Validity of four pain intensity rating scales
PAINÒ 152 (2011) 2399–2404
Validity of four pain intensity rating scales
Maria Alexandra Ferreira-Valente , José Luís Pais-Ribeiro , Mark P. Jensen
a Faculdade de Psicologia e Ciências da Educação da Universidade do Porto, Porto, Portugalb Portuguese Foundation for Science and Technology, Lisbon, Portugalc Unidade de Investigação em Psicologia e Saúde (Psychology and Health Unit), Lisbon, Portugald Department of Rehabilitation Medicine, University of Washington School of Medicine, Seattle, WA, USA
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
The Visual Analogue Scale (VAS), Numerical Rating Scale (NRS), Verbal Rating Scale (VRS), and the Faces
Received 22 February 2011
Pain Scale-Revised (FPS-R) are among the most commonly used measures of pain intensity in clinical and
Received in revised form 25 June 2011
research settings. Although evidence supports their validity as measures of pain intensity, few studies
Accepted 11 July 2011
have compared them with respect to the critical validity criteria of responsivity, and no experimenthas directly compared all 4 measures in the same study. The current study compared the relative validityof VAS, NRS, VRS, and FPS-R for detecting differences in painful stimulus intensity and differences
between men and women in response to experimentally induced pain. One hundred twenty-seven sub-
jects underwent four 20-second cold pressor trials with temperature order counterbalanced across 1°C,
ValidityNumerical Rating Scale
3°C, 5°C, and 7°C and rated pain intensity using all 4 scales. Results showed statistically significant dif-
Visual Analogue Scale
ferences in pain intensity between temperatures for each scale, with lower temperatures resulting in
higher pain intensity. The order of responsivity was as follows: NRS, VAS, VRS, and FPS-R. However, there
Verbal Rating Scale
were relatively small differences in the responsivity between scales. A statistically significant sex maineffect was also found for the NRS, VRS, and FPS-R. The findings are consistent with previous studies sup-porting the validity of each scale. The most support emerged for the NRS as being both (1) most respon-sive and (2) able to detect sex differences in pain intensity. The results also provide support for thevalidity of the scales for use in Portuguese samples.
Ó 2011 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
findings suggest that no single measure is consistently moreresponsive than any of the other measures ,
The Visual Analogue Scale (VAS), Numerical Rating Scale (NRS),
although the responsivity of the VAS, NRS, VRS, and FPS-R has
Verbal Rating Scale (VRS), and Faces Pain Scale-Revised (FPS-R) are
yet to be directly compared in the same study. Also, the validity
among the most common measures of pain intensity used by
of these scales has never been examined in a sample of individuals
clinicians and researchers. Evidence supports the reliability and
from Portugal. Evaluations of common pain measures in samples
validity of each of these measures across many populations
from different countries and cultures can help establish the
. However, each measure has strengths and weaknesses.
cross-cultural generalizability of validity findings.
For example, research indicates that VASs have more ratio scale
Perhaps the most important validity criterion for a pain measure
qualities than other pain intensity scales for groups of patients
is its ability to detect changes in pain with pain treatment or proce-
(but not necessarily for individuals) , although some
dures known to produce pain. One method for doing this would be to
authors note that VASs scales do not always have linear qualities
use an experimental design in which the amount of stimulation is
and are not always normally distributed Pain scales with
highly controlled The cold-pressor test is an experimental
more response levels (eg, the VAS or 0–10 NRS relative to the 6-
method for inducing pain that is thought to reflect many (but not
point FPS-R or 4-point VRS) have the potential to be more sensitive
all) of the critical components of clinical pain , and its advantages
although more response categories do not necessarily
are discussed in the literature . Moreover, an increase
translate to more responsivity Furthermore, research
in pain intensity as water temperature decreases is well docu-mented, with small variations in water temperature resulting in sig-nificant differences in pain intensity
⇑ Corresponding author. Address: Rua 25 de Abril, n° 5, Idanha – Belas, Belas
A number of studies have examined the influence of sex on pain
2605-119, Portugal. Tel.: +351 969082988.
perceptions and pain response to experimental pain with
E-mail address: (M.A. Ferreira-Valente).
0304-3959/$36.00 Ó 2011 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
doi:
M.A. Ferreira-Valente et al. / PAINÒ 152 (2011) 2399–2404
normally menstruating women usually being more sensitive topainful stimuli than men . Laboratory pain experi-ments that include both men and women provide an opportunityto compare the ability of pain ratings to detect these well-estab-lished sex differences.
