Cincinnati.vc.ons.org
This material is protected by U.S. copyright law. Unauthorized reproduction is prohibited. To purchase quantity reprints,
please e-mail
[email protected] or to request permission to reproduce multiple copies, please e-mail
[email protected].
An Interdisciplinary Consensus on Managing
Skin Reactions Associated With Human
Epidermal Growth Factor Receptor Inhibitors
Beth Eaby, MSN, CRNP, OCN®, Ann Culkin, RN, OCN®, and Mario E. Lacouture, MD
The use of human epidermal growth factor receptor (HER1/EGFR) inhibitors, such as erlotinib, cetuximab, and panitumumab, often is accompanied by the development of a characteristic spectrum of skin toxicities. Although these toxicities rarely are life threatening, they can cause physical and emotional distress for patients and caregivers. As a result, practitioners often withdraw the drug, potentially depriving patients of a beneficial clinical outcome. These reactions do not necessarily require any alteration in HER1/EGFR-inhibitor treatment and often are best addressed through symptomatic treatment. Although the evidence for using such therapies is limited, an interdisciplinary HER1/EGFR-inhibitor dermatologic toxicity forum was held in October 2006 to discuss the underlying mechanisms of these toxicities and evaluate commonly used therapeutic interventions. The result was a proposal for a simple, three-tiered grading system for skin toxicities related to HER1/EGFR inhibitors to be used in therapeutic decision making and as a framework for building a stepwise approach to intervention.
At a Glance
e use of human epidermal growth factor receptor (HER1/
The use of HER1/EGFR-targeted therapies, such as erlo-
tinib (Tarceva®, OSI Pharmaceuticals, Inc.), cetuximab (Erbitux®, Bristol-Myers Squibb), and panitumumab (VectibixTM, Amgen Inc.), often is accompanied by
EGFR) inhibitors often is accompanied by the development
the development of a characteristic spectrum of skin
of a characteristic class-specific spectrum of skin toxicities.
toxicities (Rhee, Oishi, Garey, & Kim, 2005). Although these
in toxicities related to HER1/EGFR inhibitors do not necessar-
events rarely are life threatening, they can cause physical and
ily require alteration in HER1/EGFR-inhibitor treatment and of-
emotional distress for patients and caregivers. Often, the rash
ten are addressed best through symptomatic management.
may be mistaken as an uncontrollable adverse effect rather than a treatable side effect. As a result, practitioners withdraw the drug,
idence-based treatment recommendations for skin tox-
potentially depriving patients of a beneficial clinical outcome.
icities related to HER1/EGFR inhibitors are not available
Oncology nurses often are the first point of contact for patients
because no data from control ed clinical studies have been
who are receiving treatment; therefore, understanding the clinical
basis for such skin reactions and offering effective and appropriate assessments and interventions are critical.
On October 29, 2006, an interdisciplinary forum was held in
Beth Eaby, MSN, CRNP, OCN®, is a nurse practitioner at the University of
Chicago, IL, to discuss skin toxicities associated with HER1/EGFR-
Pennsylvania in Philadelphia; Ann Culkin, RN, OCN®, is a clinical nurse at
targeted therapies. Oncologists, dermatologists, pharmacists, and
Memorial Sloan-Kettering Cancer Center in New York, NY; and Mario E.
nurses shared their knowledge on the underlying mechanisms
Lacouture, MD, is a dermatologist in the Department of Dermatology at
of these events and evaluated existing practices in the hope of
SERIES Clinic and the Robert H. Lurie Comprehensive Cancer Center at
reaching a consensus strategy on how best to manage them. This
Northwestern University in Chicago, IL. Eaby is a member of the Tarceva®
article provides an overview of their discussions.
speakers bureau and Culkin is a member of the Advisory Board and speak-ers bureau, both for Genentech. Lacouture received an honorarium from Genentech and is a speaker for Genentech, ImClone, and OSI Pharmaceu-
Human Epidermal Growth Factor
ticals. Mention of specific products and opinions related to those products do not indicate or imply endosement by the
Clinical Journal of Oncology
Nursing or the Oncology Nursing Society. (Submitted July 2007. Accepted
As a result of an increased understanding of the underly-
for publication September 1, 2007.)
ing molecular causes of cancer, biologic targeted agents have
Digital Object Identifier:10.1188/08.CJON.283-290
Clinical Journal of Oncology Nursing • Volume 12, Number 2 • Managing Skin Reactions
emerged since the mid-1990s as a new strategy in the treat-
tyrosine kinase (Yarden & Sliwkowski, 2001). Both prevent
ment of various malignancies. These targeted agents interact
the initiation of cell-signaling pathways that normally promote
with specific molecules that are pivotal in tumor growth and
tumor-cell proliferation, migration, adhesion and angiogenesis,
development. Traditional "cytotoxic" chemotherapies not
and inhibit apoptosis (Arteaga, 2001). A fourth agent, the TKI
only kill tumor cells by affecting processes that commonly are
gefitinib (Iressa®, AstraZeneca Pharmaceuticals), initially was
overactive or enhanced in cancerous tissue but also may be
approved in patients with non-small cell lung cancer (NSCLC)
important in affecting normal cells. Targeted agents can exert
for third-line use, based on the results of a randomized phase II
their influence by, among other things, inhibiting tumor-cell
trial (Kris et al., 2003). However, data from a phase III confir-
proliferation, inducing programmed cell death, inhibiting
matory trial failed to show a survival benefit and its use now is
angiogenesis, and enhancing antitumor immune responses
restricted to patients currently or previously benefiting from it
(Hoang & Schiller, 2002). Common targets for these agents
or to patients enrolled in a clinical study (FDA, 2005).
