Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with hiv and hepatitis c: systematic review and meta-analysis
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C
Systematic review and meta-analysis
Alexis Llewellyn, Mark Simmonds, Ginny Brunton,
Social Science Research UnitUCL Institute of EducationUniversity College London
EPPI-Centre report no. 2304October 2015
The authors are from the Centre for Reviews and Dissemination, University of York; and the EPPI-Centre, UCL Institute of Education, University College London.
This report should be cited as:
Llewellyn A, Simmonds M, Brunton G, Sowden A (2015) Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis. EPPI-Centre, Social Science Research Unit, UCL Institute of Education, University College London. ISBN: 978-1-907345-80-7
Copyright 2015 Authors of the systematic reviews on the EPPI-Centre websithold the copyright for the text of their reviews. The EPPI-Centre owns the copyright for all material on the website it has developed, including the contents of the databases, manuals, and keywording and data-extraction systems. The centre and authors give permission for users of the site to display and print the contents of the site for their own non-commercial use, providing that the materials are not modified, copyright and other proprietary notices contained in the materials are retained, and the source of the material is cited clearly following the citation details provided. Otherwise users are not permitted to duplicate, reproduce, re-publish, distribute, or store material from this website without express written permission.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Contents
Acquired immune deficiency syndrome
Chemokine receptor
Centre for Research and Dissemination, University of York
HAART Highly active antiretroviral therapy
Hepatitis C virus
Human immunodeficiency virus
Injection drug use
Interquartile range
Non-nucleoside reverse transcriptase inhibitor
Nucleoside reverse transcriptase inhibitor
Protease inhibitors
Ribonucleic acid
Sustained viral response
Abstract
Objectives
Highly active antiretroviral therapy (HAART) is the current standard treatment for individuals co-infected with HIV and hepatitis C. The impact of HAART and antiretroviral (ARV) monotherapy on liver disease in this population is unclear. This systematic review aimed to evaluate the effect of HAART and ARV monotherapy on liver disease progression and liver-related mortality in individuals co-infected with HIV and hepatitis C, including in patients with haemophilia.
MEDLINE and EMBASE bibliographic databases were searched up to June 2014 for comparative studies. A systematic review on the association between HAART and/or ARV monotherapy and liver disease progression and liver-related mortality was conducted. Study quality was assessed using a modified version of the Newcastle-Ottawa scale and the results were synthesised narratively and by meta-analysis.
Thirteen cohort studies were included. In analyses that adjusted for potential confounding factors (such as age, sex and liver disease severity), the risk of liver-related mortality was reduced by around approximately 70% in patients receiving HAART when compared to untreated patients. The results were similar in unadjusted analyses. A subgroup analysis, in which most patients had haemophilia, also found that HAART was associated with a reduction in liver-related mortality.
For other outcomes where meta-analyses could not be performed, the results were less consistent. Some studies suggested a benefit of HAART in reducing the incidence or slowing the progression of liver disease, fibrosis and cirrhosis, while others showed no evidence of benefit or harm, compared with no antiretroviral therapy.
Limitations
Only observational studies were identified, so the risks of bias and confounding cannot be excluded. Liver disease outcomes could not be pooled statistically, thereby limiting the strength of the findings on liver-disease progression.
Conclusions
The use of HAART was associated with significantly reduced liver-related mortality in patients co-infected with HIV and HCV. Evidence of an association between HAART and/or ARV monotherapy use and reduced liver-disease progression was less clear, but there was no evidence to suggest that the absence of antiretroviral therapy was preferable. Further research is required on the differential effects of HAART regimens, and on the mechanisms by which HAART reduces liver-disease mortality.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Executive summary
Background
Hepatitis C is an infectious liver disease, caused by the hepatitis C virus (HCV). Most individuals infected in Britain now acquire the virus through unsterile drug injection. Before an effective blood donor screening test was introduced in the UK in 1991, many people were infected through blood transfusion or therapy with medical products manufactured from donated human blood.
Due to shared routes of transmission, many patients infected with HIV also become infected with HCV. Cirrhosis can develop in patients with chronic HCV infection, with complications including end-stage liver disease and hepatocellular carcinoma, which are important causes of mortality. HIV co-infection has been found to accelerate the progression of chronic hepatitis C to cirrhosis and end-stage liver disease. The widespread use of antiretroviral therapy in developed countries has resulted in HIV-infected patients living longer, and so chronic HCV infection has become an important cause of liver disease in co-infected individuals.
The current standard treatment for individuals co-infected with HIV and HCV is a combination of at least three antiretroviral drugs, often called ‘highly active' or ‘combination' antiretroviral therapy (HAART). It has been suggested that HIV viral suppression and immune reconstitution, caused by HAART, could affect the rate of HCV-fibrosis progression. The most recent systematic review, however, found limited and inconsistent evidence on the association between antiretroviral therapy and liver-disease outcomes; it was published in 2007 and is now out of date.
Objectives
This systematic review aimed to evaluate the effect of HAART and ARV monotherapy on liver-disease progression and liver-related mortality in individuals co-infected with HIV and hepatitis C, including in patients with haemophilia.
This systematic review was conducted following the general principles recommended in the Guidance for Undertaking Reviews in Health Care, produced by the Centre for Reviews and Dissemination, and the reporting guidance of the PRISMA statement.
Literature search
MEDLINE and EMBASE databases were searched in June 2014 for studies published in English. No date restrictions and no study design filters were applied. The reference list of a previous systematic review on the effect of antiretroviral therapy on liver disease was checked for further relevant studies.
Selection criteria
Studies were eligible for inclusion if they evaluated the effect of HAART and/or ARV monotherapy on liver-related mortality and liver-disease progression in patients co-infected with HIV and HCV. Studies had to include a comparison group of individuals who did not receive or had discontinued HAART and/or ARV monotherapy, and had to measure exposure to treatment and outcome at more than one point in time (for example, cohort
Executive summary
and case-control studies). Studies examining HCV viral load, or transaminase/aminotransferase only, were excluded.
Appraisal and synthesis of the evidence
The quality of the included studies was assessed using a modified version of the Newcastle-Ottawa scale. Where possible and where studies provided sufficient data, these were pooled in meta-analyses. Otherwise, the results were synthesised narratively.
Quantity and quality of studies Thirteen cohort studies were included in the review. No randomised studies were identified. Most studies were conducted in Europe; none were conducted in the UK. Most patients had a history of injection-drug use, and only two studies were primarily of patients with haemophilia. All studies evaluated the effect of HAART, and about half of them also reported data for patients receiving ARV monotherapy only. Seven studies reported data on liver-related mortality, of which six were included in a meta-analysis. Nine studies reported on liver-disease progression and were summarised narratively.
The risk of confounding, due to failing to account for key confounding factors such as age, sex and liver-disease severity, was the most frequent quality concern in the included studies. The risk of bias associated with participant selection was generally considered to be either low or unclear, and the risk of bias associated with outcome measurement was mostly low.
Summary of effectiveness HAART was associated with a substantial reduction –around 60-70% – in liver-related mortality in HIV/HCV co-infected patients, depending on the analysis performed. In analyses that adjusted for potential confounding factors (such as age, sex and liver-disease severity), HAART was associated with a substantial reduction in liver-related mortality, with a hazard or odds ratio (HR/OR) of around one-third of that in untreated patients (HR/OR 0.31, 95% CI 0.14 to 0.70). The results were similar in unadjusted analyses (RR 0.40, 95% CI 0.29 to 0.55).
A subgroup analysis including nearly all patients with haemophilia also found that HAART reduced liver-related mortality, but there were too few data to provide an accurate estimate and to determine if the effect differed from that in other populations.
For other outcomes where meta-analyses could not be performed, the results were less consistent. Some studies suggested a benefit of HAART in reducing the incidence or slowing the progression of liver disease, fibrosis and cirrhosis, while others showed no evidence of benefit or harm, compared with no HAART/ARV monotherapy.
Discussion
Strengths and limitations Ideally, a review of treatment effectiveness should be based on randomised studies to reduce the risk of bias. However, no randomised trials comparing HAART/ARV monotherapy with no antiretroviral therapy were identified. Therefore, observational studies were included, and although attempts were made to address the risk of confounding in the analyses, the potential for bias cannot be excluded. A strength of this
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
systematic review was the statistical pooling of studies (where possible) on the association between HAART and liver-disease progression in patients co-infected with HIV and HCV. There were too few studies to conduct meta-regression or further subgroup analyses to explore the moderating effects of several relevant factors, including age, liver-disease severity, time since HCV/HIV infection and alcohol abuse.
Generalisability of the findings In most studies, most patients had a history of injection drug use (IDU), or their drug use was not reported. Patients with a history of IDU are likely to differ in significant ways from those with haemophilia. Although the results from studies of patients with haemophilia did not differ significantly from those from other populations, the unique circumstances of patients with haemophilia should be considered when interpreting the results from patients without haemophilia.
Most studies included in the review were conducted in Europe; none was from the UK. Potential differences in the management of HIV/HCV co-infection across different health systems may limit the applicability of the review findings to co-infected populations in the UK.
Conclusions
The use of HAART was associated with significantly reduced liver-related mortality in patients co-infected with HIV and HCV. Evidence of a positive association with liver-disease progression was less clear, but there was no evidence to suggest that the absence of HAART or ARV monotherapy was preferable.
Implications for policy and clinical practice This review supports the use of HAART in patients co-infected with HIV and HCV, and suggests that it has benefits on liver-related mortality in addition to its known impact on HIV-related morbidity and mortality.
Further research Given the common use of HAART in HIV management, a systematic review on the acute and chronic effect of different HAART regimens would be useful. Similarly, a systematic review addressing patients' experiences of HAART, for example acceptance of the intervention, would be useful. Further research is required on the impact of HAART on liver-disease progression and the mechanisms by which liver-disease mortality is reduced with HAART.
1. Background
Hepatitis C is an infectious liver disease caused by the hepatitis C virus (HCV). Hepatitis C infections occur if the virus is able to enter the bloodstream and reach the liver. Today, most individuals infected in Britain acquire the virus through unsterile drug injecting practices. HCV is also prevalent in men who have sex with men, and its incidence is rising in this population (Bradshaw et al. 2013). Historically, before an effective blood-donor screening test was introduced in the UK in 1991, many people were infected through blood transfusion or therapy with medical products manufactured from donated human blood. It is estimated that blood transfusion resulted in approximately 23,500 transmissions during the 1970s and 1980s in England (Soldan et al. 2002), and around 28,000 in the UK (Department of Health 2011). More than 4,600 patients with bleeding disorders were infected by treatment with HCV-contaminated plasma products. Since 2004, those surviving patients who acquired chronic HCV infection through contaminated blood, blood products and tissue transplantation have received financial support via the Skipton Fund.1 This provides patients infected with chronic HCV through NHS blood products with compensation payments according to prescribed criteria (House of Commons Hansard 2013). In addition, the Macfarlane Trust was set up in 1988 by the British Government to support people with haemophilia who were infected with HIV as a result of contaminated NHS blood products.2 The Eileen Trust has provided support for people infected with HIV since 1993.3
1.1 The natural history of hepatitis C infection
Two distinct stages of HCV infection are recognised; acute and chronic. Acute hepatitis occurs within six to eight weeks of infection and may or may not be symptomatic. The virus may clear from the bloodstream in 15% to 25% of those with acute HCV who are not treated during this period. Lack of viral clearance results in chronic HCV, which is marked by the presence of HCV ribonucleic acid (RNA) for more than six months (Micallef et al 2006). Cirrhosis develops in around 20% of patients with chronic infection over approximately 15 to 20 years, and the major direct complications are end-stage liver disease and hepatocellular carcinoma. Around 55% of patients treated with current therapy (pegylated interferon and ribavirin) may achieve sustained viral response (SVR) (Fried et al 2002; Manns et al 2001; Shepherd et al 2007).
1.2 Hepatitis C and HIV co-infection
Due to shared routes of transmission, many patients infected with HIV also acquire infection with hepatitis C virus (HCV); this occurs in more than 80% of in injection drug users and individuals with haemophilia (Hayashi et al. 1991; Rumi et al. 1990). Although the role of hepatitis C in the progression of HIV disease is partly unclear (Daar et al. 2001; Sulkowski et al. 2002), HIV infection has been found to accelerate the progression of chronic hepatitis C to cirrhosis and end-stage liver disease (Kramer et al. 2007). Among patients with haemophilia, there is a four- to eight-fold increase in progression to end-
1 www.skiptonfund.org
2 www.macfarlane.org.uk
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
stage liver disease in HIV-positive individuals, compared with those who are HIV-negative (Goedert et al. 2002; Graham et al. 2001; Ragni and Belle 2001). This risk may be increased by factors such as hepatitis B infection, alcohol use and increasing duration of HCV infection (Ragni and Belle 2001). HCV-related liver disease is an important cause of death in co-infected individuals (Bruno et al. 2007; Sulkowski et al. 2007), including those with haemophilia (Qurishi et al. 2003). Liver-related mortality is comparatively low in patients with HIV infection alone (Eyster et al. 1993; Rockstroh et al. 1996; Soriano et al. 1999).
