Chronic loss of noradrenergic tone produces arrestin2mediated cocaine hypersensitivity and alters cellular d2 responses in the nucleus accumbens
bs_bs_banner Addiction Biology
Chronic loss of noradrenergic tone produces
β-arrestin2-mediated cocaine hypersensitivity and
alters cellular D2 responses in the nucleus accumbens
Meriem Gaval-Cruz1*, Richard B. Goertz2*, Daniel J. Puttick1, Dawn E. Bowles3,
Rebecca C. Meyer4, Randy A. Hall4, Daijin Ko5, Carlos A. Paladini2 & David Weinshenker1
Department of Human Genetics, Emory University School of Medicine, Atlanta, GA, USA1, Department of Biology, Neurosciences Institute, University of Texasat San Antonio, San Antonio, TX, USA2, Department of Surgery, Duke University School of Medicine, Durham, NC, USA3, Department of Pharmacology,Emory University School of Medicine, Atlanta, GA, USA4 and Department of Management Science and Statistics, University of Texas at San Antonio, San Antonio,TX, USA5
Cocaine blocks plasma membrane monoamine transporters and increases extracellular levels of dopamine (DA),
norepinephrine (NE) and serotonin (5-HT). The addictive properties of cocaine are mediated primarily by DA, while NE
and 5-HT play modulatory roles. Chronic inhibition of dopamine β-hydroxylase (DBH), which converts DA to NE,increases the aversive effects of cocaine and reduces cocaine use in humans, and produces behavioral hypersensitivity
to cocaine and D2 agonism in rodents, but the underlying mechanism is unknown. We found a decrease in β-arrestin2(βArr2) in the nucleus accumbens (NAc) following chronic genetic or pharmacological DBH inhibition, andoverexpression of βArr2 in the NAc normalized cocaine-induced locomotion in DBH knockout (
Dbh −
/−) mice. TheD2/3 agonist quinpirole decreased excitability in NAc medium spiny neurons (MSNs) from control, but not
Dbh −
/−animals, where instead there was a trend for an excitatory effect. The Gαi inhibitor NF023 abolished the quinpirole-induced decrease in excitability in control MSNs, but had no effect in
Dbh −
/− MSNs, whereas the Gαs inhibitor NF449restored the ability of quinpirole to decrease excitability in
Dbh −
/− MSNs, but had no effect in control MSNs. Theseresults suggest that chronic loss of noradrenergic tone alters behavioral responses to cocaine via decreases inβArr2 and cellular responses to D2/D3 activation, potentially via changes in D2-like receptor G-protein couplingin NAc MSNs.
Cocaine, D2 receptor, dopamine, dopamine β-hydroxylase, mice, norepinephrine.
Correspondence to: David Weinshenker, Department of Human Genetics, Emory University School of Medicine, Whitehead 301, 615 Michael St., Atlanta,
GA 30322, USA. E-mail:
[email protected]
Shandilya & Kundu 2011), alters the subjective effects of
cocaine and reduces cocaine use in humans (Stanley
Dopamine β-hydroxylase (DBH) is the enzyme that
et al. 1997; Gaval-Cruz & Weinshenker 2009) (K.
converts dopamine (DA) to norepinephrine (NE) in
Cunningham, pers. comm.). Genetic (DBH knockout;
Dbh
noradrenergic neurons, thereby controlling NE produc-
−
/−) or pharmacological (disulfiram, nepicastat) DBH
tion and the DA/NE ratio (Weinshilboum 1978). DBH is
inhibition produces hypersensitivity to cocaine-induced
of clinical interest in cocaine dependence because: (1)
locomotion, stereotypy, place preference and place aver-
polymorphisms in the human DBH gene that are associ-
sion in mice; it also enhances the discriminative stimulus
ated with reduced serum DBH enzymatic activity lead to
effects of cocaine and attenuates cocaine-, cue- and
greater cocaine-induced paranoia (Cubells
et al. 2000;
stress-induced reinstatement of cocaine seeking in
Kalayasiri
et al. 2007); and (2) inhibition of DBH by the
rats (Schank
et al. 2006; Schroeder
et al. 2010, 2013;
alcoholism medication, disulfiram, or the selective DBH
Gaval-Cruz
et al. 2012; Manvich, Depoy & Weinshenker
inhibitor, nepicastat (Stanley
et al. 1997; Kapoor,
*MGC and RBG contributed equally to this work.
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
Because
Dbh −
/− mice are hypersensitive to the D2/3
mice for the experiments (Thomas, Matsumoto & Palmiter
agonist, quinpirole, but not the D1 agonist, SKF81297,
1995; Thomas
et al. 1998). Comparable numbers of male
cocaine hypersensitivity would appear to be mediated by
and female knockouts were used for each experiment, and
alterations in the D2 pathway (Weinshenker
et al. 2002;
sex-matched
Dbh +/− littermates were used as controls.
Schank
et al. 2006). These phenotypes are likely driven
Although the studies were not powered sufficiently to
by compensatory responses in DA signaling following the
rigorously detect sex differences, no obvious ones
chronic decrease in extracellular DA availability when
were observed. The
Dbh +/− mice were used as controls
noradrenergic excitatory drive on the mesocorticolimbic
because their brain catecholamine levels and behaviors
system is missing. We initially reported an increase in the
are indistinguishable from wild-type (
Dbh +/+) mice
abundance of high-affinity state D2 receptors in the
(Thomas
et al. 1998; Bourdelat-Parks
et al. 2005;
striatum of
Dbh −
/− mice, which could explain the
Mitchell
et al. 2006). Some wild-type C57BL/6J mice
cocaine and D2 hypersensitivity (Schank
et al. 2006).
(Jackson Laboratory, Bar Harbor, ME, USA) were also used
However, subsequent work failed to confirm this finding
as controls for the electrophysiology experiments.
(Skinbjerg
et al. 2010), suggesting a contribution from
All animals were treated in accordance with the
downstream signaling molecules. Indeed, the behavioral
National Institutes of Health Intramural Animal Care and
alterations in
Dbh −
/− mice were accompanied by a rise in
Use Program guidelines. The experiments described in this
striatal pERK and ΔFosB protein levels (Rommelfanger
article followed the UTSA and Emory University Division
et al. 2007).
of Animal Resources' Guide for the Care and Use of Labo-
The goals of the present study were to determine the
ratory Animals and were approved by the UTSA and
molecular and cellular mechanisms behind the D2- and
Emory Institutional Animal Care and Use Committee.
psychostimulant-induced hypersensitivity that follow
chronic DBH inhibition. First, we found a decrease of
Chronic nepicastat treatment
β-arrestin2 (βArr2), a protein involved in D2 desensitiza-
Nepicastat was administered to
Dbh +/− mice via daily i.p.
tion and signaling (Beaulieu & Gainetdinov 2011), in the
injections (Western blots) or osmotic minipumps (locomo-
nucleus accumbens (NAc) of
Dbh −
/− mice and mice
tor activity). For the i.p. administration,
Dbh +/− mice
treated chronically with nepicastat. We next used viral-
received vehicle or nepicastat (50 mg/kg, i.p. × 3, each
mediated overexpression to determine whether increas-
injection spaced 2 hours apart) for 5 consecutive days.
ing βArr2 levels in the NAc could normalize cocaine-
This dosing regimen reduces brain NE levels by ∼75 per-
induced behavior in
Dbh −
/− mice. Finally, we assessed
cent and produces cocaine hypersensitivity (Gaval-Cruz
electrophysiological responses to quinpirole in medium
et al. 2012). Mice were euthanized by CO2 asphyxiation 11
spiny neurons (MSNs) from the NAc of control and
Dbh
days later, and their brains were removed, dissected on ice
−
/− mice in the presence and absence of Gαi and Gαs
and stored at −80°C. For the minipump administration,
nepicastat was dissolved in 50 percent saline and 50
percent dimethyl sulfoxide and loaded into Alzet osmotic
minipumps (Model #2004, 0.25 μl/hour, 28 days;
MATERIALS AND METHODS
Durect, Cupertino, CA, USA) to achieve a dose of 50 mg/
kg/day. All pumps were placed in a sterile 37°C saline
bath for 1 day before implantation. Mice were anesthe-
Adult control (
Dbh +/−) and
Dbh −/− mice were generated
tized with isoflurane, and minipumps were implanted in
as previously described (Thomas
et al. 1998; Schank
the intraperitoneal cavity. Buprenorphine (2.5 mg/kg,
et al. 2006).