include growth factors such as the vascular endothelial growth factor (VEGF); intracellular signaling molecules, including
Skin Reactions Associated
cyclooxygenase-2 and the BCR-ABL tyrosine kinase; and cell-surface receptors (e.g., members of the HER family, which
With Human Epidermal Growth Factor
includes HER1/EGFR).
The HER1/EGFR is an attractive target for new biologic
targeted agents because it has been implicated in the develop-
Largely because of their specificity of action, biologic targeted
ment of a range of tumor types (Greatens et al., 1998; Ohsaki
agents often are considered to have a more favorable toxic-
et al., 2000; Salomon, Brandt, Ciardiello, & Normanno, 1995).
ity profile than traditional chemotherapy. This also is true for
Overexpression of the receptor also has been correlated with
HER1/EGFR-targeted therapies, which generally are associated
disease progression, poor prognosis, and a reduced sensitivity
with fewer hematologic adverse events than chemotherapy.
to chemotherapy (Cooke, Reeves, Lannigan, & Stanton, 2001;
However, patients treated with HER1/EGFR-targeted therapies
Nicholson, Gee, & Harper, 2001). Several HER1/EGFR inhibitors
often do present with a unique group of skin reactions (Rhee et
are in clinical development, and three (erlotinib, cetuximab,
al., 2005), which occur in more than 50% of patients receiving
and panitumumab) have demonstrated efficacy in a range of
these treatments (Segaert & Van Cutsem, 2005).
indications and received U.S. Food and Drug Administration
Clinical trials with HER1/EGFR-targeted inhibitors have
(FDA) approval. Erlotinib is an oral, reversible, tyrosine kinase
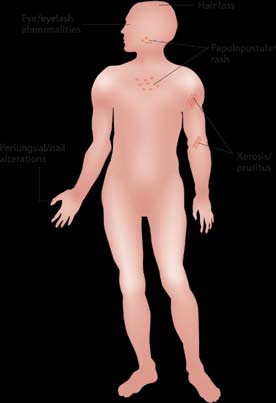
revealed a spectrum of skin toxicities of varying severity (see
inhibitor (TKI), whereas cetuximab and panitumumab are IV
Table 1 and Figure 1). The most commonly reported reactions
administered anti-HER1/EGFR monoclonal antibodies (MAbs).
are mild-to-moderate skin rashes that occur most frequently
MAbs prevent ligands, such as the EGF, from binding to the
on the face and upper trunk. Other common dermatologic
receptor, whereas TKIs block the activity of the receptor's
reactions include xerosis (dry skin), pruritus, nail changes
Table 1. Spectrum of Dermatologic Reactions Associated With Human Epidermal Growth Factor Receptor
Inhibitors
TImE couRSE
Monomorphous erythematous maculopapular, follicular,
Onset: one to three weeks of treatment;
or pustular lesions, which may be associated with mild
maximum: three to five weeks of treatment;
resolution: within four weeks of treatment
cessation but may wax and wane
Paronychia and fissuring
Painful periungual granulation-type or friable pyogenic
Onset: after two to four months of treat-
granuloma-like changes associated with erythema,
ment; resolution: persistent, several months
swelling and fissuring of lateral nailfolds, and/or distal
Curlier, finer, and more brittle hair on scalp and extremi-
Variable onset: after 7–10 weeks to many
ties; also extensive growth and curling of eyelashes and
Diffuse fine scaling
Occurs after appearance of rash
Flushing, urticaria, and anaphylaxis
Occurs on the first day of initial dosing
Mild to moderate mucositis, stomatitis, aphthous ulcers
Onset during treatment, not related to dose or
schedule; resolution without specific measures
Note. From "Epidermal Growth Factor Receptor Inhibitor–Associated Cutaneous Toxicities: An Evolving Paradigm in Clinical Management," by T.J. Lynch, Jr., E.S. Kim, B. Eaby, J. Garey, D.P. West, and M.E. Lacouture, 2007,
Oncologist, 12(5), p. 614. Copyright 2007 by AlphaMed Press. Reprinted with permission.
April 2008 • Volume 12, Number 2 • Clinical Journal of Oncology Nursing
The severity of rash also appears to vary between agents. In
general, rash associated with the use of anti-HER1/EGFR MAbs tends to be more severe than that observed with TKIs, present-ing as a more purulent and pustular reaction, which may require more aggressive interventions (Sipples, 2006).