1.3 Antiretroviral therapy in patients co-infected with hepatitis C and HIV
The first effective therapy against HIV was a nucleoside reverse transcriptase inhibitor (NRTI), which was approved by the US Food and Drug Administration in 1987. In 1996, a more effective three-drug therapy combining two NRTIs with a new class of antiretrovirals – protease inhibitors – was incorporated into clinical practice. The current standard treatment consists of a combination of at least three drugs (often called ‘highly active', ‘combination' antiretroviral therapy, or HAART). The widespread use of antiretrovirals in developed countries has substantially reduced mortality in HIV-infected individuals. Partly because of HIV-infected patients' ability to live longer, chronic HCV infection, which generally progresses to clinical disease over decades, has become an important cause of liver disease in co-infected individuals (Bica et al. 2001). A Canadian cohort study of HIV-infected patients with haemophilia found that following the introduction of HAART, the proportion of deaths due to acquired immune deficiency syndrome (AIDS) had decreased, while the proportion of deaths due to liver disease had increased (Arnold et al. 2006).
Antiretroviral therapy is currently recommended in co-infected patients, including those with cirrhosis, by national guidelines, including the British HIV Association and US National Institutes of Health (Williams et al. 2014). It has been suggested that the HIV viral suppression (Brau et al. 2006) and immune reconstitution (Lange and Lederman 2003) possible with HAART are critical factors that positively affect the rate of HCV fibrosis progression. The most recent published systematic review found limited and inconsistent evidence on the association between antiretroviral therapy and liver-disease outcomes (Kramer et al. 2007). Some studies have reported that HAART may adversely affect hepatitis C outcomes by increasing HCV viral load, liver toxicity and fibrosis progression (Bonacini 2004; Sulkowski 2005; Verma 2006; Verma et al. 2006). It is possible that HAART can attenuate liver-disease progression through the reversal or prevention of HIV-related immunosuppression, but it is also plausible that antiretroviral use may exacerbate liver disease (Ragni et al. 2009). Significant liver enzyme elevations (grade 3 or 4 hepatotoxicity) are observed in approximately 5% to 10% of people taking a new HAART regimen. In addition, the incidence of HAART-associated liver toxicity is approximately three times greater in HIV/HCV-co-infected individuals than in those without hepatitis C (Benhamou et al. 2001; Verma et al. 2006), and increases in hepatitis C viral loads have been observed in patients with haemophilia receiving HAART (Ragni and Bontempo 1999). Although many liver enzyme elevations resolve even when HAART is maintained (Brau et al. 2006), the effect of HAART on the progression of HCV-related liver disease is uncertain (Kramer et al. 2007).
A review published in 2007 (Kramer et al. 2007) addressed the association between antiretroviral therapy and liver disease outcomes, but is now out of date, and therefore, an up-to-date systematic review of the available evidence is needed.
This systematic review aimed to evaluate the effect of HAART and ARV monotherapy on liver-disease progression and liver-related mortality in individuals co-infected with HIV and hepatitis C, including in patients with haemophilia.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
2. Methods
A systematic review was conducted following the general principles recommended in the Guidance for Undertaking Reviews in Health Care (Centre for Reviews and Dissemination 2009), and the reporting guidance of the PRISMA statement (Moher et al. 2009). The review was carried out between 13 June and 31 August 2014. The short timeframe limited our ability to search for grey literature, and we limited study inclusion to those published in English.
2.1 Search strategy
A search strategy was initially developed for MEDLINE (Ovid SP). Various text words, synonyms and subject headings were identified by scanning key papers identified at the beginning of the project, by discussion with the review team and through the use of database thesauri. The final searches included terms such as ‘hepatitis C', ‘HIV', ‘antiretroviral therapy', and ‘liver disease'.
MEDLINE and EMBASE electronic databases were searched up to June 2014. The MEDLINE search strategy was adapted for EMBASE. No date restrictions and no study design filters were applied. Only studies published in English were considered, and conference abstracts were excluded. The reference list of a relevant systematic review identified by the initial searches was checked for further relevant studies (Kramer et al. 2007). The full search strategies and results for each database can be found in Appendix 2. Records were initially managed within an EndNote library (EndNote version X7, Thomson Reuters, CA, USA).
2.2 Selection criteria
The abstracts of studies identified by the searches were assessed for inclusion using the criteria outlined below. For records of potential relevance, the full papers were also assessed. Titles and abstracts were screened by one reviewer using EndNote X7 software. Full papers were assessed by two reviewers independently, with disagreements resolved by discussion. Studies were included in the review if they met the criteria listed below.
2.2.1 Participants Studies of patients co-infected with HIV and chronic HCV were included. HIV and HCV may be managed differently in developing countries; therefore, studies conducted in developing countries were excluded.
2.2.2 Interventions Any ARV monotherapy or any combination of antiretrovirals was considered to be eligible, including entry/fusion inhibitors, nucleoside reverse transcriptase inhibitors (NRTIs)/nucleoside/ nucleotide analogues, non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase inhibitors, protease inhibitors (PI) and chemokine receptor (CCR5) antagonists.
2.2.3 Comparator
Studies had to include comparison patients who did not receive or had discontinued HAART/ARV monotherapy.
2.2.4 Outcomes Liver-related mortality and liver-disease progression were the two outcomes of interest. Liver-disease progression included: progression to/of fibrosis and cirrhosis; compensated liver disease; liver decompensation (ascites, encephalopathy, bleeding varices and/or jaundice); end-stage liver disease; and hepatocellular carcinoma. Liver disease progression outcomes had to be measured using appropriate methods, such as liver biopsy or a validated non-invasive method. Studies examining HCV viral load, or transaminase/aminotransferase only were excluded. Data had to be presented as, or allow the calculation of, effect estimates such as risk ratio (RR), odds ratio (OR), hazard ratio (HR), or mean difference (MD).
2.2.5 Study design
Ideally, a review of treatment effectiveness should include long-term prospective trials with random allocation of patients to the intervention groups. Random allocation is an effective method that reduces the risk of bias and confounding (Bland 2000). However, randomised trials comparing HAART and/or ARV monotherapy with no antiretroviral therapy are not possible, primarily for ethical reasons (HAART is widely accepted for reducing HIV related morbidity and mortality). Therefore, this review included the best available non-randomised evidence.
Comparative studies that measured exposure to treatment and outcome at more than one point in time, such as cohort and case-control studies, were eligible for inclusion. Studies that measured treatment and outcome at the same point in time were excluded since they were not deemed suitable for measuring disease progression. Therefore, case series, correlation and cross-sectional studies (NICE 2006) were excluded.
2.3 Data extraction
Key study details and patient characteristics, such as age, sex, baseline liver-disease severity, mode of HCV/HIV infection, HIV/HCV treatment regimen, HIV and HCV disease history, and concomitant treatments, were extracted using EPPI-Reviewer (Thomas et al. 2010). Outcomes were extracted into a standard Excel spreadsheet. Where outcomes were reported with different levels of adjustment (for example, adjusting for age and sex only versus age, sex and time-dependent covariates), the results with the most adjustments were selected.
2.4 Quality assessment of the studies
The risk of bias was evaluated using a modified version of the Newcastle-Ottawa quality assessment tool (Wells et al. [2014]). Three main domains were considered: participant selection, confounding and outcomes. In addition, the relevance of the study participants to the key population of interest for this review (patients with haemophilia) was considered. Further details regarding the quality assessment criteria are reported in Appendix 3.
The data were extracted and study quality was assessed by one reviewer and checked by a second. Study selection was conducted using EndNote X7 software and EPPI-Reviewer. Data extraction was conducted with EPPI-Reviewer and Microsoft Excel. Quality assessment was performed using EPPI-Reviewer.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
2.5 Analysis
The results for liver-related mortality and liver-disease progression were pooled in a meta-analysis, if at least two studies reported that outcome and if data were reported consistently enough for analysis to be feasible. Otherwise, the results were summarised in a narrative synthesis. Where meta-analyses were performed, outcomes were pooled using standard random-effects DerSimonian and Laird meta-analyses (1986). Heterogeneity was assessed using I2 (Higgins and Green 2011). When pooling adjusted odds, or hazard or risk ratios, these were assumed to be equivalent regardless of the specific statistic reported and the covariates that were adjusted for.
Where participants from several studies were recruited from the same cohort and significant overlap was suspected, attempts to contact authors were made, and the data from only one study, with the most reliable reporting, were included in the main analyses. The impact of studies where substantial overlap was suspected, or where only a composite outcome was reported, was explored by including/excluding them from the main analyses (in sensitivity analyses).
Where possible, subgroup analyses including only studies with a large proportion of patients with haemophilia were conducted. Meta-regressions and other subgroup analyses were considered inappropriate due to the small number of studies.
3. Results
3.1 Overview of the evidence
Thirteen studies were included in the review. All were classed as cohort studies, with sample sizes ranging from 36 to 683 participants. Three studies were conducted in the USA. All the other studies were conducted in Europe; none were conducted in the UK. Most patients had a history of injection drug use, and only two studies were primarily of patients with haemophilia. All studies evaluated the effect of HAART, and about half of them also reported data for patients receiving ARV monotherapy only. About half of the studies evaluated the effect of HAART and/or ARV monotherapy on liver-related mortality, and nearly all reported other liver-related outcomes.
From meta-analyses, there was statistically significant evidence that HAART was associated with a substantial reduction in liver-related mortality in HIV/HCV co-infected patients, by around 60–70%, depending on the analysis performed. For other outcomes where meta-analyses could not be performed, the results were more mixed. Some studies suggested a benefit of HAART in reducing the incidence or slowing the progression of liver disease, fibrosis and cirrhosis, while others showed no evidence of benefit or harm.
3.2 Flow of studies
The database searches yielded a total of 1,748 unique titles and abstracts, including all relevant studies that had been included in the previously published systematic review by Kramer et al. (2007). From these references, 79 studies of potential relevance were identified. Based on the full text, 66 studies were rejected, and 13 studies met our inclusion criteria. Of these, six studies were included in the meta-analyses, and seven were summarised in a narrative synthesis only.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Figure 1: Flow of studies
Records identified through database searching:
2,055 (MEDLINE 559, EMBASE 1,496)
Records after duplicates were removed 1,748
Records screened by
Records excluded
author and title
Full-text articles assessed for
Full-text articles
Reasons for exclusion:
Studies included in the review
Included in meta-analysis: 6
Study design: 22
Included in narrative synthesis only: 7
3.3 Quality and assessment of bias of the included studies
The risk of confounding, due to failing to account for key factors, such as age, sex/gender and liver-disease severity, was the most frequent quality concern in the included studies. Five studies provided only unadjusted results on the relevant outcomes and were therefore considered to be at high risk of confounding (Mariné-Barjoan et al. 2004; Mehta et al. 2005; Merchante et al. 2006; Reiberger et al. 2010; Reiberger et al. 2010). Four studies used appropriate methods to adjust for potential confounding and were considered to be at a lower risk of confounding Limketkai et al. 2012; Macías et al. 2009; Qurishi et al. 2003; Ragni et al. 2009). Four other studies raised concerns because their comparison groups might have included patients exposed to HAART (for example, patients in one comparator group were classed as ‘no HAART' if they had <80% adherence to HAART, suggesting that they received some HAART) (Giron-Gonzalez et al. 2007). These four studies were, therefore, classed as at moderate risk of confounding (Bruno et al. 2007; Giron-Gonzalez et al. 2007; Macías et al. 2006; Pineda et al. 2009).
How patients were selected for inclusion in the studies was often poorly reported, suggesting that some studies could have introduced bias.1, 3-6, 9, 11 In five other studies, the selection methods were insufficiently reported to assess the risk of selection bias.2, 7 8, 10, 13 One study was considered to be at high risk of selection bias.12
Most studies measured and reported their outcomes using appropriate clinical methods, and therefore, were classed as at low risk of outcome measurement bias. Only one study was considered to be at high risk of outcome measurement bias, due to limited follow-up.7
Two studies reported including a substantial proportion of patients with haemophilia.10, 11 In all other studies, most patients had a history of injection drug use (low relevance), or their drug use or likely mode of infection was not reported (unclear relevance).
Further details of the quality criteria and judgments are reported in Table 3.1 and Appendix 3.
Table 3.1: Risk of bias
Selection bias Confounding
Relevance
measurement
bias
Giron-Gonzalez (2007) Unclear
Limketkai (2012)
Mariné-Barjoan (2004) Low
Merchante (2006)
Reiberger (2010)
Schiavini (2006)
Total risk of bias
*In all the tables, the first author only is specified.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
3.4 Characteristics of included studies
3.4.1 Study characteristics Out of 13 studies, six followed patients prospectively (Giron-Gonzalez et al. 2007; Limketkai et al. 2012; Mehta et al. 2005; Merchante et al. 2006; Pineda et al. 2009; Ragni et al. 2009); the remaining seven studies were classed as retrospective cohorts.