Dbh −
/− males were bred to
Dbh +/− females.
s.c.) was given immediately after surgery. Cocaine-
Pregnant
Dbh +/− mice were given the AR agonists
induced locomotion was recorded 21 days after pump
isoproterenol and phenylephrine (20 μg/ml each) +
vitamin C (2 mg/ml) from E9.5-E14.5, and L-3,4-
dihydroxyphenylserine (2 mg/ml) + vitamin C (2 mg/ml)
from E14.5 birth in their drinking water to rescue the
embryonic lethality associated with the homozygous
Dbh
Mice were placed in locomotion recording chambers
−
/− mutation. Because of this treatment, NE and epineph-
(transparent Plexiglas cages placed into a rack with seven
rine were present in
Dbh −
/− animals before but not after
infrared photobeams spaced 5 cm apart; San Diego
birth. They were maintained on a mixed C57BL/6J and
Instruments Inc., La Jolla, CA, USA) and allowed to
129SvEv background and group housed, and food and
habituate for 30 minutes before receiving a single injec-
water were available
ad libitum throughout the course of
tion of cocaine (10 or 15 mg/kg, i.p.). Novelty-induced
the study. Both sexes were used due to the extreme meas-
locomotion was defined as ambulations during the first
ures required to breed sufficient numbers of knockout
10 minutes of the habituation period. Ambulations
2014 Society for the Study of Addiction
Addiction Biology
Cocaine response in DBH mice
(consecutive beam breaks) were recorded for an addi-
1:1000; Cell Signaling, CS9271); Akt (anti-mouse;
tional 1–2 hours following drug administration.
1:500; Santa Cruz Biotechnology, SC5298); pGSK3β-Ser9 (anti-rabbit; 1:1000; Cell Signaling, CS9322);
pGSK3β (anti-rabbit; 1:1000; Cell Signaling, CS9315);FosB (anti-rabbit; 1:1000; Cell Signaling, CS9890).
Mouse brain tissue was homogenized in 500 μlharvest
β
Arr2 viral vectors
piperazineethanesulfonic acid (HEPES), 50 mM NaCl,
5 mM ethylenediaminetetraacetic acid, pH 7.4, supple-
The original βArr2 plasmid (rat sequence) was obtained
mented with protease inhibitors] using a sonicator.
from Sudha Shenoy in the laboratory of Dr. Robert
Laemmli sample buffer containing sodium dodecyl sulfate,
Lefkowitz. The Duke Neurotransgenic Laboratory then
β-mercaptoethanol, glycerol, Tris-Cl and bromophenol
removed the βArr2 open reading frame, and the insert
blue was added to samples after measuring protein con-
was cloned into a pCMVShuttle plasmid (AdEasy System,
centrations with a bicinchoninic acid assay (Thermo
Stratagene, Santa Clara, CA, USA). The AdEasy βArr2
Fisher Scientific, Rockford, IL, USA). Samples were
recombinant plasmid was generated per Stratagene
resolved by sodium dodecyl sulfate–polyacrylamide gel
instructions, and the βArr2 adenoviral vector was
electrophoresis on 4–20 percent Tris-glycine precast gels
expanded and purified. The viruses were harvested with a
followed by transfer to nitrocellulose membranes. Follow-
titer of 2 × 1012/μl (βArr2) and 5 × 109/μl [green fluo-
ing transfer, membranes were incubated with Ponceau
rescent protein (GFP) control].
staining to assess even protein loading, then rinsed with
distilled water. Membranes were then incubated in block-
β
Arr2 viral infusions
ing buffer (10 mM HEPES, 50 mM NaCl, 1 percent Tween-
(
n = 16 for each treatment group: βArr2
20, 2 percent dry milk, pH 7.4, for most antibodies; 1X
overexpression adenovirus and GFP adenovirus) were
TBS, 0.1 percent Tween-20 with 5 percent w/v non-fat
anesthetized using isoflurane and placed in a stereotaxic
dry milk, for pAKT, GSK3β and pGSK3β) for 30 minutes,
frame with a nose bar. The animal's scalp was opened and
and then incubated with primary antibody overnight at
bregma and lambda aligned to flat-skull position. The
4°C. The primary incubation buffer was the same as block-
stereotaxic arm was then lowered to the NAc core.
ing buffer for all antibodies except pAKT, GSK3β and
The core subregion was chosen because it has been impli-
pGSK3β. For these, the primary incubation buffer was 1X
cated in cocaine-induced locomotion and behavioral
TBS, 0.1 percent Tween-20 with 5 percent bovine serum
sensitization to cocaine. The anteroposterior (AP) and
albumin (BSA). The membranes were washed three times
mediolateral (ML) coordinates of the NAc core in relation
in blocking buffer and incubated with either a fluorescent
to bregma were AP
= 1.4 mm and ML = ±1.0 mm, and
(1:10 000) or horseradish peroxidase-conjugated second-
a small hole was drilled in the skull at these coordinates.
ary (1:4000) antibody (Invitrogen, Carlsbad, CA, USA) for
A 5-μl Hamilton microsyringe was lowered to target
30 minutes, washed three more times, and then visualized
the NAc core (dorsoventral coordinate = −4.2 mm). The
using either the Odyssey imaging system (Li-Cor, Lincoln,
26-gauge beveled tip of the Hamilton needle was
NE, USA) or via enhanced chemiluminescence reagent
precoated with 2 percent BSA prior to loading the virus to
(Thermo Fisher Scientific), followed by exposure to film.
prevent molecular interactions between the syringe and
Membranes were stripped for 20 minutes at 37°C and 10
the viral vectors. Animals received 1 μl of virus per side,
minutes at room temperature with stripping buffer and
injected at a rate of 0.2 μl
/minute, and the needle
re-probed for α-actin to confirm equal loading of samples.
remained in place for 5 minutes after the injection and
Blots were analyzed by densitometry using ImageJ Soft-
removed slowly. The skin was glued together using
ware (National Institutes of Health, Bethesda, MD, USA).
Vetbond tissue glue (Henry Schein, Roswell, GA, USA).
A mean density value was calculated for the ‘control'
All animals received meloxicam (0.5 mg/kg) for postop-
group (i.e.
