Corrected_copy_accept_and_print

May-July. 2012, Vol. 2, No. 3, 1257-1266. e- ISSN: 2249 –1929
Journal of Chemical, Biological and Physical Sciences
An International Peer Review E-3 Journal of Sciences
Available online at www.jcbsc.org
Section A: Chemical Sciences
CODEN (USA): JCBPAT
Research Article
Synthesis of Rabeprazole–Na for Hydrotropes as a
Reaction Media
Jagannath J Kadam1, Ajay Patil, K.George A 3, Vijay Bhawe and Manohar V Lokhande2
Department of Chemistry, Bhavan's College, Andheri (W),Mumbai 400058( India).
1Department of Chemistry, Bharati Vidyapeeth College of Engineering, Navi Mumbai-400614(India).
2 Department of Chemistry, Sathaye College, Vile Parle (E) Mumbai-400057(India).
Department of Chemistry, SIES College, SION (W) Mumbai-400022(India).
Received: 10 June 2012; Revised: 30 June 2012; Accepted: 3 July 2012
The paper describes the synthesis of Rabeprazole–Na. It is used as an Antiulcerative agent. The
molecules in the lattice are held together by intermolecular hydrogen bonds between the NH group of
benzimidazole and sulfinyl oxygen atom. The synthesis of Rabeprazole–Na. was carried out using four
different hydrotropes. The effect of various hydrotropes on yield, rate constant and activation energy
at various temperatures and concentrations are studied.
Keywords: Hydrotropes, Xylene sulfonic acid (XSA), Cumene sulfonic acid (CSA), (n-BBSA): n-Butyl
benzene sulfonic acid (n-BBSA), Isobutyl benzene sulfonic acid (I-BBSA), 1H NMR, IR.
Almost a Century ago Carl Neuberg1 conceptualized one such area in the form of hydrotropy. After a
dormancy period of about eight decades this exciting field sprung back into the chemical limelight and
today it is regarded as one of the frontiers in the field of applied organic chemistry. The pioneer Carl
Neuberg baptized this phenomenon as Hydrotropy or Hydrotropism.1,2 It is enhancement in the solubility
of organic molecules in water, which otherwise are sparingly soluble or totally insoluble.3-6 Hydrotropes
or hydrotropic agents are defined as the compounds which possess the property of solubility enhancement
of other compounds.
Hydrotropes are surface active, highly water soluble organic salts, which when present at high
concentration, can solubilise the otherwise insoluble or sparingly soluble organic compounds in water.
Hydrotropes can be differentiated from common surfactants in terms of their hydrofobicity i.e. hydrotopes
1257 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
are poorly hydrophobic as compared to surfactants. The performance of hydrotropic solutions is found to
be efficient, usually at higher concentration ranging from 0.2M to 1.0M. At concentration above 1.0M
‘salting out effect' is observed.
In the present study, the researchers intend to study the aromatic hydrotropes, especially the aromatic
sulphonates which are considered to be superior to the aliphatic counterparts as they are thermally stable
and have higher affinity. Hydrotrops are readily biodegradable in water under areobic conditions studies
with cummene, tolune and xylene7. This ecofriendly methodology where hydrotropes demonstrate a low
level of toxicity on aquatic life Xylene and cumene sulfonates ( ammonium , calcium and sodium salts)
have no acute toxicity towards fish and invertebrates at concentrations tested (> 318 mg/L )7. The
scientists around the globe are adopting environment friendly techniques to conserve flora (environment)
& fauna (animal life), also to manufacture & synthesize molecules useful to mankind. Carcinogenicity
studies reported for both rats and mice exposed to sodium Xylene sulfonates hydrotropes demonstrated
no carcinogenic reponse.7
One of the great advantage of Hydrotopes is the reusability of solvent media without operations such as
distillation etc. there by reducing operation cost, Hence it is an alternate media to organic solvents include
water, ionic liquids, supercritical solvents ,hydrotropic solutions etc.8 Hydrotropic solution are non toxic
shows no hazards of flammability hence consider as safer solvents. The compatibility of aqueous
hydrotropic solutions as safer solvents for microwave assisted reactions has been studied.9 So there is now
a realization that more benign chemical synthesis' is required as an integral part of developing sustainable
technologies 10-13. Efforts have been made to carry out studies on hydrotropes as effective reaction media
for the certain organic reactions.