Five studies were conducted in Spain, three in the USA, two in Italy, one in France, one in Germany and one in Austria. There was some overlap across the Spanish cohorts in their recruitment centres and study dates, and it was likely that some participants were included in more than one of the these studies. However, we felt that there were sufficient differences in the reporting of outcomes and liver-disease severity to treat them as distinct studies. See Appendix 4 table 6 for further details.
Nearly all the included studies were published after 2005 (end of searches by Kramer et al. 2007), except for three studies which were also included in the review by Kramer et al. (Mariné-Barjoan et al. 2004; Mehta et al. 2005; Qurishi et al. 2003).
Study dates ranged from 1970 to 2011. Six studies were conducted across the pre-post HAART era (before and after 1996) (Limketkai et al. 2012; Macías et al. 2006, 2009; Qurishi et al. 2003; Ragni et al. 2009; Schiavini et al. 2006), including the two studies in which most patients had haemophilia (Qurishi et al. 2003; Ragni et al. 2009). Only one study reported receiving industry funding (Merchante et al. 2006).
All the included studies evaluated the impact of HAART, and seven also included patients treated with ARV monotherapy only. Most patients who received HIV treatment and who were included in cohorts after 1996 received HAART. Where reported, the most common ‘base' for HAART was protease inhibitors, followed by nucleoside analogues and NNRTIs. Only one study clearly reported time of HIV treatment initiation, which took place after a first event of decompensation (Bruno et al. 2007). Only one study clearly reported HIV treatment duration, which ranged from 87 to 364 weeks (Macías et al. 2006).
Eight studies reported HAART/ARV monotherapy exposure prior to baseline. All stated that most participants in the intervention group had received HAART and/or ARV monotherapy before the study started (Bruno et al. 2007; Giron-Gonzalez et al. 2007; Limketkai et al. 2012; Macías et al. 2006; Mehta et al. 2005; Merchante et al. 2006; Pineda et al. 2009; Qurishi et al. 2003).
Five studies reported treating between 8% and 92% of their sample with HCV therapy (interferon with or without ribavirin) (Giron-Gonzalez et al. 2007; Macías et al. 2009; Pineda et al. 2009; Ragni et al. 2009; Schiavini et al. 2006), while four stated that no patients received HCV therapy during the study (Macías et al. 2006; Mariné-Barjoan et al. 2004; Qurishi et al. 2003; Reiberger et al. 2010). Liver transplants were reported in only two studies: Ragni et al. (2009) reported that 6% of their participants underwent liver transplant during the study, and Giron-Gonzalez et al. (2007) reported a similarly low rate (8%).
The reason for absence of antiretroviral therapy in the comparison group was only provided in one study; patients included in the no-HAART group in Ragni et al. (2009) were either ‘unwilling or died before drugs were available.' Further intervention characteristics are reported in Appendix 4.
Table 3.2: Study characteristics
Author (year)
HAART, ARV monotherapy, Concomitant treatment
or both
HCV treatment unknown. None for 33% with genotype 3
8% liver transplant
Limketkai (2012) HAART and/or ARV
HAART and/or ARV
HAART and/or ARV
44% HCV treatment (across groups)
HAART and/or ARV
Merchante (2006) HAART only
43% of total population HCV therapy at follow-up
HAART and/or ARV
No HCV treatment
HAART and/or ARV
1% HCV, 6% liver transplant
Reiberger (2010) HAART only
No HCV treatment
Schiavini (2006)
HAART and/or ARV
Interferon 92% across groups (of which
NR: Not reported. Further study characteristics can be found in Appendix table 4.
Participant characteristics Most participants were male (67% to 100% across studies). Only two studies reported including patients with haemophilia. In Qurishi et al. (2003), 81% of patients had haemophilia, and in Ragni et al. (2009), all patients were recruited from a haemophilia clinic. Only three studies reported the participant's age at HIV/HCV infection, or data from which this could be inferred. Age at HIV/HCV infection ranged from birth (in the two studies including patients with haemophilia) (Qurishi et al. 2003; Ragni et al. 2009) to over 26 years (Mariné-Barjoan et al. 2004). Only two studies reported how long participants had been infected with HCV (Mariné-Barjoan et al. 2004; Ragni et al. 2009). Where reported, the median age at HAART/ARV monotherapy initiation ranged from 28 to 45.6 years. Only three studies reported data on ethnicity. Nearly all participants in Ragni et al. (2009) were
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Caucasian (96%). Limketkai et al. (2012) and Mehta et al. (2005) included 80% and 85% Black/African American participants respectively.
Baseline liver damage severity varied across the studies. Eight studies included no or few patients with cirrhosis (Limketkai et al. 2012; Macías et al. 2006, 2009; Mariné-Barjoan et al. 2004; Mehta et al. 2005; Ragni et al. 2009; Reiberger et al. 2010; Schiavini et al. 2006); four studies included only patients with compensated cirrhosis at baseline. Two of these tracked liver disease progression from the first event of decompensation (Giron-Gonzalez et al. 2007; Merchante et al. 2006). One reported no symptomatic liver disease at ARV initiation (Qurishi et al. 2003).
Nearly all patients were HCV RNA positive, although two studies reported rates of patients who tested anti-HCV positive only (Giron-Gonzalez et al. 2007; Ragni et al. 2009). Where reported, baseline CD4 cell count ranged from a median of 202 to 460 cells/mm3, and the percentage of patients with current active HBV infection ranged from zero to 17.
Where reported, the percentage of patients abusing alcohol at baseline ranged from 12 to 47. Seven studies reported a percentage of participants with current or past injection drug use of 72 or above. Further participant characteristics are reported in Table 3.3.
Table 3.3: Participant characteristics
Age (years)1
Comorbidities Baseline liver
CD4 cell count
Current or past
disease severity
(cells/mm3) at
substance
baseline
Median 261 (IQR 150 to Alcohol: 46
Limketkai Median 45.6
18% <200. Median 381 Alcohol: 47
NR. At follow-up:
median 504 (IQR 343 to current
Mean 37 (SD 5.5) 68
Median 460 (IQR 319 to Alcohol:
Median 440 (IQR 321 to Alcohol: 14
15yrs, IQR 12 to 20)
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Age (years)1
Comorbidities Baseline liver
CD4 cell count
Current or past
disease severity
(cells/mm3) at
substance
baseline
Median 366 (IQR 219 to Alcohol: 39.5
(current or past)
Merchante Median 38
Median 202 (IQR 109 to Alcohol:
Median 403 (IQR 255 to Alcohol: 21
572). HIV contracted
between 1982 and 1985 or previous
in patients with
Median CD4 count
HAART: 0.243 (0·108 to
ARV monotherapy: 0.279 (0·122 to 0·414)
untreated: 0.255 (0·079 to 0·473)
100, of which from HCV
HAART: 351 (SD 56,
range 64 to 948)
Age (years)1
Comorbidities Baseline liver
CD4 cell count
Current or past
disease severity
(cells/mm3) at
substance
baseline
ARV monotherapy: 90
(SD 19, range 4 to 412)
untreated: 145 (SD 43, range 2 to 610) (time of measurement unclear)
Mean 510 (SD 203): 12% Alcohol: 29
>500; 45% 499 to 201; current
advanced fibrosis 43% <200
Median 429 (256.5 to
1 At the start of the study, unless otherwise stated; 2 Proportion of patients with active HCV not reported
IDU: Injection drug user; IQR: Interquartile range; NR: Not reported; NA: Not applicable
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
3.5 Findings
Seven studies reported data on liver-related mortality (Bruno et al. 2007; Giron-Gonzalez et al. 2007; Limketkai et al. 2012; Merchante et al. 2006; Pineda et al. 2009; Qurishi et al. 2003; Ragni et al. 2009), and nine studies reported on liver disease progression (Giron-Gonzalez et al. 2007; Macías et al. 2006, 2009; Mariné-Barjoan et al. 2004; Mehta et al. 2005; Pineda et al. 2009; Ragni et al. 2009; Reiberger et al. 2010; Schiavini et al. 2006). Three studies reported data on liver-related mortality and other liver-related outcomes (Giron-Gonzalez et al. 2007; Pineda et al. 2009; Ragni et al. 2009).
3.5.1 Liver-related mortality Findings from six of the seven studies on liver-related mortality were combined in meta-analyses (Bruno et al. 2007; Giron-Gonzalez et al. 2007; Limketkai et al. 2012; Pineda et al. 2009; Qurishi et al. 2003; Ragni et al. 2009). To avoid the risk of double counting the participants from one study (Giron-Gonzalez et al. 2007), the results from the study by Merchante et al. (2006) were not included in the main analyses; their impact on the pooled estimates was explored in a sensitivity analysis. One study presented its results specifically at one and three years; all the others reported total mortality over the study period (ranging from a median of 20 months to 35 years).
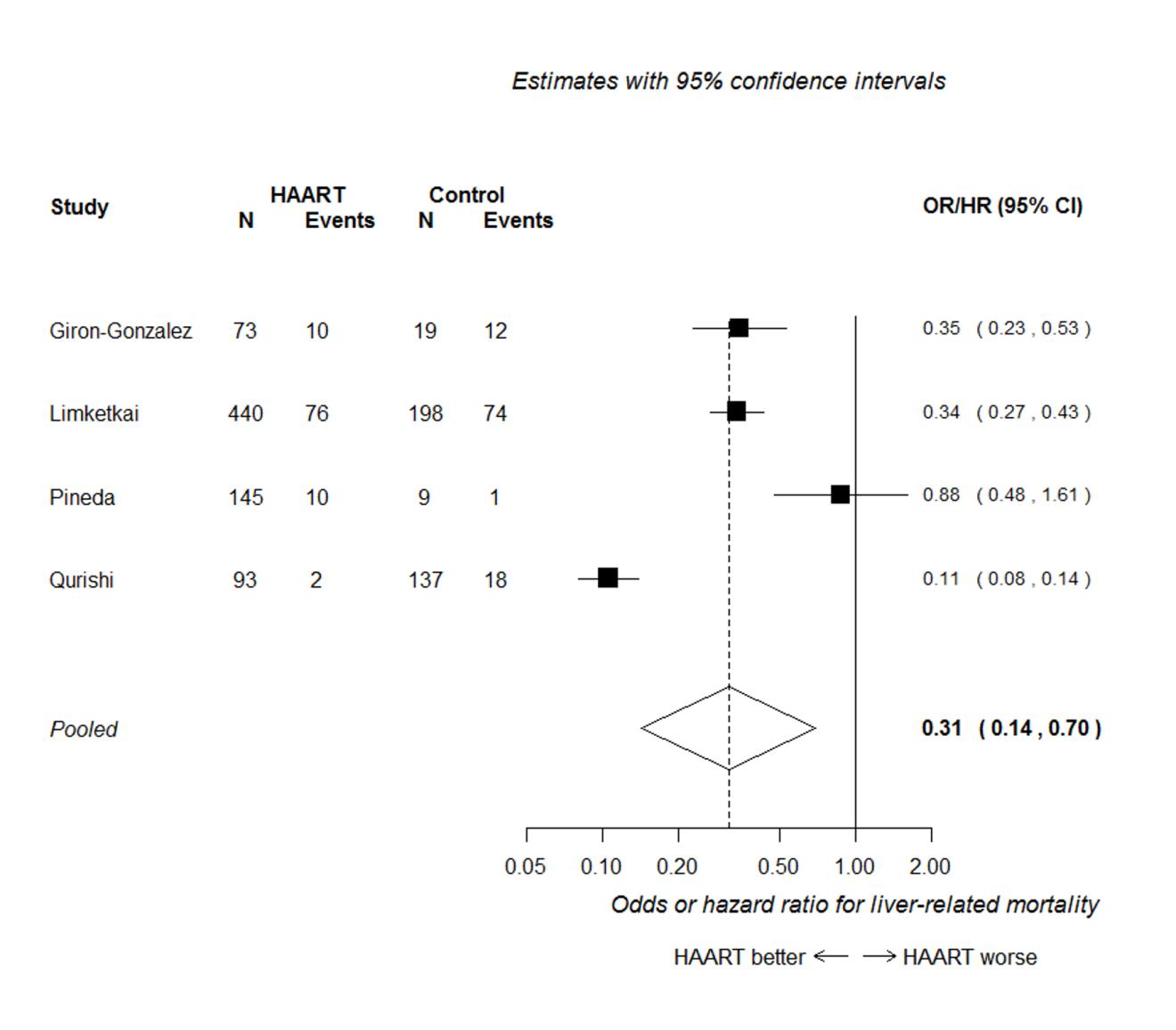
Four out of these seven studies presented analyses adjusted for potential confounding factors, and reported an odds risk or hazard ratio (Giron-Gonzalez et al. 2007; Limketkai et al. 2012; Pineda et al. 2009; Qurishi et al. 2003). Figure 2 presents the forest plot of the meta-analysis of these four studies, assuming that odds, risk and hazard ratios are equivalent. This analysis shows that HAART is associated with a substantial reduction in liver-related mortality, with a hazard/odds ratio of around one-third of that in untreated patients (HR/OR 0.31, 95% CI 0.14 to 0.70). Heterogeneity was high (I2=95%), apparently because of the discordant result between the Qurishi et al. (2003) and the Pineda et al. (2009) studies. The Qurishi et al. study showed a much larger benefit; most participants in this study were patients with haemophilia, whereas in the other studies, most patients had a history of injection drug use.
