Med-pace.com
Am J Physiol Gastrointest Liver Physiol 306: G796–G801, 2014.
First published March 13, 2014; doi:10.1152/ajpgi.00130.2013.
Prokinetic effects of mirtazapine on gastrointestinal transit
Jieyun Yin,1 Jun Song,1 Yong Lei,3 Xiaohong Xu,3 and Jiande D. Z. Chen1,2,3
1
Division of Gastroenterology, University of Texas Medical Branch, Galveston, Texas; 2
Ningbo Pace Translational Medical
Research Center, Ningbo, China; and 3
Veterans Research and Education Foundation, VA Medical Center, Oklahoma City,
Oklahoma
Submitted 26 April 2013; accepted in final form 24 February 2014
Yin J, Song J, Lei Y, Xu X, Chen JD. Prokinetic effects
tion (4). Currently, there is lack of effective medications for
of mirtazapine on gastrointestinal transit.
Am J Physiol Gastro-
treating both upper and lower GI symptoms.
intest Liver Physiol 306: G796 –G801, 2014. First published March
Antidepressants are often prescribed in patients with IBS or
13, 2014; doi:10.1152/ajpgi.00130.2013.—Mirtazapine is a norad-
FD (7, 17, 24). Their efficacy on treating IBS may be attributed
renergic and specific serotonergic antidepressant. The aim of this
to a reduction in visceral sensation and a relief of depression
study was to investigate the effects of mirtazapine on gastrointestinal
(5). The rationale for the use of antidepressants in FD was
motility in dogs, including solid gastric emptying, antral and small
considered to reduce the severity of psychological symptoms:
intestinal contractions, and small intestinal and colonic transit. Six
central analgesic actions and local pharmacological actions on
dogs were implanted with two cannulas located at the duodenum and
the upper gut (38). However, the effects of antidepressants on
the ascending colon; another six dogs were implanted with gastric
the GI motility have not been reported frequently. Mirtazapine
cannula 6 cm proximal to the pylorus. Mirtazapine 45 mg wasadministered orally 90 min before the study. We found that
1)
was reported to act on both noradrenergic and serotonergic
Mirtazapine accelerated gastric emptying during the entire 3 h in
systems, suggesting a brand-new antidepressive mechanism
normal dogs (
P ⬍ 0.04) and accelerated delayed gastric emptying
compared with other antidepressants. It increases the release of
induced by rectal distention (
P ⬍ 0.04).
2) Mirtazapine restored
norepinephrine (NE) and serotonin (5-hydroxytryptamine,
impaired gastric tone and accommodation induced by rectal distention
5-HT) by blocking presynaptic ␣2-adrenergic receptors (11,
(
P ⬍ 0.05).
3) No significant changes were noted in small intestinal
16). Recently, it was reported that mirtazapine reduced visceral
contractions or transit with mirtazapine (
P ⬎ 0.1).
4) Mirtazapine
hypersensitivity and accelerated liquid gastric emptying in rats
accelerated colonic transit at 2 and 4 h but not 6 h. The geometric
with neonatal colon sensitivity (42). Clinic case report showed
center was increased from 1.9 ⫾ 0.6 to 3.0 ⫾ 0.5 and 3.9 ⫾ 0.5 to
that mirtazapine was effective in treating severe gastroparesis
4.7 ⫾ 0.1 at 2 and 4 h respectively (
P ⫽ 0.04 vs. corresponding
unresponsive to the conventional prokinetic treatment (29); in
control). In conclusion, mirtazapine improves gastric emptying in
a patient with diabetic gastroparesis who is recalcitrant to
healthy dogs and normalizes rectal distention-induced delay in gastric
first-line treatment, mirtazapine improved gastroparetic symp-
emptying and accelerates colon but not small intestinal transit in
toms (20). We therefore hypothesized that mirtazapine might
healthy dogs. Clinical studies are warranted to assess the effects of
have a prokinetic effect on GI motility.
mirtazapine on gastrointestinal motility and sensory functions in
The aim of this study was to investigate the effect of
patients with functional gastrointestinal diseases.
mirtazapine on GI motility in a canine model under normal
antidepressant; gastrointestinal motility; gastric emptying; colonic
conditions and during rectal distention. Experiments were
transit; rectal distention
designed to assess the possible prokinetic effects of mirtazap-ine on solid gastric emptying and on small intestinal andcolonic transit.
DELAYED GASTRIC EMPTYING IS reported frequently in variousgastrointestinal (GI) motility disorders, such as gastroesopha-
MATERIALS AND METHODS
geal reflux disease, functional dyspepsia, and gastroparesis (25,28, 35). Delayed gastric emptying is associated with upper
Animal Model and Surgical Procedures
gastric symptoms such as nausea, vomiting, fullness, bloating,
Twelve healthy female hound dogs (22–26 kg) were used. Under
and early satiety (25). Prokinetics are the first-line medicine for
general anesthesia, in six of the dogs, two cannulas were implanted in
treating delayed gastric emptying. However, there is a poor
each animal: one at the duodenum, 20 cm distal to the pylorus for the
correlation between the acceleration of gastric emptying and
assessment of solid gastric emptying and small intestinal motility, and
the improvement of gastric symptoms with prokinetics (27).