(i)aq.NaOH and aq.HT. Solution(ii)aq.H2O2
Synthesis
sulfinyl]-1
H-benzimidozole: The reaction of 2–Mercaptobenzeneidazole with 2–bromomethyl–3–methyl–
4–(3–methoxy propoxy) pyridine was performed in aqueous hydrotropic solutions followed by
oxidation using aqueous H2O2 to afford the titled product which was tested for purity. Hydrotropes Used:
i) (Na-XS): Sodium xylene sulfonate
1258 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
ii) (Na-CS): Sodium Cumene sulfonates
iii) (Na-BBS): Sodium Butyl benzene sulfonate
iv) (Na-IBBS): Sodium Isobutyl benzene sulfonate
Melting points are uncorrected. 1H NMR spectra were recorded at 300 MHz on a Varian spectrometer and
IR spectra on a Shimadzu FT/IR-4200 instrument. Column chromatography 100 – 200 mesh Acme grade
silica gel was used. The crude reaction mixture was concentrated under reduced pressure to yield crude
mass which was preadsorbed on silica gel and purified by column chromatography with increase in
concentration of Ethyl acetate in Petroleum ether. The fractions having similar ‘Rf" values were pooled together, concentrated and subjected for characterization using various spectroscopic techniques. TLC
plates were prepared using silica gel G (ACME, Mumbai ). Pet. Ether : EtOAc (85 : 15) was used as the
solvent system. Radial chromatography : The circular glass plates of thickness 1 mm, were prepared by
using silica gel (PF254, E. MERCK, 50 g) in cold distilled water (105 ml). For elution, gradually
increasing concentrations of EtOAc in pet ether were employed.
In a 250 cm3 3-necked flask fitted with a stirrer, thermowell and an addition funnel, were added 0.01
moles of 2-mercaptobenzimidazole at room temperature followed by the addition of (0.01 moles) 2–
bromomethyl–3–methyl–4-(3–methoxy propoxy) pyridine dissolved in aqueous solution of hydrotrope
sodium Xylene sulfonate. The reaction mixture was stirred and 0.15N (10 ml) NaOH added and after 3
hours 50% H2O2 (20 cm3 ) were added then the reaction mixture was heated at 303K and 323K for 6 hours. The progress of the reaction was monitored by TLC for the completion of reaction.
On cooling at room temperature the product precipitated out from the reaction medium and was washed
with demineralized water in order to make it free from the traces of the hydrotropic solution adhering to it.
The product was then purified and dried in a vacuum drier. The qualitative estimation of the product was
done by TLC using the following system: Chloroform: Toluene (8:2)
The product was found to be pure without the traces of either of the starting materials. This is because of
the selective solubilization of the reactants which helps maintain them in the hydrotropic medium. The
product 2–[[[4–(3–Methoxy propoxy)–3–methyl–2–pyridinyl] methyl] sulfinyl]–1H–benzimidazole has a
melting point of 99 - 100°C. The similar reactions are carried out using other hydrotropes such as
Sodium Cumene Sulfonate, Sodium Benzene Sulfonate and Sodium Isobutyl Benzene Sulfonate. The
concentration range utilized for these hydrotropes was from 0.2 Mol/dm³ to 1.0 Mol/dm³. Higher
concentration of hydrotropes was avoided due to the salting out of the hydrotropes from the water which
is an inherent property of these salts.