All six studies included in the meta-analysis presented the numbers of patients with and without liver-related mortality, from which relative risks could be calculated (these relative risks were not adjusted for potential confounders). The forest plot of the meta-analysis of relative risks from these six studies is shown in Figure 3. This analysis shows a clear association in favour of HAART for preventing liver-related mortality (RR 0.40, 95% CI 0.29 to 0.55). The summary effect estimate is similar to that seen in Figure 2, but more precise (as indicated by the narrower confidence intervals). There was no evidence of significant heterogeneity in this analysis (I2=24%).
3.5.1.1 Subgroup and sensitivity analyses
Figure 4 presents the forest plot for the two studies conducted primarily in patients with
haemophilia (Qurishi et al. 2003; Ragni et al. 2009). This shows that HAART was associated
with a reduced risk of liver-related mortality (RR 0.28, 95% CI 0.09 to 0.83), but there
were too few data to accurately estimate the effect, and to determine if the effect
differed in patients with a history of injection drug use.
One study presented the results only as a Kaplan-Meier survival curve (Merchante et al. 2006). By extracting the data presented in this curve, it was possible to estimate liver-
related mortality at 40 months. These data excluded patients with censored (i.e. unknown) disease status. If these censored patients had different disease status (for example, if they dropped out because they could not tolerate HAART) then the results may be biased. The study found that liver-related mortality was significantly lower in patients with compensated cirrhosis on HAART, compared with no treatment (unadjusted HR 0.5; 95% CI 0.3 to 0.9). Adding this study to the meta-analysis did not affect the conclusions, and had a limited effect on the overall results (RR 0.46, 95% CI 0.28 to 0.75). The number of liver-related deaths per group was not reported in the Limketkai study, but at least 63% of the events reported across the two study groups were liver-related deaths. Removing this study from the analyses had only a limited effect on the pooled estimates (RR 0.35, 95% CI 0.21 to 0.57). Further details on liver-related mortality and adjustments are provided in Appendix 5.
Figure 3.2: Hazard ratio of liver-related mortality in HIV/HCV co-infected patients
according to HAART use*
*Only Limketkai et al. (2012) included a small proportion of patients who received ARV monotherapy. All other patients in the intervention group received HAART
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Figure 3.3: Unadjusted odds ratios of liver-related mortality in HIV/HCV co-infected
patients according to HAART use*
*Only Limketkai et al. (2012) included a small proportion of patients who received ARV monotherapy. All other patients in the intervention group received HAART
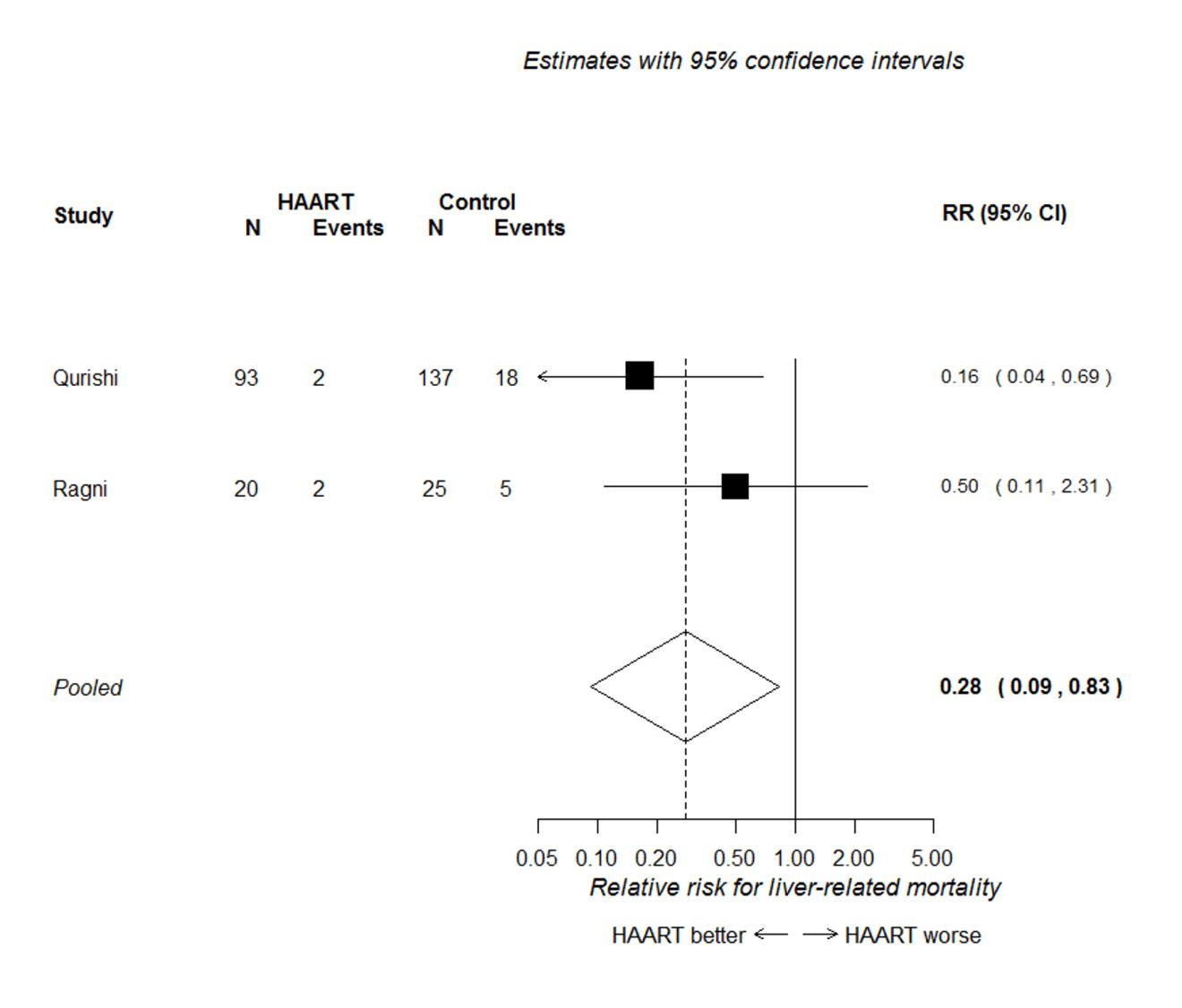
Figure 3.4: Unadjusted odds ratios of liver-related mortality in HIV/HCV co-infected
haemophiliac patients according to HAART use
3.5.2 Liver disease Liver disease outcomes were reported too diversely, or in too few studies for meta-analysis. Therefore all nine studies that reported liver disease are summarised in a narrative synthesis (Giron-Gonzalez et al. 2007; Macías et al. 2006, 2009; Mariné-Barjoan et al. 2004; Mehta et al. 2005; Pineda et al. 2009; Ragni et al. 2009; Reiberger et al. 2010; Schiavini et al. 2006). A summary of the findings from these studies is presented in Table 4.
3.5.2.1 End-stage liver disease and decompensation events
Three studies reported data on end-stage liver disease or liver decompensation events
(Giron-Gonzalez et al. 2007; Pineda et al. 2009; Ragni et al. 2009). Two of these studies
found at least one statistically significant effect in favour of HAART (Giron-Gonzalez et al.
2007; Ragni et al. 2009).
Ragni et al. (2009), investigating the risk of developing end-stage liver disease over 35 years, found no difference between patients with haemophilia receiving HAART and/or ARV monotherapy and untreated patients (RR 1.00, 95% CI 0.37 to 2.71). However, the study found that compared with patients on ARV monotherapy or no treatment, patients receiving HAART survived longer before progressing to end-stage liver disease (30.3 years for HAART, 20.0 years for ARV monotherapy/no treatment; HR 3.14, 95% CI 1.27 to 7.08).
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Giron-Gonzalez et al. (2007) and Pineda et al. (2009) both reported on the risk of liver decompensation in patients with liver cirrhosis. Giron-Gonzalez et al. found a significantly lower risk of a new event of decompensation in HAART patients with stable cirrhosis who had experienced decompensation in the past (HR 0.376, 95% CI 0.161 to 0.883). However, no statistically significant difference was found in the subgroup of patients with no previous decompensation at baseline. Similarly, Pineda et al. found no significant difference between HAART and no treatment in the risk of decompensation in patients with cirrhosis.
3.5.2.2 Advanced fibrosis or cirrhosis, and necroinflammatory activity
Only one study reported on the odds of advanced fibrosis or cirrhosis. Mehta et al. (2005)
found no statistically significant association between HAART or ARV monotherapy and this
outcome in patients with less severe or no fibrosis. ARV monotherapy and HAART were
both associated with lower necroinflammatory activity compared with untreated
individuals at follow-up, but the association was only statistically significant for the HAART
group (OR 0.27, 95% CI 0.14 to 0.5).
3.5.2.3 Fibrosis progression
Five studies reported on liver damage, expressed as fibrosis progression, in patients with
no cirrhosis at baseline (Macías et al. 2006, 2009; Mariné-Barjoan et al. 2004; Reiberger et
al. 2010; Schiavini et al. 2006). Of these, three reported the odds of fibrosis progression
(dichotomous outcome) (Macías et al. 2006, 2009; Schiavini et al. 2006), and three
reported the progression rate (continuous outcome) (Macías et al. 2006; Mariné-Barjoan et
al. 2004; Reiberger et al. 2010). One study reported both continuous and dichotomous
outcomes (Macías et al. 2006).
Of the three studies that reported the odds of fibrosis progression, only one reported a statistically significant difference between intervention and control. Macías et al. (2006) found significantly lower odds of liver fibrosis progression, over up to 49 years, in patients on HAART with protease inhibitors (OR 0.4, 95% CI 0.2 to 0.7) and in patients who switched from a protease inhibitors-based regimen to efavirenz during their treatment (OR 0.3, 95% CI 0.1 to 0.7), but not with other regimens. Macías et al. (2009) and Schiavini et al. (2006) found no significant association between HAART/ARV monotherapy and fibrosis progression.
Of the three studies that reported fibrosis progression rates, two found a difference in favour of HAART (Macías et al. 2006; Mariné-Barjoan et al. 2004), and one found no difference between HAART and no treatment (Reiberger et al. 2010). Macías et al. (2006) found slower median rates of fibrosis progression in patients treated with HAART, compared with no treatment, regardless of the regimen used. However, the difference was only statistically significant for some regimens (zidovudine/lamivudine and stavudine/lamivudine). Mariné-Barjoan et al. (2004) found a slower mean rate of fibrosis progression, over approximately 15 years, in patients taking HAART at follow-up, but the difference did not reach statistical significance. Reiberger et al. (2010) found no difference in fibrosis progression rate and time to cirrhosis, over 25 years, between HAART and no treatment. Further details are reported in Table 3.4.
Table 3.4: Liver disease progression: study results
Follow-up
Statistically Adjustments
duration
estimate
End-stage liver disease and decompensation events Giron-Gonzalez
HR 0.376 (95% Yes. Favours Liver disease
decompensation at baseline)
(subgroup without
decompensation at baseline)
CI 0.37 to 2.71)
7.08) (30.3 vs. Favours 20.0 Years)
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Follow-up
Statistically Adjustments
duration
estimate
Advanced fibrosis, cirrhosis and necroinflammatory activity Mehta (2005)
Advanced fibrosis or
Advanced fibrosis or
Necroinflammatory
Necroinflammatory
Yes. Favours None
CI 0.14 to 0.5) treatment
Liver fibrosis progression (dichotomous) Macías (2009)
Age, undetectable
genotype 3, baseline ALT, baseline necroinflammatory activity, time between liver biopsies, HCV treatment response
Schiavini (2006)
OR 2.5 (95% CI No
Follow-up
Statistically Adjustments
duration
estimate
OR 0.4 (95% CI Yes
Age at infection,
Favours treatment
OR 0.3 (95% CI Yes
Age at infection,
Favours treatment
Liver fibrosis progression (continuous) Macías (2006)
Fibrosis progression
with PI switched
Fibrosis progression
Fibrosis progression
difference -0.06 (95% CI -0.14 to 0.01)
Reiberger (2010)
Fibrosis progression
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Follow-up
Statistically Adjustments
duration
estimate
0.01 (95% CI -0.01 to 0.04)
Reiberger (2010)
Time to cirrhosis from Mean 24 to
initial HCV exposure
difference -1.00 (95% ci -7.26 to 5.26)
1 p<0.05 threshold; 2 Compared with ARV monotherapy and no ARV combined; ARV monotherapy patients formed 62% of the comparator group in this analysis. 3 Ishak score ≥F3. 4 Modified Hepatic Activity Index (MHAI) ≥5. 5 At least 1 Scheuer stage (scale 0 to 4) (modified Knodell-Ishak). 6 ≥1 Knodell-Ishak stage increase between two biopsies spaced by at least one year. 7 METAVIR Fibrosis stage (0 to 3)/length of HCV infection
PI: protease inhibitors; NVP: nevirapine; ESLD: end-stage liver damage
4. Discussion
4.1 Summary of findings
This systematic review aimed to evaluate the effect of HAART and ARV monotherapy on liver-disease progression and liver-related mortality in patients co-infected with HIV and hepatitis C.