the other at the ascending colon, 6 cm distal to the cecum for the
Upper GI symptoms are common in patients with lower GI
measurement of colonic motility. In the other six dogs, a gastric
motility disorders. It is reported that 30 –50% of patients with
cannula was implanted at the anterior wall of the stomach 6 cmproximal to the pylorus for assessing gastric tone and accommodation.
functional GI disorders had both functional dyspepsia (FD) and
The study was initiated after the dogs were completely recovered from
irritable bowel syndrome (IBS) (23, 37). Patients with consti-
the surgery, usually 2 wk after the operation. The study was approved
pation-dominant IBS reported significantly more overall GI
by the Animal Care and Use Committee at both the University of
symptoms compared with the patients with diarrhea-dominant
Texas Medical Branch at Galveston, Texas, and VA Medical Center,
IBS (37). One study has reported that 90% patients with
Oklahoma City, Oklahoma.
overlapping symptoms exhibited rectal intolerance to disten-
Experiment 1: assessment of complete gastric emptying of solid.
Address for reprint requests and other correspondence: J. Yin, Division
of Gastroenterology, Route 0655, Galveston, TX 77555-0655 (e-mail:
This experiment was performed in six dogs with a duodenal cannula.
Each dog was studied in one session. The purpose of this session was
0193-1857/14 Copyright 2014 the American Physiological Society
MIRTAZAPINE ON GASTROINTESTINAL MOTILITY
to measure the total gastric emptying of solids and to determine the
The appearance of phenol red in the colonic collection was verified by
percentage of gastric chyme we were able to collect for complete
spectrophotometer after the experiment.
gastric emptying. The experiment was needed because some gastric
Experiment 5: effects of mirtazapine on colon transit. Each dog was
chyme might bypass the duodenal cannula and flow distally to the
studied in two randomized sessions with an interval of 3–5 days:
small intestine. After an overnight fast, each dog was fed with 375 g
control and mirtazapine. After an overnight fast, mirtazapine 45 mg
of solid dog food (413 kcal, Pedigree, Chopped Chicken). Immedi-
was administered orally 1.5 h prior to the experiment. The experiment
ately after the ingestion of the test meal, the duodenal cannula was
was initiated immediately after the insertion of a capsule containing
opened for collecting gastric chyme emptied from the stomach. The
24 radiopaque markers (Sitzmarks, Konsyl Pharmaceuticals, Easton,
chyme was collected every 15 min for the first hour and every 30 min
MD) through the colonic cannula. Before inserting the Sitzmarks, we
for subsequent hours. The collection was continued for about 8 h until
flushed the colon gently with 20 ml saline through the colonic
the completion of gastric emptying (nothing except secretion was
cannula. The capsule was dissolved in the colon within 10 min.
collected from the cannula for a period of 30 min). The amount of
Abdominal X-ray was performed at 2, 4, and 6 h after the insertion of
total collections was defined as 100% gastric emptying.
the capsule. To avoid possible stress effects, the animals were accli-
Experiment 2: effects of mirtazapine on solid gastric emptying. The
mated to X-ray film taking several times a week before the initiation
same six dogs from
experiment 1 were used in this experiment. Each
of the colon transit test.
dog was studied in four sessions on separate days in a randomizedorder: control, mirtazapine (Remeron, Rockford, IL), rectal distention
Measurements and Analyses
(RD), and RD ⫹ mirtazapine. In the control session, no medicationwas given. Since our aim was to study the effect of mirtazapine on GI
Measurement of solid gastric emptying. The collected chyme sam-
transit, and antidepressants are often used to treat patients with
ples from the duodenal cannula were centrifuged for 20 min (3,000
functional GI diseases. Clinically, the tablet of mirtazapine is mixed
rpm). After centrifugation, each sample was placed in a ⫺20°C
with the vehicle; it is therefore more relevant for us to compare the
refrigerator for 4 h and then kept for 30 – 45 min at room temperature;
difference between the use of mirtazapine tablets and no medication at
in this way, the total sample in each tube was completely transferred.
all. The method of gastric emptying was the same as in
experiment 1
The entire precipitate of each collection was then taken out, placed in
except that the total measurement time was 3 h; the duration was
a paper plate, and dried in air for a few days until there were no
chosen on the basis of a previous study indicating that more than 80%
changes in weight. The percentage of gastric emptying for each
of the solids were emptied during the first 3 h (40). Mirtazapine (45
collection was calculated by using the following formula: % gastric
mg) was administered orally 1.5 h prior to the meal. The dose of 45
emptying for each collection ⫽ Wn/Wt⫻100%, where Wn was the
mg was chosen on the basis of the dosage used in clinic (30 mg/day)
dried weight of the collection and Wt was the total dried weight of all
(7); since the dosage of 30 mg/day was for chronic treatment and the
samples collected in the same dog in
experiment 1 (40). This normal-
present study was acute, we chose a higher dose of 45 mg. The
ized percentage of gastric emptying compensated for any possible loss
half-life of mirtazapine was reported to be 14 ⫾ 3 h with a single oral
of ingested food, such as bypass of gastric chyme to the distal small
dose of 45 mg (19). In the two sessions with RD, RD was performed
intestine and any absorption that might have occurred before the
60 –90 min after the meal. From preliminary studies, we observed that
collection from the duodenal cannula.