The different modes of administration for these agents might
be one reason: TKIs (e.g., erlotinib, gefitinib) are administered orally on a daily basis, whereas MAbs (e.g., cetuximab, panit-mumumab) are given intravenously once a week or every two weeks. The pharmacokinetic properties of these agents are therefore different, with potentially greater differences in peak and trough concentrations for MAbs than for TKIs. This may ex-plain the differing incidence and manifestation of rash observed
To view this image,
with these agents, particularly because preclinical data support a correlation between the appearance of rash and concentration
please see the print version.
for these agents (Bruno, Mass, Jones, Lu, & Winer, 2003).
Some evidence suggests that the appearance of skin rash may
be useful as a marker of efficacy for HER1/EGFR-targeted agents. Several studies with erlotinib have demonstrated a relationship between severity of skin reaction and treatment efficacy. Phase II studies in NSCLC, head and neck cancer, and ovarian cancer sug-gest that survival rates are significantly increased in patients with skin reactions, compared to those without (Clark, Perez-Soler, Siu, Gordon, & Santabarbara, 2003; Perez-Soler et al., 2004). This observation was repeated in the pivotal phase III trial of erlotinib
Figure 1. Dermatologic Toxicities Secondary
monotherapy for NSCLC, in which the patients who developed
to Human Epidermal Growth Factor Receptor
a rash (75%) survived significantly longer than those who did
not: 8.5 months for patients with grade 1 rash and 19.6 months
Note. From "Mechanisms of Cutaneous Toxicities to EGFR Inhibitors,"
for patients with grade 2 or 3 rash versus 1.5 months for patients
by M.E. Lacouture, 2006, Nature Reviews Cancer, 61(1), p. 804. Copy-
who did not develop any rash (p < 0.0001) (Perez-Soler, 2006).
right 2006 by Nature Publishing Group. Reprinted with permission.
Overall, these observations support the consensus that patients who develop rash should be treated for the reaction while being maintained on anti-HER1/EGFR therapy; those patients may ob-
(usually manifested as paronychia), and hair changes (usually
tain the greatest benefit from the drugs.
manifested as mild hair loss) (Lacouture, 2006; Segaert & Van Cutsem, 2005). These adverse events are observed during
pathobiology of Skin Reactions
treatment with all HER1/EGFR-targeted agents, MAbs, and TKIs, and their etiology suggests that such skin toxicities are
Associated With Human Epidermal
a class effect of HER1/EGFR-targeted therapies. However, dif-
Growth Factor Receptor Inhibitors
ferences may exist in the incidence and manifestation of these events with different treatments.
Although the pathobiology of HER1/EGFR-targeted inhibitor-
The papulopustular rash generally develops along a char-
associated skin reactions is not understood fully, drug-induced
acteristic course. Within the first week, patients experience
inhibition of the HER1/EGFR is believed to affect keratinocyte
a sensory disturbance on the face with erythema and edema,
proliferation, differentiation, migration, and attachment (Jost,
followed by a papulopustular eruption (in weeks 1–3). In
Kari, & Rodeck, 2000; Woodworth et al., 2005). HER1/EGFR is
week 4, a crusting of the rash develops. Provided that the rash
expressed in epidermal keratinocytes, sebaceous and eccrine
is treated successfully, patients may continue to experience
glands, and the epithelium of the hair follicle (Nanney, Sto-
erythema and dry skin in the areas previously affected by the
scheck, King, Underwood, & Holbrook, 1990). Such expression
papulopustular eruption.
is particularly high in proliferating and undifferentiated kerati-nocytes, which commonly are found in the basal and suprabasal
layers of the epidermis, and in the outer root sheath of the hair
Directly comparing the incidence of skin toxicities (and, in
follicle (Fox, 2006). Inhibition of the HER1/EGFR signaling
particular, rash) for different agents is challenging. Descriptive
pathway is believed to play a critical role in inflammatory cell
terms often differ in gradation schemes between trials, and in
recruitment and subsequent cutaneous injury, which can lead
some instances skin toxicities are recorded as a single event
to the development of dry skin, papulopustules, and periungual
(e.g., dermatitis), whereas in others, they may be broken down
inflammation (Lacouture, 2006). The potential effects of HER1/
into separate categories (e.g., rash, acne, dry skin).
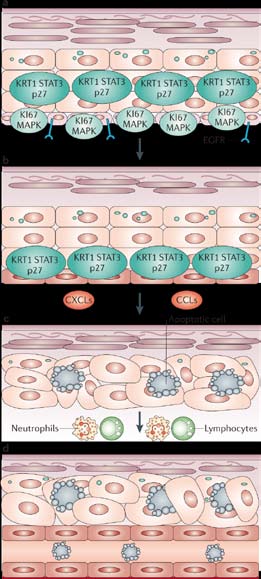
EGFR inhibition in the skin are illustrated in Figure 2.