CHARACTERISATION DATA
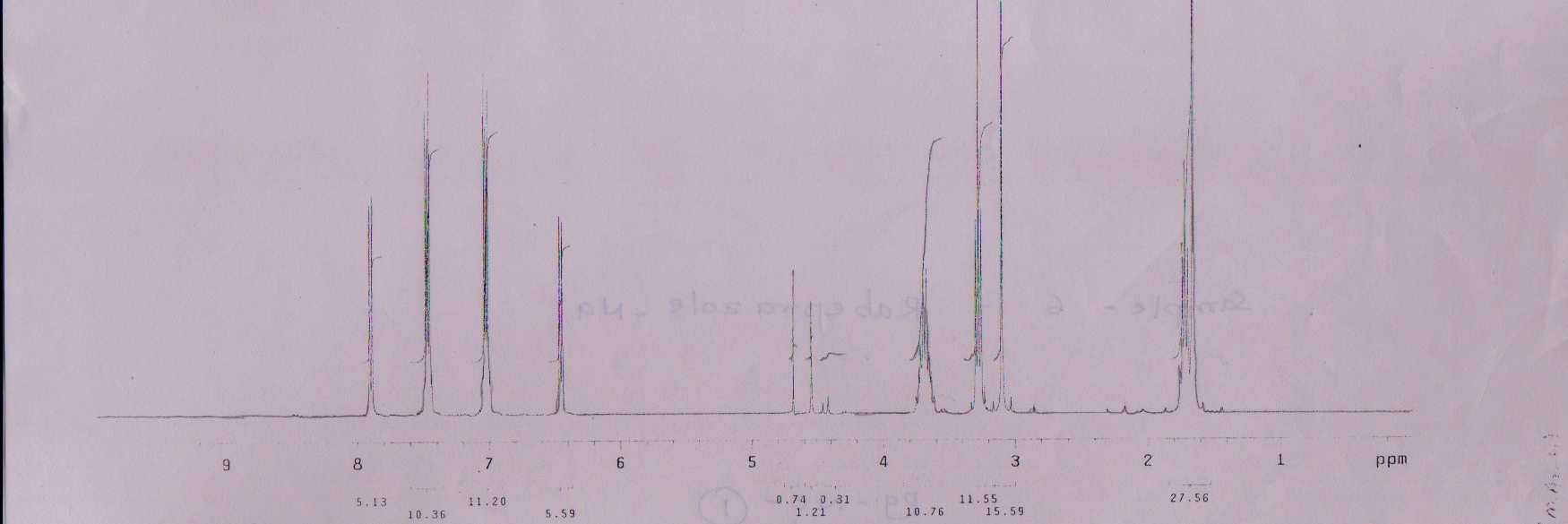
1HNMR δppm
1.66 (S, 3H, - CH
1HNMR (300MHz, D2O)
3), 1.69 - 1.75 (m, 2H, - CH2), 3.1 (S, 3H, - OCH3), 3.3
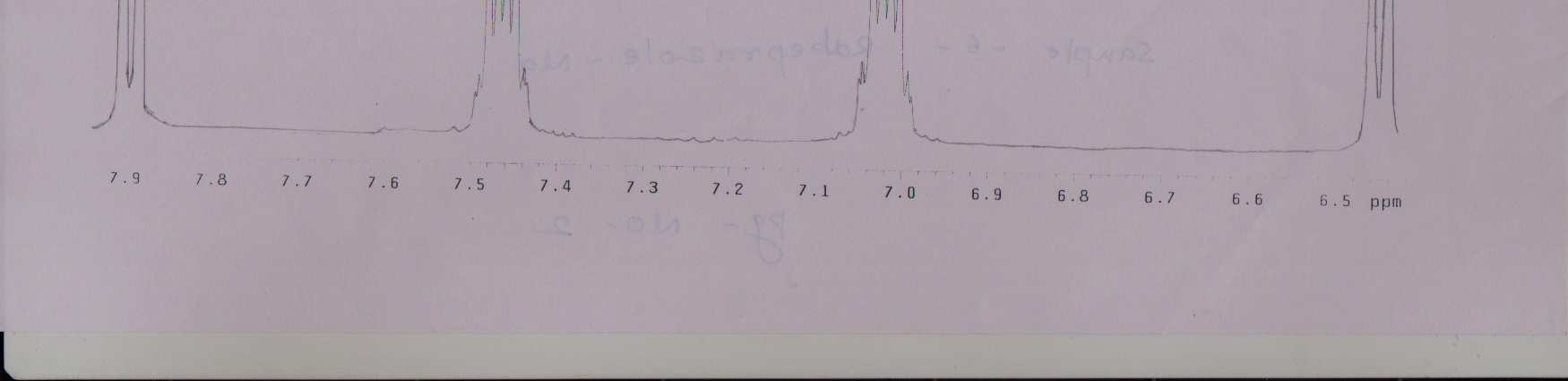
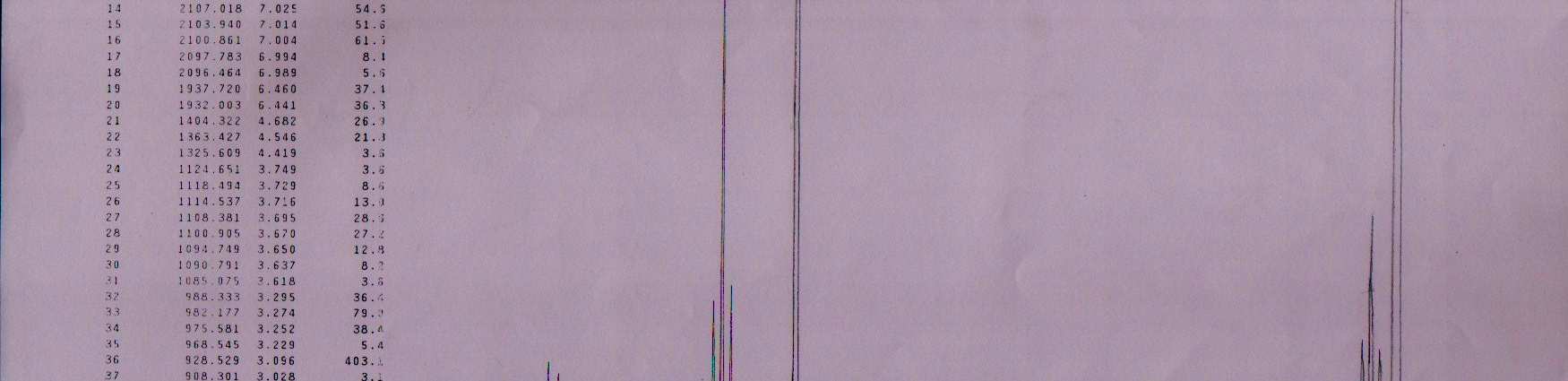
2), 3.6 - 3.8 (m, 2H, CH2), 4.7 (S, 1H, > NH), 6.4 - 8.0
IR עmax cm-¹.
IR values cm-¹.
3461, 3446, 3421, 3396, 3383 (- NH), 2923 (- CH
IR (KBr) עmax cm-¹
3, - CH2, - CH), 1582,
1458 (Ar - H), 745.
1259 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
Melting Range = 99 - 100°C,rf = 0.6 (Chloroform: Toluene (8:2).
RESULTS AND DISCUSSION
The Effect of hydrotrope concentration and temperature on the yield of 2 – [[[4 – (3 – Methoxy propoxy)
– 3 – methyl – 2 – pyridinyl] methyl] sulfinyl] – 1H – benzimidazole (Rabeprazole–Na) was depicted in
Table I a to Table I d.
Table - I a:Hydrotrope Used: Na-XS
Hydrotrope Concentration % Yield Obtained & Time in hours
Mole/dm3
303 K (6hrs)
323 K (6hrs)
Table - I b: Hydrotrope Used: Na-CS
Hydrotrope Concentration % Yield Obtained & Time in hours
Mole/dm3
303 K (6hrs)
323 K (6hrs)
Table - I c: Hydrotrope Used: Na-BBS
Hydrotrope Concentration Mole/dm3
% Yield Obtained & Time in hours
303 K (6hrs)
323 K (6hrs)
Table - I d: Hydrotrope Used: Na-IBBS
Hydrotrope Concentration % Yield Obtained & Time in hours
Mole/dm3
303 K (6hrs)
323 K (6hrs)
Similarly the effect of hydrotrope concentrations on the rate constants (k1, k2) and activation energies
(Ea) was listed in Table I e to Table I h.