gastric emptying of solid was the fastest 60 –90 min after the meal and
Measurement of gastric tone and accommodation. Gastric tone was
therefore RD was performed during that period (40). RD was achieved
assessed from the measured gastric volume. At the constant operating
by the inflation of a latex balloon inserted into the rectum with 120 ml
pressure, an increase in gastric volume reflects a decrease in gastric
tone, and vice versa. Gastric accommodation was calculated by the
Experiment 3: effects of mirtazapine on gastric tone and
difference in gastric volume before and after a meal.
accommodation. The experiment was performed in six dogs with the
Measurement and analysis of small intestinal contractions. Small
gastric cannula. Each dog was studied for four sessions in a random-
intestinal contractions were measured by the same manometric sys-
ized order: control, mirtazapine (45 mg), RD, and RD ⫹ mirtazapine
tem. All pressure sensors were located in the jejunum with the most
(45 mg). Gastric tone and accommodation were assessed using an
proximal one 15 cm distal to the duodenal cannula. Intestinal con-
established method of Barostat (Distender Series IIR, G & J Electron-
tractions measured from the middle sensor, which was 25 cm distal to
ics, Willowdale, Ontario, Canada) (36, 41, 45). Gastric volume was
the duodenal cannula, showed the best quality and were chosen to be
recorded for 30 min in the fasting state and 30 min after a liquid meal
analyzed (43), from 0 to 60 min.
(237 ml, 240 kcal, Boost) in the sessions without RD, and for 60 min
Measurement of colonic transit. Colonic transit was assessed by
in the fasting state and 60 min after the liquid meal in the sessions
counting the numbers of radiopaque markers from X-ray films in
with RD, in which RD was performed during the second 30-min
different segments of the colon at different time points. The colon was
fasting period and first 30-min postprandial period.
divided into five segments, including ascending colon (
segment 1),
Experiment 4: effects of mirtazapine on small intestinal contrac-
transverse colon, splenic flexure, descending colon, and rectum (
seg-
tions and transit. This experiment was performed in the same six dogs
ment 5); the markers expelled out from the anus were considered to be
as in
experiment 1. It consisted of two sessions on 2 separate days in
in
segment 6. The geometric center (GC) of the markers was used to
a randomized order: control and mirtazapine 45 mg. After an over-
represent colon transit and calculated as follows:
night fast, each dog was fed with a 375-g solid meal to induce
postprandial small intestinal contractions. Small intestinal contrac-
n n) ⁄ 24 for
n ⫽ 1, 2, 3, 4, 5, 6
tions were then recorded for 60 min after the meal.
where P
n was the number of radiopaque markers in the
nth segment
Small intestinal transit was assessed simultaneously with gastric
and
n was the number of the segment.
emptying in
experiment 1 in two of the sessions: control and mir-tazapine. Immediately after the meal, a 30-ml solution with phenol red
(0.5 mg/ml, dissolved in saline) was injected through the duodenalcannula, then 50 ml saline was injected every 30 min until the end of
All data are presented as means ⫾ SE. One-way ANOVA was used
the experiment; these saline injections were used to offset the diver-
to investigate differences in gastric emptying and GC at different time
sion of gastric secretion and chyme from the duodenum cannula.
points among various sessions. Paired
t-test was used to evaluate
Samples through the colonic cannula were collected every 15 min
differences in antral contractions and small intestinal motility between
during the experiment and small intestinal transit was determined by
control and mirtazapine. A
P value of ⬍ 0.05 was considered
the time of first appearance of phenol red from the colonic cannula.
AJP-Gastrointest Liver Physiol • doi:10.1152/ajpgi.00130.2013 • www.ajpgi.org
MIRTAZAPINE ON GASTROINTESTINAL MOTILITY
Effects of Mirtazapine on Small Intestinal Transit and
* P < 0.04: RD vs. Mirt.+RD
# P < 0.04: Control vs. Mirt.
Mirtazapine had no effect on either small intestinal transit or
contractions. The small intestinal transit, represented by the
first appearance of phenol red in the colon cannula, was not
different between the control and mirtazapine sessions (137.8 ⫾
19.3 min vs. 150.0 ⫾ 17.4 min,
P ⫽ 0.3, Fig. 4). Neither was
there a difference in the small intestinal contractions between
the two sessions. The postprandial small intestinal contractile
index was 10.6 ⫾ 1.4 mmHg in the control session and 13.6 ⫾
3.7 mmHg with mirtazapine (
P
⫽ 0.2, Fig. 4).
Effects of Mirtazapine on Colonic Transit
Fig. 1. Effects of mirtazapine (Mirt.) on solid gastric emptying. Mirtazapinesignificantly accelerated gastric emptying in the normal condition (
P ⬍ 0.04).
Mirtazapine accelerated colonic transit at 2 and 4 h after the
In addition, mirtazapine normalized delayed gastric emptying in dogs with
insertion of the markers from the colon cannula. As shown in
rectal distention (RD) (
P ⬍ 0.04).
Fig. 5, the GC was increased from 1.9 ⫾ 0.6 to 3.0 ⫾ 0.5 at 2h and 3.9 ⫾ 0.5 to 4.7 ⫾ 0.1 at 4 h (
P ⫽ 0.04, control vs.
mirtazapine at both time points). At 6 h, the GC became similar
between the two sessions since most of the markers were in the
Effects of Mirtazapine on Solid Gastric Emptying and Antral
rectum (5.0 ⫾ 0.1 vs. 5.0 ⫾ 0.1,
P ⫽ 0.5).