Clinical Journal of Oncology Nursing • Volume 12, Number 2 • Managing Skin Reactions
Guidelines for the Treatment
of Skin Reactions Associated
With Human Epidermal Growth Factor
Inhibitors
As yet, no data from controlled clinical studies investigating
treatment options for HER1/EGFR-targeted inhibitor-associated skin reactions have been published; therefore, no peer-reviewed, evidence-based treatment recommendations are available. To date, proposed treatments are based mostly on qualitative rather than quantitative evidence (Lacouture, Basti, Patel, & Benson, 2006; Rhee et al., 2005). However, patients still require treatment and advice for skin reactions, and in the absence of suitable clini-cal data, best practice offers the best treatment advice.
The aim of the forum in Chicago was to bring a number of
experts together in an attempt to amalgamate their interdisci-plinary knowledge (published or otherwise) as well as to distill the common practices employed by their institutions into a consensus approach for the treatment of dermatologic adverse events associated with HER1/EGFR-targeted inhibitor therapy. Discussion at the forum focused primarily on the clinical man-agement of cutaneous toxicities; however, the experts also advocated a proactive approach for patients that could minimize the occurrence and severity of toxicities.
To view this image,
practical Guidelines for patients
please see the print version.
Two key patient education recommendations were made at the
forum: Patients should regularly use a thick, alcohol-free emollient for dry skin (alcohol can dry the skin). A number of recommended emollients are listed in Table 2. Application of high-sun protection factor (≥ 15) physical sunscreen (i.e., containing zinc oxide or tita-nium dioxide) each morning and prior to sun exposure is recom-mended for all patients receiving HER1/EGFR-targeted agents.
Based on the existing literature, a range of other practical
guidelines also might be considered to help prevent rash and
a. Normal expression of HER1/EGFR-dependent molecular markers. Be-
other cutaneous toxicities, which include the following (Herb-
fore treatment, the basal layer shows expression of phosphorylated
st, Fox, Viele, & Messner, 2006; O'Keeffe, Parrilli, & Lacouture,
HER1/EGFR, MAPK (mitogen-activated protein kinase) and the prolif-
2006; People Living With Cancer, 2006).
eration marker KI67, and suprabasal expression of phopsphorylated
• Patients should take care to remain hydrated.
HER1/EGFR, the cyclin-dependent-kinase inhibitor p27, KRT1 (keratin
• Patients should ensure that they apply an adequate amount
1) and STAT3 (signal transducer and activator of transcription 3).
of sunscreen: more than half a teaspoon of sunscreen to
b. During HER1/EGFR inhibitor therapy, phosphorylated HER1/EGFR
each arm, the face, and neck, and a little more than one
is abolished in all epidermal cells and the expression of MAPK is
teaspoon to the chest and abdomen, back, and each leg. The
reduced. Inhibition of HER1/EGFR in basal keratinocytes leads to
use of a broad-brimmed hat also is advised if going outside.
growth arrest and premature differentiation, as demonstrated by upregulated p27 KIP1, KRT1 and STAT3 in the basal layer.
• Patients should avoid long, hot showers. Instead, patients
c. Subsequently, the release of inflammatory cell chemoattractants
should use lukewarm water and mild (preferably scent-free)
(such as CXCLs and CCLs) recruits leukocytes that release enzymes,
soap, ensuring that genital, rectal, and skin-fold areas are
causing apoptosis and tissue damage, with consequent apoptotic
cleaned thoroughly. A moisturizer should be applied within 15
keratinocytes and ectatic (dilated) vessels.
minutes of showering or bathing (to prevent skin drying).
d. A decrease in epidermal thickness is observed, with a thin stratum
• Patients should avoid laundry detergent with strong per-
corneum that lacks its characteristic basket weave configuration,
fumes and use only hypoallergenic makeup.
indicating abnormal differentiation.
• The use of saline nasal spray followed by petroleum jelly
may reduce risk of nosebleeds.
Figure 2. The Effects of Human Epidermal Growth
• Patients should use personal lubricant for intercourse.
Factor Receptor (HER1/EGFR) Inhibition in Skin
• To prevent nail problems, patients should keep finger and toe-
Note. From "Mechanisms of Cutaneous Toxicities to EGFR Inhibitors,"
nails clean and trimmed; avoid biting of nails, pushing back cu-
by M.E. Lacouture, 2006, Nature Reviews Cancer, 61(1), p. 806. Copy-
ticles, tearing the skin around the nail bed, or applying artificial
right 2006 by Nature Publishing Group. Reprinted with permission.
nails; and avoid tight-fitting shoes. Patients also are advised to
April 2008 • Volume 12, Number 2 • Clinical Journal of Oncology Nursing
therapeutic decision making, forum attendees felt that a simple,
Table 2. Recommended Agents for Dry Skin,
more specific grading system would be the most appropriate rash
Itching, and Sun Exposure
associated with HER1/EGFR-targeted inhibitors. Thus, classifying skin reactions as mild, moderate, or severe was believed to be the
most effective system for assigning therapeutic intervention.
The following is a stepwise approach to intervention, employ-
• Vanicream® (Pharmaceutical Specialties, Inc.),
ing the suggested three-tiered grading system developed by the
Eucerin® (Beiersdorf AG), Aquaphor® (Beiersdorf
consensus group.