1260 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
Table - I e: Hydrotrope Used - Na-XS
Hydrotropic Concentration K1 x 10-4
Moles/dm3
Table - I f:Hydrotrope Used - Na-CS
Hydrotropic
Concentration K1 x 10-4
Moles/dm3
Table - I g: Hydrotrope Used - Na-BBS
Hydrotropic Concentration K1 x 10-4
Moles/dm3
Table - I h: Hydrotrope Used – Na-IBBS
Hydrotropic
Concentration K1 x 10-4
Moles/dm3
The graphical Representation of effect of Hydrotrope Concentrations on Energy of activation (Ea) was
summarized below:
1261 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
Hydrotropic Concentration Moles/dm3
IR Spectra for Rabeprazole–Na.:
1262 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.




Synthesis. Jagannath J Kadam et al.
NMR Spectra for Rabeprazole–Na.:
1263 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
The reaction of 2–mercaptobenzimidazole with 2–bromomethyl–3 –methyl–4–(3–methoxy propoxy)
pyridine was performed in aqueous hydrotropic solutions followed by oxidation using aqueous H2O2 which shows the following mechanism:
The peroxide (H2O2) electrons directly attach across the C-S bond to produce the sulfinyl compound or S = O product. The phenomenon of hydrotropy was applied to this synthesis and its effect on yield and
reaction dynamic was studied. The hydrotropes used are (Na-XS) Sodium Xylene Sulfonate, (Na-CS)
Sodium Cumene Sulfonate, (Na-BBS) Sodium-Butyl Benzene Sulfonate, (Na-IBBS) Sodium-Isobutyl
Benzene Sulfonate. The percentage yield obtained for all the four hydrotropes were tabulated in tables I
(a) to I (d) respectively. It was observed that percentage yield increased from 12% to 51% at 303K and
from 17.2% to 56% at 323K for hydrotrope Na-XS. It further increased from 14.1% to 55% at 303K and
from 20% to 61% at 323K for hydrotrope Na-CS.
1264 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
For hydrotrope Na-BBS percentage yield increased from 17% to 70% at 303K and from 23% to 75.5% at
323K while for Na-IBBS percentage yield increased from 19.9% to 72% at 303K and from 25.5% to
77.7% at 323K. These were in accordance with the fact that the hydrophobicity of Na-IBBS was more
than that of Na-XS, Na-CS and Na-BBS and the reactants are soluble to a greater extent in Na-IBBS. The
Kinetics of this reaction was studied and the rate constant K1 and K2 for temperatures 303K and 323K were calculated for all four hydrotropic solutions. The values of K1 and K2 obtained are recorded in the table I(e) to I(h).
For the hydrotrope Na-XS the value of K1 and K2 increased from 0.0591 to 0.3163 at 303K and from 0.0873 to 0.3801 at 323K respectively. Similarly for the hydrotrope Na-CS the value of K1 and K2 increased from 0.0703 to 0.3697 at 303K and from 0.1033 to 0.4360 at 323K respectively. Similarly for
the hydrotrope Na-BBS the value of rate constant K1 and K2 increased from 0.0862 to 0.5574 at 303K and from 0.1210 to 0.6512 at 323K, while for the hydrotrope Na-IBBS the value of rate constant were more
than that of Na-XS, Na-CS and Na-BBS. They are increased from 0.1027 to 0.5894 at 303K and from
0.1363 to 0.6948 at 323K, with increasing hydrotrope concentration from 0.2 Mol/dm³ to 1.0 Mol/dm³.