Thirteen cohort studies met the inclusion criteria for this review. All studies evaluated HAART, and about half also included patients on ARV monotherapy. Seven studies reported data on liver-related mortality, of which six were included in a meta-analysis. Nine studies reported other liver-disease-related outcomes, which were reported in a narrative synthesis. In most studies, most patients had a history of injection drug use; in two studies, all or most patients had haemophilia.
Most studies were at a moderate-to-high risk of confounding. The risk of bias associated with participant selection was generally considered to be either low or unclear, and the risk of bias associated with outcome measurement was mostly low.
HAART was found to be associated with a substantial reduction in liver-related mortality, with a chance/hazard ratio of around one-third of that in patients not receiving treatment. The pooled estimates from unadjusted analyses also showed a clear association in favour of HAART for preventing liver-related mortality. A subgroup analysis, in which nearly all patients had haemophilia, also found that HAART was associated with reduced liver-related mortality, but there were too few data to provide an accurate estimate, and to determine if the effect differed from that in other populations.
The findings for other liver-related outcomes were less consistent. One study found a statistically significant lower risk of repeated decompensation in patients on HAART. Another study found no difference between treated and untreated patients in the risk of decompensation, but it found that patients with haemophilia receiving HAART progressed significantly less rapidly to end-stage liver disease, compared with untreated patients. One study found no statistically significant association between HAART and the odds of developing advanced fibrosis or cirrhosis in patients with less severe or no fibrosis. Only two of the five studies that estimated liver-fibrosis progression found a statistically significant result, which favoured HAART. No studies reported that a lack of treatment was associated with significantly better liver-disease outcomes.
4.2 Strengths and limitations of the review
This systematic review was conducted following the general principles recommended in Centre for Reviews and Dissemination (2009) Guidance for Undertaking Reviews in Health Care, and the reporting guidance of the PRISMA statement Moher et al. 2009). Study quality and the risk of bias were assessed systematically and considered when interpreting the results. Rigorous methods were used to minimise reviewer bias and error. Wherever possible, data on the treatment effects in individual studies were extracted or calculated, even where quantitative synthesis was not undertaken.
Only English-language studies were included, so it is possible that studies published in languages other than English were missed. The fact that only published studies were
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
considered means that the risk of publication bias, where studies with statistically significant results are more likely to be published, cannot be excluded.
Bibliographic searches identified only one previous systematic review of direct relevance. This review, which searched for studies up to 2005, reported limited and inconclusive evidence on the association between ARVs and liver disease. The systematic review reported here has identified significantly more relevant cohort studies, most of which were published after 2005. Therefore, this review presents a valuable update of the evidence on the association between HAART/ARV monotherapy and liver-disease progression in patients co-infected with HIV and HCV.
Liver disease outcomes were reported too diversely, or in too few studies for statistical pooling. This was unfortunate as several of the included studies had relatively few participants and may have been underpowered to identify significant effects on liver disease. This limits the strength of the findings on liver-disease progression.
Most studies on liver-related mortality were pooled in a meta-analysis. Adjusted and unadjusted results were pooled separately to address the limitations associated with the risk of confounding.
There were too few studies to conduct meta-regression or further subgroup analyses to explore the moderating effects of several relevant factors, including age, liver-disease severity, baseline CD4 count, time since HCV/HIV infection and alcohol abuse.
4.3 Limitations of the evidence and uncertainties
Ideally, survival should be assessed in a long-term prospective study, with randomised allocation of patients to the treatment groups. However, no randomised controlled trials were identified and all the included studies were observational. Nearly half of the studies did not attempt to adjust for potential confounders, such as age and sex. In those studies that did adjust, the factors accounted for varied across studies. For instance, only two studies controlled for alcohol misuse in their analyses. As mentioned above, attempts were made to address the risk of confounding in the analyses, but given the varied level of adjustment in the studies, the risk of confounding should not be ruled out, even for those studies classed as being at a lower risk of bias due to better adjustment.
The studies might have been affected by a survivorship bias if patients in the treatment group who survived long enough to receive HAART/ARV monotherapy had slower HCV progression, and therefore may have had better HCV-related outcomes (Kramer et al. 2007). The use of a time-dependent variable or Cox proportional hazards modelling, taking HCV duration or progression into account, might have remedied this bias. However, no studies reported using this technique. On the other hand, it is possible that comparison groups had levels of immunosuppression that were considered sufficiently high for their HIV treatment to be delayed, following treatment guidance (Brook et al. 2010; Nuñez 2005; Williams et al. 2014). In this case, patients in the treatment group may in fact have had poorer health at treatment initiation, and may therefore have been more vulnerable and prone to liver-disease progression. It is also possible that co-infected patients, who did not survive before the advent of ARV monotherapy, died of non-liver-related causes. Unfortunately, there were insufficient data on the characteristics of the study participants at baseline to support or reject these assumptions.
The reasons for not receiving HAART/ARV monotherapy were generally not reported. However, given that HIV treatment was likely to have been recommended to most HIV/HCV co-infected patients, particularly those with a high viral load, the reasons for not receiving treatment were likely to be influenced by patient choice. For example, those receiving HAART/ARV monotherapy might have been less likely to be active injection drug users, such as ex-injection drug users on methadone programmes who acquired HIV/HCV through this route, and might have had different lifestyles, such as less alcohol and substance abuse, compared with those who did not receive treatment. The reporting of baseline differences between treatment and comparator groups was variable and limited in several studies. Although no studies reported significant differences between groups, such as in current alcohol, injection drug or other substance abuse, and although some cohorts adjusted for these variables in their analyses, it is still possible that those who received HAART/ARV monotherapy were different from those who did not for reasons that may have influenced their liver-related outcomes.
There was significant evidence of a positive effect on liver disease favouring HAART in patients with haemophilia, who were the primary population of interest in this review. However, this finding was based on only two studies, and there were too few data to provide an accurate effect estimate and to determine if the effect differed from that in other populations. There were too few studies to explore the effects of other variables (such as age, liver-disease severity, CD4 count, ARV regimen, alcohol abuse, and time since HCV infection), using appropriate statistical methods.
4.4 Generalisability of the findings
Most studies included in this review were conducted in Europe; none were from the UK. Potential differences in the management of HIV/HCV co-infection across different health systems may limit the applicability of the review findings to co-infected populations in the UK. Most participants included in the studies were under 50 years old. The burden of other co-morbidities is likely to be higher in an older population. This, in addition to the toxicity of other treatments, may impact differently upon liver disease. This limits the applicability of the results to older populations, especially given the increasing life expectancy of people with HIV and HCV, and the growing proportion of people with HIV aged 50 years or older. The applicability of the findings to Black and minority ethnic groups affected by different strains of HIV, who are likely to have been under-represented in the included cohorts, is also unclear, although results from the two studies that included mostly Black participants (Limketkai et al. 2012; Mehta et al. 2005) were consistent with those of other included cohorts.
Two studies included a substantial proportion of patients with haemophilia (Qurishi et al. 2003; Ragni et al. 2009), so these studies are of greater relevance for this review. All other studies either mostly included patients with a history of injection drug use, or did not report on their drug use or likely mode of infection. Participants with a history of injection drug use are likely to differ in significant ways from patients with haemophilia. For instance, co-infected patients with haemophilia are more likely to have contracted HCV and HIV through repeated exposure to infected blood products from early childhood, whereas injection drug users may be more likely to have been infected in adolescence or adulthood. For this reason, liver damage and progression to AIDS may have occurred from a younger age in patients with haemophilia. It is also possible that comorbidities and
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
interactions with therapies specific to patients with haemophilia may affect liver-disease outcomes. Although the results of studies of patients with haemophilia did not differ significantly from those of other populations, the unique circumstances of patients with haemophilia should be considered when interpreting the results from populations without haemophilia.
4.5 Conclusions
The use of HAART is associated with a significantly reduced liver-related mortality in patients co-infected with HIV and HCV. Evidence of a positive association between HAART and/or ARV monotherapy with liver-disease progression is less clear, although there is no evidence to suggest that the absence of antiretroviral therapy is preferable.
4.5.1 Implications for policy and clinical practice The findings of this review support the use of HAART in patients co-infected with HIV and HCV as recommended in the EACS and BHIVA guidelines (Brook et al. 2010; Rockstroh et al. 2008). However, given the increased risk of liver-related morbidity in patients co-infected with HIV and HCV and the limited evidence on the impact of HAART and liver disease progression, the need for monitoring liver-disease progression in this population clearly remains. Future management of co-infected patients is likely to evolve with the advent of new directly acting antivirals (DAAs), and several are currently being reviewed by NICE (2014a, b; 2015a, b).
4.5.2 Further research Few included studies reported data separately for different antiretroviral classes and combinations. Several studies comparing different regimens did not compare HAART with untreated people and were therefore excluded from our review. Given the common use of HAART in HIV management, a systematic review on the acute and chronic effects of different HAART regimens would be useful. In addition, the mechanisms by which liver-disease mortality is reduced with HAART are still largely unknown (Ragni et al. 2009). It may be that the effects of HAART on liver-disease progression and mortality occur through immune reconstitution, viral suppression or a combination of both (Kramer et al. 2007), but further research in this area is required.
Once further data on the impact of HAART on liver-disease progression in co-infected patients are available, an update of this systematic review may be appropriate. Further research on the impact of HAART on liver-disease outcomes in specific populations (such as patients with haemophilia) would also help to clarify the applicability of the review findings to different subgroups.
There is little long term evidence on the impact of HAART on health-related quality of life in HIV/HCV co-infected patients (Jin et al. 2014), and exploring qualitative evidence on patient experience and perception of HAART/ARV monotherapy in this population was beyond the scope of this review. A systematic review of qualitative studies on patient perspectives (and possibly further primary qualitative studies) may help to provide a more complete picture of the evidence on the effect of HAART/ARV monotherapy in patients co-infected with HIV and HCV.
5. References
References included in the in-depth review are marked with an asterisk*
Arnold DM, Julian JA, Walker IR, Association of Hemophilia Clinic Directors of Canada (2006) Mortality rates and causes of death among all HIV-positive individuals with hemophilia in Canada over 21 years of follow-up. Blood 108:460-464. Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, Katlama C, Poynard T, MultivirC Group (2001) Factors affecting liver fibrosis in human immunodeficiency virus- and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology 34:283-287. Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR (2001) Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clinical Infectious Diseases 32:492-497. Bland M (2000) An introduction to medical statistics. 3rd ed. Oxford: Oxford University Press. Bonacini M (2004) Liver injury during highly active antiretroviral therapy: the effect of hepatitis C coinfection. Clinical Infectious Diseases 38 (Suppl 2):S104-108. Bradshaw D, Matthews G, Danta M (2013) Sexually transmitted hepatitis C infection: the new epidemic in MSM? Current Opinion in Infectious Diseases 26:66-72. Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, Rodríguez-Torres M (2006) Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. Journal of Hepatology 44:47-55. Brook G, Main J, Nelson M, Bhagani S, Wilkins E, Leen C, Fisher M, Gilleece Y, Gilson R, Freedman A, Kulasegaram R, Agarwal K, Sabin C, Deacon-Adams C (2010) British HIV Association guidelines for the management of coinfection with HIV-1 and hepatitis B or C virus 2010. HIV Medicine 11:1-30. *Bruno R, Sacchi P, Puoti M, Maiocchi L, Patruno S, Carosi G, Filice G (2007) Natural history of compensated viral cirrhosis in a cohort of patients with HIV infection. Journal of Acquired Immune Deficiency Syndromes 46:297-303. Centre for Reviews and Dissemination (2009) Systematic reviews: CRD's guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York.(accessed 18 June 2015). Daar ES, Lynn H, Donfield S, Gomperts E, O'Brien SJ, Hilgartner MW, Hoots WK, Chernoff D, Arkin S, Wong WY, Winkler CA; Hemophilia Growth and Development Study (2001) Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. Journal of Infectious Diseases 183:589-595. De Bona A, Sitia G, Uberti-Foppa C, Galli L, Ciuffreda D, Gallotta G, Paties C, Lazzarin A (2003) Impact of HAART on liver histology of HIV/HCV coinfected patients. Journal of Biological Regulators and Homeostatic Agents 17:195-197. Department of Health (2011) Review of the support available to individuals infected with hepatitis C and/or HIV by NHS supplied blood transfusions or blood products, and their dependants. London: Department of Health. (accessed 18 June 2015).