The primary aim of the current study was to compare the relative
validity of VAS, NRS, VRS, and FPS-R for detecting differences in pain-ful stimulation and for detecting sex effects in response to painfulstimulation. Based on previous research, we hypothesized that all4 scales would be able to detect a sex effect and differences in painresulting from 4 temperatures (1°C, 3°C, 5°C, and 7°C). Given the lar-ger number of response levels of the VAS and NRS, we also antici-pated that these would evidence greater responsivity than the VRSor FPS-R. Finally, this study also sought to evaluate the validity ofthe pain intensity rating scales in a Portuguese sample.
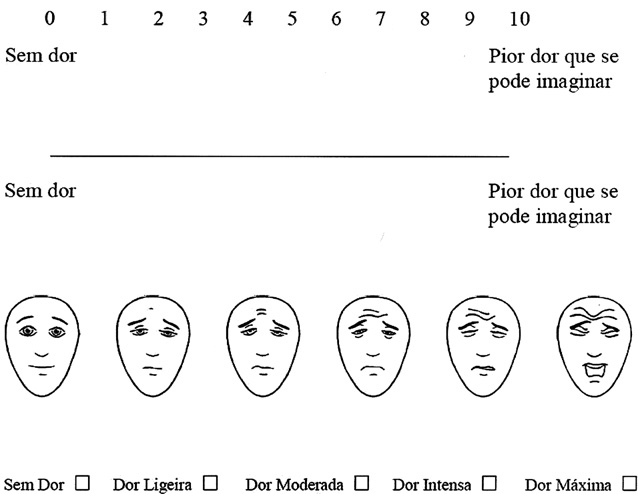
Fig. 1. The Numerical Rating Scale, Visual Analogue Scale, Faces Pain Scale –
2.1. Participants
Revised, and Verbal Rating Scale; Faces Pain Scale – Revised, copyright Ó2001,International Association for the Study of Pain, reproduced with permission,
Participants were 127 volunteer university students. Exclusion
criteria included: (1) being under 18 years of age; (2) reporting a his-tory of any of the following diagnoses or medical problems: muscu-
with the end points ‘‘No pain'' and ‘‘Worst imaginable pain'' placed
loskeletal problems, cancer, heart disease, stroke, epilepsy, diabetes
at each end of the line. Respondents are asked to make a mark on
or Raynaud syndrome; (3) having an open wound, cut, or fracture in
the line that best represents the level of pain intensity that they are
any of the upper limbs; (4) having a cognitive or physical disability
experiencing. The NRS is an 11-point scale consisting of integers
that could prevent participation; or (5) refusal to participate.
from 0 through 10; 0 representing ‘‘No pain'' and 10 representing
Of the 127 subjects who expressed an interest in participating in
‘‘Worst imaginable pain.'' Respondents select the single number
the study, 112 were eligible and completed the entire experimental
that best represents their pain intensity. Although validity studies
procedures. Of the 112 completers, 3 subjects were excluded from
for the Portuguese versions of these measures have not been pub-
the analyses because they were unable to understand how to use
lished, to our knowledge, both the VAS and the NRS used in this
the VAS. Thus, complete data were available for 109 subjects, 56 of
study have been previously used in research with Portuguese sam-
whom were female (51.4%). The ages of the participants ranged from
ples , and are recommended for use by the Portuguese
18 to 40 years old (M = 22.27, SD = 3.92; Female: M = 21.24,
Ministry of Health (Normative Circular n° 9/DGCG of June 14,
SD = 3.45; Male: M = 23.34, SD = 4.13). Most of the sample had their
2003). The VRS is a 5-point scale consisting of a list of phrases
permanent residence in an urban area (74.3%), and the remainder
(no pain, mild pain, moderate pain, intense pain, maximum pain)
(25.7%) lived in a rural area. Ninety-nine participants were under-
that describe increasing levels of pain intensity. Respondents select
graduate college students (33.0%, 17.4%, 21.1%, 11.0%, and 8.3% in
the single phrase that best characterizes their pain intensity. The
their first, second, third, fourth, and fifth year, respectively). Ten
VRS used in this study is commonly used by Portuguese research-
(9.2%) of the participants were in graduate school.