From above data it was observed that rate constant went on increasing as the concentration of hydrotropes
increased. The activation energy for various concentrations of the hydrotropes is tabulated in table I(e) to
I(h). The activation energy dropped from the 1.5874 to 0.7476 for hydrotrope Na-XS and from 1.5660 to
0.6712 for the hydrotrope Na-CS. Similarly for the hydrotrope Na-BBS the activation energy dropped
from 1.3799 to 0.6333 and for hydrotrope Na-IBBS it dropped from the 1.1517 to 0.6696 as the
concentration of hydrotrope increased. As the hydrotrope concentration increases the activation energy
goes on decreasing, suggest that these hydrotropes also provide shifting of the catalytic equilibrium
towards the product.
CONCLUSION
It is evident from the above scheme that at lower hydrotrope concentration, the solubility of organic
solutes is less thereby yielding less product. At lower concentration of hydrotrope, the quantity of water is
substantially large thereby the reaction are not favorable also resulting in less yields. At higher
concentration of hydrotrope the quantity of water is less and the reaction solubility is more and hence the
yields are much better. It is also seen in the above experiment that for lower hydrotrope concentrations
the time required for the completion of all reaction is more than the time required for a higher hydrotrope
We are thankful for Principals for provided Laboratory facilities by Sathaye College ,
MUMBAI-400057 , Bhavan's College, Andheri (West), Mumbai-400058 and Bharati
Vidyapeeth College of Engineering, Navi Mumbai.
REFERENCES
1. C.Neuberg; Biochem.Z, 1916, 76,107.
2. C.Neuberg, J.Chem.soc., 1916,110,155.
3. D.Balasubramanian,V.Srinivas,,V.G.Gaikarand M.M.Sharma,; J.Phys.Chem., 1989,93,3865.
4. H.Freundichand G.V.Slottaman;, Biochem.Z., 1927,188,101.
5. J.Traube,I. Schoning and L. Webber; J.Ber.Deutsch.Chem.Ges., 1927,60,180,8.
1265 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Synthesis. Jagannath J Kadam et al.
6. F.V.Hahn; Kolloid.Z.,1933,62,202.
7. HERA hydrotropes September 2005
8. H.C.Hailes; Org. Process Res. Dev., 2007, 11 ,114.
9. M.J. Earle,P.B. MaCormac,K.R. Seddon ; Green Chem .1999,1, 23.
10. M.K. Khadilkar,V.G.Gaikar,A.A.Chitnavis; Tetrahedron Lett. 1995,36, 8083
11. V.G. Sadvilkar,S.D.Samant,V.G.Gaikar; J.Chem.Technol. Biotechnol .1999,69,405
12. G.Rashinkar, S.Kamble, A.Kumbhar,R.Salunkhe; Transition Met.Chem.,2010,35, 185
13. Pt.Anastas, J.C. Warner; Green chemistry: theory and practice, Oxford University Press, 2009.
*Correspondence Author: Jagannath J Kadam, Department of Chemistry, Bharati Vidyapeeth
College of Engineering, Navi Mumbai-400614(India).
1266 J. Chem. Bio. Phy. Sci. Sec.A, 2012, Vol.2, No.3, 1257-1266.
Source: http://www.jcbsc.org/admin/get_filechem.php?id=86
AJCS 10(2):161-168 (2016) ISSN:1835-2707 Influence of row spacing and plant population density on management of "white mould" in soybean in southern Brazil David de Souza Jaccoud-Filho1, Felipe Fadel Sartori1,2, Miguel Manosso-Neto1, Cláudio Maurício Vrisman1,3, Marcelo L. da Cunha Pierre1, Ayrton Berger-Neto1, Hamilton Edemundo Túllio1, Altair Justino1, Adriel Ferreira da Fonseca1, Sérgio Zanon2
2011 Graduate Research Symposium Abstract Erika Briesacher Eileen Alexander Franklin B. Lebo Jessica Van Meter James C. Redfearn Matthew C. Hudnall Master's Program Justin J. Montemarano Ashlie Louie Sochor Kurtis Eisermann Lisa Regula Meyer Madhabi Majumdar Inoka H. Widanagamage Jennifer Burrell Megan Hendrickson Michael A. Sulak