Under normal condition without RD, mirtazapine acceler-
In the present study, we have found that mirtazapine accel-
ated gastric emptying during the entire 3-h experimental period
erated solid gastric emptying in dogs with or without RD.
(Fig. 1). Compared with the control session, gastric emptying
Mirtazapine also accelerated colonic transit but showed no
was accelerated with mirtazapine by 48.1% at 90 min (
P ⬍
effects on small intestinal transit or contractions.
0.01), 38.2% at 120 min (
P ⫽ 0.02), and 17.7% at 180 min
Delayed gastric empting is common in patients with GI
(
P ⫽ 0.03).
motility disorders, such as FD and gastroparesis. Treatment
RD during 60 –90 min substantially delayed gastric empty-
options for FD and gastroparesis are very limited. Although
ing (Fig. 1). Gastric emptying was reduced with RD by 46.2%
prokinetics stimulate peristalsis and improve gastric pump
at 90 min (
P ⬍ 0.01), 45.2% at 120 min (
P ⬍ 0.001), and
functions by enhancing antral contractility, electrical rhythm,
36.3% at 180 min (
P ⬍ 0.01). As shown in Fig. 1, mirtazapine
and/or antroduodenal coordination, sometimes they do not
accelerated gastric emptying during 45– 60 min without RD
result in significant symptom improvement (27, 34). This is
and normalized RD-induced delayed gastric emptying during
because patients with delayed gastric emptying often have
90 –180 min: the percentages of gastric emptying during 90 –
impaired gastric accommodation and/or visceral hypersensitiv-
180 min in the session with RD and mirtazapine were compa-
ity. The failure of certain prokinetics on improving symptoms
rable to those in the control session without RD. The acceler-
may be attributed to their detrimental effects on gastric accom-
ation before the administration of RD confirmed that mirtazap-
modation and/or ineffectiveness in improving visceral hyper-
ine was able to increase gastric emptying even under the
normal condition.
Because of the lack of effective therapies on functional GI
Consistent with the findings on gastric emptying data, mir-
motility disorders, antidepressants are commonly prescribed in
tazapine enhanced antral contractions in the fed state. Asshown in Fig. 2, the contractile index (sample-by-sampleaverage of manometric data) was increased from 8.0 ⫾ 0.7
mmHg in the control session to 12.9 ⫾ 3.0 mmHg with
mirtazapine (
P ⫽ 0.03).
mHg 14
(m 12
Effects of Mirtazapine on Gastric Tone and Accommodation
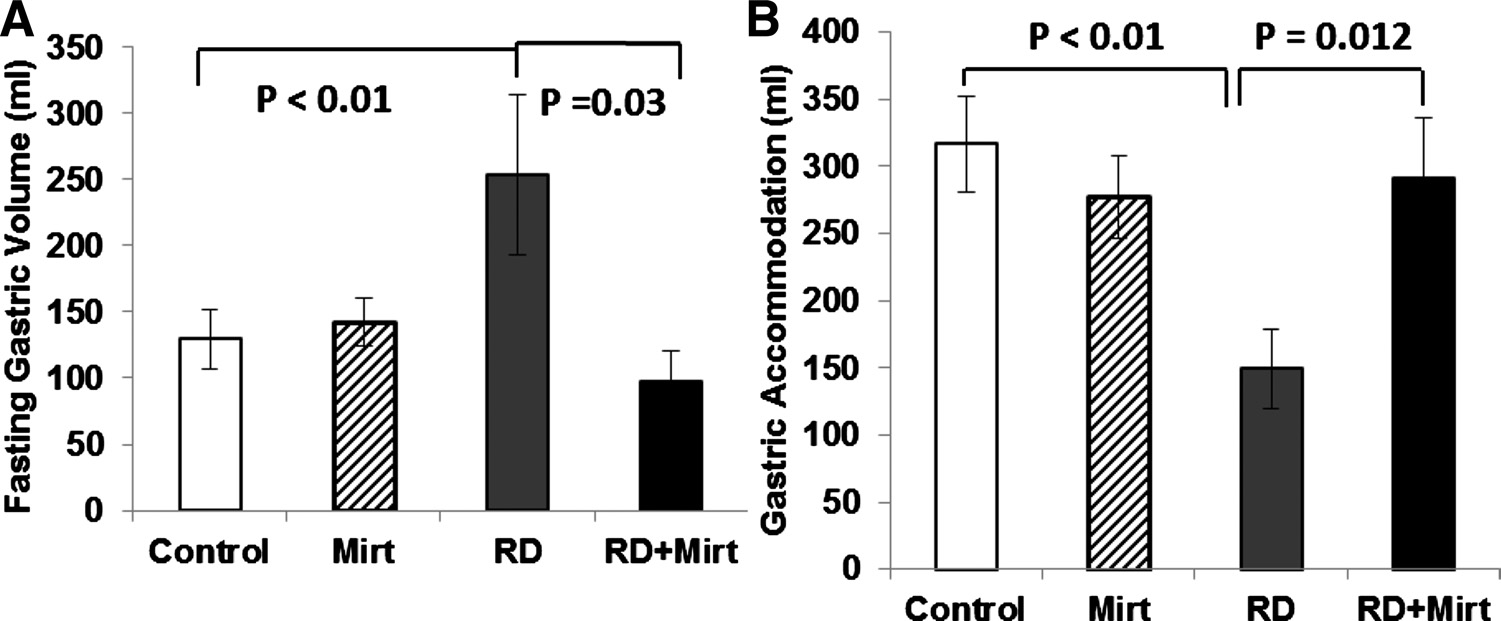
Mirtazapine had no effects on gastric tone or gastric accom-
modation in the normal physiological condition (without RD).