ers (eg, The FPS-R is a 6-point scale, with 6 differentfaces that represent increasing levels of pain intensity. Respon-
dents are asked to select the one expression that best characterizeshis or her pain intensity, from the left-most face (‘‘No pain''), to the
The cold-pressor apparatus used consisted of 4 thermal insu-
right-most face (‘‘Very much pain''). Each illustration corresponds
lated containers with 18.1 L of capacity containing water chilled
to a numeric score (0, 2, 4, 6, 8, or 10). Research supports the valid-
to 4 different temperatures. The apparatus was capable of main-
ity of each of the pain measures used in this study as measures of
taining water temperatures within ±0.5°C of the desired tempera-
pain intensity . Although the FPS-R was ini-
tures throughout the experimental procedures. Each container
tially developed for use with children, researchers also use the
had 2 compartments separated by a metal filter, one of which held
measure in samples of individuals with cognitive and communica-
water, ice, and a water pump, and the other (the immersion tank)
tion impairment. The Portuguese (Portugal) translation of the FPS-
contained water alone with an armrest; the participants' hands
R was performed by Batalha and is available online
therefore never came in direct contact with the ice. Four water
pumps (JAD, model SP-602, 200 L/h, Guangdong, China) made thewater flow continuously between the 2 compartments of each con-
tainer to prevent warm water pockets from forming near the partic-ipants' hands The temperature of the hand and water was
The cold-pressor procedures closely followed the guidelines for
monitored and controlled by asking the participants to hold a mer-
this task described in the literature, and were adapted to fit the
cury thermometer in the palm of their hands and immersing a ther-
study aims The study had Institutional Review Board
mometer in the water, respectively. One extra thermal insulated
approval. The study procedures were described to all potential par-
container without divisions contained an armrest and tepid water.
ticipants and each was given a written consent form to read andsign before any measures were administered. After signing the
consent form, participants completed a demographic and medicalhistory questionnaire in order to identify potential medical condi-
presents all the pain intensity rating scales used in this
tions that would prevent participation. Participants who met any
study. The VAS consists of a horizontal line 100 mm in length,
of the exclusion criteria were then excluded from participation.
M.A. Ferreira-Valente et al. / PAINÒ 152 (2011) 2399–2404
The nondominant hand temperature was measured in all par-
Pain ratings, as measured by VAS, NRS, VRS, and FPS-R, showed
ticipants, followed by hand washing to the wrist. The nondominant
normal distributions for each of the 4 temperatures (Sk <1 and Ku
hand was then immersed to the wrist in the container with tepid
<1). However, for each measure, we noted a violation of the
water (36°C ± 1°C) for 2 minutes, in order to reduce preexisting dif-
ferences in hand temperature Hand temperature was again
p < 0.001; NRS: W = 0.59, X2 (5) = 55.53, p < 0.001; VRS: W = 0.82,
measured, and participants were instructed to immerse the hand
X2 (5) = 20.54, p < 0.01; FPS-R: W = 0.81, X2 (5) = 21.74, p < 0.01].
to the wrist in the first cold water container for 20 seconds. Partic-
We therefore used Huynh-Feldt epsilon to determine the degrees
ipants were also told they could take the hand from the cold water
of freedom in the analyses .
at any moment if it felt too uncomfortable to continue. For each of
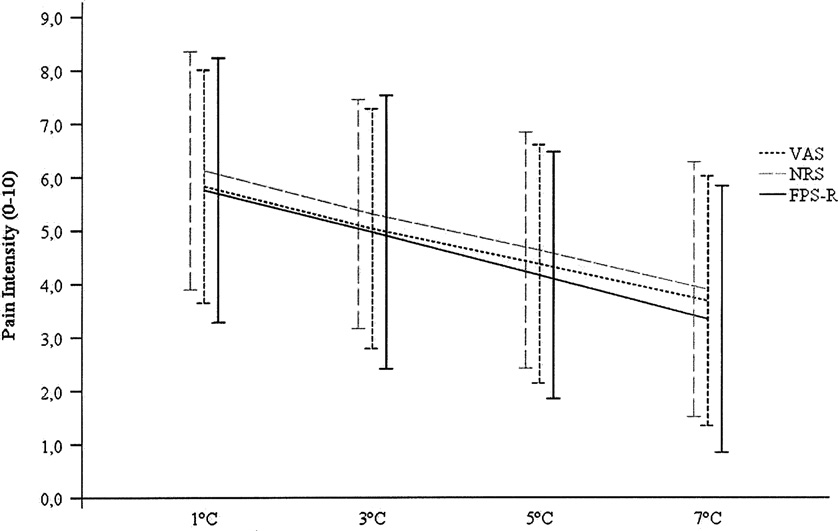
As hypothesized, there were statistically significant tempera-
the 4 study conditions, only 3 participants took their hands out
ture main effects for each of the 4 scales used in the study [VAS:
of the water before the established tolerance time (20 seconds).