Dbh +/− mice were the control for
Dbh −
/− mice,
erative pain and water/liquid ibuprofen (0.1 mg/ml).
vehicle was the control for nepicastat), and data were
Ten days after the infusion of βArr2 overexpres-
expressed as % control.
sion and GFP control vectors, all mice were placed in
locomotor chambers, and their basal locomotion was
recorded for 30 minutes before receiving an injection of
The antibodies used and their working dilutions were as
cocaine (15 mg/kg, i.p.), and cocaine-induced locomo-
follows: βArr2 (anti-rabbit; 1:2500; Cell Signaling
tion was recorded for 2 hours. Mice were anesthetized
Technology, Danvers, MA, USA, CS3857); α-actin
and transcardially perfused with saline and 4 percent
(anti-mouse; 1:1000; Santa Cruz Biotechnology, Santa
paraformaldehyde 24–48 hours later, their brains were
Cruz, CA, USA, SC58671); pAkt-Ser473 (anti-rabbit;
removed, stored in 4 percent paraformaldehyde for 4
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
days, and then transferred to 30 percent sucrose. Brains
(< 350 MΩ), and delayed spiking upon current injection.
were sectioned and stained with antibodies against GFP
Drugs were applied to the slice by superfusion at the indi-
or βArr2, and expression in the NAc was assessed. Three
cated concentration. All experiments were performed in
mice that received the βArr2 virus and two mice that
the presence of 5 μM NBQX (AMPA antagonist), 25 μM
received GFP virus were removed from the analysis due to
D-APV (NMDA antagonist), 100 μM picrotoxin (GABAa
incorrect placement of viral infusion.
antagonist) and 10 μM SCH 23390 (D1 antagonist). Thedrug NF023 (10 μM) was applied internally. For NF 449
Electrophysiological recordings of NAc neurons
(1 μM) application, the slices were incubated in the Gαs
C57BL/6J,
Dbh +/− and
Dbh −
/− mice were used for
antagonist for 1 hour prior to recording, and then con-
electrophysiological recordings. C57BL/6J mice were
tinuously exposed to NF449 throughout the recording
used to: (1) confirm that
Dbh +/− mice NAc MSNs were
process. All drugs were obtained from Tocris Bioscience
similar to wild-type NAc MSNs; and (2) increase the
(Bristol, UK) or Sigma-Aldrich (St. Louis, MO, USA). In
number of cells in a few experiments when not enough
current-clamp configuration, current was injected for
appropriately sex- and age-matched
Dbh +/− control
200 ms at 100-pA step intervals (100–500 pA) with 5
animals were available. Mice were anesthetized with a
seconds between each pulse, until the cell was depolarized
lethal dose of isoflurane and decapitated. The brains were
and spikes were evoked. An input/output curve was
quickly removed and placed into an ice-cold, oxygenated
obtained under baseline conditions before and after
cutting solution containing (in mM): 110 choline Cl, 2.5
superfusing 10 ml of a 5 μM solution of quinpirole for
KCl, 1.25 NaH2PO4, 4 MgCl2, 2 CaCl2, 10 dextrose, 25
approximately 5 minutes. Action potentials were detected
NaHCO3, 1.3 ascorbic acid, 2.4 sodium pyruvate and
using an amplitude threshold, and spike frequency was
0.05 glutathione. Parasagittal brain slices containing the
calculated as the reciprocal of the interspike interval.
NAc (250 μm) were cut using a vibrating tissue slicer(Microm HM 650V, Thermo Fisher Scientific). The slices
were then transferred to an incubation chamber contain-
Western blot data were analyzed by
t-test using GraphPad
ing warm (35°C) artificial cerebral spinal fluid (ACSF) for
Prism 6.0 (La Jolla, CA, USA) for Macintosh (Apple,
1 hour prior to recordings, and then stored at room tem-
Cupertino, CA, USA). Behavioral data were analyzed by
perature. The slices were transferred to a recording
two-way repeated measures ANOVA (RMANOVA), fol-
chamber for the experiments, where they were sub-
lowed by Bonferroni
post hoc tests, where appropriate,
merged in oxygenated ACSF. The ACSF was equilibrated
using Prism. Electrophysiological data were analyzed by
with 95 percent O2-5 percent CO2, had a pH of 7.2, and
RMANOVA with a generalized estimating equation (GEE).
contained (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2
In our data, the number of observations differed between
MgCl2, 2 CaCl2, 10 dextrose, 25 NaHCO3, 1.3 ascorbic
different current steps across cells. Because of the unbal-
acid and 2.4 sodium pyruvate. The slices were superfused
anced design, the classic RMANOVA was therefore not an
with 34–36°C ACSF at a rate of 2 ml/minute.
appropriate test. We used the RMANOVA with a GEE
The cells were visualized using gradient contrast illu-
approach with an exchangeable correlation structure to
mination through a 40X water-immersion lens attached
take into account the unbalanced design, as well as cor-
to an Olympus BX51 (Olympus, Center Valley, PA, USA)
related observations. For each test, GEE uses a robust test
upright microscope. Patch pipettes were pulled from
(Wald χ2 test based on robust variance estimators) for
borosilicate glass (o.d. 1.5 mm, i.d. 0.84 mm) using a
each effect. These analyses were performed using R
P-97 Flaming/Brown electrode puller (Sutter Instru-
ments, Novato, CA USA). Pipettes were filled with a solu-
tion containing (in mM): 138 K-gluconate, 10 HEPES,
0.0001 CaCl2, 0.2 ethylene glycol tetraacetic acid, 4
NaATP, 0.4 NaGTP and 2 MgCl2, with an osmolarity of
Dbh −
/−
mice have decreased β
Arr2 in the NAc
270–275 mOsm and adjusted to a pH of 7.3 with potas-
sium hydroxylase (KOH). Recordings were made using a
We showed previously that ΔFosB, which is induced in
MultiClamp 700B amplifier (Molecular Devices, Sunny-
the NAc by chronic drug exposure and is known to
vale, CA, USA). Signals were digitized at 15–30 kHz and
promote psychostimulant-induced behaviors (Kelz
et al.
saved to a hard drive for analysis using the software
1999), is elevated in the striatum of drug-naïve
Dbh −
/−
program AxoGraph X (AxoGraph Scientific, Berkeley, CA,
mice (Rommelfanger
et al. 2007). As part of a larger
survey to identify potential upstream mediators of the
Spiny neurons in the NAc core were identified as
cocaine hypersensitivity that follows chronic DBH inhibi-
having the following properties: a hyperpolarized mem-
tion, we found that
Dbh −
/− mice had significantly less
brane potential (< −70 mV), a low input resistance
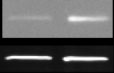
βArr2 in the NAc (
t6 = 3.493,
P < 0.05) (Fig. 1a). Besides
2014 Society for the Study of Addiction
Addiction Biology







Cocaine response in DBH mice
β-arrestin2/actin ratio
Dbh +/–
Dbh –/–
0 0 30 60 90 120 150 180 210 240 270 300 330 360
βarrestin-2/actin ratio
pAkt/tAkt ratio (% control)
Dbh +/–
Dbh –/–
ΔfosB/actin ratio
Dbh +/–
Dbh –/–
Figure 1 Behavioral and neurochemical phenotypes of mice with chronic DBH deficiency. Western blot data (mean ± SEM above,
representative blot below) for (a) βArr2 : actin ratio, (b) protein kinase B (Akt; phospho : total ratio), and (c) glycogen synthase kinase-3β
(GSK-3β; phosphor : total ratio) in the nucleus accumbens (NAc) of Dbh +/− and Dbh −/− mice (n = 8 per group). *P < 0.05 compared
with Dbh +/− mice. (d) Cocaine-induced (10 mg/kg, i.p.) locomotion, (e) βArr2 : actin ratio, and (f) ΔFosB : actin ratio in the NAc of Dbh
+/− control mice treated chronically with the selective DBH inhibitor nepicastat. *P < 0.05, **P < 0.01, ****P < 0.0001 compared with
vehicle
its role in G-protein-coupled receptor desensitization,
Nepicastat-treated mice are hypersensitive to cocaine
βArr2 can signal through a protein kinase B/glycogen
and have decreased βArr2 and increased ΔFosB
synthase kinase-3β (Akt/GSK3β) pathway (Del'guidice,
in the NAc
Lemasson & Beaulieu 2011); however, we detected no
genotype differences in the proportion of phosphorylated
We next determined whether the cocaine hypersensitivity
Akt and GSK3β proteins compared with the total protein
observed in Dbh −/− mice could be mimicked by chronic
levels when comparing Dbh −/− mice to control Dbh +/−
pharmacologic DBH inhibition in control mice. Dbh +/−
mice (Fig. 1b & c).