RD significantly reduced both gastric tone and gastric accom-
modation, whereas mirtazapine partially blocked the inhibitoryeffects of RD on gastric tone and gastric accommodation. The
Contractile Index
RD-induced increase in gastric volume in the fasting state was
253.6 ⫾ 61.1% without mirtazapine and 97.8 ⫾ 23.1% with
mirtazapine (
P ⫽ 0.03). Gastric accommodation was 149.4 ⫾
Fig. 2. Effects of mirtazapine on postprandial antral contractions in dogs under
29.8 ml with RD and 290.9 ⫾ 45.7 ml with RD plus mirtazap-
normal condition. Mirtazapine significantly enhanced antral contractions com-
ine (
P ⫽ 0.012) (Fig. 3).
pared with the control session (
P ⫽ 0.03).
AJP-Gastrointest Liver Physiol • doi:10.1152/ajpgi.00130.2013 • www.ajpgi.org
MIRTAZAPINE ON GASTROINTESTINAL MOTILITY
Fig. 3. Effects of mirtazapine on gastricsensitivity to rectal distention. Rectal disten-tion significantly reduced gastric tone andaccommodation. Mirtazapine restored rectaldistention induced reduced gastric tone (A)and accommodation (B) (P ⬍ 0.05).
clinic practices, such as tricyclic antidepressants and selective
rectal distention was not suitable for such a study; rectal
serotonin reuptake inhibitor. It is conceivable to treat func-
distention was performed to induce delayed gastric emptying;
tional GI disorders with antidepressants because these drugs
however, it was only convenient for assessing acute effects but
are used to treat depression and reduce chronic pain. However,
not adequate for a chronic study.
the evidence is conflicting and there is lack of large controlled
Overlapping syndrome is common in clinic practice; upper
trials. Mirtazapine is a noradrenergic and specific serotonergic
GI dyspepsia symptoms are often present in patients with lower
antidepressant, a newer class of antidepressant that is charac-
GI tract disorders, such as constipation. The mechanisms have
terized by a potent antagonism of presynaptic ␣2 adrenergic
not been elucidated and there is lack of satisfactory treatment
receptors on both NE and serotonin neurons (11). While
options for overlapping syndromes. Fecal or gas distention
treating depression, mirtazapine was noticed to be beneficial
might be one of the major contributing factors in producing
for bowel movement in IBS patients (39). In patients with
upper GI symptoms in patients with lower GI dysmotility.
severe gastroparesis unresponsive to the conventional proki-
Rectal balloon distention was reported to induce upper GI
netic treatment, refractory nausea and vomiting that persisted
symptoms, inhibit gastric tone and accommodation, and delay
for over 7 mo were improved dramatically within a few days of
gastric emptying (2, 31, 44), and it is considered one of the
once-daily mirtazapine dosing (29). In a previous study, we
constipation models commonly used in the study of upper GI
observed improvement of gastric emptying with mirtazapine in
motor activities. In the present study, in addition to testing the
colonic sensitized "IBS-like" rats (42). In the present canine
effect of mirtazapine on solid gastric emptying in normal dogs,
study, we found that mirtazapine improved gastric emptying,gastric accommodation, and colon transit; this was different
RD was used to mimic fecal or gas stasis in the colorectal
from what was reported in a clinical study in which mirtazap-
region. We found that RD at 60 –90 min substantially delayed
ine showed no effects on gastric emptying in healthy volun-
gastric emptying from 90 min to 180 min, whereas mirtazapine
teers (7). The difference might be attributed to the differences
normalized RD-induced delayed in gastric emptying. More-
in species (dogs vs. humans), dosage (45 mg vs. 30 mg), and/or
over, mirtazapine restored RD-induced reduced gastric tone
duration of the study (acute vs. chronic).
and accommodation. The changes of gastric sensitivity and
There were at least two limitations in the present study: lack
gastric emptying may explain the improvements of symptoms
of dose-response study and absence of chronic repetitive study.
in patients with gastroparesis reported in the clinic practice.
A dose-responsive study was, however, performed in our
The normalization of RD-induced delay in gastric emptying
previous study investigating the ameliorating effect of mir-
and the acceleration of colon transit suggest that mirtazapine
tazapine on neonatal rectal distention-induced gastric hyper-
may have a therapeutic potential for treating patients with
sensitivity. The absence of any data on repetitive chronic study
symptoms attributed to both upper and lower GI motility
was attributed to the fact that the present canine model with
Fig. 4. Effects of mirtazapine on small intestinal
motility. The small intestinal transit was not dif-
ferent between the control and mirtazapine ses-
sions (P ⫽ 0.3) (A); neither was there a difference
in small intestinal contractions between the con-
trol and mirtazapine sessions (P ⫽ 0.2) (B).
AJP-Gastrointest Liver Physiol • doi:10.1152/ajpgi.00130.2013 • www.ajpgi.org
MIRTAZAPINE ON GASTROINTESTINAL MOTILITY
1A receptor agonist has been shown to in-
crease esophageal motility and colonic migrating motor com-
plexes (14, 15). Accordingly, we speculate that the prokinetic
effects of mirtazapine observed in this study are attributed to
increased release of 5-HT (mainly via the actions of 5-HT2Cand 5-HT1A) and the blockage of ␣2 adrenergic receptors.