FHuynh-Feldt(2.48, 265.24) = 85.74; p < 0.001, g2 = 0.45; p = 1; NRS:
After the 20-second cold immersion, when we anticipated that
FHuynh-Feldt(2.28, 243.64) = 93.49; p < 0.001, g2 = 0.47; p = 1; VRS:
pain would be at its most intense, we administered paper-and-
g2 = 0.42; p = 1;
pencil versions of the 4 pain measures (VAS, NRS, VRS, and FPS-
FHuynh-Feldt(2.70, 286.42) = 72.62; p < 0.001, g2 = 0.32;
R). The measures were presented in a random order using a Latin
p = 1]. Moreover, all of the effect sizes associated with the temper-
Square design. After a 3-minute break, the participants were asked
ature main effects in the omnibus ANOVAs for each measure were
to immerse his or her hand into the tepid warm water again for
large. Effect sizes for the comparisons for each pair of temperature
2 minutes, and hand temperature was again assessed. Participants
differences ranged from medium (0.17) to large (0.59), consistent
underwent 4 trials with 4 different water temperatures (1°C, 3°C,
with the differences between temperatures. These differences also
5°C, and 7°C), in counterbalanced order, as proposed by Mitchell
follow the same pattern for each scale (and ).
and colleagues Each participant experienced each water tem-
Statistically significant sex main effects emerged for the NRS
perature only once, and provided ratings using each of the 4 scales
[F(1, 107) = 4.40; p < 0.05, g2 = 0.04; p = 0.55], VRS [F(1, 107) =
for each temperature; thus, each participant provided 16 ratings.
8.38; p < 0.01, g2 = 0.07; p = 0.82], and FPS-R [F(1, 107) = 14.13;
Participants were not given any information regarding the water
p < 0.001, g2 = 0.12; p = 0.96], and a nonsignificant trend emerged
temperature in each container.
for the main effect of gender on pain ratings on VAS[F(1, 107) = 3.49; p = 0.07, g2 = 0.03; p = 0.46], with women report-
2.4.1. Data analysis
ing higher pain ratings in every experimental condition.
We first computed medians, means, and SDs for demographic
Post hoc Fisher's least significant difference paired tempera-
and study variables for descriptive purposes. We next computed
tures comparisons for each pain scale found statistically significant
Pearson correlations between the VAS, NRS, FPS-R, and VRS, for
differences between all observations (p < 0.001). As would be ex-
descriptive purposes. In order to compare the ability of VAS, NRS,
pected, in every case, lower temperatures resulted in higher pain
FPS-R, and VRS to detect differences in pain stimuli resulting from
intensity ratings for all 4 scales.
4 different temperatures, as well as the hypothesized sex main ef-
The NRS and VAS evidenced slightly higher effect sizes (0.47
fect on pain intensity ratings, we then performed 4 mixed-design
and 0.44, respectively) and higher F statistics (93.49 and 85.74,
repeated-measures analyses of variance (ANOVAs), with the pain
respectively) than VRS (0.42 effect size and F statistic of 76.36)
intensity ratings as the dependent variables, and sex and temper-
and FPS-R (0.32 effect size and F statistic of 72.62). Power analyses
ature as the independent variables. Prior to these analyses, we
based on these effect sizes indicated that the number of partici-
evaluated test assumptions, namely normality and sphericity of
pants needed to be able to detect an overall difference between
the variance–covariance matrix, by analysing skewness (Sk) and
temperatures, as tested by an ANOVA, would be 5, 5, 5, and 7 for
kurtosis (Ku), with values of Sk and Ku lower than 1 indicating ab-
the NRS, VAS, VRS, and FPS-R, respectively. Power analyses to
sence of severe violation of normality assumption and Mauchlytest, respectively . If a violation of the assumption of spheric-ity was found, we planned to use Huynh-Feldt epsilon to set the
Table 1Means and SDs of the pain ratings for each temperature condition.
degrees of freedom In the event that a significant tempera-ture effect was found, we planned to perform between-tempera-
ture comparisons using post hoc Fisher's least significant
difference tests. Effect sizes were estimated using g2, and, along
with P values and F statistic magnitudes, were used to compare
the hypothesized differences in responsivity of the 4 pain mea-
sures, with larger g2 and F statistics, as well as smaller P values,
indicating greater sensitivity Finally, we performed
power analyses to determine the sample size required to obtain
significant effects for each of the 4 measures, both for the omnibus
ANOVA and for each planned temperature paired comparison. Al-
pha was set at 0.05 and power at 0.95 for these analyses. Statistical
analyses were computed using software PASW Statistics 18 (v. 18,
SPSS Inc. Chicago, IL, USA) and G⁄Power (v. 3.1) .
lists the descriptive statistics of the study variables, and
presents the correlation coefficients between the pain mea-
sures. As can be seen, the pain scales showed strong to very strong
and statistically significant inter-scale correlations (rs ranging
VAS, Visual Analogue Scale; NRS, Numerical Rating Scale; FPS-R, Faces Pain Scale-
from 0.79 to 0.96) for all 4 water temperatures.
Revised; VRS, Verbal Rating Scale.
M.A. Ferreira-Valente et al. / PAINÒ 152 (2011) 2399–2404
Table 2Inter-scale correlation coefficients between the VAS, NRS, FPS-R, and VRS.