mice with normal NE content that received chronic
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
nepicastat (via osmotic minipump or daily i.p. injections)
Dbh −/− mice overexpressing GFP or βArr2 had decreased
had no change in locomotion induced by a novel environ-
novelty-induced locomotion compared with Dbh +/− con-
ment, but displayed increased cocaine-induced stereo-
trols with normal NE content at the 10-minute time
et al.
point. As expected, Dbh −/− mice that were infused with
(Fig. 1d), reminiscent of Dbh −/− mice (Weinshenker
the GFP virus were hypersensitive to cocaine-induced
et al. 2002; Schank et al. 2006; Gaval-Cruz et al. 2012).
locomotion compared with mice with normal NE
Two-way ANOVA revealed a main effect of
content. By contrast, overexpression of βArr2 in the NAc
(F11,110 = 17.55, P < 0.0001) and a treatment × time
core of Dbh −/− mice completely normalized their cocaine
interaction (F11,110 = 2.64, P < 0.01). Post hoc tests
response (Fig. 2d). Two-way ANOVA revealed a main
showed that peak cocaine-induced locomotion was sig-
effect of time (F11,286 = 9.62, P < 0.0001), genotype
nificantly enhanced by chronic nepicastat administra-
(F2,26 = 5.20, P < 0.05), and a time × genotype interac-
tion. Acute DBH inhibition, in contrast, does not
tion (F22,286 = 2.88, P < 0.0001). Post hoc tests showed
augment cocaine responses and can even inhibit them
that Dbh −/− mice overexpressing GFP displayed
(Maj, Przegalinski & Wielosz 1968; Haile et al. 2003;
increased locomotion compared with Dbh +/− mice and
Schroeder et al. 2013). These results indicate that the
Dbh −/− overexpressing βArr2 at the 20-, 30- and
hypersensitivity to psychostimulants seen in Dbh −/−
40-minute time points following cocaine administration,
mice cannot be attributed to developmental alterations
whereas there were no apparent differences between Dbh
produced solely by DBH knockout, but likely results from
+/− mice and Dbh −/− mice overexpressing βArr2 in the
downstream changes in the signaling pathways that
NAc at any time point. These results suggest that the
occur following prolonged deficits in NE.
cocaine hypersensitivity conferred by chronic DBH inhi-
Because Dbh −/− mice have decreased βArr2 in the
bition is mediated, at least in part, by reduced βArr2
NAc and increased ΔFosB in the striatum, we measured
levels in the NAc.
the relative levels of these proteins in the NAc of control
mice following chronic treatment with nepicastat. We
Dbh −/− NAc MSNs have aberrant responses
found that nepicastat-treated mice had decreased βArr2
(t14 = 3.49, P < 0.01; Fig. 1e) and increased ΔFosB(t14 = 2.69, P < 0.05; Fig. 1f), confirming that genetic
To uncover the cellular underpinnings of the D2 and
and pharmacological inhibition of NE synthesis produces
cocaine hypersensitivity following chronic DBH inhibi-
similar alterations in DA signaling proteins in the ventral
tion, we measured the spike frequency of trains of action
potentials elicited by the injection of current steps in NAc
MSNs both at baseline and following bath application of
quinpirole (5 μM) from control and Dbh −/− mice. The
Overexpression of βArr2 in the NAc reverses cocaine
control group consisted of both Dbh +/− and wild-type
hypersensitivity in Dbh −/− mice
C57Bl/6J mice because results comparing baseline and
While ΔFosB is induced in the NAc by chronic drug expo-
quinpirole responses in these groups were not signifi-
sure and is known to promote psychostimulant-induced
cantly different [n = 22 Dbh −/−, 24 C57Bl/6J; χ2
behaviors (Kelz et al. 1999), the role of βArr2 is less clear.
(d.f. = 1) = 0.1, P = 0.75] (Fig. 3). We found no genotype
To determine whether the decreased βArr2 in the NAc of
differences between control and Dbh −/− mice in baseline
mice with chronic NE deficiency contributes to their
firing rate in untreated MSNs (F4,160 = 0.43; P = 0.79;
behavioral hypersensitivity to cocaine, we overexpressed
data not shown), and activation of D2 receptors by
GFP or βArr2 in the NAc of Dbh −/− mice using
quinpirole did not significantly change the input resist-
adenoviral vectors and assessed novelty- and cocaine-
ance or the resting membrane potential in either control
induced locomotor activity. High levels of
or Dbh −/− mice (control ΔRin = 5.84 ± 4.28 MΩ, Dbh −/−
immunoreactivity were evident along the needle track
ΔRin = 6.57 ± 8.55 MΩ, P = 0.54; control ΔVrest = −0.71
and in both the core and shell subregions (Fig. 2a), and
± 1.80 mV, Dbh −/− ΔVrest = −1.36 ± 1.04 mV, P = 0.43).
βArr2 protein levels were doubled in the Dbh −/− NAc
As reported previously and expected for a Gαi/o-coupled
as assessed by Western blot (Fig. 2b) 7–10 days fol-
receptor (Surmeier & Kitai 1993; Zamponi & Snutch
lowing viral vector injection, indicating that βArr2
1998; Yasumoto et al. 2002; Perez, White & Hu 2006),
overexpression was achieved and that the antibody we
activation of D2 receptors by quinpirole (5 μM) reduced
used was detecting βArr2. βArr2 overexpression had no
evoked mean spike frequency in MSNs from control
effect on the reduced novelty-induced locomotor activity
animals [χ2 (d.f. = 1) = 5.2, P = 0.02] (Fig. 4a & c).
of Dbh −/− mice (Fig. 2c). Two-way ANOVA revealed a
However, the D2-mediated reduction in MSN excitability
main effect of time (F2,66 = 99.44, P < 0.0001) and geno-
seen in control mice was absent in MSNs recorded from
type (F2,33 = 4.46, P < 0.05). Post hoc tests showed that
Dbh −/− mice. Instead, quinpirole tended to have an
2014 Society for the Study of Addiction
Addiction Biology
Cocaine response in DBH mice
β-arrestin/actin ratio
Dbh –/– βArr2
Dbh –/– βArr2
10 20 30 40 50 60 70 80 90 100 110 120
Figure 2 β-arrestin2 overexpression in the nucleus accumbens restores normal cocaine sensitivity to Dbh −/− mice. (a) Representative
picture of βArr2 overexpression in the NAc of a Dbh −/− mouse that received the βArr2 virus. (b) Western blot data (mean ± SEM above,
representative blot below) for βArr2 : actin ratio in the NAc of Dbh −/− mice that received GFP virus (n = 6) or βArr2 virus (n = 7).