AG), Aveeno® (Johnson & Johnson Consumer Com-
panies, Inc.), and Cutemol® (Summers Laboratories
mild Skin Toxicity
Mild skin toxicity (papulopustular rash) is defined as generally
Exfoliants (for scaly areas)
localized, minimally symptomatic, with no sign of superinfection,
• Ammonium lactate12% (Am-Lactin®, Upsher-Smith
and no impact on daily activities. Some attendees suggested that,
Laboratories, Inc.) for body
in many instances, no intervention was necessary, whereas others
• Urea 20%–40% cream for palms, soles, and fissures
felt that intervention of either topical hydrocortisone (1% or 2.5%
cream) or clindamycin (1% gel) would be beneficial. Dose reduc-
• Body: Sarna Ultra® (PharmaDerm) cream and Re-
tion of the HER1/EGFR-targeted agent was not recommended.
genecare® (MPM Medical, Inc.) gel as needed
• Scalp: Fluocinonide 0.05% shampoo or clobetasol
moderate Skin Toxicity
Moderate rash is defined as papulopustules with mild pruritus
or tenderness, with minimal impact on activities of daily living
• Antihistamines (diphenhydramine 25–50 mg twice
and no signs of superinfection. The suggested therapeutic inter-
daily and/or cetirizine 10–20 mg per day)
vention is hydrocortisone (2.5% cream), clindamycin (1% gel), or
• Pregabalin (Lyrica®, Pfizer) 75–100 mg twice daily
pimecrolimus (Elidel®, Novartis) (1% cream), with the addition of
• Any broad-spectrum sunscreen containing zinc ox-
doxycycline (100 mg PO BID) or minocycline (100 mg PO BID)
ide or titanium dioxide
(Micantonio et al., 2005; Molinari, De Quatrebarbes, Andre, & Aractingi, 2005; Sapadin & Fleischmajer, 2006). Dose reduction of the HER1/EGFR-targeted agent was not recommended.
wear gloves when washing dishes or using chemical clean-ing agents. Hands and feet should be moisturized frequently;
Severe Skin Toxicity
petroleum jelly is particularly effective and should be applied to the skin around the nails periodically throughout the day.
Severe skin toxicity is defined as a rash that may be generalized
At night, applying a thick coat to hands and feet and covering
and accompanied by severe pruritus or tenderness; this level of
with white cotton gloves and socks may be helpful.
toxicity has a significant impact on activities of daily living and
• If a patient develops trichomegaly (i.e., excessive eyelash
has the potential for superinfection. The dose of the HER1/EGFR
growth) or eye complaints, the eyelashes should be trimmed
inhibitor should be reduced (according to the principal investiga-
and the patient should have an ophthalmologic consultation.
tor). The skin toxicity should be treated the same as for moderate
Most importantly, patients should be educated to call a health-
rash but with the addition of a methylprednisolone dose pack. In
care professional as soon as they develop any symptoms of
the event that severe rash symptoms fail to respond to the above-
cutaneous toxicity. Early intervention is critical in the clinical
mentioned interventions, interruption of the HER1/EGFRI-tar-
management of these events, so an early (< 14 days from EGFR-
geted therapy is recommended. However, treatment may resume
inhibitor therapy onset) follow-up is advisable.
(most likely at a lower dose) once skin reactions have diminished in severity at a recommended follow-up of two weeks.
clinical management
The use of pimecrolimus or tacrolimus (Protopic®, Astellas
Pharma, Inc.) for HER1/EGFR-associated skin reactions is being
The treatment algorithm presented in Figure 3 represents the
investigated at a number of institutions; however, it is an immu-
key output from the forum. The effective treatment of skin rashes
nosuppressant, which should be taken into account when con-
associated with HER1/EGFR-targeted inhibitors depends largely
sidering treatment options (Novartis, 2006). Although a causal
on accurate grading of skin reactions to allow for appropriate
relationship has not been established, rare cases of skin malig-
intervention. The National Cancer Institute (2006) Common
nancies and lymphoma have been observed in patients treated
Toxicity Criteria (NCI-CTC) is used most commonly to grade
with calcineurin inhibitors, such as pimecrolimus (Novartis).
adverse events in clinical trials with HER1/EGFR-targeted agents
Such incidences most likely are associated with long-term usage;
(see Table 3); it is designed, primarily, as a surveillance tool and
pimecrolimus is not recommended, however, in immunosup-
is of limited use as an aid to selecting intervention. Although cer-
presed patients (Novartis). As a result, great care should be
tain categories are relevant to events associated with HER1/EGFR
taken when considering its use in patients with cancer.
inhibitors, including rash and desquamation, they often are not sufficiently specific. For example, within the NCI-CTC, rash sever-
ity is based on body surface area coverage; this can be misleading because rash associated with HER1/EGFR inhibitors generally
The skin toxicities associated with HER1/EGFR-targeted
are confined to the face and upper trunk. For the purposes of
inhibitors can cause patients physical and psychological symp-
Clinical Journal of Oncology Nursing • Volume 12, Number 2 • Managing Skin Reactions
• Employ a proactive approach in managing skin reactions.