VAS, Visual Analogue Scale; NRS, Numerical Rating Scale; FPS-R, Faces Pain Scale-Revised; VRS, Verbal Rating Scale.
Table 3Effect sizes (and number of participants required to obtain significant effects) for the VAS, NRS, FPS-R, and VRS.
VAS, Visual Analogue Scale; NRS, Numerical Rating Scale; FPS-R, Faces Pain Scale-Revised; VRS, Verbal Rating Scale.
Fig. 2. Average pain intensity ratings across temperatures. Error bars represent SD.
compute the number of participants needed to detect differences
and FPS-R. This is consistent with previous studies demonstrating
between each pair of temperatures for each of the pain scales are
the superiority of VAS and NRS responsiveness, due perhaps to the
presented in , and range from 5 to 19. These are consistent
larger number of response levels that these 2 scales provide
with the distance between temperatures, with larger distances
In our study, the NRS has shown to be slightly more
(and effect sizes) corresponding to fewer participants needed.
responsive than the VAS, as indicated by its larger effect size and Fstatistic value. This is consistent with a group of studies showing a
similar sensitivity between the NRS and VAS or a slight superiorityof the NRS over the VAS . Nevertheless, the results of
The results of this study provide strong support for the validity
power analyses to determine the number of subjects needed to de-
of all 4 scales studied for detecting changes in pain intensity in Por-
tect a significant effect indicate that the 4 measures have very sim-
tuguese university students. All of the scales were able to detect
ilar levels of responsivity, with very small differences between
differences in pain resulting from 4 different temperatures, with
scales in the numbers of subjects needed to detect differences. This
variations in temperature resulting in statistically significant dif-
finding is consistent with the strong to very strong associations we
ferences in pain intensity ratings, and lower temperatures result-
found among the study measures (a finding also consistent with
ing in higher pain ratings for each of the 4 scales studied. These
previous research, eg, indicating that all 4 measures
results are consistent with previous studies that support each
tap into the same overall dimension (ie, pain intensity).
scale's validity and show that small variations
Thus, the results indicate that all else being equal, any of the 4
in water temperature result in significant differences in pain inten-
scales could be used for detecting changes in pain, although the
sity ratings .
NRS and VAS might be considered first when particularly sensitive
As predicted, we found some differences in the relative respon-
and responsive measures of pain intensity are needed. Based on
sivity, with NRS being the most responsive, followed by VAS, VRS,
other considerations, however, researchers and clinicians may
M.A. Ferreira-Valente et al. / PAINÒ 152 (2011) 2399–2404
elect to choose the NRS over the VAS in many settings. First,
the pain is not associated with any tissue damage, whereas pa-
although the NRS has not consistently been shown to have ratio
tients with clinical pain cannot always be so sure of this. For these
properties its scores can provide data for parametric anal-
reasons, clinical pain has an emotional significance and quality-of-
ysis . Also, the NRS has been shown to be at least as
life implications that may influence pain perception There-
sensitive as the VAS, whether a 0–10 NRS or a 0–100 NRS is used
fore, the study findings do not necessarily generalize to patients
. Third, the NRS is preferred over the VAS by patients
with clinical pain conditions. It would be useful to examine the rel-
and clinicians for its relative simplicity and ease of administration
ative responsivity of the 4 pain measures in response to treatments
or procedures known to impact clinical pain conditions to help
Fourth, the VAS tends to have higher failure rates
determine their generalizability.
than the NRS or VRS, probably because both the NRS and the VRS
Nevertheless, the findings provide support for the validity and
sensitivity of all 4 pain scales studied, with the exception that it
. This latter point was also supported in the
might be best to avoid the use of VAS in studies seeking to examine
current study, given our finding that the only participants excluded
sex effects. The findings also suggest that the NRS may be (very)
from the sample for not being able to understand the measures
slightly more sensitive than the other measures in our sample of
were excluded because they were unable to understand the VAS.
individuals from Portugal; a finding consistent with some other
The VRS is a categorical measure that might not have ratio prop-
studies that have compared the NRS to other pain measures, and
erties As a result, VRSs do not necessarily have equal intervals
supporting their cross-cultural reliability. Research is needed to
between levels, which limits the conclusions that can be drawn
compare these measures in clinical settings to confirm the general-
about the magnitude of differences over time or between patient
izability of the current findings to clinical populations.
groups Also, when differences in responsivity are found,VRSs, as well as the FPS-R, tend to be less sensitive than VAS and
Conflict of interest statement
NRS, consistent with the results from our study. This lower levelof responsivity may be related to the lower number of response
None of the authors have any conflicts of interest with respect
categories of these measures. However, we did find that both of
to this study.
