007. SINTIF report 2011.
Oncology. 2004 Mar 15;22(6):1055-62).
16. Analyse économique des coûts du cancer en France. Institute Nacional
36. Fisher, B., et al., Tamoxifen for prevention of breast cancer: report of
du Cancer, 2007.
the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl
17. Federal Health Monitoring Germany. [Online at: http://www.
Cancer Inst, 1998. 90 (18): p. 1371- 88.
37. Freedman, A.N., et al., Estimates of the number of US women who could
benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer
Inst, 2003. 95 (7): p. 526- 32.
Accessed 30 July, 2013.
38. Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS,
18. Mäklin, S. and P. Rissanen, Kostnader för cancer.
Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER Jr, Wade JL 3rd, Robidoux
Cancerorganisationernas publikationer 2006
A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE,
19. B. and N. Wilking, A global comparison regarding patient access to
McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N; National Surgical
cancer drugs. Ann Oncol, 2007. 18 Suppl 3 : p. iii1- iii77.
Adjuvant Breast and Bowel Project (NSABP)). Effects of tamoxifen vs
20. Dahlberg, L., J. Lundkvist, and H. Lindman, Health care costs for
raloxifene on the risk of developing invasive breast cancer and other
treatment of disseminated breast cancer. Eur J Cancer, 2009. 45 (11): p.
disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2
trial. JAMA. 2006 Jun 21;295(23):2727-41. Epub 2006 Jun 5. JAMA. 2006 Jun
Prevention and the economic burden of breast cancer
21;295(23):2727-41. Epub 2006 Jun 5. JAMA. 2006 June 21;295(23):2727-41.
Research Institute Professor N.N.Petrov, St Petersburg, Russia, 2009.
Epub 2006 Jun 5.
54. Report Ministry of Health of Moscow region 2008. Personal
39. Gail, M.H., The estimation and use of absolute risk for weighing the risks
communication: Prof. MariaMikhailovna Konstantinova, Deputy Head of
and benefits of selective estrogen receptor modulators for preventing breast Moscow Region Oncology Hospital, Moscow, Russia,cancer. Ann N Y Acad Sci, 2001. 949 : p. 286-91.
55. Tomsk city and region (annual results) 2008. Personal communication:
40. H. D. Nelson, M.E . Beth Smith; J.C. Griffin, R. Fu. Use of Medications to
Prof. Elena Mikhailovna Slonimskaya, Head of General Surgery Department,
Reduce Risk for Primary Breast Cancer: A Systematic Review for the U.S.
Tomsk Cancer Research Center, Tomsk,Russia, 2009.53.
Preventive Services Task Force. Ann Intern Med. 2013;158:604-614
56. Programa Nacional de Salud 2007- 2012 - Por un México sano:
41. NICE Clinical Guidelines CG164. Familial breast cancer: classification
construyendo alianzas para una mejor salud. Secretaria de Salud, 2007
and care of people at risk of familial breast cancer and management
(National Health Plan Mexico)
of breast cancer and related risks in people with a family history of
57. Hujun, L., The Plight of China's Breast Cancer Screening Program. China
breast cancer, issued June, 2013 http://www.nice.org.uk/guidance/index.
Today - Explaining China to the World, [Online at: www.chinatoday.com.cn,
Accessed September 2009].
42. Kala Visvanathan, Patricia Hurley, Elissa Bantug, Powel Brown, Nananda
58. Wang, S.-m., Concerns on diagnosis and treatment of breast cancer in
F. Col, Jack Cuzick, Nancy E. Davidson, Andrea DeCensi, Carol Fabian, Leslie
China. Chinese Medical Journal, 2007. 120(20): p. 1741-1742
Ford, Judy Garber, Maria Katapodi, Barnett Kramer, Monica Morrow, Barbara
59. Jorgensen, K.J. and P.C. Gotzsche, Overdiagnosis in publicly organised
Parker, Carolyn Runowicz, Victor G. Vogel III, James L. Wade and Scott
mammography screening programmes: systematic review of incidence
M. Lippman. Use of Pharmacologic Interventions for Breast Cancer Risk
trends. BMJ, 2009. 339 : p. b2587
Reduction: American Society of Clinical Oncology Clinical Practice Guideline.