• Suggest patients use a thick, alcohol-free emollient cream.
• Suggest patients use a sunscreen of SPF 15 or higher, preferably containing zinc oxide or titanium dioxide.
• If a patient presents with rash, verify appropriate administration and follow the proceeding algorithm in a step-wise manner.
Continue EGFR inhibitor at current dose and monitor for change in severity.
• Generally localized• Minimally symptomatic• No impact on ADL
Topical hydrocortisone 1% or 2.5% cream* and/or clindamycin 1% gel
• No sign of superinfection
Reassess after 2 weeks (either by healthcare professional or patient self-reported); if reactions worsen or do not improve, proceed to next step.
Continue EGFR inhibitor at current dose and monitor for change in severity; continue treatment of
• Mild symptoms (e.g.,
skin reaction with the following:
pruritus, tenderness)
• Minimal impact on ADL• No sign of superinfection
Hydrocortisone 2.5% cream* or Clindamycin 1% gel or pimecrolimus 1% cream PLUS doxycycline
100 mg BID or minocycline 100 mg BID
Reassess after 2 weeks (either by healthcare professional or patient self-reported); if reactions worsen or do not improve, proceed to next step.
Severe• Generalized• Severe symptoms (e.g.,
pruritus, tenderness)
Reduce EGFR-inhibitor dose as per label and monitor for change in severity; continue treatment of
• Significant impact on
skin reaction with the following:
• Potential for
Hydrocortisone 2.5% cream* or clindamycin 1% gel or pimecrolimus 1% cream PLUS doxycycline
100 mg BID or minocycline 100 mg BID PLUS medrol dose pack
Reassess after 2 weeks; if reactions worsen, dose interruption or discontinuation may be necessary.
* The use of topical steroids should be employed in a pulse manner based on your institution's guidelines.
ADL—activities of daily living; BID—twice daily; EGFR—epidermal growth factor receptor; SPF—sun protection factor
Figure 3. proposed Therapy Algorithm for the management of Skin Reaction Associated
With Human Epidermal Growth Factor Receptor Inhibitors
Note. From "Epidermal Growth Factor Receptor Inhibitor–Associated Cutaneous Toxicities: An Evolving Paradigm in Clinical Management," by T.J. Lynch, Jr., E.S.
Kim, B. Eaby, J. Garey, D.P. West, and M.E. Lacouture," 2007, Oncologist, 12(5), p. 618. Copyright 2007 by AlphaMed Press. Reprinted with permission.
toms, particularly given that rash characteristically occurs
The algorithm suggested by the consensus group and pre-
on exposed areas such as the face. Although the concerns of
sented in this article represents current best practice for the
patients (and caregivers) must be addressed sympathetically,
treatment of rash associated with HER1/EGFR inhibitors. Con-
in most cases, these adverse events can be managed without
trolled studies remain necessary to fully evaluate the efficacy
the need for dose modification or interruption to the HER1/
of the strategies presented in the algorithm, but this approach
EGFR-inhibitor regimen. Moreover, in refractory cases, the
hopefully will be a useful guide for nurses and other healthcare
suspension of HER1/EGFR inhibitor therapy is temporary,
professionals, ensuring that patients receive the maximum pos-
allowing for appropriate intervention and diminution of skin-
sible clinical benefits from the continued and uninterrupted use
toxicity severity.
of the HER1/EGFR inhibitors.
April 2008 • Volume 12, Number 2 • Clinical Journal of Oncology Nursing
Table 3. classification of Dermatologic Toxicities Associated With Human Epidermal Growth Factor
Receptor Inhibitors
Symptomatic, not interfering with Interfering with ADL
Discoloration, ridging, Partial or complete loss of nails;
Interfering with ADL
pain in nailbed(s)
Mild or localized
Intense or widespread
Intense or widespread and inter-
Macular or papular
Macular or papular eruption or
Severe, generalized erythro-
Generalized exfolia-
eruption or erythema
erythema with pruritus or other
derma or macular, papular, or
tive, ulcerative, or
without associated
associated symptoms; localized
vesicular eruption; desquamation bullous dermatitis
desquamation or other lesions
covering > 50% BSA
covering < 50% BSA
Intervention not
Intervention indicated
Associated with pain, disfigure-
ment, ulceration, or desquamation
Life threatening;
ADL—activities of daily living; BSA—body surface areaNote. From Common Terminology Criteria for Adverse Events (CTCAE) [v.3.0], by National Cancer Institute, 2006. Retrieved February 20, 2008, from http://ctep.cancer.gov/forms/CTCAEv3.pdf. Adapted with permission.