these scales were able to detect changes in pain associated withdifferences in water temperature, indicating that they are valid
and could be used when responsivity is not a critical issue. Also, statisticians note that it is possible to draw va-
The authors gratefully acknowledge José Elísio Pereira for his
lid conclusions using parametric analysis with ordinal data, such as
contribution in the construction of the apparatus, and to Rita Ferre-
data from VRSs, especially if number of categories in the scale is 5
ira and Vera Melo for their assistance in data collection. M. Alexan-
or more Thus, the VRS can be considered a viable choice
dra Ferreira-Valente received PhD grant SFRH/BD/40956/2007 in
in settings with patients or research subjects who might be less
the past year from the Portuguese Foundation for Science and
able to use the NRS (eg, very young individuals or individuals with
Technology. José L. Pais Ribeiro received a sabbatical grant from
significant cognitive impairment). Likewise, our findings provide
FCT (SFRH/BSAB/982/2010) between January and April 2010. Mark
support for the validity of the FPS-R in adults, supporting its use
P. Jensen received research support, consulting fees, or honoraria in
in clinical and research settings. Although the FPS-R has been
the past year from Analgesic Research, Consultants in Behavioral
developed for use with children and proven useful with people
Research, Endo, Fralex, Medtronic, Merck, Pfizer, Smith & Nephew,
with cognitive and communication disabilities, there may be situ-
US Department of Education, US Department of Veterans Affairs,
ations or settings in which it might be useful for other populations.
and the US National Institutes of Health.
For example, the FPS-R might be useful for samples that includeboth adults without cognitive impairment and children (or the el-
derly) in the same study, and a measure is needed that all partici-pants can complete. The FPS-R might also be considered for use in
[1] Arantes S, Ferreira C, Lobo S, Moutinho R, Correia J, Carvalho J, Marcos A. Low
cross-cultural studies with adults, where researchers cannot be
back pain: the reality of our pain treatment unit. Dor 2007;15:22–7.
[2] Batalha L. Instructions for administering the Faces Pain Scale-Revised in
certain of the meaning equivalence of the verbal endpoint descrip-
tors. In this situation, a measure based on facial expressions might
show a greater cultural equivalence.
[3] Bergh I, Sjöström B, Odén A, Steen B. An application of pain rating scales in
geriatric patients. Aging (Milano) 2000;12:380–7.
Regarding sex effect on pain intensity ratings, our results are in
[4] Bieri D, Reeve R, Champion GD, Addicoat L, Ziegler J. The Faces Pain Scale for
line with previous research showing significant sex effects on
the self-assessment of the severity of pain experienced by children:
intensity ratings following painful stimulation , with
development, initial validation and preliminary investigation for ratio scaleproperties. Pain 1990;41:139–50.
women reporting higher pain intensity ratings across tempera-
[5] Bollen KA. Structural equation with latest variables. New York: Wiley; 1989.
tures. The sex effects were statistically significant for 3 of the scales
[6] Bolton JE, Wilkinson RC. Responsiveness of pain scales: a comparison of three
(NRS, VRS, FPS-R), and showed a nonsignificant trend for the VAS. It
pain intensity measures in chiropractic patients. J Manipulative Physiol Ther1998;21:1–7.
is interesting to note that other researchers have not found sex
[7] Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb
main effects when they used the VAS to test for these effects
pain: a randomized, double-bind, placebo-controlled, cross-over study. Pain
. These findings suggest the possibility that for experiments
specifically designed to test for or explain sex effects in pain inten-
[8] Box GEP. Some theorems on quadratic form applied to the study of analysis of
variance problems: II. Effects of inequality of variance and the correlation
sity, it might be best to avoid using the VAS.
between errors in the two-way classification. Ann Math Stat 1954;25:484–98.
One significant limitation of the study is that it was performed
[9] Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by
with healthy young participants. Although it has been argued by
sampling from clinical trial data. Clin J Pain 2000;16:22–8.
[10] Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, Kennedy P,
some that the cold-pressor test mimics the effects of chronic pain
Lundeberg T, Richards JS, Rintala DH, Siddall P, Widerstrom-Noga E. Pain after
conditions due to its unpleasantness and to the fact that
spinal cord injury: an evidence-based review for clinical practice and research.
the painful stimuli is conducted by the C fibers, which are impli-
Report of the National Institute on Disability and Rehabilitation ResearchSpinal Cord Injury Measures meeting. J Spinal Cord Med 2007;30:421–40.
cated in chronic pain, experimental pain is different from clinical
[11] Chanques G, Viel E, Constantin JM, Jung B, de Lattre S, Carr J, Cissé M, Lefrant JY,
pain. Clinical pain, for example, is less predictable and controllable.