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clinical Trials 7:177-188. Eyster ME, Diamondstone LS, Lien JM, Ehmann WC, Quan S, Goedert JJ (1993) Natural-history of hepatitis-c virus-infection in multitransfused haemophiliacs: effect of coinfection with human-immunodeficiency-virus: The Multicenter Hemophilia Cohort Study. Journal of Acquired Immune Deficiency Syndromes 6:602-610. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J (2002) Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine 347:975-982. Fuster D, Planas R, Muga R, Ballesteros AL, Santos J, Tor J, Sirera G, Guardiola H, Salas A, Cabré E, Ojanguren I, Barluenga E, Rey-Joly C, Clotet B, Tural C (2004) Advanced liver fibrosis in HIV/HCV-coinfected patients on antiretroviral therapy. AIDS Research and Human Retroviruses 20:1293-1297. *Giron-Gonzalez JA, Brun F, Terron A, Vergara A, Arizcorreta A (2007) Natural history of compensated and decompensated HCV-related cirrhosis in HIV-infected patients: a prospective multicentre study. Antiviral Therapy 12:899-907. Goedert JJ, Eyster ME, Lederman MM, Mandalaki T, de Moerloose P, White GC, Angiolillo AL, Luban NL, Sherman KE, Manco-Johnson M, Preiss L, Leissinger C, Kessler CM, Cohen AR, DiMichele D, Hilgartner MW, Aledort LM, Kroner BL, Rosenberg PS, Hatzakis A (2002) End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood 100:1584-1589. Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ (2001) Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clinical Infectious Diseases 33:562-569. Hayashi PH, Flynn N, McCurdy SA, Kuramoto IK, Holland PV, Zeldis JB (1991) Prevalence of hepatitis-C virus-antibodies among patients infected with human-immunodeficiency-virus. Journal of Medical Virology 33:177-180. Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0.(accessed 18 June 2015). House of Commons Hansard. Hepatitis C (haemophiliacs). 29 October 2013, cols 197-222WH. (accessed 18 June 2015). Jin Y, Liu Z, Wang X, Liu H, Ding G, Su Y, Zhu L, Wang N (2014) A systematic review of cohort studies of the quality of life in HIV/AIDS patients after antiretroviral therapy. International Journal of STD and AIDS 25:771-777. Kramer JR, Giordano TP, El-Serag HB (2007) Effect of human immunodeficiency virus and antiretrovirals on outcomes of hepatitis C: a systematic review from an epidemiologic perspective. Clinical Gastroenterology and Hepatology 5:1321-1328.e7. Lange CG, Lederman MM (2003) Immune reconstitution with antiretroviral therapies in chronic HIV-1 infection. Journal of Antimicrobial Chemotherapy 51:1-4. *Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, Moore RD, Thomas DL, Sulkowski MS (2012) Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 308:370-378. Macías J, Castellano V, Merchante N, Palacios RB, Mira JA, Saez C, García-García JA, Lozano F, Gómez-Mateos JM, Pineda JA (2004) Effect of antiretroviral drugs on liver
fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS 18:767-774. *Macías J, Mira JA, Lopez-Cortes LF, Santos I, Giron-Gonzalez JA, Gonzalez-Serrano M, Merino D, Hernández-Quero J, Rivero A, Merchante N, Trastoy M, Carrillo-Gómez R, Arizcorreta-Yarza A, Gómez-Mateos J, Pineda JA (2006) Antiretroviral therapy based on protease inhibitors as a protective factor against liver fibrosis progression in patients with chronic hepatitis C. Antiviral Therapy 11:839-846. *Macías J, Berenguer J, Japon MA, Giron JA, Rivero A, Lopez-Cortes LF, Moreno A, González-Serrano M, Iribarren JA, Ortega E, Miralles P, Mira JA, Pineda JA (2009) Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology 50:1056-1063. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK (2001) Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. *Mariné-Barjoan E, Saint-Paul MC, Pradier C, Chaillou S, Anty R, Michiels JF, Sattonnet C, Ouzan D, Dellamonica P, Tran A, Registre des Ponctions-Biopsies Hépatiques (2004) Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus co-infected patients. AIDS 18:2163-2170. Martin-Carbonero L, Benhamou Y, Puoti M, Berenguer J, Mallolas J, Quereda C, Arizcorreta A, Gonzalez A, Rockstroh J, Asensi V, Miralles P, Laguno M, Moreno L, Girón JA, Vogel M, García-Samaniego J, Nuñez M, Romero M, Moreno S, de la Cruz JJ, Soriano V (2004) Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clinical Infectious Diseases 38:128-133. *Mehta SH, Thomas DL, Torbenson M, Brinkley S, Mirel L, Chaisson RE, Moore RD, Sulkowski MS (2005) The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection. Hepatology 41:123-131. *Merchante N, Giron-Gonzalez JA, Gonzalez-Serrano M, Torre-Cisneros J, Garcia-Garcia JA, Arizcorreta A, Ruiz-Morales J, Cano-Lliteras P, Lozano F, Martínez-Sierra C, Macías J, Pineda JA, Grupo Andaluz para el Estudio de las Enfermedades Infecciosas (2006) Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS 20:49-57. Micallef JM, Kaldor JM, Dore GJ (2006) Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. Journal of Viral Hepatitis 13:34-41. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. NICE (2006) Methods for development of NICE public health guidance. London: National Institute for Health and Clinical Excellence. (accessed 18 June 2015). NICE (2014a) Hepatitis C (chronic): ledipasvir-sofosbuvir [ID742]. London: National Institute for Health and Care Excellence. (accessed 1 December 2014). NICE (2014b) Hepatitis C (chronic): daclatasvir [ID766]. London: National Institute for Health and Care Excelle(accessed 1 December 2014).
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
NICE (2015a) Simeprevir in combination with peginterferon alfa and ribavirin for treating genotypes 1 and 4 chronic hepatitis C. London: National Institute for Health and Care Excellence.(accessed 18 June 2015). NICE (2015b) Sofosbuvir for treating chronic hepatitis C. National Institute for Health and Care Excellence.(accessed 18 June 2015). Nuñez M (2005) British HIV guidelines for the management of hepatitis B and C in HIV-coinfected patients. AIDS Reviews 7:181-183. *Pineda JA, Aguilar-Guisado M, Rivero A, Giron-Gonzalez JA, Ruiz-Morales J, Merino D, Ríos-Villegas MJ, Macías J, López-Cortés LF, Camacho A, Merchante N, Del Valle J, Grupo para el Estudio de las Hepatitis Víricas (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas (2009) Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clinical Infectious Diseases 49:1274-1282. *Qurishi N, Kreuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh JK, Spengler U (2003) Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 362:1708-1713. Ragni MV, Belle SH (2001) Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C infection. Journal of Infectious Diseases 183:1112-1115. Ragni MV, Bontempo FA (1999) Increase in hepatitis C virus load in hemophiliacs during treatment with highly active antiretroviral therapy. Journal of Infectious Diseases 180:2027-2029. *Ragni MV, Nalesnik MA, Schillo R, Dang Q (2009) Highly active antiretroviral therapy improves ESLD-free survival in HIV-HCV co-infection. Haemophilia 15:552-558. *Reiberger T, Ferlitsch A, Sieghart W, Kreil A, Breitenecker F, Rieger A, Schmied B, Gangl A, Peck-Radosavljevic M (2010) HIV-HCV co-infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. Journal of Viral Hepatitis 17:400-409. Rockstroh JK, Spengler U, Sudhop T, Ewig S, Theisen A, Hammerstein U, Bierhoff E, Fischer HP, Oldenburg J, Brackmann HH, Sauerbruch T (1996) Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. American Journal of Gastroenterology 91:2563-2568. Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, Puoti M, Soriano V, Tural C, EACS Executive Committee (2008) European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Medicine 9:82-88. Rumi MG, Colombo M, Gringeri A, Mannucci PM (1990) High prevalence of antibody to hepatitis-C virus in multitransfused hemophiliacs with normal transaminase levels. Annals of Internal Medicine 112:379-380. *Schiavini M, Angeli E, Mainini A, Zerbi P, Duca PG, Gubertini G, Vago L, Fociani P, Giorgi R, Cargnel A (2006) Risk factors for fibrosis progression in HIV/HCV coinfected patients from a retrospective analysis of liver biopsies in 1985-2002. HIV Medicine 7:331-337. Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N (2007) Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technology Assessment 11(11):1-205.
Soldan K, Ramsay M, Robinson A, Harris H, Anderson N, Caffrey E, Chapman C, Dike A, Gabra G, Gorman A, Herborn A, Hewitt P, Hewson N, Jones DA, Llewelyn C, Love E, Muddu V, Martlew V, Townley A (2002) The contribution of transfusion to HCV infection in England. Epidemiology and Infection 129:587-591. Soriano V, Garcia-Samaniego J, Valencia E, Rodriguez-Rosado R, Munoz F, Gonzalez-Lahoz J (1999) Impact of chronic liver disease due to hepatitis viruses as cause of hospital admission and death in HIV-infected drug users. European Journal of Epidemiology 15:1-4. Sterling RK, Wilson MS, Sanyal AJ, Luketic VA, Stravitz RT, Contos MJ, Mills AS, Shiffman ML (2004) Impact of highly active antiretroviral therapy on the spectrum of liver disease in HCV-HIV coinfection. Clinical Gastroenterology and Hepatology 2:432-439. Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL (2002) Hepatitis C and progression of HIV disease. JAMA 288:199-206. Sulkowski MS, Mehta SH, Torbenson M, Afdhal NH, Mirel L, Moore RD, Thomas DL (2005) Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS 19:585-592. Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, Moore RD, Afdhal NH, Thomas DL (2007) Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS 21:2209-2216. Thomas J, Brunton J, Graziosi S (2010) EPPI-Reviewer 4.0: software for research synthesis. EPPI-Centre software. London: Social Science Research Unit, Institute of Education, University of London. Tural C, Fuster D, Tor J, Ojanguren I, Sirera G, Ballesteros A, Lasanta JA, Planas R, Rey-Joly C, Clotet B (2003) Time on antiretroviral therapy is a protective factor for liver fibrosis in HIV and hepatitis C virus (HCV) co-infected patients. Journal of Viral Hepatitis 10:118-125. Vento S, Garofano T, Renzini C, Casali F, Ferraro T, Concia E (1998) Enhancement of hepatitis C virus replication and liver damage in HIV-coinfected patients on antiretroviral combination therapy. AIDS 12:116-117. Verma S (2006) HAART attenuates liver fibrosis in patients with HIV/HCV co-infection: fact or fiction? Journal of Antimicrobial Chemotherapy 58:496-501. Verma S, Wang CH, Govindarajan S, Kanel G, Squires K, Bonacini M (2006) Do type and duration of antiretroviral therapy attenuate liver fibrosis in HIV-hepatitis C virus-coinfected patients? Clinical Infectious Diseases 42:262-270. Wells G, Shea B, O'Connell D, Peterson J, Welch J, Losos M, Tugwell P ([2014]) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. (accessed 6 August 2014). Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, Cwynarski K, Edwards S, Fidler S, Fisher M, Freedman A, Geretti AM, Gilleece Y, Horne R, Johnson M, Khoo S, Leen C, Marshall N, Nelson M, Orkin C, Paton N, Phillips A, Post F, Pozniak A, Sabin C, Trevelion R, Ustianowski A, Walsh J, Waters L, Wilkins E, Winston A, Youle M (2014) British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013. All changed text is cast in yellow highlight). HIV Medicine 15 (Suppl. 1):1-85.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Appendices
Appendix 1: Authorship
The authors of this report are: A Llewellyn,1 M Simmonds,1 G Brunton,2 A Sowden1
1Centre for Reviews and Dissemination, University of York
2EPPI-Centre, UCL Institute of Education, University College London
Author contributions Alexis Llewellyn: Contributed to the protocol development. Performed the study selection, data extraction, quality assessment and narrative synthesis, and contributed to the analyses; wrote all sections of the report.
Mark Simmonds: Contributed to the protocol development. Performed the statistical analyses and drafted the analysis results, performed full-text study selection, data extraction and quality assessment; contributed generally to the writing of the report.
Ginny Brunton: Contributed to the protocol development and provided comments on drafts of the report.
Amanda Sowden: Contributed to the protocol development. Took overall managerial responsibility for the project, contributed to all aspects of the project, and provided comments on drafts of the report.