The authors gratefully the participants of the October 2006 inter-
Greatens, T.M., Niehans, G.A., Rubins, J.B., Jessurun, J., Kratzke, R.A.,
disciplinary forum: Jean Pierre DeLord, Jody Gary, Giuseppe Giaccone,
Maddaus, M.A., et al. (1998). Do molecular markers predict survival
Patricia LoRusso, Thomas J. Lynch, Barbara Melosky, Martin Reck,
in non-small-cell lung cancer? American Journal of Respiratory
Roman Perez-Soler, Jennifer Temel, and Dennis P. West. The authors
and Critical Care Medicine, 157(4, Pt. 1), 1093–1097.
also acknowledge third-party medical writing support from Gardiner
Herbst, R., Fox, L.P., Viele, C.S., & Messner, M. (2006). Managing
Caldwell US, funded by Genentech, Inc., OSI Pharmaceuticals, Inc.,
rash and other skin reactions to targeted treatment. Retrieved
and F. Hoffmann-La Roche Ltd.
February 20, 2008, from http://www.cancercare.org/pdf/book-lets/ccc_managing_rash.pdf
Author contact: Beth Eaby, MSN, CRNP, OCN®, can be reached at eabyb@
Hoang, T., & Schiller, J.H. (2002). Advanced NSCLC: From cytotoxic
uphs.upenn.edu with copy to editor at [email protected].
systemic chemotherapy to molecularly targeted therapy. Expert Review of Anticancer Therapy, 2(4), 393–401.
Jost, M., Kari, C., & Rodeck, U. (2000). The EGF receptor—An essen-
tial regulator of multiple epidermal functions. European Journal
Arteaga, C.L. (2001). The epidermal growth factor receptor: From mu-
of Dermatology, 10(7), 505–510.
tant oncogene in nonhuman cancers to therapeutic target in human
Kris, M.G., Natale, R.B., Herbst, R.S., Lynch, T.J., Jr., Prager, D., Be-
neoplasia. Journal of Clinical Oncology, 19(18, Suppl.), 32S–40S.
lani, C.P., et al. (2003). Efficacy of gefitinib, an inhibitor of the
Bruno, R., Mass, R.D., Jones, C., Lu, J., & Winer, E. (2003). Preliminary
epidermal growth factor receptor tyrosine kinase, in symptomatic
population pharmacokinetics (PPK) and exposure-safety (E-S) rela-
patients with non-small cell lung cancer: A randomized trial. JAMA,
tionships of erlotinib HCI in patients with metastatic breast cancer
(MBC) [Abstract 823]. Proceedings of the American Society of
Lacouture, M.E. (2006). Mechanisms of cutaneous toxicities to EGFR
Clinical Oncology, 22, 205.
inhibitors. Nature Reviews Cancer, 6(10), 803–812.
Clark, G.M., Perez-Soler, R., Siu, L., Gordon, A., & Santabarbara, P.
Lacouture, M.E., Basti, S., Patel, J., & Benson, A., III. (2006). The
(2003). Rash severity is predictive of increased survival with erlo-
SERIES clinic: An interdisciplinary approach to the management
tinib HCI [Abstract 786]. Proceedings of the American Society of
of toxicities of EGFR inhibitors. Journal of Supportive Oncology,
Clinical Oncology, 22, 196.
Cooke, T., Reeves, J., Lannigan, A., & Stanton, P. (2001). The value of
Micantonio, T., Fargnoli, M.C., Ricevuto, E., Ficorella, C., Marchetti,
the human epidermal growth factor receptor-2 (HER2) as a prognos-
P., & Peris, K. (2005). Efficacy of treatment with tetracyclines to
tic marker. European Journal of Cancer, 37(Suppl. 1), S3–S10.
prevent acneiform eruption secondary to cetuximab therapy.
Fox, L.P. (2006). Pathology and management of dermatologic
Archives of Dermatology, 141(9), 1173–1174.
toxicities associated with anti-EGFR therapy. Oncology, 20(5,
Molinari, E., De Quatrebarbes, J., Andre, T., & Aractingi, S. (2005).
Suppl. 2), 26–34.
Cetuximab-induced acne. Dermatology, 211(4), 330–333.
Clinical Journal of Oncology Nursing • Volume 12, Number 2 • Managing Skin Reactions
Nanney, L.B., Stoscheck, C.M., King, L.E., Jr., Underwood, R.A., &
Salomon, D.S., Brandt, R., Ciardiello, F., & Normanno, N. (1995).
Holbrook, K.A. (1990). Immunolocalization of epidermal growth
Epidermal growth factor-related peptides and their receptors in
factor receptors in normal developing human skin. Journal of
human malignancies. Critical Reviews in Oncology/Hematology,
Investigative Dermatology, 94(6), 742–748.
National Cancer Institute. (2006). Common terminology criteria
Sapadin, A.N., & Fleischmajer, R. (2006). Tetracyclines: Nonantibiotic
for adverse events (CTCAE) [v.3.0]. Retrieved February 20, 2008,
properties and their clinical implications. Journal of the American
Academy of Dermatology, 54(2), 258–265.