Jaber S. The measurement of pain in intensive care unit: comparison of 5 self-
Also, participants in experimental pain studies can be assured that
report intensity scales. Pain 2010;151:711–21.
M.A. Ferreira-Valente et al. / PAINÒ 152 (2011) 2399–2404
[12] Cruz M, Gomes M, Sevivas N, Borralho N, Torres A, Silva B. Astragalus fracture –
[36] Kucuk O, Fisher E, Moinpour CM, Coleman D, Hussain MH, Sartor AO, Chatta
what's new – methods of diagnosis and treatment: 10 years experience. Rev
GS, Lowe BA, Eisenberger MA, Crawford ED. Phase II trial of bicalutamide in
Portuguesa Ortopedia Traumatol 2009;17:193–4 [Portuguese].
patients with advanced prostate cancer in whom conventional hormonal
[13] Dijkers M. Comparing quantification of pain severity by verbal rating and
therapy failed: a Southwest Oncology Group study (SWOG 9235). Urology
numeric rating scales. J Spinal Cord Med 2010;33:232–42.
[14] Edens JL, Gil KM. Experimental induction of pain: utility in the study of clinical
[37] Lund I, Lundeberg T, Sandberg L, Budh CN, Kowalski J, Svensson E. Lack of
pain. Behav Ther 1995;26:197–216.
interchangeability between visual analogue and verbal rating pain scales: a
[15] Faul F, Erdfelder E, Lang AG, Buchner A. G⁄Power 3: a flexible statistical power
cross sectional description of pain etiology groups. BMC Med Res Methodol
analysis program for the social, behavioral, and biomedical sciences. Behav Res
[38] Maroco J. Análise estatística com utilização do SPSS (3ª Edição). Lisboa: Edições
[16] Ferreira-Valente MA, Pais-Ribeiro JL, Jensen M. Coping with chronic
Sílabo; 2007 [Portuguese].
muskuloskeletal pain: preliminary validation of the Portuguese version of
[39] Miró J, Huguet A. Evaluation of reliability, validity, and preference for a
two two item measures. Psychol Health 2009;24:171.
pediatric pain intensity scale: the Catalan version of the faces pain scale-
[17] Ferreira-Valente MA, Pais-Ribeiro JL, Jensen M. Adjustment and quality of life
revised. Pain 2004;111:59–64.
in persons with chronic musculoskeletal pain: importance of self-efficacy and
[40] Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J
social support. In: Livro das 18ª Jornadas da unidade de dor do Hospital Garcia
de Orta; 2011. p. 30 [Portuguese].
[41] Moore MJ, Osoba D, Murphy K, Tannock IF, Armitage A, Findlay B, Coppin C,
[18] Finney SJ, DiStefano C. Non-normal and categorical data in structural equation
Neville A, Venner P, Wilson J. Use of palliative end points to evaluate the
modeling. In: Hancock GR, Mueller RO, editors. Structural equation modeling:
effects of mitoxantrone and low-dose prednisone in patients with hormonally
a second course. Greenwich, CT: Information Age Publishing (IAP); 2006. p.
resistant prostate cancer. J Clin Oncol 1994;12:689–94.
[42] Myles PS, Urquhart N. The linearity of the visual analogue scale in patients
[19] Frost S, Grossfeld S, Kirkley A, Litchfield B, Fowler P, Amendola A. The efficacy
with severe acute pain. Anaesth Intensive Care 2005;33:54–8.
of femoral nerve block in pain reduction for outpatient hamstring anterior
[43] Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement
cruciate ligament reconstruction: a double-blind, prospective, randomized
characteristics of mechanical visual analogue and simple numerical rating
trial. Arthroscopy 2000;16:243–8.
scales. Pain 1994;56:217–26.
[20] Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain
[44] Price D, McGrath P, Rafii A, Buckingham B. The validation of Visual Analogue
measurement: a ratio measure? Pain Pract 2003;3:310–6.
Scales as ratio scale measure for chronic and experimental pain. Pain
[21] Hicks CL, von Baeyer CL, Spafford P, van Korlaar I, Goodenough B. The Faces
Pain Scale – Revised: toward a common metric in pediatric pain measurement.
[45] Price DD, Patel R, Robinson MR, Staud R. Characteristics of electronic visual
analogue and numerical scales for ratings of experimental pain in healthy
[22] Hirsch MS, Liebert RM. The physical and psychological experience of pain: the
subjects and fibromyalgia patients. Pain 2008;140:158–66.
effects of labelling and cold pressor temperature on three pain measures in
[46] Ramer L, Richardson JL, Cohen MZ, Bedney C, Danley KL, Judge EA.
college women. Pain 1998;77:41–8.