60. Gotzsche, P.C. and O. Olsen, Is screening for breast cancer with
Published online before print July 8, 2013, doi: 10.1200/JCO.2013.49.3122
mammography justifiable? Lancet, 2000. 355 (9198): p. 129- 3
43. Pharoah, P.D.P., et al., Polygenes, risk prediction, and targeted prevention
61. Humphrey, L.L., et al., Breast cancer screening: a summary of the
of breast cancer. The New England Journal of Medicine, 2008.
evidence for the U.S. Preventive Services Task Force. Ann Intern Med, 2002.
44. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast
137 (5 Part 1): p. 347- 60.
cancer. JAMA 2005;293(10):1245–56. Tabar L, Vitak B, Chen HH, Duffy SW,
62. Mette Kalager, M.D., Marvin Zelen, Ph.D., Fr.ydis Langmark, M.D., and
Yen MF, Chiang CF, et al. The Swedish Two-County Trial twenty years later.
Hans-Olov Adami, M.D., Ph.D.Effect of Screening Mammography on Breast-
Updated mortality results and new insights from long-term follow-up. Radiol
Cancer Mortality in Norway. NEJM 2010;363:1203-10.
Clin North Am 2000;38(4):625–51.
63. Adam Lieive and Thomas Stratmann Cancer Screening Guidelines
45. Soerjomataram I, Louwman M, Ribot J, Roukema J, Coebergh J. An
and Mortality, March, 2013 (Electronic copy available at: http://ssrn.com/
overview of prognostic factors for long-term survivors of breast cancer.
Breast Cancer Res Treat 2008;107(3):309–30. Foulkes WD, Reis-Filho JS,
64. Berry DA, Cronin KA, Plevritis SK, et al.; Cancer Intervention and
Narod SA. Tumor size and survival in breast cancer—a reappraisal. Nat Rev
Surveillance Modeling Network (CISNET) Collaborators. Effect of screening
Clin Oncol 2010;7(6):348–53
and adjuvant therapy on mortality from breast cancer. N Engl J Med.
46. Webb, M. L., Cady, B., Michaelson, J. S., Bush, D. M., Calvillo, K. Z., Kopans,
D. B. and Smith, B. L. (2013), A failure analysis of invasive breast cancer.
65. EUROSCREEN Working Group. Summary of the evidence of breast
Cancer. doi: 10.1002/cncr.2819.
cancer service screening outcomes in Europe and first estimate of the
47. Perry et al European guidelines for quality assurance in breast cancer
benefit and harm balance sheet. J Med Screen 2012;19 Suppl1:5–13
screening and diagnosis: F o u r t h E d i t i o n http://www.euref.org/
66. M G Marmot, D G Altman, D A Cameron, J A Dewar, S G Thompson,
M Wilcox – The Independent UK Panel on Breast Cancer Screening. The
48. Screening for Breast Cancer. WHO, [Online at:http://www.who.int/cancer/
benefits and harms of breast cancer screening: an independent review. A
detection/breastcancer/en/, Accessed September 2009].
report jointly commissioned by Cancer Research UK and the Department of
49. Union, C.o.t.E.,Council recommendation of 2 December 2003 on cancer
Health (England) October 2012
67. A. Sarvazyan, V. Egorov, J.S. Son and C.S. Kaufman Cost-Effective
(2003/878/EC). Official Journal of the European Union, 2003: p. available at:
Screening for Breast Cancer Worldwide: Current State and Future Directions
Breast Cancer: Basic and Clinical Research 2008:1
68. Chubak J, Boudreau DM, Fishman PA, Elmore JG. Cost of breast-related
50. Knaul, F.M., et al., Breast cancer in Mexico: a pressing priority. Reprod
care in the year following false positive screening mammograms. Medical
Health Matters, 2008.16 (32): p. 113- 23.
Care. 2010;48(9):815-20
51. Nigenda, G., M. Caballero, and L. González, Social process of breast
69. Weaver DL, Rosenberg RD, Barlow WE, Ichikawa L, Carney PA,
cancer in Mexico. Research protocol. National Institute of Public
Kerlikowske K, et al. Pathologic findings from the Breast Cancer Surveillance
Consortium: population-based outcomes in women undergoing biopsy
52. Chua, M.S., et al., Knowledge, perceptions, and attitudes of Hong Kong
after screening mammography. Cancer. 2006;106(4):732-42.).