Nicholson, R.I., Gee, J.M., & Harper, M.E. (2001). EGFR and cancer
Segaert, S., & Van Cutsem, E. (2005). Clinical signs, pathophysiology
prognosis. European Journal of Cancer, 37(Suppl. 4), S9–S15.
and management of skin toxicity during therapy with epidermal
Novartis. (2006). Elidel® (pimecrotimus) [Package insert]. Re-
growth factor receptor inhibitors. Annals of Oncology, 16(9),
trieved February 20, 2008, from http://www.pharma.us.novartis
Sipples, R. (2006). Common side effects of anti-EGFR therapy: Acne-
Ohsaki, Y., Tanno, S., Fujita, Y., Toyoshima, E., Fujiuchi, S., Nishigaki,
form rash. Seminars in Oncology Nursing, 22(1, Suppl. 1), 28–34.
Y., et al. (2000). Epidermal growth factor receptor expression cor-
U.S. Food and Drug Administration. (2005). Patient information
relates with poor prognosis in non-small cell lung cancer patients
sheet gefitinib (marketed as Iressa). Retrieved February 20,
with p53 overexpression. Oncology Reports, 7(3), 603–607.
O'Keeffe, P., Parrilli, M., & Lacouture, M.E. (2006). Toxicity of targeted
therapy: Focus on rash and other dermatologic side effects. Oncol-
Woodworth, C.D., Michael, E., Marker, D., Allen, S., Smith, L., &
ogy Nurse Edition, 20(13), 1–6.
Nees, M. (2005). Inhibition of the epidermal growth factor recep-
People Living With Cancer. (2006). Skin reactions to targeted thera-
tor increases expression of genes that stimulate inflammation,
pies. Retrieved February 20, 2008, from http://www.plwc.org/
apoptosis, and cell attachment. Molecular Cancer Therapeutics,
Yarden, Y., & Sliwkowski, M.X. (2001). Untangling the ErbB signalling
Perez-Soler, R. (2006). Rash as a surrogate marker for efficacy of epi-
network. Nature Reviews, Molecular Cell Biology, 2(2), 127–137.
dermal growth factor receptor inhibitors in lung cancer. Clinical Lung Cancer, 8(Suppl. 1), S7–S14.
Perez-Soler, R., Chachoua, A., Hammond, L.A., Rowinsky, E.K., Huber-
Receive free continuing nursing education credit
man, M., Karp, D., et al. (2004). Determinants of tumor response
for reading this article and taking a brief quiz on-
and survival with erlotinib in patients with non-small-cell lung
line. To access the test for this and other articles,
cancer. Journal of Clinical Oncology, 22(16), 3238–3247.
visit www.cjon.org, select "CE from CJON," and
Rhee, J., Oishi, K., Garey, J., & Kim, E. (2005). Management of rash
choose the test(s) you would like to take. You
and other toxicities in patients treated with epidermal growth fac-
will be prompted to enter your Oncology Nurs-
tor receptor-targeted agents. Clinical Colorectal Cancer, 5(Suppl.
ing Society profile username and password.
2), S101–S106.
April 2008 • Volume 12, Number 2 • Clinical Journal of Oncology Nursing
Source: http://cincinnati.vc.ons.org/file_depot/0-10000000/0-10000/6741/associatedFiles/Eaby+Rash+Management+ArticleforVC.pdf
XYLAN & ARABINOXYLAN (100 Assays per Kit) or (1000 Microplate Assays per Kit) or (1300 Auto-Analyser Assays per Kit) © Megazyme International Ireland 2012 INTRODUCTION:In nature, D-xylose occurs mainly in the polysaccharide form as xylan, arabinoxylan, glucuronoarabinoxylan, xyloglucan and xylogalacturonan. Mixed linkage D-xylans are also found in certain seaweed species and a similar polysaccharide is thought to make up the backbone of psyllium gum. Free D-xylose is found in guava, pears, blackberries, loganberries, raspberries, aloe vera gel, kelp, echinacea, boswellia, broccoli, spinach, eggplant, peas, green beans, okra, cabbage and corn. In humans, D-xylose is used in an absorption test to help diagnose problems that prevent the small intestine from absorbing nutrients, vitamins and minerals in food. D-Xylose is normally easily absorbed by the intestine. When problems with absorption occur, D-xylose is not absorbed and blood and urine levels are low. A D-xylose test can help to determine the cause of a child's failure to gain weight, especially when the child seems to be eating enough food. If in a polysaccharide, the ratio of D-xylose to other sugars etc. is known, then the amount of the polysaccharide can be quantitated from this knowledge plus the determined concentration of D-xylose in an acid hydrolysate. Xylans are a major portion of the polysaccharides that could potentially be hydrolysed to fermentable sugar for biofuel production.
Fighting counterfeit phones Making communication easier UgANDA CoMMUNICATIoNS UCC LAUNCHED RURAL CoMMUNICATIoN CoMMISSIoN ADVISES oN HoW DEVELoPMENT FUND To BRIDgE THE To BUY gENUINE PHoNES P. 7 DIgITAL RURAL DIVIDE P. 3 Daily Monitor FRIDAY, MAY 30, 2014 www.monitor.co.ug Journal UGANDA DIGITAL MIGRATION DEADLINE June next year is the global deadline for replacing analogue with digital TV