Multimeasure pain assessment in an ethnically diverse group of patients
[23] Huskisson E. Visual analogue scales. In: Melzack R, editor. Pain measurement
with cancer. J Transcult Nurs 1999;10:94–101.
and assessment. New York: Raven Press; 1983. p. 33–7.
[47] Shannon MM, Ryan MA, D'Agostino N, Brescia FJ. Assessment of pain in
[24] Jensen MP. The validity and reliability of pain measure in adults with cancer. J
advanced cancer patients. J Pain Symptom Manage 1995;10:274–8.
Pain 2003;4:2–21.
[48] Silveira H, Soares J, Lima T, Sousa A, Brasil R, Soares C, Lima H, Carreiro P. Pain
[25] Jensen MP. Pain assessment in clinical trials. In: Wittink H, Carr D, editors. Pain
treatment. Amsterdam: Elsevier; 2008. p. 57–88.
[49] Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the
[26] Jensen MP. Measurement of pain. In: Fishman SM, Ballantyne JC, Rathmell JP,
psychometric properties, interpretability and feasibility of self-report pain
editors. Bonica's management of pain. Media, PA: Williams & Wilkins; 2010. p.
intensity measures for use in clinical trials in children and adolescents. Pain
[27] Jensen MP, Chen C, Brugger AM. The relative validity of three pain treatment
[50] Stockler MR, Osoba D, Goodwin P, Corey P, Tannock IF. Responsiveness to
outcome measures in post-surgical pain. Pain 2003;99:101–9.
change in health-related quality of life in a randomized clinical trial: a
[28] Jensen MP, Miller L, Fisher LD. Assessment of pain during medical procedures:
comparison of the Prostate Cancer Specific Quality of Life Instrument
a comparison of three scales. Clin J Pain 1998;14:343–9.
(PROSQOLI) with analogous scales from the EORTC QLQ-C30 and a trial
[29] Jensen MP, Turner JA, Romano J. What is the maximum number of levels
specific module. European Organization for Research and Treatment of Cancer.
needed in pain intensity measurement? Pain 1994;58:387–92.
J Clin Epidemiol 1998;51:137–45.
[30] Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and
[51] Tashani OA, Alabas OA, Johnson MI. Cold pressor pain responses in healthy
validity of chronic pain intensity measures. Pain 1999;83:157–62.
[31] Joyce CR, Zutshi DW, Hrubes V, Mason RM. Comparison of fixed interval and
visual analogue scales for rating chronic pain. Eur J Clin Pharmacol
[52] Trapanotto M, Pozziani G, Perissinotto E, Barbieri S, Zacchello F, Benini F. The
cold pressor test for the pediatric population: refinement of procedures,
[32] Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the
development of norms, and study of psychological variables. J Pediatr Psychol
McGill Pain Questionnaire: an overview of psychometric properties. Phys Ther
[53] von Baeyer CL, Piira T, Chambers CT, Trapanotto M, Zeltzer LK. Guidelines for
[33] Kim EJ, Buschmann MT. Reliability and validity of the Faces Pain Scale with
the cold pressor task as an experimental pain stimulus for use with children. J
older adults. Int J Nurs Stud 2006;43:447–56.
[34] Klatzkin RR, Mechlin B, Girdler SS. Menstrual cycle phase does not influence
[54] Walsh NE, Schoenfeld L, Ramamurthy S, Hoffman J. Normative model for cold
gender differences in experimental pain sensitivity. Eur J Pain 2010;14:77–82.
pressor test. Am J Phys Med Rehabil 1989;68:6–11.
[35] Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences
[55] Williamson A, Hoggart B. Pain: a review of three commonly used pain rating
and hormonal influences on response to cold pressor pain in humans. J Pain
scales. J Clin Nurs 2005;14:798–804.
Source: http://files.mafvalente.webnode.com.pt/200000202-df205e01aa/PAIN%202011%20152%202399%E2%80%932404.pdf
KHYBER MEDICAL UNIVERSITY, PESHAWAR Pharmacokinetic profile of Carbamazepine and valproic acid therapy in management of epilepsies with reference to single nucleotide polymorphisms in EPHX1, SCN1A and SCN2A genes in population of Khyber Pakhtunkhwa Principal Investigator: Dr. Niaz Ali
Market Outlook 17th Nov, 2015 NIFTY HELDS ITS SUPPORT OF 7750-7800; THANKS TO IMPRESSIVE GAINS IN BANK NIFTY; AVOID TRADING THE NIFTY INDEX AND FOCUS ON BLUE CHIPS; LONG POSITIONS SHOULD BE PROTECTED IN BANK NIFTY WITH A TRAILING STOP LOSS. It was a trend day in the Bank Nifty where prices opened with a gap down of 70 points at 17681 and went further lower to









