Pnas201214605 1.5

Pain relief produces negative reinforcement throughactivation of mesolimbic reward–valuation circuitryEdita Navratilovaa,1, Jennifer Y. Xiea,1, Alec Okuna, Chaoling Qua, Nathan Eydea, Shuang Cia, Michael H. Ossipova,Tamara Kingb, Howard L. Fieldsc, and Frank Porrecaa,2
aDepartment of Pharmacology, Arizona Health Sciences Center, University of Arizona, Tucson, AZ 85724; bDepartment of Physiology, University of NewEngland, Biddeford, ME 04005; and cErnest Gallo Clinic and Research Center, University of California at San Francisco, Emeryville, CA 94608
Edited by Leslie Lars Iversen, University of Oxford, Oxford, United Kingdom, and approved November 2, 2012 (received for review August 22, 2012)
Relief of pain is rewarding. Using a model of experimental post-
(avoidance of touching the floor with the injured area) and
surgical pain we show that blockade of afferent input from the
thermal hypersensitivity (decreased response latencies to a nox-
injury with local anesthetic elicits conditioned place preference,
ious thermal stimulus). As demonstrated previously (22), evoked
activates ventral tegmental dopaminergic cells, and increases dopa-
pain hypersensitivity was prominent at 24 h and still present,
mine release in the nucleus accumbens. Importantly, place prefer-
although diminished, at 96 h postincision. Peripheral nerve block
ence is associated with increased activity in midbrain dopaminergic
(PNB) with popliteal fossa (PF) lidocaine injection given 24 h
neurons and blocked by dopamine antagonists injected into the
postincision resulted in strong preference for the chamber paired
nucleus accumbens. The data directly support the hypothesis that
with PNB, demonstrating negative reinforcement. In contrast, in
relief of pain produces negative reinforcement through activation of
sham-operated animals or at 96 h postincision, when evoked
the mesolimbic reward–valuation circuitry.
hypersensitivity is still present, pairing PNB with the context didnot produce CPP (Fig. 1 A and B). PNB at the site contralateral
motivated behavior incision in vivo microdialysis
to the injured hind paw did not result in CPP con-
immunohistochemistry ventral tegmental area
firming that lidocaine at the dose used for PF injection does not
produce systemic effects on pain relief, as demonstrated pre-
Reinforcement of behaviors that maximize benefit (positive re- viously (23). These data suggest that relief of postsurgical, on-
inforcement) and reduce loss or injury (negative reinforcement)
going (i.e., spontaneous) pain is rewarding.
is crucial for survival. Whereas positive reinforcement can be
To determine whether the mesolimbic reward circuit is neces-
produced by activation of mesolimbic dopaminergic pathways,
sary for PNB-induced CPP, we investigated whether CPP would
the neural circuits that underlie negative reinforcement are not
be prevented by inactivation of dopaminergic neurons in the
well understood. Ongoing pain can be "unmasked" in animals
VTA. Microinjection of lidocaine (4%, wt/vol; 0.5 μL per side) to
using conditioned place preference (CPP). Thus, in the presence of
block neuronal activity, including inhibition of fibers of passage, in
ongoing pain, pairing manipulations that are not rewarding in the
the VTA 10 min before PNB prevented PNB-induced CPP (Fig.
absence of pain, such as peripheral nerve block (PNB) or in-
1C). Moreover, VTA baclofen, a GABAB receptor agonist (25 ng/
trathecal administration of ω-conotoxin or clonidine, with a pre-
0.2 μL per side), known to inhibit firing of dopaminergic neurons
viously neutral context elicits CPP (1–3). CPP resulting from pain
and reduce NAc dopamine release (24–26), also abolished PNB-
relief is a measure of negative reinforcement.
induced CPP. In the absence of PNB, pairing VTA baclofen
Human functional imaging studies have shown that offset of an
treatment with a chamber had no effect on place preference (
acute noxious stimulus (4, 5) or placebo analgesia (6) activates
). In contrast, although endogenous opioids in the VTA un-
brain regions that overlap extensively with those implicated in
derlie the positive reinforcing effects of addictive drugs (27),
appetitive rewards, in particular the ventral tegmental area (VTA),
pretreatment of the VTA with a nonselective opioid receptor
and its dopaminergic projections to the nucleus accumbens (NAc)
antagonist, naloxone (3 μg/0.5 μL per side), did not prevent PNB-
(5, 6). Manipulations that disrupt mesolimbic dopamine trans-
induced CPP (Fig. 1C).
mission attenuate food or drug reward-induced CPP (7, 8). Elec-
Activation of VTA dopaminergic neurons in injured rats fol-
trophysiological recordings from dopaminergic neurons in the
lowing PNB was investigated using immunohistochemistry and
VTA demonstrate phasic neuronal activation by primary food or
expression of c-FOS, a marker of neuronal activity (28). In cor-
liquid rewards, by rewarding drugs, and reward-predicting cues (9).
onal brain sections the number of FOS-positive cells was counted
Similarly, immunohistochemical studies show increased expression
along the anteroposterial axes of the VTA identified by staining
of the immediate early gene cFOS in the VTA in response to re-
of catecholaminergic (dopaminergic) neurons with tyrosine hy-
warding drugs, providing further support for an enhanced neuronal
droxylase (TH) (Fig. 2 and ). PNB in injured rats increased
activity (10–13). The NAc can be anatomically and functionally
the number of FOS-expressing cells in posterior regions of the
divided into core and shell regions that respectively receive pro-
VTA at −5.8 to −6.3 mm from bregma. Incision injury itself, or
jections from the lateral and medial VTA (14). In vivo micro-
PNB in sham rats, did not change FOS expression (sham/saline,
dialysis measurements or fast-scan voltammetry demonstrate that
37 ± 8; sham/lidocaine, 41 ± 10; incision/saline, 39 ± 6; incision/
appetitive rewards promote an efflux of dopamine in the NAc (15,
lidocaine, 62 ± 8; n = 4–5 rats). Confocal images acquired within
16). It has been suggested that NAc neurons signal reward valueand participate in behavioral decision making (17–21).