***P < 0.001 compared with Dbh −/− with GFP virus. (c) Novelty-induced (drug-free state) and (d) cocaine-induced (15 mg/kg, i.p.,
administered after 30 minutes in chamber) locomotor activity in Dbh +/− mice (n = 8), Dbh −/− mice that received GFP virus (n = 10), and Dbh
−/− mice that received βArr2 virus (n = 11). *P < 0.05, **P < 0.01 compared with Dbh +/− controls. AC = anterior commissure; core = NAc
core; shell = NAc shell; arrow = needle track
excitatory effect in the knockout neurons, but it did not
abolished the quinpirole-induced decrease in excitability
quite reach significance [χ2 (d.f. = 1) = 3.5, P = 0.06]
[χ2 (d.f. = 1) = 1.2, P = 0.27] and resulted in a significant
(Fig. 4b & c). There was a highly significant difference
difference in the spike frequency difference curve com-
between the spike frequency difference curves between
pared with the spike frequency difference curve for MSNs
MSNs from controls compared with MSNs from Dbh −/−
treated with quinpirole alone [χ2 (d.f. = 1) = 16.5;
animals [χ2 (d.f. = 1) = 20.99, P < 0.0001] (Fig. 4c),
P < 0.0001] (Fig. 5a & c). By contrast, the spike fre-
indicating the excitability of MSNs is increased in Dbh −/−
quency difference curve obtained from Dbh −/− MSNs
animals compared with controls.
after application of NF023 was not significantly different
Because D2-like receptor abundance is normal in Dbh
from the spike frequency difference curve obtained
−/− mice but the cellular response to quinpirole is altered,
from Dbh −/− MSNs treated with quinpirole alone
we suspected that D2 receptors might be aberrantly
[χ2 (d.f. = 1) = 1.57, P = 0.21] (Fig. 5b & d).
coupled in NAc MSNs of Dbh −/− mice. To test this idea,
Application of the Gαs inhibitor, NF449, did not
we assessed the effects of quinpirole on MSN excitability
occlude the inhibitory effects of D2 activation in MSNs
in the presence of either a Gαi (NF023; 10 μM) or a
from control mice; no changes in spike frequency
Gαs (NF449; 1 μM) inhibitor (Freissmuth et al. 1996;
were observed when compared with quinpirole alone
Hohenegger et al. 1998). As expected for a Gαi-coupled
[χ2 (d.f. = 1) = 0.14, P = 0.71] (Fig. 6a & c). However,
receptor like D2, application of NF023 to control MSNs
application of NF449 in Dbh −/− MSNs significantly
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
Figure 4 Quinpirole inhibits evoked firing of NAc MSNs from
Figure 3 Activation of D2 receptors similarly inhibits evoked firing
control, but not Dbh −/− mice. Example traces of a MSN from a
of NAc MSNs from Dbh +/− and C57BL/6J mice. Example traces of
control (a) and Dbh −/− (b) animal at baseline, and following bath
a MSN from a Dbh +/− (a) and a wild-type C57BL/6J (b) animal at
application of the D2/3 agonist, quinpirole (5 μM), while action
baseline and following bath application of the D2/3 agonist,
potentials were evoked with a series of current steps. Dashed line
quinpirole (5 μM), while action potentials were evoked with a series
indicates −70 mV. (c) Population data (mean ± SEM) showing the
of current steps. Dashed line indicates −70 mV. (c) Population data
effects of D2-like receptor activation on evoked spike frequency
(mean ± SEM) showing the effects of D2 receptor activation on spike
differences (quinpirole − baseline) from cells recorded from control
frequency differences (quinpirole − baseline) from cells recorded
(n = 46) and Dbh −/− (n = 15) mice. *Indicates significant difference
from Dbh +/− (n = 22) and C57BL/6J (n = 24) mice
(P < 0.05) between control and Dbh −/− population data
2014 Society for the Study of Addiction
Addiction Biology
Cocaine response in DBH mice
Figure 5 Gαi inhibition abolishes quinpirole-mediated inhibition of MSN spike excitability in control mice, but has no effect in Dbh −/− mice.
Example traces from MSNs recorded from a control (a) and a Dbh −/− (b) animal at baseline or following bath application of quinpirole (5 μM)
with the Gαi inhibitor NF023 (10 μM) applied internally, while action potentials were evoked with a series of current steps. Dashed line
indicates −70 mV. (c) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences
(quinpirole − baseline) in the presence of vehicle (n = 46) or NF023 (n = 10) from cells recorded from control mice. (d) Population data
(mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole − baseline) in the presence
of vehicle (n = 15) or NF023 (n = 17) from cells recorded from Dbh −/− mice. *Indicates significant difference (P < 0.05) between vehicle and
NF023 population data
reduced spike frequency compared with quinpirole alone
including psychostimulant drugs of abuse (Weinshenker
[χ2 (d.f. = 1) = 14.99, P = 0.0001] (Fig. 6b & d). Com-
et al. 2002, 2008; Haile et al. 2003; Schank et al. 2006;
bined, these results suggest that D2 receptors on NAc
Gaval-Cruz et al. 2012). We identified a decrease in
MSNs primarily couple to Gαi and suppress firing rate in
βArr2 and an increase in ΔFosB in the NAc
control mice, but that D2-G-protein coupling is altered
following chronic genetic or pharmacological DBH
and quinpirole-induced inhibition is lost in Dbh −/− NAc
inhibition, and overexpression of βArr2 normalized
cocaine responses in Dbh −/− mice. Slice electro-physiology experiments revealed that MSNs from
control mice are inhibited by quinpirole in a Gαi-
dependent manner, while the effects of quinpirole are
Pharmacological and genetic DBH inhibition leads
altered and become sensitive to Gαs blockade in Dbh −/−
to behavioral hypersensitivity to dopaminergic drugs,
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
Figure 6 Gαs inhibition has no effect on quinpirole-mediated inhibition in control mice, but restores quinpirole-mediated inhibition of MSN
spike frequency in Dbh −/− mice. Example traces from MSNs recorded from a control (a) and a Dbh −/− (b) animal at baseline or following
bath application of quinpirole (5 μM) with the Gαs inhibitor, NF449 (10 μM; slices pre-incubated in the Gαs antagonist for 1 hour prior to
recording, and then continuously exposed to NF449 throughout the recording process), while action potentials were evoked with a series of
current steps. Dashed line indicates −70 mV. (c) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evokedspike frequency differences (quinpirole − baseline) in the presence of vehicle (n = 46) or NF449 (n = 11) from cells recorded from controlmice. (d) Population data (mean ± SEM) showing the effects of D2-like receptor activation on evoked spike frequency differences (quinpirole− baseline) in the presence of vehicle (n = 15) or NF449 (n = 22) from cells recorded from Dbh −/− mice. *Indicates significant difference(P < 0.05) between vehicle and NF449 population data
Chronic DBH inhibition alters the abundance of DA
Weinshenker & Schroeder 2007), producing a compen-
receptor signaling proteins in the NAc
satory up-regulation of D2 signaling and hypersensitivity
to psychostimulants and quinpirole (Weinshenker et al.
Because DBH catalyzes the conversion of NE to DA in
2002, 2008; Schank et al. 2006). Indeed, neurotoxic
noradrenergic neurons, DBH inhibition decreases NE
ablation of brain NE neurons, which reduces NE without
production, with a concomitant increase in tissue DA
an increase in tissue DA, confers a similar pattern of drug
et al.
responses (Harro et al. 2000; Weinshenker et al. 2008;
Bourdelat-Parks et al. 2005). However, because NE pro-
Nowak et al. 2009).
vides direct and indirect excitatory drives onto midbrain
We originally reported increased high-affinity state D2
DA neurons, basal and stimulant-evoked DA overflow is
receptors in the striatum of Dbh −/− mice, which we
actually reduced in Dbh −/− mice (Schank et al. 2006;
speculated might underlie the behavioral hypersensitivity
2014 Society for the Study of Addiction
Addiction Biology
Cocaine response in DBH mice
of the knockouts to psychostimulants (Schank et al.
frequency in MSNs. By contrast, Gαs-coupled receptors,
2006). However, subsequent in vitro radioligand compe-
such as D1, have a facilitatory effect on MSN responses
tition experiments failed to confirm these results
(Hu & Wang 1988; West & Grace 2002; Surmeier et al.