Chinese women on screening mammography and early breast cancer
70. Bensink et al A Diagnostic Test to Reduce Benign Biopsies after
management. Breast J, 2005. 11 (1): p. 52- 6.
Screening Mammography: Costs, Benefits and Consequences. Technical
53. Merabishvili, V.M. and S. Y A, Current development of information
report Research and Economic Assessment in Cancer and Healthcare
systems of Oncological Service. Published in St Petersburg, personal
(REACH) Group Fred Hutchinson Cancer Research Center
communcation: Prof. Vladimir Fedorovich Semiglazov,General Director of
Prevention and the economic burden of breast cancer
71. Van Breest Smallenburg V, Nederend J, Voogd AC, Coebergh JW, van
Beek M, Jansen FH, Louwman WJ, Duijm LE. Trends in breast biopsies for abnormalities detected at screening mammography: a population-based study in the Netherlands Br J Cancer. 2013 Jul 9;109(1):242-8
For further information visit: newsroom.gehealthcare.com
72. Hrung J, Sonnad S, Schwartz J, Langlotz C (1999). "Accuracy of MRimaging in the work-up of suspicious breast lesions: a diagnostic metaanalysis". Acad Radiol 6 (7): 387–9773. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-040951.pdf' Government Department of Health and Ageing, 2009 74. Paul D P Pharoah, Deborah, Hayley S Bennett, Nora Pashayan Cost effectiveness of the NHS breast screening programme: life table model. BMJ 2013;346:f261875. BreastScreen Australia Evaluation - Economic evaluation and modellingstudy. Prepared by IMS Health Pty Ltd Australia for the AustralianGovernment Department of Health and Ageing, 200976. Paul D P Pharoah, Deborah, Hayley S Bennett, Nora Pashayan Costeffectiveness of the NHS breast screening programme: life table model. BMJ2013;346:f261877. Value Based Pricing in Sweden: Lessons for Design? Ulf Persson, SeminarBriefing, November 201278. American Cancer Society: Breast Cancer Facts and Figures 2013-201479. Tosteson AN, et al. (2008). Cost-effectiveness of digital mammographybreast cancer screening. Ann Intern Med, 148, 1-1080. Socialstyrelsen (The National Board of Health and Welfare), Sweden,.
Cancer statistics81 Groot et al Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North Americaand Asia. Breast J 2006; 12 (suppl 1): S81; 82 Okonkwo et al Breast cancer screening policies in developing countries: a cost-effectiveness analysis for India. JNCI 2008; 100:129083 De Gelder et al (2009). Cost-effectiveness of opportunistic versus organised mammography screening in Switzerland.Eur J Cancer, 45(1):127-38
Prevention and the economic burden of breast cancer
Source: http://www3.gehealthcare.com.au/~/media/downloads/anz/products/mammography/prevention-and-economic-burden-of-breast-cancer-white-paper-jb16682au.pdf?Parent=%7B76EB7741-6A5F-46F2-94E0-7DB1FA101974%7D
Instructions for Use Erythropoietin ELISA Enzyme immunoassay for the quantitative determination of Erythropoietin (EPO) in human serum. Flughafenstrasse 52a Phone: +49 (0)40-53 28 91-0 D-22335 Hamburg, Germany Fax: +49 (0)40-53 28 91-11 Erythropoietin ELISA (NM56011) INTENDED USE
Journal of Modern Medicinal Chemistry, 2014, 2, 1-9 1 Synthesis, Characterization and Microbial Evaluation of Metal Complexes of Molybdenum with Ofloxacin (Levo (S-form) and Dextro (R-form)) Isomers Qadeer K. Panhwar1,2 and Shahabuddin Memon2,* 1Dr. M. A. Kazi Institute of Chemistry, University of Sindh, Jamshoro, Pakistan 2National Center of Excellence in Analytical Chemistry, University of Sindh, Jamshoro 76080, Pakistan