The ameliorating effects of mirtazapine on rectal distention-
induced impairment in gastric motility (tone, accommodation,
contractions, and emptying) are believed to be attributed to
both its prokinetic effects and its inhibitory effects on rectaldistention-induced hypersensitivity. In the present study, mir-
tazapine blocked the rectal distention-induced inhibitory ef-
fects on gastric motility. In a previous study, mirtazapine was
Fig. 5. Effects of mirtazapine on colonic transit. Mirtazapine accelerated
found to reduce rectal distention-induced gastric hypersensi-
colonic transit 2 and 4 h after the insertion of the radiopaque markers (P ⫽
tivity (42).
Interestingly, although mirtazapine was found to have the
prokinetic effect on both gastric emptying and colonic transit,it showed no effects on small intestinal contractions or transit
The effect of mirtazapine on colonic transit had not been
in the normal dogs. In this study, intestinal transit was per-
studied before. It is known that many IBS patients display
formed simultaneously with gastric emptying, and gastric
visceral hypersensitivity during colorectal distention (26, 30,
chyme emptied from the stomach was diverted out through the
33). In an earlier study we reported improvement on visceral
duodenal cannula. One might argue that this was not physio-
hypersensitivity with the administration of mirtazapine in co-
logical because the intestine was not exposed to nutrients. Our
lonic sensitized rats (42); however, its effect on the colonic
rationale for such a special design was based on following
transit was not investigated. In the present study, we found that
considerations. Under normal physiological condition, intesti-
mirtazapine accelerated colonic transit in normal dogs. The
nal transit is influenced by gastric emptying, i.e., how fast
combined effects of mirtazapine on visceral hypersensitivity
nutrients are delivered from the stomach to the small intestine.
and colon transit may play a role in treating symptoms of IBS,
While designing the study, we anticipated that mirtazapine
especially in constipation-dominant IBS. It has to be men-
would alter gastric emptying and, therefore, the effect of
tioned that in the present study acceleration of colonic transit
mirtazapine on gastric emptying would be a confounding factor
by mirtazapine was observed in normal dogs rather than a
in studying the effect of mirtazapine on intestinal transit. To
canine model of constipation.
eliminate this confounding factor, we decided to divert all
Mirtazapine is an adrenoceptor antagonist with preferential
gastric chyme and perform the test simultaneously with gastric
affinity for presynaptic ␣2 adrenoceptors and low affinity for
emptying by ingesting saline mixed with phenol red to the
1 adrenoceptors (10). Blockade of ␣2 receptors by mirtazap-
small intestine via the duodenal cannula. Although gastric
ine enhances both noradrenergic and serotonergic transmission
chyme was diverted, the small intestinal motility pattern ob-
(11). Although the increase in NE potentially inhibits GI
served during the test was similar to the postprandial pattern.
motility, mirtazapine may enhance GI motility for the follow-
The mechanisms underlying why mirtazapine had no effect on
ing reasons: 1) Blockage of ␣2 receptors is expected to increaseGI motility. In one previous study, it was shown that pretreat-
small bowel transit or contractions was not clear. It could be
ment of mirtazapine antagonized the effect of clonidine (21),
due to the differences in the coupling of ␣2 receptors to
whereas clonidine is known to delay gastric emptying and
adrenergic and serotonergic neurons in the small bowel. Fur-
induce constipation. 2) Enhancement of serotonin transmission
ther mechanistic studies are needed to investigate why there
due to blockade of ␣
were differences in the effects of mirtazapine among different
2 receptors by mirtazapine increases 5-HT
release. Mirtazapine has a high affinity for serotonergic 5-HT
organs of the gut.
receptor subtypes, especially 5-HT
In conclusion, mirtazapine improves gastric emptying in
2A and 5-HT2C (9). 5-HT2C
was known to regulate GI motor activity and it was reported to
healthy dogs, normalizes rectal distention-induced delay in
stimulate phasic contractions and phase III activity in canine
gastric emptying and accelerates colon transit in healthy dogs.
jejunum (19, 22). Antidepressants with a strong affinity for
Clinical studies are warranted to assess the effects of mirtazap-
ine on GI motility and sensory functions in patients with
2C receptor are reported to be associated with weight gain
(13). Studies have shown that more than 10% of patients
functional GI diseases.
experience substantial weight gain during mirtazapine treat-ment and more than 10% patients have been noticed to have an
increased of appetite (1). 3) Facilitation of NE transmission
No conflicts of interest, financial or otherwise, are declared by the author(s).
due to blockade of ␣2 receptors by mirtazapine may furtherenhance serotonin transmission due to enhancement of 5-HT1A
function (3, 32) mediated via ␣2 heteroreceptors (12); blockageof ␣
J.Y. and J.D.C. conception and design of research; J.Y., J.S., Y.L., and X.X.
2-adrenergic heteroreceptors located on the 5-HT terminals
performed experiments; J.Y., J.S., Y.L., and X.X. analyzed data; J.Y. and
removes the tonic inhibition caused by endogenous NE. Fur-
J.D.C. interpreted results of experiments; J.Y. prepared figures; J.Y. drafted
thermore, mirtazapine has been reported to block 5-HT3 recep-
manuscript; J.Y. and J.D.C. edited and revised manuscript; J.Y. and J.D.C.
tors with a potency similar to its effects on ␣2 receptors (8, 18).
approved final version of manuscript.