(Skinbjerg et al. 2010) (our unpublished data). The dis-
2010). Our electrophysiological recordings from NAc
crepancy between studies measuring D2 affinity states
neurons confirmed that the D2/3 agonist, quinpirole,
may be due to some differences in the radioligands
suppressed evoked MSN firing in slices from control mice.
and approaches employed, but more concerning was
By contrast, the inhibitory effects of quinpirole were abol-
our failure to observe a genotype difference in the abun-
ished in Dbh −/− MSNs, and in fact there was a trend for
dance of high-affinity state D2 receptors in vivo using
quinpirole to be excitatory.
positron emission tomography imaging (Skinbjerg et al.
There are several potential explanations for this
change in cellular response to D2/3 activation. For
Because of these issues and inconsistencies, we sus-
example, the involvement of βArr2 in receptor desensiti-
pected that changes in downstream signaling molecules,
zation and endocytosis suggests a possible contribution of
rather than D2 receptor affinity state, were responsible for
altered D2 trafficking and localization. In addition,
cocaine hypersensitivity following chronic NE deficiency.
because βArr2 recruits cAMP-degrading phospho-
Both genetic and pharmacological DBH inhibition pro-
diesterase to the membrane upon receptor binding (Perry
duced a decrease of βArr2 and an increase of ΔFosB in the
et al. 2002; Kendall & Luttrell 2009), reduction of βArr2
NAc. ΔFosB is a transcription factor that is induced by
could dampen inhibition and promote excitation by
chronic exposure to drugs or other environmental stimuli
potentiating cAMP abundance. The reduction in βArr2
and enhances behavioral responses to cocaine (Kelz et al.
could also affect neuronal firing by altering G-protein-
1999). We chose to pursue the contribution of βArr2
independent GSK3β/Akt signaling, although we did not
because it is upstream of ΔFosB in the DA receptor
detect any differences in these proteins in the NAc of Dbh
signaling pathway, and because it had not been implicated
−/− mice. It is also possible that the decrease in βArr2 and
in cocaine-induced behaviors; locomotor activity and
the altered quinpirole response are unrelated. Future
conditioned place preference following cocaine adminis-
experiments to determine whether loss of βArr2 directly
tration are unchanged in βArr2 knockout mice (Bohn
causes aberrant D2/3 signaling will help identify the
et al. 2003). We found that viral-mediated overexpression
of βArr2 in the NAc suppressed the cocaine hypersensi-
Given that NF023 abolished quinpirole-induced inhi-
tivity in Dbh −/− mice. This effect was not due to a general
bition in MSNs from control mice, but had no effect on
motor activity because ambulatory
quinpirole response in Dbh −/− MSNs, while NF449 had
behavior in a novel environment was unaffected by
no effect on quinpirole-induced inhibition in control
βArr2 overexpression, and cocaine-induced locomotion
MSNs, but suppressed firing in the presence of quinpirole
was normalized to, but not below, control levels. Because
in Dbh −/− mice, it is possible that at least some D2 recep-
we examined and used a non-selective CMV promoter to
tors are coupled to Gαs instead of Gαi in Dbh −/− MSNs. In
drive overexpression of βArr2, we cannot attribute its
vivo Gαi-to-Gαs switching has been reported for μ-opioid
effects on cocaine-induced locomotion specifically to
receptors and CB1 cannabinoid receptors following
changes in D2 signaling. Future experiments using D1-
chronic agonist exposure (Wang et al. 2005; Paquette
and D2-specific promoters will be required to delineate the
et al. 2007), and a reduction in βArr2 promotes Gαs-to-
importance of βArr2 in direct versus indirect pathway
Gαi switching of β-adrenergic receptors (Baillie et al.
MSNs. The discrepancies between the βArr2-mediated
2003). However, these data are associated with several
phenotypes in Dbh −/− and βArr2 knockout mice may be
limitations and must be interpreted with caution.
due to the complete lack of global βArr2 in the βArr2
Quinpirole is an agonist of both D2 and D3 DA receptors,
knockout mice versus the partial reduction of βArr2
and both subtypes are present in the NAc. Thus, the Gαs-
specifically in the NAc of Dbh −/− mice.
to-Gαi switch could be solely or preferentially affectingone of these two subtypes. D3 receptors and D2-D3
heterodimer receptors have been reported to couple to
Aberrant cellular responses to quinpirole in NAc MSNs
Gαq, and thus promote neuronal excitation. However,
of Dbh −/− mice
increased D3-Gαq or D2/D3-Gαq heterodimer signaling is
Because normal D2 autoreceptor function is preserved in
unlikely to underlie quinpirole-induced excitation in Dbh
Dbh −/− mice (Paladini, Beckstead & Weinshenker 2007),
−/− MSNs for two reasons. First, Dbh −/− mice are hyper-
we focused our attention on potential changes in D2
sensitive to quinpirole but not the preferential D2/D3
receptor signaling in accumbal MSNs. Activation of D2
heterodimer agonist SKF83959 (our unpublished data).
and other Gαi/o-coupled receptors typically inhibits
Second, the altered response of Dbh −/− MSNs to
evoked action potentials, reducing firing and spike
quinpirole is blocked by the Gαs inhibitor NF449, which
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
does not interfere with Gαq signaling. NF023 and NF449
providing the nepicastat, and C. Strauss for helpful
are not totally selective for Gαi and Gαs; e.g. both com-
editing of the manuscript. This work was supported by
pounds are also P2X and P2Y receptor antagonists
the National Institute of Drug Abuse, National Institute
(Lambrecht 1996; Braun et al. 2001). A contribution of
of Mental Health, and National Institute of Neurological
purine/pyrimidine receptors is nevertheless unlikely
Disorders and Stroke (NIDA grants DA017963 and
given our results because, as a ligand-gated ion channel,
DA027535 to D.W., DA25040 and DA015040 to M.G.C.,
would have a direct effect on membrane potential and
and DA030530 to C.A.P.; NIMH grant MH079276 to
firing rate, which we did not observe. NF023 and NF449
C.A.P.; and NINDS grant NS060658 to C.A.P.).
also had different effects in our different groups of
animals, their inhibition which is inconsistent with these
Disclosure/Conflict of Interest
drugs acting as purinergic/pyrimidinergic antagonists.
DW is coinventor on a patent concerning the use of selec-
Finally, our methods did not allow us to distinguish
tive DBH inhibitors for the treatment of cocaine depend-
between D1 and D2 MSNs, which precludes assigning
ence (US-201-0274303-A1; ‘Methods and Compositions
direct effects of quinpirole and could also account for the
for Treatment of Drug Addiction'). MGC, RBG, DJP, DEB,
high variability in some of our experiments, particularly
RCM, RAH, DJ and CAP declare no conflict of interest.
the ones involving Dbh −/− recordings. In addition, itwill be important to test alterations in D2-G-protein
associations using other techniques such as co-
immunoprecipitation. Unfortunately, D2 antibodies of
MGC, RBG, CAP and DW participated in the research
sufficient quality and specificity for this approach are not
design. MGC, DJP and RCM conducted the Western blot
experiments. MGC and DJP conducted the behavioral
experiments. RBG conducted the electrophysiology
experiments. DK conducted the statistical analysis of the
electrophysiology experiments. RAH and DEB contrib-
The consequences of chronic reduction in DBH function
uted analytic tools. MGC, RBG, CAP and DW wrote the
may be relevant to drug addiction. Non-selective DBH
inhibitors, like disulfiram, have shown promise in human
laboratory studies and clinical trials for the treatment of
stimulant dependence (Gaval-Cruz & Weinshenker
2009), and a large phase II trial of nepicastat for cocaine
Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ,
Houslay MD (2003) Beta-arrestin-mediated PDE4 cAMP
NCT01704196). Genetic or chronic pharmacological
phosphodiesterase recruitment regulates beta-adrenoceptor
reduction of DBH activity enhances some interoceptive
switching from Gs to Gi. Proc Natl Acad Sci U S A 100:940–945.
properties of cocaine, particularly its aversive effects,
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling,
such as paranoia and anxiety (Hameedi et al. 1995;
and pharmacology of dopamine receptors. Pharmacol Rev
McCance-Katz, Kosten & Jatlow 1998a,b; Schank et al.