Acknowledgements The authors would like to thank members of our advisory group, including Joseph Peaty and Mark Ward from Tainted Blood for providing testimonies on living with HIV, Hepatitis C and haemophilia and on undergoing antiretroviral therapy, for suggesting references for inclusion, and for commenting on the draft report. We thank Professor Will Irving, Professor of Virology, Queen's Medical Centre, Nottingham, for his assistance and advice in the interpretation of the studies and for commenting on the draft report. We also thank Professor James Thomas, Professor of Social Research and Policy, UCL Institute of Education, University College London, for his advice and support.
Funding This is an independent report commissioned and funded by the Policy Research Programme in the UK Department of Health. The views expressed are not necessarily those of the Department.
Conflicts of interest There were no conflicts of interest in the writing of this report.
Contributions The opinions expressed in this publication are not necessarily those of the University of York, the EPPI-Centre or the funders. Responsibility for the views expressed remains solely with the authors.
Appendix 2: Search strategy
Medline Searched 18/06/14 via OVID interface.
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present> No date or language limits applied. Search strategy: 1 exp HIV/ (84307) 2 exp HIV Infections/ (233038) 3 exp Hepatitis C/ (47865) 4 exp Hepacivirus/ (24124) 5 (1 or 2) and (3 or 4) (7098) 6 exp Antiretroviral Therapy, Highly Active/ (17442) 7 Anti-Retroviral Agents/ (5840) 8 Antiviral Agents/ (56460) 9 Anti-HIV Agents/ (34699) 10 6 or 7 or 8 or 9 (106310) 11 5 and 10 (2269) 12 exp Fibrosis/ (50801) 13 Liver Cirrhosis/ (56237) 14 Liver Diseases/ (58223) 15 Liver/ (373600) 16 Drug-Induced Liver Injury/ (23066) 17 (liver adj2 (fibrosis or cirrhosis)).ti,ab. (29669) 18 12 or 13 or 14 or 15 or 16 or 17 (530439) 19 11 and 18 (559)
Embase
Searched 18/06/14 via OVID interface.
Database: Embase <1974 to 2014 June 17>
No date or language limits applied.
Search strategy:
1 exp Human immunodeficiency virus/ (133543)
2 exp Human immunodeficiency virus infection/ (298276)
3 exp hepatitis C/ (73811)
4 exp Hepatitis C virus/ (43388)
5 (1 or 2) and (3 or 4) (16222)
6 exp highly active antiretroviral therapy/ (29310)
7 antiretrovirus agent/ (33456)
8 antivirus agent/ (53721)
9 anti human immunodeficiency virus agent/ (13395)
10 6 or 7 or 8 or 9 (113132)
11 5 and 10 (4430)
12 exp fibrosis/ (153092)
13 liver cirrhosis/ (94465)
14 liver disease/ (83604)
15 liver/ (400588)
16 toxic hepatitis/ (8742)
17 liver injury/ or liver toxicity/ (92069)
18 (liver adj2 (fibrosis or cirrhosis)).ti,ab. (41464)
19 12 or 13 or 14 or 15 or 16 or 17 or 18 (738045)
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
20 11 and 19 (1573)
21 limit 20 to Embase (1496)
Appendix Table 1: Results from bibliographic searches
After deduplication
Appendix 3: Quality assessment and risk of bias
Risk of bias was evaluated using a modified version of the Newcastle-Ottawa quality assessment tool (Wells et al. [2014]). Following this tool, three main domains were considered: participant selection, confounding and outcomes. However, two main modifications were made for the purposes of this review. First, questions were devised to inform a judgement about risk of bias for each of these three domains within each study, whereas the Newcastle-Ottawa tool is designed to provide an overall quality score for each study. We felt that the use of quality scores was potentially misleading. For instance, a study with high risk of confounding due to lack of adjustment in its analysis may still receive a relatively high quality score if it meets all other criteria. Secondly, the relevance of the study participants to the key population of interest for this review was considered, whereas the Newcastle-Ottawa tool does not address this question.
Appendix Table 2: Quality assessment criteria
Participant selection
Question 1
Was the sample representative of study population of interest? (Yes/No/Unclear)
Was there no presence of outcome at baseline? (Yes/No/Unclear)
Was HCV assessed using valid methods (e.g. polymerase chain reaction)? (Yes/No/Unclear)
Risk of selection
Risk of bias associated with participant selection (High/Low/Unclear)
bias
Confounding
Question 4
Group differences: Were there significant differences in population characteristics between intervention and comparison groups? (Yes/No/Unclear)
Was there adjustment for relevant confounders in the analyses? (e.g. age, sex/gender, duration of HCV infection, alcohol abuse, HCV treatment, baseline liver damage) (Yes/No/Unclear)
Was risk of cross-over addressed? (i.e. risk that comparison group received HAART/ARV monotherapy during the study) (Yes/No/Partly)
Risk of bias associated with confounding
confounding bias
Outcome measurement
Question 7
Was the outcome assessed using valid methods? (e.g. using hospital registry data) (Yes/No/Unclear)
Was follow-up duration adequate/was follow-up sufficient to assess the impact of the intervention on the outcome of interest? (report assessment separately if the answer differs per outcome) (Yes/No/Unclear)
Was loss to follow-up acceptable? (Yes/No/Unclear/Not applicable)
Risk of bias associated with outcome measurement
measurement bias (High/Low/Unclear)
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Relevance Relevance to the
Were there any concerns about the relevance of this study in the
context of the review? (e.g. low proportion of patients with haemophilia) (High/Moderate/Low relevance)
Appendix Table 3: Quality assessment and risk of bias results
Baseline
Confounding
Follow-up
Relevance
assessment
Representative: Yes.
Outcome at
Adjustment for relevant
Adequate
All eligible patients in a baseline: No
confounders? Unclear. May not
assessed using
follow-up
be adjusted analyses
valid methods? duration?
Selection bias: Low
Cross-over risk addressed?
assessed
Partly. HAART only recorded
Limited loss
Differences between
from 1st event of
measurement
to follow-
groups: Unclear
bias: Low
up? NA.
Only
Confounding bias: Moderate.
Insufficient information on
methods of adjustment used.
Unclear how participants were
assigned to groups
Outcome at
Adjustment for relevant
Adequate
Unclear if consecutive
baseline: No.
confounders? Yes
assessed using
follow-up
Child-Pugh index and MELD score valid methods? duration?
Selection bias: Unclear compensated
(decompensation outcome);
Plasma HCV viral load; Child-
Limited loss
Differences between
Pugh index =OR>9; Child-Pugh
measurement
to follow-
groups: Yes. Higher
index progression; MELD score
bias: Low
decompensation ≥14, previous or occurring
frequency before
decompensation at follow-up; >1
baseline in untreated
decompensation at follow-up
group (p=0.006). No
(mortality outcome). Other
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Baseline
Confounding
Follow-up
Relevance
assessment
significant differences
assessed
variables measured at baseline
in alcohol abuse, IDU
not adjusted for because not
independently associated with
Cross-over risk addressed?
Partly. No. HAART= <80%
adherence. Some patients in the
comparison group were exposed
to HAART
Confounding bias: Moderate.
Some patients in comparison
group were exposed to HAART
Outcome at
Adjustment for relevant
Adequate
Unclear. 80% Black
baseline: Yes
confounders? Yes. Adjusted for
assessed using
follow-up
11% at baseline age, sex, race, IDU, time-varying valid methods? duration?
population was aimed
with F4 Fibrosis CD4 cell count and current
Selection bias: Low
Cross-over risk addressed? Yes. measurement
Limited loss
HAART exposure addressed as a
bias: Low
to follow-
Differences between
assessed
time-varying measure; current
up? Unclear.
groups: Unclear
HAART exposure adjusted for in
Confounding bias: Low.
Adjusted for age, sex, race,
Baseline
Confounding
Follow-up
Relevance
assessment
injection drug use, time-varying CD4 cell count and current HAART exposure.
Representative: Yes.
Outcome at
Adjustment for relevant
Adequate
>1 liver biopsy. May be
baseline: No
confounders? Yes. Age,
assessed using
follow-up
different from other
undetectable HIV viraemia,
valid methods? duration?
co-infected patients
assessed
genotype 3, baseline ALT,
baseline necroinflammatory
Selection bias: Low
activity, time between liver
Differences between
biopsies, HCV treatment
measurement
groups: Unclear
bias: Low
Limited loss
Cross-over risk addressed?
to follow-
Partly. Only at follow-up
up? NA Only
participants
Confounding bias: Moderate
adjustment for relevant
confounders, but risk of cross-
Representative: Yes
Outcome at
Adjustment for relevant
Adequate
baseline: No
confounders? Yes. Age,
assessed using
follow-up
Selection bias: Low
undetectable HIV viraemia,
valid methods? duration?
Differences between
assessed
genotype 3, baseline ALT,
groups: Unclear. NR
baseline necroinflammatory
Limited loss
activity, time between liver
measurement
to follow-
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Baseline
Confounding
Follow-up
Relevance
assessment
biopsies, HCV treatment
bias: Unclear.
Cross-over risk addressed?
Partly. Comparator included ARV reported, out of up data
monotherapy and untreated
Confounding bias: Low.
Multivariate adjusted model used
Representative: Yes.
Outcome at
Adjustment for relevant
Adequate
Appears to be all
baseline: No
confounders? No
assessed using
follow-up
eligible; reasonable
valid methods? duration?
Cross-over risk addressed?
inclusion criteria
assessed
Yes. Median through
Partly. Exposure only measured
Selection bias: Low
at time of biopsy
Differences between
Confounding bias: High. No
measurement
groups: Unclear
adjustments for relevant
bias: Low
Limited loss
to follow-
up? NA.
Only
participants
with follow-
up data
were
Baseline
Confounding
Follow-up
Relevance
assessment
Outcome at
Adjustment for relevant
Adequate
Unclear. Partly random baseline:
confounders? No. Multivariate
assessed using
follow-up
cohort, partly not.
models used, but not reported
valid methods? duration?
Unclear if consecutive
for HAART exposure
Selection bias: Unclear
Cross-over risk addressed?
Limited loss
Partly. Only about half of the
to follow-
Differences between
comparison group were
up? Unclear.
groups: Unclear.
assessed
completely naive to HAART.
Though most no-HAART
appeared to come from
Confounding bias: High. No
the clinical care
cohort, which was less
likely to have abused
alcohol and had higher
ALT levels at biopsy
measurement
bias: High
Merchante Representative:
Outcome at
Adjustment for relevant
Adequate
Unclear. Insufficient
baseline: No
confounders? No. Only raw data assessed using
follow-up
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Baseline
Confounding
Follow-up
Relevance
assessment
reported for outcome of interest valid methods? duration?
assessed
Selection bias:
Cross-over risk addressed? No.
data, definition 70 months
Unclear. Insufficient
58% with prior HAART history,
unclear if all were in
Limited loss
intervention group
to follow-
measurement
up? Unclear.
Differences between
Confounding bias: High.
bias: Low
groups: Unclear. NR
Unadjusted data and unclear risk of cross-over
Representative: Yes.
Outcome at
Adjustment for relevant
Adequate
Clear selection criteria
baseline: No.
confounders? No. Not for
assessed using
follow-up
decompensation. Unclear for
valid methods? duration?
Selection bias: Low
liver-related mortality (variables Yes. Definition
Differences between
Limited loss
groups: Unclear.
Cross-over risk addressed?
to follow-
Comparison group very
assessed
Partly. Mixed intervention group measurement
(some with HAART history at
bias: Low
baseline). History of comparison group unknown. Criteria for exposure/non-exposure unreported.
Confounding bias: High.
Especially for unadjusted
decompensation results
Baseline
Confounding
Follow-up
Relevance
assessment
Outcome at
Adjustment for relevant
Adequate
Unclear. Unclear if
baseline: No
confounders? Yes. Sex, age, risk assessed using
follow-up
category, alcohol misuse, HBV,
valid methods? duration?
assessed
CD4 count, AAT, AST, GGT,
Yes. Beyond 94% men
Selection bias: Unclear appropriately? cholinesterase, bilirubin,
Differences between
platelets count, immunoglobulin assessor blinded Limited loss
groups: Yes.
concentration. No adjustment
to follow-
Statistically significant
for viral loads (not available
higher bilirubin and
before 1996). Fixed and time-
measurement
dependent covariates addressed
bias: Low.
transpeptidase levels
separately. Risk of over-
Assessor blinded have been
adjustment due to correlations
untreated; statistically
between covariates?
significant higher
Cross-over risk addressed? Yes.
Comparison group was untreated
transpeptidase for
throughout. HAART and ARV
HAART vs untreated. No
monotherapy clearly defined
statistically significant differences in
Confounding bias: Low.
haemophilia, substance abuse, HBV and HIV viral load at baseline
Representative: Yes.
Outcome at
Adjustment for relevant
Adequate
All eligible patients
baseline: No
confounders? No.
assessed using
follow-up
Cox model was adjusted, but
valid methods? duration?
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Baseline
Confounding
Follow-up
Relevance
assessment
assessed
incidence of ESLD not adjusted
Selection bias: Low
Cross-over risk addressed? Yes.
All HAART group had previously
Limited loss
Differences between
received ARV monotherapy.
to follow-
groups: Yes. Mean age
positive; active Untreated group received no
measurement
up? Unclear.
at HIV seroconversion:
treatment, with reasons
bias: Unclear.
significantly older in
untreated group (35.7),
compared with HAART
Confounding bias: Low (high
group (20.8) and ARV
for unadjusted results, with
monotherapy (26.6),
several important between-
p=0.03. HAART group
group differences)
significantly older than ARV monotherapy and untreated combined (p=0.05). CD4 count significantly lower in HAART group, followed by untreated and then ARV monotherapy (p<0.01). HBV (HBsAg+) significantly higher in untreated and ARV monotherapy vs HAART (p=0.002). Mean age at ESLD older in untreated (53.0), followed by
Baseline
Confounding
Follow-up
Relevance
assessment
HAART (43.4) and ARV monotherapy (34.5) (p-value NR)
Outcome at
Adjustment for relevant
Adequate
Unclear. Unclear if
baseline: Yes.
confounders? No. No
assessed using
follow-up
consecutive, limited
valid methods? duration?
reporting on sampling
fibrosis stage 4
Cross-over risk addressed? No.
Selection bias: High.
Unclear when exposure was
Limited loss
19% with fibrosis stage
assessed
to follow-
4 at baseline. Reporting appropriately?
Confounding bias: High. No
clinical history
of selection limited
adjustment and risk of cross-
Differences between
measurement
groups: Unclear. NR.
bias: Low
Unclear how patients
were assigned to groups
Representative: Yes.
Outcome at
Adjustment for relevant
Adequate
Appears to be all
baseline:
confounders? No. No
assessed using
follow-up
patients in stated time
adjustments for HAART
valid methods? duration?
Cross-over risk addressed?
Selection bias: Unclear
Partly. Reported absence of ARV
highest possible therapy, though definition NR
Limited loss
Differences between
measurement
to follow-
groups: Unclear. NR
Confounding bias: High.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Baseline
Confounding
Follow-up
Relevance
assessment
Unadjusted values only
bias: Low
assessed
up data were included
NA: Not applicable; NR: Not reported; AOR: Adjusted odds ratio; ALT: alanine aminotransferase; IDU: Injection drug use
Appendix 4: Characteristics of included studies
Appendix Table 4: Study characteristics
Author (year) Country Industry Prospective/ Inclusion criteria
Exclusion
Start End
Total N Intervention Comparator
funding retrospective?
criteria
date date
Bruno (2007) Italy
Retrospective >18 years; HCV or HBV
Cirrhosis (compensated)
Elevated alphafetoprotein levels (0.200 ng/mL)
Anti-HCV antibodies;
liver cancer; 2004 Oct
cirrhosis; previous
decompensation eligible
if stable and compensated at baseline
HIV/HCV co-infection;
liver biopsy between
Macías (2006) Spain
Retrospective HIV/HCV who underwent HCV treatment 1991 2005
self-described liver biopsy
attempts to assess progression
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Author (year) Country Industry Prospective/ Inclusion criteria
Exclusion
Start End
Total N Intervention Comparator
funding retrospective?
criteria
date date
between two time points
Macías (2009) Spain
Retrospective HCV (RNA); >1 liver
biopsy, separated by at concomitant
HAART, 3 ARV suggests 26,
monotherapy) table 4)
suggests 109, table 4)
Retrospective HCV RNA; liver biopsy
Self-described before July 2000
cohort because it attempts to capture disease progression based on two separate time points
Mehta (2005) USA
Co-infected patients with ESLD; lost to 2001 NR
at least 2 years of
HAART; HCV treatment
Author (year) Country Industry Prospective/ Inclusion criteria
Exclusion
Start End
Total N Intervention Comparator
funding retrospective?
criteria
date date
HIV-HCV presenting with Metabolic or
1997 2004(?) 153
1st decompensation
previous decompensation
Pineda (2009) Spain
Decompensatio 1996 2006
Turcotte (CPT) class A
decompensation of liver disease
Qurishi (2003) German No
Retrospective Co-infected patients
regularly treated at
hospital department
Ragni (2009) USA
HCV positive since 1978, NR
85 (HIV HAART: 20; 25
with or without HIV
Retrospective HIV–HCV co-infected
patients with available history;
data on portal pressure HBsAg+; AIDS-and liver histology
condition; TB history
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Author (year) Country Industry Prospective/ Inclusion criteria
Exclusion
Start End
Total N Intervention Comparator
funding retrospective?
criteria
date date
Retrospective HIV-HCV co-infection;
1985 NR (last 36
liver biopsy specimen
recruite (subgroup
January paired 2002, liver median biopsies) f-u 4.5 years)
ESLD: end-stage liver disease; NR: not reported; f-u: follow-up; IQR: interquartile range
Appendix Table 5: Treatment regimens and concomitant treatment
Author (year)
ARV monotherapy HAART regimens
"No antiretroviral
Concomitant
regimens
therapy" group inclusion treatment
HAART or both
criteria
After 1st event of
No HAART after 1st event
of decompensation
unknown. None for
33% with genotype 3
2 NNRTIs +PI (51%) or
Absence of HAART = <80%
+efavirenz (34%) or
adherence during follow-
+abacavir. No nevirapine
up (definition from
related study), rather than permanent discontinuation
Limketkai (2012)
Including multiple agents
with at least 1 PI, NNRTI or
monotherapy at time of
Author (year)
ARV monotherapy HAART regimens
"No antiretroviral
Concomitant
regimens
therapy" group inclusion treatment
HAART or both
criteria
integrase inhibitor
Based on PI; nevirapine;
Mixed drug naive (67%)
analogue or dual
efavirenz; PI switched to
and ARV monotherapy
nevirapine; PI switched to
efavirenz; backbone
nucleoside analogues; others.
Nucleoside analogues and PI most frequent. Median exposure from 87 to 364 weeks depending on regimens.
NR (3 patients at f- At f-u. Only PI-based (43);
Lack of antiretroviral
44% HCV treatment
ARV monotherapy u)
only nevirapine based (14),
only efavirenz based (20);
At time of biopsy. Non-
At time of biopsy
nucleoside analogue (28
patients), nucleoside analogue (89), PI (72) (some patients received more than one class).
Cumulative HAART exposure none at biopsy (of which
ARV monotherapy PI/NNRTI +NRTIs
about half were totally
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Author (year)
ARV monotherapy HAART regimens
"No antiretroviral
Concomitant
regimens
therapy" group inclusion treatment
HAART or both
criteria
Merchante (2006)
Received during follow-up.
No HAART during follow-
Nucleoside analogues: 100%; up
PIs: 70%; non-nucleoside
analogues: 43%; 42% stavudine+lamivudine
72 (50% of intervention)
No HAART from baseline
received PI throughout f-u.
therapy at follow-
combinations. 29 switched
(from PI to NNRTI or vice
versa) during f-u.
PI (76%) or NNRTI (24%)
Did not receive any
No HCV treatment
ARV monotherapy analogues only (35% based. Transient interruption HAART/ARV monotherapy
in 6%. Available from 1995
interruption in 7%. Available from 1992
HAART: protease inhibitor
Unwilling or died before
(95%), NNRTI (5%). All had
drugs were available
liver biopsies pre-
previously received
transplant or pre-
HAART/ARV monotherapy
Reiberger (2010)
NR. Unclear when exposure
No HCV treatment
Author (year)
ARV monotherapy HAART regimens
"No antiretroviral
Concomitant
regimens
therapy" group inclusion treatment
HAART or both
criteria
Schiavini (2006)
Single or dual drug 0
Absence of antiretroviral
ARV monotherapy therapy
across groups (of which 6% has SVR)
NA: not applicable; NR: not reported; f-u: follow-up; SVR: systemic vascular resistance
Appendix Table 6: Overlap across Spanish cohort studies
Key patient
Cirrhosis (compensated
Decompensation (subgroup), Liver
Possible overlap with
and decompensated)
related mortality
Pineda (2009) and
Merchante (2006).
10 tertiary care
All fibrosis stages
Advanced Fibrosis and Fibrosis
Possible partial
progression rate (FPR: ratio between
overlap with Macías
liver damage at f-u and estimated
duration of HCV infection)
Fibrosis progression
4 southern Spain
Probable overlap with
cirrhosis (no previous
Giron-Gonzalez (2007)
but separate from Pineda
7 southern Spain
Cirrhosis class A, no
Decompensation; Liver mortality
Possible overlap with
Giron-Gonzalez (2007)
but separate from Merchante
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Appendix 5: Detailed results of studies on liver-related mortality
Appendix Table 7: Liver-related mortality study results
Follow-up
Adjustments
Included in
duration
estimate
Liver-related mortality
(95% CI 0.30 to 0.85)
Liver-related mortality
(95% CI 0.58 to 0.91)
Liver-related mortality
HCV viral load, liver disease
(95% CI 0.23 severity, liver disease
progression, decompensation during or before follow-up
(95% CI 0.11 to 0.42)
HAART (mostly) Liver-related mortality,
Age, sex, race, injection drug use, Yes
end stage liver disease
(95% CI 0.27 time-varying CD4 cell count and
and hepatocellular
current HAART exposure
(95% CI 0.35 to 0.61)
Liver-related mortality
Up to at least HR 0.5 (95% None
Liver-related mortality
Yes (covariates NR)
Follow-up
Adjustments
Included in
duration
estimate
(95% CI 0.09 to 4.33)
Liver-related mortality
Sex, age, risk category, alcohol
(95% CI 0.08 misuse, HBV, CD4 count, AAT, to 0.14)
AST, cholinesterase bilirubin, platelets count, immunoglobulin
(95% CI 0.04 to 0.69)
Liver-related mortality
Sex, age, risk category, alcohol
misuse, HBV, CD4 count, AAT,
AST, bilirubin, platelets count,
immunoglobulin concentration
Liver-related mortality
(95% CI 0.11 to 2.31)
Liver-related mortality
(95% CI 0.26 to 2.20)
1 One year follow-up was preferred for the meta-analysis because there were no survivors at three years in the control group. Once there are no survivors left, the relative risk becomes increasingly biased towards unity, because HAART group people continue to die but control group do not.
2 The number of liver-related mortality events per group was unclear, although at least 63% of events were liver-related mortality.
Impact of antiretroviral therapy on liver disease progression and mortality in patients co-infected with HIV and hepatitis C: Systematic review and meta-analysis
Appendix 6: Studies included in Kramer et al. (2007)
Appendix Table 8: Selection decision for studies included in the Kramer et al. (2007)
review
Decision
Exclude- comparator (comparing different regimens)
Exclude- design (cross-sectional study)
Exclude- design (cross-sectional study)
Exclude- comparator (not comparing HAART vs no treatment)
Mariné-Barjoan (2004
Martin-Carbonero (2004) Exclude- design (cross-sectional study)
Exclude- design (cross-sectional study)
Exclude- design (cross-sectional study)
Exclude- comparator (not comparing HAART vs no treatment)
The Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre) is part of the Social Science Research Unit (SSRU), UCL Institute of Education, University College London. The EPPI-Centre was established in 1993 to address the need for a systematic approach to the organisation and review of evidence-based work on social interventions. The work and publications of the Centre engage health and education policy makers, practitioners and service users in discussions about how researchers can make their work more relevant and how to use research findings.
Founded in 1990, the Social Science Research Unit (SSRU) is based at the UCL Institute of Education, University College London. Our mission is to engage in and otherwise promote rigorous, ethical and participative social research as well as to support evidence-informed public policy and practice across a range of domains including education, health and welfare, guided by a concern for human rights, social justice and the development of human potential.
The views expressed in this work are those of the authors and do not necessarily reflect the views of the EPPI-Centre or the funder. All errors and omissions remain those of the authors.
First produced in 2015 by:
Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre) Social Science Research UnitUCL Institute of Education, University College London18 Woburn SquareLondon WC1H 0NR
Tel: +44 (0)20 7612 6397
ISBN: 978-1-907345-80-7
Cover image NIAID
This document is available in a range of accessible formats including large print.
Please contact the UCL Institute of Education for assistance: telephone: +44 (0)20 7947 9556 email: [email protected]
Source: https://eppi.ioe.ac.uk/cms/LinkClick.aspx?fileticket=XDlY_U_h_DI%3D&tabid=3481
The bioMérieux solution Did you know? VITEK2 has been challenged with ESBL in http://www.lahey.org/studiesSite of Lahey Clinic, where tables are updated for several studies.The broader scope has been B-lactamases with amino-acid sequences published by Livermore et al.
Extracts from the following websites have been used in compiling this • Cardiac Infarction information. Web links are provided below, however, original textsare zipped up into this file: • Heartbeat (irregular) Liver (fatty degeneration) • Lungs (reduces bronchial spasms) • Intestines (regulates activity)• Stomach Ulcers (normalizes gastric juices) The Budwig Diet by