We hypothesized that relief of ongoing pain would activate the
Author contributions: T.K., H.L.F., and F.P. designed research; E.N., J.Y.X., A.O., C.Q., N.E.,
mesolimbic dopamine pathway and that such activation is nec-
and S.C. performed research; E.N., J.Y.X., M.H.O., and T.K. analyzed data; and E.N., J.Y.X.,
essary for negative reinforcement. We tested this hypothesis di-
H.L.F., and F.P. wrote the paper.
rectly in rats with incisional injury-induced pain (22) subsequently
The authors declare no conflict of interest.
relieved by peripheral nerve block.
This article is a PNAS Direct Submission.
1E.N. and J.Y.X. contributed equally to this work.
2To whom correspondence should be addressed. E-mail:
Incision of the skin and underlying hind-paw tissue induced
This article contains supporting information online at
time-dependent, observable pain behaviors, including guarding
PNAS Early Edition 1 of 5

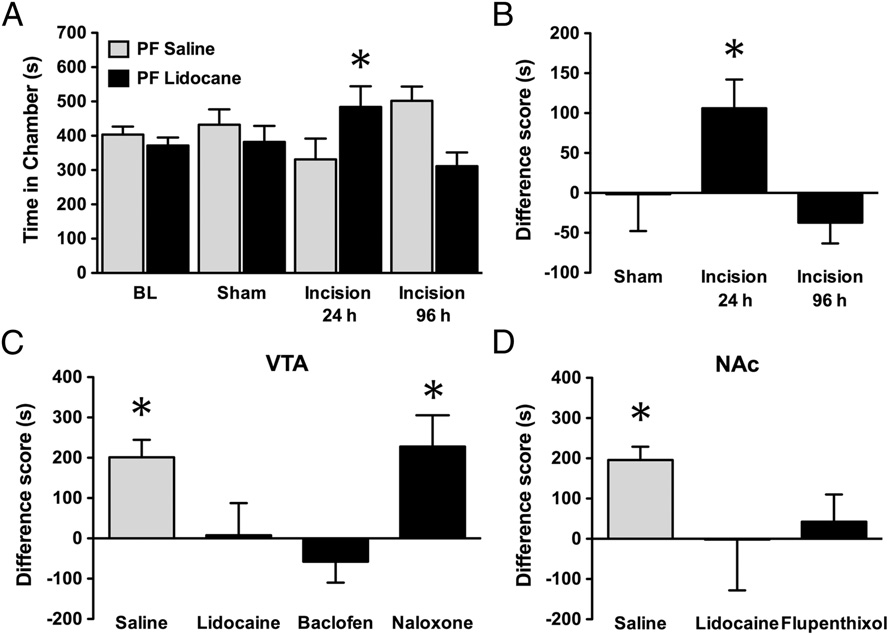
PNB in rats with incisional pain produced VTA- and NAc-dependent CPP. PNB in injured rats 24 h, but not 96 h, following incision of the hind paw
induced CPP as demonstrated by (A) increased time spent in chambers paired with popliteal fossa (PF) lidocaine and (B) difference scores. (C) Pretreatmentwith lidocaine or baclofen, but not naloxone, in the VTA 10 min before PNB abolished CPP. (D) Pretreatment with NAc lidocaine or flupenthixol blocked CPP.
Mean ± SEM, n = 9–13, *P < 0.05, Student's paired t test.
the posteromedial VTA at bregma −5.8 mm (rectangle in Fig.
difficult to evaluate in laboratory animals. In agreement with our
2A) demonstrate that increased FOS immunoreactivity occurred
previous findings in other models of experimental pain (1, 3, 30,
preferentially in TH-positive (dopaminergic) neurons (sham/sa-
31), our current behavioral data demonstrate that the removal of
line, 20 ± 5; sham/lidocaine, 17 ± 7; incision/saline, 18 ± 2; in-
the aversive state resulting from ongoing postsurgical nociceptive
cision/lidocaine, 32 ± 8; n = 4–5 rats).
input by PNB elicits CPP. Importantly, no CPP was observed
In vivo microdialysis in awake and freely moving rats was
following PNB in sham-treated rats or 96 h after the incision
performed to investigate potential efflux of dopamine in the NAc
surgery, when injury-induced ongoing pain was likely to be greatly
shell of incised rats following PNB. Basal NAc dopamine levels
diminished. Thus, as we have previously shown, ongoing (i.e.,
did not differ between incised and sham-treated animals [4.68 ±
spontaneous) pain can be unmasked by demonstrating that its
0.45 pg/30 μL (n = 15) and 4.71 ± 0.45 pg/30 μL (n = 13), re-
removal is rewarding using CPP (1, 3, 30, 31).
spectively]. An increase from baseline was detected in injured rats
Findings that relief of ongoing incisional, neuropathic, osteo-
during the 120-min time period following PNB, with peak levels
arthritic, and inflammatory pain (1, 3, 30) produces CPP confirms
in the 30- to 60-min fraction elevated by 63 ± 24% (Fig. 3A). The
that pain relief elicits reward. However, direct neurochemical
area under the curve of percent change from baseline was sig-
evidence for the activation of the brain reward circuitry during
nificantly higher only in incised rats given PNB 24 h, but not 96 h,
pain relief has not previously been established directly. Here, we
postincision (Fig. 3B). Administration of cocaine (20 mg/kg, i.p.)
show that (i) peripheral nerve block at 24 h, but not 96 h, pro-
after the testing induced a robust increase (about 500%) in ex-
duces CPP in animals with hind-paw incision consistent with
tracellular dopamine in both incised and sham animals and served
ongoing surgery-induced pain; (ii) activation of dopaminergic
as a positive control.
projections from the VTA to NAc is necessary for PNB-induced
In line with dopamine efflux in the NAc following pain relief,
CPP; (iii) dopaminergic transmission in the NAc is required for
we further investigated whether inhibiting the dopaminergic ac-
PNB-induced CPP; and (iv) in contrast, PNB-induced CPP does
tivity of the NAc would prevent PNB-induced CPP (Fig. 1D).
not require endogenous opioids in the VTA. These findings are
Microinjection of lidocaine (4%, wt/vol; 0.5 μL per side) into the
consistent with functional magnetic resonance imaging studies in
NAc shell 10 min before PNB in injured rats abolished CPP.
humans showing increased activation of the NAc at the offset of
More specifically, blockade of dopamine signaling by a non-
an acute noxious stimulus (pain relief) (5).
selective dopamine receptor antagonist, flupenthixol (3 μg/0.5 μL
Behavioral, electrophysiological (9), and in vivo microdialysis
per side) (29), also prevented CPP. This directly demonstrates
(15, 16) studies have consistently demonstrated activation of
that dopaminergic transmission in the nucleus accumbens is re-
mesolimbic dopaminergic neurons by natural rewards, rewarding
quired for the negative reinforcement (manifested as CPP) that
drugs, and reward-predicting cues (11, 32, 33). Distinct patterns
results from pain relief. In the absence of PNB, pairing NAc
of neuronal activity are consistent with the role of the VTA in
flupenthixol treatment with a chamber had no effect on place
positive reinforcement and learned appetitive behavior (34). Our
preference (These results implicate mesolimbic dopa-
data showing activation of VTA dopaminergic neurons and do-
minergic signaling in pain-relief–induced CPP.
pamine efflux in the NAc indicate that a population of midbraindopamine neurons is activated following termination of an aver-
sive state and may underlie negative reinforcement. Importantly,
Pain is a subjective and multidimensional experience with sen-
because PNB-induced CPP is prevented by blockade of meso-
sory, affective, and cognitive components. The subjective un-
limbic dopamine neurotransmission, activation of VTA neurons
pleasantness of pain is essential to the human experience but
appears to be necessary for reinforced learning.
Navratilova et al.

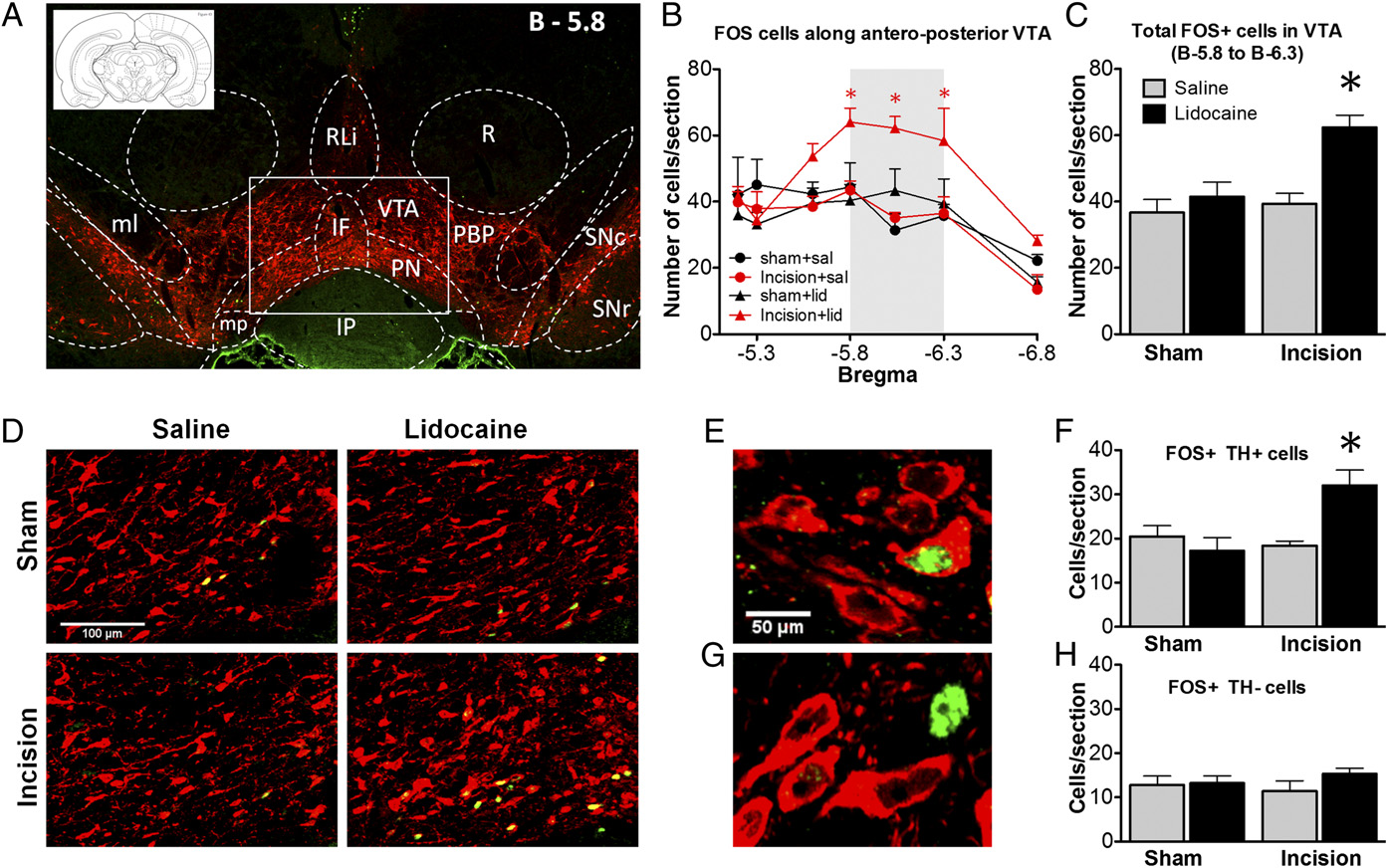
PNB increased the number of FOS-positive dopaminergic neurons in the VTA. (A) A coronal brain section at bregma −5.8 mm outlining the VTA
identified by tyrosine hydroxylase labeling (red). (B) The average number of FOS+ cells (green) per section was calculated between bregma −5.2 and −6.8 mm.
(C) The number of FOS+ cells increased significantly at bregma −5.8 to −6.3 mm only in incised rats following PNB. (D) Confocal images within the medial VTA(rectangle in Fig. 2A). The number of dopaminergic FOS+/TH+ cells (E) increased in incised rats after PNB (F); the number of nondopaminergic FOS+/TH- cells(G) did not change (H). Mean ± SEM, n = 4–5 rats/group, *P < 0.05; two-way ANOVA with Bonferroni multiple comparisons test (B), one-way ANOVA withTukey's multiple comparisons test (C and F).
The midbrain VTA is an anatomically and functionally heteroge-
these neurons, dopamine levels were increased in the medial shell of
neous region composed of several nuclei including the parabrachial
the NAc. Thus, pain relief reward appears to share many similarities
pigmented area (PBP), paranigral nucleus (PN), rostral linear nu-
with appetitive rewards. However, as indicated by our findings with
cleus raphe (RLi), interfascicular nucleus (IF), and caudal linear
VTA naloxone, activation of a reward circuit by pain relief does not
nucleus raphe (CLi) (Fig. 2A). The activation of posteromedial VTA
appear to involve an endogenous opioid system in the VTA, sug-
dopaminergic neurons, which primarily target the medial accumbens
gesting that there may be important differences as well (27, 35, 36).
shell, appears to promote positive affective states (14). Accordingly,
Acute noxious stimuli have been reported to excite some and
the present study found enhanced cFOS expression in the poster-
inhibit other midbrain dopamine neurons (37), suggesting multi-
omedial division of the VTA. In agreement with cellular targets of
ple populations of neurons support different aspects of aversive
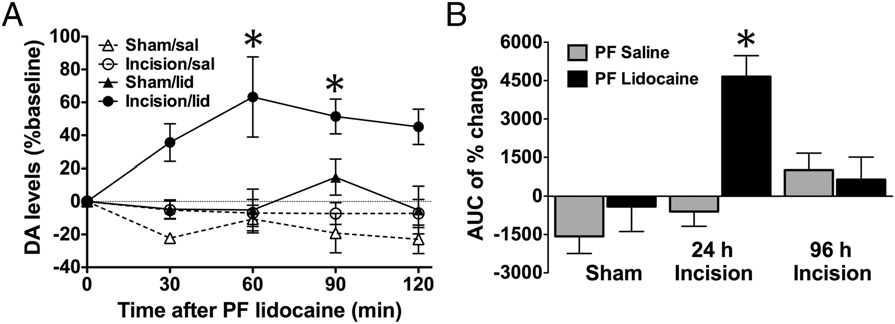
PNB increased the extracellular dopamine (DA) levels in the NAc shell of rats with incisional pain. (A) Extracellular dopamine levels increased above
basal levels following PNB only in incised animals. (B) Area under the curve (AUC) of percent change from baseline demonstrated significant dopamine releasewithin 120 min after PNB given 24 h, but not 96 h, following incision. Mean ± SEM, n = 3–8 rats/group, *P < 0.05; one-way ANOVA with Tukey's multiplecomparisons test.
Navratilova et al.
PNAS Early Edition 3 of 5

processing (38). These diverse patterns of neuronal activity are
200 μL of saline or lidocaine (4%, wt/vol; Qualitest Pharmaceuticals) into PF
likely reflected in the observed complexity of dopaminergic
under light isofluorane anesthesia (30).
signaling in experimental models of tonic pain (39, 40). In thepresent study, we found no change in the number of FOS-posi-
Brain Microinjection. Bilateral intracranial microinjections of 4% (wt/vol) li-docaine hydrochloride (0.5 μL per side), baclofen (25 ng/0.2 μL per side;
tive neurons in incised versus sham rats. Further, the basal do-
Sigma), flupenthixol (3 μg/0.5 μL per side; Sigma), or naloxone hydrochloride
pamine levels in the NAc of incised and sham rats did not differ.
(3 μg/0.5 μL per side; Tocris) in the VTA or NAc were done using injectors
Thus, 24 h following incisional surgery, before PNB-induced pain
extended 1 mm beyond the guide cannula. All animals were euthanized via
relief, we saw no net change in mesolimbic dopamine transmission.
CO2 overdose at the end of the experiments and the placement of the guide
Numerous factors may account for this observation, including
cannulas/injection sites was confirmed with histology methods. Data from
(i) the sensitivity of our analytical technique, (ii) the non-
animals with misplaced cannulas were removed from the analyses.
chronic nature of the injury, or (iii) involvement of VTA neu-rons that project to other sites beyond the NAc.
Guarding Behavior. Assessment of guarding behavior was done as previously
How afferent nociceptive pathway(s) modulate the reward cir-
described (30). Rats were observed for 10 s each at 1-min intervals and scored
cuit is not known. In the rat, major ascending nociceptive pathways
0–2 (0, injured hind-paw area was touching the mesh and the area wasblanched or distorted by the mesh; 1, the injured hind paw touched the mesh
from lamina I of the lumbar spinal cord terminate in the lateral
without blanching or distortion; 2, the injured hind paw was completely off
parabrachial nucleus (PBN) or in the thalamic nuclei (41, 42).
the mesh). For each rat a cumulative score was obtained by adding 30 scores
Midbrain dopaminergic neurons receive direct nociceptive inputs
during the 30-min testing period. All testing was done by an experimenter
from projections in the PBN (43). The anterior cingulate cortex
blinded to the treatment conditions.
(ACC) receives prominent inputs from nociceptive neurons in thethalamus, and activation of the ACC through the spinothalamic
Thermal Hypersensitivity. Nociceptive withdrawal thresholds to noxious ra-
pathway contributes to the aversiveness of nociceptive stimuli.
diant heat were determined using the Hargreaves test. Rats were allowed to
Accordingly, functional imaging studies have implicated the ACC
acclimate within a Plexiglas enclosure on a clear glass plate for 30 min. A
in processing the unpleasant affective aspects of pain in humans
radiant heat source was directed onto the plantar surface of the left hindpaw. A motion detector halted both heat lamp and timer when the paw was
(44). The ACC projects directly or via other limbic regions such as
withdrawn. Baseline latencies were established at 20 s. A maximal cutoff of
the amygdala to the mesolimbic reward system (45, 46). Recent
30 s was used to prevent tissue damage.
work has identified a circuit that includes the lateral habenulaneurons that indirectly inhibit midbrain dopamine neurons (47).
Conditioned Place Preference Procedures. A single trial conditioning protocol
Inhibition of this input pathway would disinhibit VTA dopa-
was used for CPP as previously described (2, 30). All rats underwent handling
mine neurons.
by the experimenter before the preconditioning phase. On preconditioning
In conclusion, we demonstrate that relief of ongoing post-
day, rats were placed into the CPP boxes with access to all chambers; time
surgical pain following PNB produces CPP and activates the mes-
spent in each chamber was determined by an automated process and ana-
olimbic dopaminergic circuit implicated in positive reinforcement.
lyzed across 15 min to verify no preconditioning chamber preference. Fol-lowing preconditioning, rats received incision or sham surgeries and were
PNB elicits efflux of dopamine in the NAc shell only in the setting
placed back into their home cages overnight. On conditioning day (24 h
of injury-induced, ongoing pain. Additionally, inhibition of do-
postincision), rats received a saline (200 μL) injection into the PF and were
paminergic neurons in the VTA and dopamine signaling in their
immediately (within 2 min) placed into the appropriate pairing chamber.
projection target in the NAc shell prevented PNB-induced CPP,
Four hours later, rats received a lidocaine injection (4%, wt/vol; 200 μL) into
providing direct evidence for a causal relationship between acti-
the PF and were placed into the opposite chamber. Chamber pairings were
vation of this mesostriatal circuit and the negative reinforcing
counterbalanced. To determine the role of the VTA or NAc in PNB-induced
effect of pain relief. These data indicate that activation of the
CPP, rats with VTA or NAc cannulas received saline injection into the VTA or
VTA to NAc dopamine signaling contributes to both positively
NAc followed 10 min later by PF saline injection and immediate placement
and negatively reinforced behavior.
into the appropriate pairing chambers. Four hours later, rats received treat-ment drug injection into the VTA or NAc followed in 10 min by PF lidocaine
Materials and Methods
injection and placement into the opposite chambers. The conditioning timewas 30 min in each chamber. On test day, 20 h following the afternoon
Animals. Adult, male Sprague-Dawley rats (250–350 g; Harlan) were used. All
pairing, rats were placed in the CPP box with access to all chambers and
procedures were performed in accordance with the policies of the National
behavior was recorded for 15 min for analysis for chamber preference. Dif-
Institutes of Health guidelines for laboratory animals under protocols ap-
ference scores were calculated as test time minus preconditioning time spent
proved by the University of Arizona Institutional Animal Care and Use
in the PF–lidocaine paired chamber.
Committee. Rats were housed three per cage on a 12-h light–dark cycle withfood and water provided for ad libitum consumption.
Immunohistochemistry. Two hours after treatment rats were anesthetizedby i.p. ketamine/xylazine and transcardially perfused with 4% (wt/vol)
Intracranial VTA and NAc Cannulation. Stereotaxic surgeries were performed
paraformaldehyde. Coronal brain sections (30 μm thick) were cut in a Microm
in anesthetized rats (i.p. ketamine/xylazine 80/12 mg/kg; Western Medical
HM 525 cryostat and mounted on Superfrost Plus microscope slides. Brain
Supply/Sigma) according to the brain atlas. Two 26-gauge guide cannulas
tissue was permeabilized with 0.2% Triton X-100, blocked with 5% (wt/vol)
(Plastics One) were directed toward the following coordinates: VTA [ante-
normal goat serum and incubated overnight with the mixture of primary
roposterior (AP), bregma −5.8 mm; mediolateral (ML), midline ±0.6 mm;
antibodies of rabbit polyclonal anti-cFOS (sc-52, 1:25,000; Santa Cruz) and
dorsoventral (DV), skull −8.0 mm], NAc shell (AP, bregma +1.5 mm; ML,
mouse monoclonal anti-tyrosine hydroxylase (MAB55280, 1:3,000; Milli-
midline ±1.0 mm; DV, skull −6.5 mm). For microdialysis, a single guide can-
pore). The sections were incubated with biotinylated anti-rabbit antibody
nula (AG-8; EICOM Corp.) was implanted into the left NAc (AP, bregma +1.7
followed by the ABC complex (Vectastain Elite ABC kit; Vector Laboratories,
mm; ML, midline −1.0 mm; DV, skull −6.0 mm). Stainless steel dummy can-
Inc.) and tyramide signal amplification detection (TSA Plus Fluorescein Kit;
nulas were inserted to keep the guide free of debris. After surgery, rats were
Perkin-Elmer). TH was visualized with anti-mouse Alexa Fluor 555 (1:1,000;
housed individually and allowed to recover for 5–7 d.
Molecular Probes, Invitrogen). Slides were mounted in Vectashield mountingmedium (Vector Laboratories, Inc.) and examined under an Olympus BX51
Incisional Injury Pain Model. Incision injury of the skin plus deep tissue, in-
microscope equipped with a Hamamatsu C8484 digital camera. Confocal
cluding fascia and underlying muscle, was done as described by Brennan et al.
images were obtained with a Zeiss LSM520 laser scanning confocal micro-
(22). Rats were anesthetized with 2% (vol/vol) isoflurane, a 1-cm longitudi-
scope using the 488- and 543-nm excitation wavelengths. Micrographs of
nal incision was made through the skin of the left hind paw, and the
10–15 sections per rat 150 μm apart within the bregma −5.20 to −6.80 mm
plantaris muscle was elevated and incised longitudinally. The cut skin was
were analyzed for FOS expression. In the ImageJ software the VTA area
stitched with two 5–0 nylon sutures and the wound site treated with neo-
including the PBP, PN, RLi, IF, and CLi was outlined according to the TH
mycin. Sham animals were anesthetized and the left hind paw was cleaned,
staining. FOS-positive nuclear puncta within the outlined area were counted
but no incision was made. Peripheral nerve block was achieved by injecting
manually by an observer blinded to the treatment conditions. Coexpression
Navratilova et al.

of FOS and TH was evaluated within the medial VTA at bregma −5.80 mm
column and Coulochem III 5014B electrochemical detector (ESA). The guard
(rectangle in Fig. 2A) in two to four sections per each rat using the Zeiss
cell was set at 350 mV, electrode 1 at −150 mV, and electrode 2 at 250 mV.
LSM520 confocal microscope images. Cells with nuclear FOS staining and
Standard curve was obtained from seven serial dilutions of dopamine
a visible cytoplasmic TH staining (Fig. 2E) were counted as dopaminergic;
(2.5–160 pg in 20 μL aCSF plus antioxidant mixture). The limit of detection
FOS-positive cells with no clear cytoplasmic TH staining (Fig. 2G) were
(LOD) and limit of quantification (LOQ) were calculated according to the
counted as nondopaminergic.
formulas LOD = 3.3 (SDr/S) and LOQ = 10 (SDr/S), where the SD of the responseSDr (SD of y intercepts of regression lines) and the slope of the standard curve
In Vivo Microdialysis and HPLC Quantification of Dopamine. Microdialysis was
S was determined from the measurements of 10 independent standard curves
done in awake, freely moving animals. The microdialysis probe (A-I-8-02;
(The data from rats that failed to generate dopamine efflux following
Eicom) was inserted into the NAc with 2 mm of semipermeable membrane
cocaine treatment were excluded. Dopamine concentrations were expressed
projecting beyond the guide cannula (and perfused at 1.25 μL/min
as percent of the corresponding baseline level.
with artificial cerebrospinal fluid (aCSF: 147.0 mM NaCl, 2.8 mM KCl, 1.2mM MgCl2, and 1.2 mM CaCl2). After a 90-min washout period, two base-line and four treatment fractions (30 min/fraction) were collected into
Statistical Analysis. Statistical analyses were calculated using GraphPad Prism
prechilled (4 °C) Eppendorf tubes containing 1.0 μL of 40× antioxidant so-
5 software. Results were expressed as mean ± SEM. Two-way ANOVA with
lution [6.0 mM L-cysteine, 2.0 mM oxalic acid, and 1.3% (vol/vol) glacial
Bonferroni multiple comparisons test or one-way ANOVA with Tukey's
acetic acid] (48). All rats were then injected with cocaine (20 mg/kg, i.p.)
multiple comparison post hoc tests was used for between-groups compari-
and dialysates were collected for additional 90 min. Fractions were ana-
son. Student's paired t test was used to analyze the difference scores for the
lyzed using an Agilent 1100 HPLC system with a 5020 guard cell, MD-150
CPP data. Significance was set at P < 0.05.
1. King T, et al. (2011) Contribution of afferent pathways to nerve injury-induced
27. Olmstead MC, Franklin KB (1997) The development of a conditioned place preference
spontaneous pain and evoked hypersensitivity. Pain 152(9):1997–2005.
to morphine: Effects of microinjections into various CNS sites. Behav Neurosci 111(6):
2. King T, et al. (2009) Unmasking the tonic-aversive state in neuropathic pain. Nat Neu-
28. Garcia MM, Brown HE, Harlan RE (1995) Alterations in immediate-early gene proteins
3. Liu P, et al. (2011) Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett
in the rat forebrain induced by acute morphine injection. Brain Res 692(1-2):23–40.
29. Laviolette SR, Nader K, van der Kooy D (2002) Motivational state determines the
4. Baliki MN, Geha PY, Fields HL, Apkarian AV (2010) Predicting value of pain and an-
functional role of the mesolimbic dopamine system in the mediation of opiate reward
algesia: Nucleus accumbens response to noxious stimuli changes in the presence of
processes. Behav Brain Res 129(1-2):17–29.
chronic pain. Neuron 66(1):149–160.
30. Okun A, et al. (2011) Transient inflammation-induced ongoing pain is driven by
5. Becerra L, Borsook D (2008) Signal valence in the nucleus accumbens to pain onset
TRPV1 sensitive afferents. Mol Pain 7:4.
and offset. Eur J Pain 12(7):866–869.
31. Qu C, et al. (2011) Lesion of the rostral anterior cingulate cortex eliminates the
6. Zubieta JK, Stohler CS (2009) Neurobiological mechanisms of placebo responses. Ann
aversiveness of spontaneous neuropathic pain following partial or complete ax-
N Y Acad Sci 1156:198–210.
otomy. Pain 152(7):1641–1648.
7. Kaplan GB, Leite-Morris KA, Joshi M, Shoeb MH, Carey RJ (2003) Baclofen inhibits
32. Liu ZH, Shin R, Ikemoto S (2008) Dual role of medial A10 dopamine neurons in af-
opiate-induced conditioned place preference and associated induction of Fos in cor-
fective encoding. Neuropsychopharmacology 33(12):3010–3020.
tical and limbic regions. Brain Res 987(1):122–125.
33. Zellner MR, Ranaldi R (2010) How conditioned stimuli acquire the ability to activate
8. Moaddab M, Haghparast A, Hassanpour-Ezatti M (2009) Effects of reversible in-
VTA dopamine cells: A proposed neurobiological component of reward-related
activation of the ventral tegmental area on the acquisition and expression of mor-
learning. Neurosci Biobehav Rev 34(5):769–780.
phine-induced conditioned place preference in the rat. Behav Brain Res 198(2):
34. Fields HL, Hjelmstad GO, Margolis EB, Nicola SM (2007) Ventral tegmental area
neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neu-
9. Schultz W (2010) Dopamine signals for reward value and risk: Basic and recent data.
rosci 30:289–316.
Behav Brain Funct 6:24.
35. Shippenberg TS, Herz A, Spanagel R, Bals-Kubik R, Stein C (1992) Conditioning of
10. Bajic D, Commons KG (2010) Acute noxious stimulation modifies morphine effect in
opioid reinforcement: Neuroanatomical and neurochemical substrates. Ann N Y Acad
serotonergic but not dopaminergic midbrain areas. Neuroscience 166(2):720–729.
Sci 654:347–356.
11. Soderman AR, Unterwald EM (2008) Cocaine reward and hyperactivity in the rat: Sites
36. Sticht M, Mitsubata J, Tucci M, Leri F (2010) Reacquisition of heroin and cocaine place
of mu opioid receptor modulation. Neuroscience 154(4):1506–1516.
preference involves a memory consolidation process sensitive to systemic and intra-
12. Allen KV, McGregor IS, Hunt GE, Singh ME, Mallet PE (2003) Regional differences in
ventral tegmental area naloxone. Neurobiol Learn Mem 93(2):248–260.
naloxone modulation of Delta(9)-THC induced Fos expression in rat brain. Neuro-
37. Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009) Phasic excitation of do-
pamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 106(12):
13. Hargreaves GA, Hunt GE, Cornish JL, McGregor IS (2007) High ambient temperature
increases 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy")-induced Fos ex-
38. Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational
pression in a region-specific manner. Neuroscience 145(2):764–774.
control: Rewarding, aversive, and alerting. Neuron 68(5):815–834.
14. Ikemoto S (2007) Dopamine reward circuitry: Two projection systems from the ventral
39. Ozaki S, et al. (2004) Role of extracellular signal-regulated kinase in the ventral
midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Brain Res
tegmental area in the suppression of the morphine-induced rewarding effect in mice
Rev 56(1):27–78.
with sciatic nerve ligation. J Neurochem 88(6):1389–1397.
15. Di Chiara G, et al. (2004) Dopamine and drug addiction: The nucleus accumbens shell
40. Austin PJ, Beyer K, Bembrick AL, Keay KA (2010) Peripheral nerve injury differentially
connection. Neuropharmacology 47(Suppl 1):227–241.
regulates dopaminergic pathways in the nucleus accumbens of rats with either ‘pain
16. Roitman MF, Wheeler RA, Wightman RM, Carelli RM (2008) Real-time chemical re-
alone' or ‘pain and disability' Neuroscience 171(1):329–343.
sponses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat
41. Polgár E, Wright LL, Todd AJ (2010) A quantitative study of brainstem projections
17. Fields HL (2007) Understanding how opioids contribute to reward and analgesia. Reg
from lamina I neurons in the cervical and lumbar enlargement of the rat. Brain Res
Anesth Pain Med 32(3):242–246.
18. Montague PR, King-Casas B, Cohen JD (2006) Imaging valuation models in human
42. Gauriau C, Bernard JF (2002) Pain pathways and parabrachial circuits in the rat. Exp
choice. Annu Rev Neurosci 29:417–448.
19. Platt ML, Huettel SA (2008) Risky business: The neuroeconomics of decision making
43. Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG (2010) The parabrachial
under uncertainty. Nat Neurosci 11(4):398–403.
nucleus is a critical link in the transmission of short latency nociceptive information to
20. O'Doherty J, et al. (2004) Dissociable roles of ventral and dorsal striatum in in-
midbrain dopaminergic neurons. Neuroscience 168(1):263–272.
strumental conditioning. Science 304(5669):452–454.
44. Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D (2001) Reward circuitry acti-
21. O'Doherty JP (2004) Reward representations and reward-related learning in the hu-
vation by noxious thermal stimuli. Neuron 32(5):927–946.
man brain: Insights from neuroimaging. Curr Opin Neurobiol 14(6):769–776.
45. Carr DB, Sesack SR (2000) Projections from the rat prefrontal cortex to the ventral
22. Brennan TJ, Vandermeulen EP, Gebhart GF (1996) Characterization of a rat model of
tegmental area: Target specificity in the synaptic associations with mesoaccumbens
incisional pain. Pain 64(3):493–501.
and mesocortical neurons. J Neurosci 20(10):3864–3873.
23. Okun A, et al. (2012) Afferent drive elicits ongoing pain in a model of advanced
46. Stuber GD, et al. (2011) Excitatory transmission from the amygdala to nucleus ac-
osteoarthritis. Pain 153(4):924–933.
cumbens facilitates reward seeking. Nature 475(7356):377–380.
24. Yun IA, Wakabayashi KT, Fields HL, Nicola SM (2004) The ventral tegmental area is
47. Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O (2011) Negative reward signals
required for the behavioral and nucleus accumbens neuronal firing responses to in-
from the lateral habenula to dopamine neurons are mediated by rostromedial teg-
centive cues. J Neurosci 24(12):2923–2933.
mental nucleus in primates. J Neurosci 31(32):11457–11471.
25. Kalivas PW, Duffy P, Eberhardt H (1990) Modulation of A10 dopamine neurons by
48. Hubbard KE, et al. (2010) Determination of dopamine, serotonin, and their metab-
gamma-aminobutyric acid agonists. J Pharmacol Exp Ther 253(2):858–866.
olites in pediatric cerebrospinal fluid by isocratic high performance liquid chroma-
26. Xi ZX, Stein EA (1999) Baclofen inhibits heroin self-administration behavior and
tography coupled with electrochemical detection. Biomed Chromatogr 24(6):
mesolimbic dopamine release. J Pharmacol Exp Ther 290(3):1369–1374.
Navratilova et al.
PNAS Early Edition 5 of 5
Source: http://neurology.arizona.edu/sites/default/files/narrative_porreca_feb_5_journal_club.pdf
January—April 2013 AfCiC News Action for Children in Conflict (AfCiC) "Working towards a world of equal and sustainable opportunities for every Child From street freedom to a life of Hope Who is AfCiC? The Interim Care Centre for street chil- sional authority. They are incorporated in streets, things were not different. We
Metagenomic study of single-nucleotide polymorphism within candidate genes associated with type 2 diabetes in an Indian population P.N. Mukhopadhyaya1, A. Acharya1, Y. Chavan1, S.S. Purohit1 and A. Mutha2 1Medical Genetics Division, geneOmbio Technologies, Pashan, Pune, Maharashtra, India2Diabetes Care and Research Foundation, Rasta Peth, Pune,