2006; Kalayasiri et al. 2007; Sofuoglu et al. 2008;
Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF,
Gaval-Cruz & Weinshenker 2009; Mutschler, Diehl &
Gremel CM, Christensen CH, Adrover MF, Alvarez VA (2013)Strengthening the accumbal indirect pathway promotes resil-
Kiefer 2009), suggesting that the clinical efficacy of DBH
ience to compulsive cocaine use. Nat Neurosci 16:632–
inhibitors is related to an increase in cocaine aversion.
Interestingly, optogenetic inhibition of D2 MSNs drives,
Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO,
while excitation of D2 MSNs inhibits, compulsive cocaine
Lefkowitz RJ, Dykstra LA, Caron MG (2003) Enhanced
seeking in mice (Bock et al. 2013). Thus, under normal
rewarding properties of morphine, but not cocaine, inbeta(arrestin)-2 knock-out mice. J Neurosci 23:10265–
conditions, cocaine may facilitate its own use by promot-
ing DA signaling via the D2 receptor and suppressing D2
Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM,
MSN activity; however, our data suggest that, under con-
Bonsall RW, Emery MS, Liles LC, Weinshenker D (2005)
ditions of chronic DBH inhibition, cocaine may actually
Effects of dopamine beta-hydroxylase genotype and disulfiram
limit its own use because D2-mediated inhibition is
Psychopharmacology (Berl) 183:72–80.
absent, leading to increased cell excitability.
Braun K, Rettinger J, Ganso M, Kassack M, Hildebrandt C,
Ullmann H, Nickel P, Schmalzing G, Lambrecht G (2001)
NF449: a subnanomolar potency antagonist at recombinantrat P2X1 receptors. Naunyn Schmiedebergs Arch Pharmacol
We thank Dainippon-Sumitomo Pharmaceuticals Inc.
(Osaka, Japan) for providing the DOPS needed to
Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM,
maintain our Dbh mouse colony, Synosia Therapeutics for
Malison RT, Price LH, Gelernter J (2000) A haplotype at the
2014 Society for the Study of Addiction
Addiction Biology
Cocaine response in DBH mice
DBH locus, associated with low plasma dopamine beta-
McCance-Katz EF, Kosten TR, Jatlow P (1998a) Chronic disulfi-
hydroxylase activity, also associates with cocaine-induced
ram treatment effects on intranasal cocaine administration:
paranoia. Mol Psychiatry 5:56–63.
initial results. Biol Psychiatry 43:540–543.
Del'guidice T, Lemasson M, Beaulieu JM (2011) Role of beta-
McCance-Katz EF, Kosten TR, Jatlow P (1998b) Disulfiram
arrestin 2 downstream of dopamine receptors in the Basal
effects on acute cocaine administration. Drug Alcohol Depend
Ganglia. Front Neuroanat 5:58.
Freissmuth M, Boehm S, Beindl W, Nickel P, Ijzerman AP,
Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D
Hohenegger M, Nanoff C (1996) Suramin analogues as
(2006) The effects of norepinephrine transporter inactivation
subtype-selective G protein inhibitors. Mol Pharmacol
on locomotor activity in mice. Biol Psychiatry 60:1046–
Musacchio JM, Goldstein M, Anagnoste B, Poch G, Kopin IJ
disulfiram-induced cocaine abstinence: antabuse and cocaine
(1966) Inhibition of dopamine-beta-hydroxylase by disulfi-
relapse. Mol Interv 9:175–187.
ram in vivo. J Pharmacol Exp Ther 152:56–61.
Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D (2012)
Mutschler J, Diehl A, Kiefer F (2009) Pronounced paranoia as a
Chronic inhibition of dopamine beta-hydroxylase facilitates
result of cocaine-disulfiram interaction: case report and mode
behavioral responses to cocaine in mice. PLoS ONE 7:e50583.
of action. J Clin Psychopharmacol 29:99–101.
Goldstein M (1966) Inhibition of norepinephrine biosynthesis at
Nowak P, Nitka D, Kwiecinski A, Josko J, Drab J, Pojda-Wilczek D,
the dopamine-beta-hydroxylation stage. Pharmacol Rev
Kasperski J, Kostrzewa RM, Brus R (2009) Neonatal co-lesion
by DSP-4 and 5,7-DHT produces adulthood behavioral
Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA (2003)
sensitization to dopamine D(2) receptor agonists. Pharmacol
Disulfiram facilitates the development and expression of loco-
Rep 61:311–318.
motor sensitization to cocaine in rats. Biol Psychiatry 54:915–
Electrophysiological properties of catecholaminergic neurons
Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price
LH, Jatlow PI, Woods SW, Kosten TR (1995) Behavioral,
physiological, and pharmacological interaction of cocaine
Paquette JJ, Wang HY, Bakshi K, Olmstead MC (2007)
and disulfiram in humans. Biol Psychiatry 37:560–563.
Cannabinoid-induced tolerance is associated with a CB1
Harro J, Merikula A, Lepiku M, Modiri AR, Rinken A, Oreland L
receptor G protein coupling switch that is prevented by
(2000) Lesioning of locus coeruleus projections by DSP-4
ultra-low dose rimonabant. Behav Pharmacol 18:767–
hyperlocomotion and dopamine D2 receptor binding in rats.
Perez MF, White FJ, Hu XT (2006) Dopamine D(2) receptor
Pharmacology & Toxicology 86:197–202.
modulation of K(+) channel activity regulates excitability of
Hohenegger M, Waldhoer M, Beindl W, Boing B, Kreimeyer A,
nucleus accumbens neurons at different membrane poten-
Nickel P, Nanoff C, Freissmuth M (1998) Gsalpha-selective G
tials. J Neurophysiol 96:2217–2228.
protein antagonists. Proc Natl Acad Sci U S A 95:346–351.
Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL,
Hu XT, Wang RY (1988) Comparison of effects of D-1 and D-2
Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ
dopamine receptor agonists on neurons in the rat caudate
(2002) Targeting of
cyclic AMP degradation to beta
putamen: an electrophysiological study. J Neurosci 8:4340–
2-adrenergic receptors by beta-arrestins. Science 298:834–
Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V,
Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller
Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT
GW, Weinshenker D (2007) Norepinephrine loss produces
(2007) Dopamine beta-hydroxylase gene (DbetaH) -1021C–
more profound motor deficits than MPTP treatment in mice.
>:T influences self-reported paranoia during cocaine self-
Proc Natl Acad Sci U S A 104:13804–13809.
administration. Biol Psychiatry 61:1310–1313.
Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD,
Kapoor A, Shandilya M, Kundu S (2011) Structural insight of
Liles LC, Seeman P, Weinshenker D (2006) Dopamine
dopamine beta-hydroxylase, a drug target for complex traits,
beta-hydroxylase knockout mice have alterations in dopamine
and functional significance of exonic single nucleotide
signaling and are hypersensitive to cocaine. Neuropsycho-
polymorphisms. PLoS ONE 6:e26509.