AJP-Gastrointest Liver Physiol • doi:10.1152/ajpgi.00130.2013 • www.ajpgi.org
MIRTAZAPINE ON GASTROINTESTINAL MOTILITY
22. Graf S, Sarna SK. 5-HT-induced jejunal motor activity: enteric locus of
action and receptor subtypes. Am J Physiol Gastrointest Liver Physiol 270:
1. Anttila SA, Leinonen EV. A review of the pharmacological and clinical
G992–G1000, 1996.
profile of mirtazapine. CNS Drug Rev 7: 249 –264, 2001.
23. Halder SL, Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR,
2. Azpiroz F, Malagelada JR. Perception and reflex relaxation of the
Melton LJ 3rd. Impact of functional gastrointestinal disorders on health-
stomach in response to gut distention. Gastroenterology 98: 1193–1198,
related quality of life: a population-based case-control study. Aliment
Pharmacol Ther 19: 233–242, 2004.
3. Berendsen HH, Broekkamp CL, Van Delft AML. Downregulation of
24. Halpert A, Dalton CB, Diamant NE, Toner BB, Hu Y, Morris CB,
5-HT1A receptors after chronic treatment with Remeron [letter]. Eur
Bangdiwala SI, Whitehead WE, Drossman DA. Clinical response to
Neuropsychopharmacol 5: 306, 1995.
tricyclic antidepressants in functional bowel disorders is not related to
4. Bouin M, Lupien F, Riberdy M, Boivin M, Plourde V, Poitras P.
dosage. Am J Gastroenterol 100: 664 –671, 2005.
Intolerance to visceral distension in functional dyspepsia or irritable bowel
25. Hasler WL. Gastroparesis: symptoms, evaluation, and treatment. Gastro-
syndrome: an organ specific defect or a pan intestinal dysregulation?
enterol Clin North Am 36: 619 –647, ix, 2007.
Neurogastroenterol Motil 16: 311–314, 2004.
26. Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable
5. Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable
bowel syndrome. J Gastroenterol Hepatol 26, Suppl 3: 119 –121, 2011.
bowel syndrome: end points and regulatory hurdles. Gastroenterology
27. Karamanolis G, Caenepeel P, Arts J, Tack J. Determinants of symptom
135: 1877–1891, 2008.
pattern in idiopathic severely delayed gastric emptying: gastric emptying
6. Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture
rate or proximal stomach dysfunction? Gut 56: 29 –36, 2007.
improves impaired gastric motility and slow waves induced by rectal
28. Keshavarzian A, Bushnell DL, Sontag S, Yegelwel EJ, Smid K. Gastric
distension in dogs. Am J Physiol Gastrointest Liver Physiol 295: G614 –
emptying in patients with severe reflux esophagitis. Am J Gastroenterol
86: 738 –742, 1991.
7. Choung RS, Cremonini F, Thapa P, Zinsmeister AR, Talley NJ. The
29. Kim SW, Shin IS, Kim JM, Kang HC, Mun JU, Yang SJ, Yoon JS.
effect of short-term, low-dose tricyclic and tetracyclic antidepressant
Mirtazapine for severe gastroparesis unresponsive to conventional proki-
treatment on satiation, postnutrient load gastrointestinal symptoms and
netic treatment. Psychosomatics 47: 440 –442, 2006.
gastric emptying: a double-blind, randomized, placebo-controlled trial.
30. Klooker TK, Leliefeld KE, Van Den Wijngaard RM, Boeckxstaens
Neurogastroenterol Motil 20: 220 –227, 2008.
GE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does
8. Costall B. The breadth of action of the 5-HT
not affect visceral sensitivity to rectal distension in healthy volunteers and
3 receptor antagonists. Int
Clin Psychopharmacol 8, Suppl 2: 3–9, 1993.
IBS patients. Neurogastroenterol Motil 23: 30 –35.e2
9. de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry
31. Lei Y, Xing J, Chen JD. Effects and mechanisms of implantable gastric
57, Suppl 4: 19 –25, 1996.
stimulation on gastric distention in conscious dogs. Obes Surg 15: 528 –
10. de Boer TH, Maura G, Raiteri M, de Vos CJ, Wieringa J, Pinder RM.
Neurochemical and autonomic pharmacological profiles of the 6-aza-
32. Norman TR, McGrath CJ, Burrows GD. The neurochemical and
analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology
behavioural effects of the isomers of mirtazapine in normal rats [letter].
27: 399 –408, 1988.
Eur Neuropsychopharmacol 5: 316, 1995.
11. de Boer TH, Nefkens F, van Helvoirt A, van Delft AM. Differences in
33. Nozu T, Okumura T. Visceral sensation and irritable bowel syndrome;
modulation of noradrenergic and serotonergic transmission by the alpha-2
with special reference to comparison with functional abdominal painsyndrome. J Gastroenterol Hepatol 26, Suppl 3: 122–127, 2011.
adrenoceptor antagonists, mirtazapine, mianserin and idazoxan. J Phar-
34. Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol
macol Exp Ther 277: 852–860, 1996.
Ther 27: 724 –740, 2008.
12. De Boer TH, Nefkens F, Van Helvoirt A. The ␣2-adrenoceptor antago-
35. Shay SS, Eggli D, McDonald C, Johnson LF. Gastric emptying of solid
nist Org 3770 enhances serotonin transmission in vivo. Eur J Pharmacol
food in patients with gastroesophageal reflux. Gastroenterology 92: 459 –
253: R5–R6, 1994.
13. De Luca V, Mueller DJ, de Bartolomeis A, Kennedy JL. Association of
36. Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of
the HTR2C gene and antipsychotic induced weight gain: a meta-analysis.
impaired gastric accommodation to a meal in functional dyspepsia. Gas-
Int J Neuropsychopharmacol 10: 697–704, 2007.
troenterology 115: 1346 –1352, 1998.
14. Di Stefano M, Papathanasopoulos A, Blondeau K, Vos R, Boecxstaens
37. Talley NJ, Dennis EH, Schettler-Duncan VA, Lacy BE, Olden KW,
V, Farre R, Rommel N, Tack J. Effect of buspirone, a 5-HT1A receptor
Crowell MD. Overlapping upper and lower gastrointestinal symptoms in
agonist, on esophageal motility in healthy volunteers. Dis Esophagus 25:
irritable bowel syndrome patients with constipation or diarrhea. Am J
Gastroenterol 98: 2454 –2459, 2003.
15. Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3,
38. Talley NJ, Herrick L, Locke GR. Antidepressants in functional dyspep-
and 5-HT7 receptor subtypes in the initiation, generation, and propagation
sia. Expert Rev Gastroenterol Hepatol 4: 5–8, 2010.
of the murine colonic migrating motor complex. Am J Physiol Gastrointest
39. Thomas SG. Irritable bowel syndrome and mirtazapine. Am J Psychiatry
Liver Physiol 299: G144 –G157, 2010.
157: 1341–1342, 2000.
16. Fawcett J, Barkin RL. Review of the results from clinical studies on the
40. Yin J, Chen JD. Electroacupuncture improves rectal distension-induced
efficacy, safety and tolerability of mirtazapine for the treatment of patients
delay in solid gastric emptying in dogs. Am J Physiol Regul Integr Comp
with major depression. J Affect Disord 51: 267–285, 1998.
Physiol 301: R465–R472, 2011.
17. Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Effi-
41. Yin J, Ouyang H, Chen JD. Potential of intestinal electrical stimulation
cacy of antidepressants and psychological therapies in irritable bowel
for obesity: a preliminary canine study. Obesity (Silver Spring) 15:
syndrome: systematic review and meta-analysis. Gut 58: 367–378, 2009.
1133–1138, 2007.
18. Frazer A. Antidepressant drugs. Depression 2: 1–19, 1994.
42. Yin J, Wang W, Winston JH, Zhang R, Chen JD. Ameliorating effects
19. Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H,
of mirtazapine on visceral hypersensitivity in rats with neonatal colon
Kojima S, Fujimiya M, Inui A. Selective serotonin reuptake inhibitors
sensitivity. Neurogastroenterol Motil 22: 1022–1028.e267, 2010.
modify physiological gastrointestinal motor activities via 5-HT2c receptor
43. Yin J, Zhang J, Chen JD. Inhibitory effects of intestinal electrical
and acyl ghrelin. Biol Psychiatry 65: 748 –759, 2009.
stimulation on food intake, weight loss and gastric emptying in rats. Am J
20. Gooden JY, Takahashi PY. Mirtazapine treatment of diabetic gastropa-
Physiol Regul Integr Comp Physiol 293: R78 –R82, 2007.
resis as a novel method to reduce tube-feed residual: a case report. J Med
44. Youle MS, Read NW. Effect of painless rectal distension on gastrointes-
Case Rep 7: 38, 2013.
tinal transit of solid meal. Dig Dis Sci 29: 902–906, 1984.
21. Gower AJ, Broekkamp CL, Rijk HW, Van Delft AM. Pharmacological
45. Zhao X, Yin J, Chen J, Song G, Wang L, Zhu H, Brining D, Chen JD.
evaluation of in vivo tests for alpha 2-adrenoceptor blockade in the central
Inhibitory effects and mechanisms of intestinal electrical stimulation on
nervous system and the effects of the enantiomers of mianserin and its
gastric tone, antral contractions, pyloric tone, and gastric emptying in
aza-analog ORG 3770. Arch Int Pharmacodyn Ther 291: 185–201, 1988.
dogs. Am J Physiol Regul Integr Comp Physiol 296: R36 –R42, 2009.
AJP-Gastrointest Liver Physiol • doi:10.1152/ajpgi.00130.2013 • www.ajpgi.org
Source: http://www.med-pace.com/down/?240.html
Low-level laser therapy for tinnitus (Protocol) Peng Z, Chen XQ, Gong SS, Chen CF This is a reprint of a Cochrane protocol, prepared and maintained by The Cochrane Collaboration and published in The CochraneLibrary 2012, Issue 4 Low-level laser therapy for tinnitus (Protocol)Copyright © 2012 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
INF RMATION MINDS MAKE MOLECULES – MOLECULES MAKE SENSE Årgang 6, nr. 4 April 2014 http://inf.ku.dk FRA NEUROBIOLOGISK KAFFE TIL NY INDSIGT Af Albert Gjedde dimittend fra den neurobiologiske kaf- emne for neurovidenskaben i Køben- feklub, Martin Lauritzen, og hans med- havn var enheden genstand for ud- Den neurobiologiske kaffeklub var kæ-