Kelz MB, Chen J, Carlezon WA Jr., Whisler K, Gilden L, Beckmann
Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M,
AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T,
Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM,
Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto
Edwards GL, Holmes PV, Weinshenker D (2010) Disulfiram
MR, Nestler EJ (1999) Expression of the transcription factor
attenuates drug-primed reinstatement of cocaine seeking via
deltaFosB in the brain controls sensitivity to cocaine. Nature
dopamine beta-hydroxylase. Neuropsycho-
Kendall RT, Luttrell LM (2009) Diversity in arrestin function.
Schroeder JP, Epps SA, Grice TW, Weinshenker D (2013) The
Cell Mol Life Sci 66:2953–2973.
selective dopamine beta-hydroxylase inhibitor nepicastat
Lambrecht G (1996) Design and pharmacology of selective
attenuates multiple aspects of cocaine-seeking behavior.
P2-purinoceptor antagonists. J Auton Pharmacol 16:341–
Skinbjerg M, Seneca N, Liow JS, Hong J, Weinshenker D, Pike
Maj J, Przegalinski E, Wielosz M (1968) Disulfiram and the drug-
VW, Halldin C, Sibley DR, Innis RB (2010) Dopamine beta-
induced effects on motility. J Pharm Pharmacol 20:247–248.
hydroxylase-deficient mice have normal densities of D(2)
Manvich DF, Depoy L, Weinshenker D (2013) Dopamine beta-
dopamine receptors in the high-affinity state based on in vivo
hydroxylase inhibitors enhance the discriminative stimulus
PET imaging and in vitro radioligand binding. Synapse
effects of cocaine in rats. J Pharmacol Exp Ther 347:564–573.
2014 Society for the Study of Addiction
Addiction Biology
Meriem Gaval-Cruz et al.
Sofuoglu M, Poling J, Waters A, Sewell A, Hill K, Kosten T (2008)
Weinshenker D, Schroeder JP (2007) There and back again: a
Disulfiram enhances subjective effects of dextroamphetamine
tale of norepinephrine and drug addiction. Neuropsycho-
in humans. Pharmacol Biochem Behav 90:394–398.
Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S,
Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter
Walker K, Martinez G, Eglen RM, Whiting RL, Hegde SS (1997)
RD (2002) Mice with chronic norepinephrine deficiency
Catecholamine modulatory effects of nepicastat (RS-25560-
resemble amphetamine-sensitized animals. Proc Natl Acad Sci
197), a novel, potent and selective inhibitor of dopamine-beta-
U S A 99:13873–13877.
hydroxylase. Br J Pharmacol 121:1803–1809.
Weinshenker D, Ferrucci M, Busceti CL, Biagioni F, Lazzeri G,
Surmeier DJ, Kitai ST (1993) D1 and D2 dopamine receptor
Liles LC, Lenzi P, Pasquali L, Murri L, Paparelli A, Fornai F
modulation of sodium and potassium currents in rat
(2008) Genetic or pharmacological blockade of noradre-
neostriatal neurons. Prog Brain Res 99:309–324.
naline synthesis enhances the neurochemical, behavioral,
Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin
and neurotoxic effects of methamphetamine. J Neurochem
JL (2010) The role of dopamine in modulating the structure
and function of striatal circuits. Prog Brain Res 183:149–
Weinshilboum RM (1978) Serum dopamine beta-hydroxylase.
Pharmacol Rev 30:133–166.
Thomas SA, Matsumoto AM, Palmiter RD (1995) Noradrenaline
West AR, Grace AA (2002) Opposite influences of endogenous
is essential for mouse fetal development. Nature 374:643–
dopamine D1 and D2 receptor activation on activity states and
electrophysiological properties of striatal neurons: studies
Thomas SA, Marck BT, Palmiter RD, Matsumoto AM (1998)
combining in vivo intracellular recordings and reverse
Restoration of norepinephrine and reversal of phenotypes in
microdialysis. J Neurosci 22:294–304.
mice lacking dopamine beta-hydroxylase. J Neurochem
Yasumoto S, Tanaka E, Hattori G, Maeda H, Higashi H (2002)
Direct and indirect actions of dopamine on the membrane
Wang HY, Friedman E, Olmstead MC, Burns LH (2005) Ultra-
potential in medium spiny neurons of the mouse neostriatum.
low-dose naloxone suppresses opioid tolerance, dependence
J Neurophysiol 87:1234–1243.
and associated changes in mu opioid receptor-G protein cou-
Zamponi GW, Snutch TP (1998) Modulation of voltage-
pling and Gbetagamma signaling. Neuroscience 135:247–
dependent calcium channels by G proteins. Curr Opin
2014 Society for the Study of Addiction
Addiction Biology
Source: http://www.pharm.emory.edu/rhall/GavalCruz2014.pdf
Neuroscience Letters 290 (2000) 137±140 Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain Christopher J. LaBuda, Perry N Fuchs* Department of Psychology, University of Texas at Arlington, PO Box 19528, Arlington, TX 76019, USA Received 3 April 2000; received in revised form 30 June 2000; accepted 5 July 2000
Ergebnisdarstellung des Experten-Arbeitskreises „Verdauung und Ausscheidung" Der dritte Expertenarbeitskreis des Forum Wartaweil hat sich zum Ziel gesetzt, das meist nur am Rande behandelte und in der Fachliteratur eher vernachlässigte Thema der Verdauung und Ausscheidung von Menschen mit schwersten Behinderungen und Lebenseinschränkungen transdisziplinär aufzuarbeiten. Folgende Ergebnisse können festgehalten werden: Begegnung mit dem Thema im Alltag Für Eltern ist das Thema „Verdauung und Ausscheidung" ein sehr zentrales, über das sie sich untereinander (z.B. in Elterninitiativen) regelmäßig und intensiv austauschen. Professionelle in Einrichtungen der Behindertenhilfe, die in ihrem pflegerischen Arbeitsalltag mit diesem Thema konfrontiert sind, sprechen ebenso offen und häufig mit Kollegen über dieses Thema. Ein Defizit in der literarischen Aufarbeitung des Themas bestätigen und beklagen alle Teilnehmer der Gesprächsrunde. Die Erschwernisse bei der Nahrungsaufnahme sind ausreichend wissenschaftlich aufgearbeitet und publiziert, die oftmals problematische Ausscheidung, medizinisch im Fachgebiet der (pädiatrischen) Gastroenterologen angesiedelt, findet jedoch nur im Kontext persönlicher Betroffenheit Beachtung. Auch die sonderpädagogische Fachrichtung (Schwerstbehindertenpädagogik) hat sich in noch nicht ausreichendem Maße dieser Thematik angenommen. Gerade Jugendliche mit Verdauungsproblemen aufgrund schwerster Behinderungen können dieses Thema als sehr belastend erleben. Entsprechend finden Gespräche zwischen Pflegenden und zu Pflegenden statt. Die Problematik eines nicht täglich funktionierenden Stuhlganges wird von medizinischer Seite u.U. seltener gesehen, jede Darmausscheidung zwischen drei mal täglich bis zwei mal wöchentlich liegt im Bereich des Vertretbaren. Auch die dauerhafte Einnahme von dosierten Abführmitteln gilt als unbedenklich. In Einrichtungen für Menschen mit schwersten Mehrfachbehinderungen sind die wenigsten Betreuten kontinent, fast alle haben Probleme mit der Ausscheidung, gleichzeitig müssen Wege der (unterstützten) Kommunikation gefunden werden, um den Betroffenen den Ausdruck von Wünschen und Bedürfnissen auch in diesem Bereich zu ermöglichen. Ursachen von Verdauungs- und Ausscheidungsproblemen bei Kindern mit Behinderungen Verdauungs- und Ausscheidungsprobleme von Kindern mit schweren Körper- und Mehrfachbehinderungen können durch die Addition unterschiedlicher Ursachen zustande kommen:















