Nuvaring-ci_e
IMPORTANT: PLEASE READ
PART III: CONSUMER INFORMATION
Reported Pregnancies per 100 Women per Year
Combination pill
less than 1 to 2
NUVARING®
Contraceptive vaginal ring
etonogestrel/ethinyl estradiol slow release vaginal ring
Intrauterine device (IUD)
less than 1 to 6
Condom with spermicidal foam or gel
CONTRACEPTIVE VAGINAL RING
This leaflet is part III of a three-part "Product Monograph"
Diaphragm with spermicidal foam or gel
published when NuvaRing® was approved for sale in Canada, and is
designed specifically for Consumers. This leaflet is a summary and
Sponge with spermicide
will not tell you everything about NuvaRing®. Contact your doctor
Cervical cap with spermicide
or pharmacist if you have any questions about the use of this
Periodic abstinence (rhythm), all types
No birth control
Pregnancy rates vary widely because people differ in how
READ THIS PAMPHLET CAREFULLY BEFORE YOU
consistently and/or correctly they use each method. (This does not
START USING NUVARING®
.
apply to IUDs since they are implanted in the uterus). When used as
directed, users may achieve pregnancy rates in the lower ranges.
Others may expect pregnancy rates more in the middle ranges.
ABOUT THIS MEDICATION
Hormonal contraceptives (such as NuvaRing®) have important
What the medication is used for:
advantages over other methods of birth control. They also have
NuvaRing® (NEW-vah-ring) is a flexible contraceptive vaginal ring
certain risks that other methods do not. Your doctor is the best
used to prevent pregnancy.
person to explain the consequences of any possible risks.
When it should not be used:
Since NuvaRing® releases two different types of hormones, an
Hormonal contraceptives are not suitable for every woman. You
estrogen and a progestin, it is called a combination hormonal
should not use combination hormonal contraceptives (including
contraceptive (CHC). NuvaRing® delivers 120 mcg of the progestin
NuvaRing®) if you have or have had any of the following
etonogestrel and 15 mcg/day of the estrogen, ethinyl estradiol.
NuvaRing® works by releasing a steady dose of progestin and
blood clots in the legs, lungs, eyes, or elsewhere. For additional
estrogen into the body. The ring is inserted into the vagina and left
information, see section "RISKS OF USING HORMONAL
in place for 3 weeks in a row.
CONTRACEPTIVES – Circulatory disorders"
a stroke, heart attack, chest pain (angina pectoris) or other
Like other combination hormonal contraceptives, NuvaRing® works
blood circulatory disorders in the brain
disease of the heart valves with complications
1. By inhibiting the monthly release of an egg by the ovaries.
know abnormalities of the blood clotting system that increase
2. By changing the mucus produced by the cervix. This slows the
your risk for developing blood clots
movement of the sperm through the mucus into the uterus further
severe high blood pressure
reducing the chance of fertilization.
diabetes with damaged blood vessels
NuvaRing® has been shown to be highly effective in preventing
very high blood cholesterol or triglyceride levels
pregnancy when used as prescribed.
you smoke and are over age 35
if you have major surgery (e.g. an operation) and your ability to
When used according to directions, NuvaRing® is 98 to 99% effective
move around is limited for a long period of time (see "RISKS
at preventing pregnancy. This means that, for every 100 women who
OF USING HORMONAL CONTRACEPTIVES – Circulatory
use NuvaRing® for a year, about one or two will become pregnant.
Your chance of getting pregnant increases if NuvaRing® is not used
known or suspected cancer of the breast or sex organs
exactly according to the directions.
liver tumor associated with the use of the pill or other estrogen-
containing products
Other ways to prevent pregnancy
jaundice (yellowing of the eyes or skin), liver disease or liver
Other methods of birth control are available to you.
cancers which are caused by or enhanced by estrogen
The following table gives reported pregnancy rates for various
eye diseases, eye lesions or defects or loss of vision
forms of birth control, including no birth control. The reported rates
have (had) a type of migraine called ‘migraine with aura'
represent the number of women out of 100 who would become
unusual vaginal bleeding that has not yet been diagnosed
pregnant in one year.
pancreatitis (inflammation of the pancreas) associated with high
levels of fatty substances in your blood
if you are pregnant or think you might be pregnant
allergic reactions or hypersensitivity to the hormones found in
the contraceptives or to any of the other components found in NuvaRing®
What the medicinal ingredient is:
an abdominal exam and a pelvic exam, including a Pap smear. Visit
etonogestrel and ethinyl estradiol
your doctor three months or sooner after the initial examination.
Afterward, visit your doctor at least once a year. Use NuvaRing®
What the important nonmedicinal ingredients are:
only on the advice of your doctor and carefully follow all directions
ethylene vinylacetate copolymers and magnesium stearate.
given to you. Use NuvaRing® exactly as prescribed or you could
NuvaRing® does not contain any latex.
become pregnant.
What dosage forms it comes in:
If you see another doctor, inform him or her that you are using
Slow-release vaginal ring – 11.4 mg etonogestrel/2.6 mg ethinyl
estradiol to deliver 120 mcg etonogestrel/15 mcg ethinyl estradiol
NuvaRing® may not be suitable for women with conditions that
make the vagina more susceptible to vaginal irritation or ulceration.
NuvaRing® is available in boxes of 1 sachet and 3 sachets.
In some cases, vaginal (fibrous) tissue may grow over the ring, necessitating removal by a doctor.
WARNINGS AND PRECAUTIONS
Pregnancy is almost always more risky than using combination
Serious Warnings and Precautions
hormonal contraceptives. However, this risk with hormonal
contraceptives can be higher if you are over 35 and you smoke.
Cigarette smoking increases the risk of cardiovascular side
effects (heart and blood vessel problems) associated with the use
If you and your doctor decide that, for you, the benefits of
of hormonal contraceptives. This risk increases with age,
NuvaRing® outweigh the risks, you should be aware of the
particularly in women over 35 years of age, and with the
number of cigarettes smoked. For this reason, NuvaRing®
should not be used by women who are over 35 years of age and
RISKS OF USING HORMONAL CONTRACEPTIVES
Specific studies with vaginal administration of contraceptive
hormones (as in NuvaRing®) are limited. The information given
NuvaRing®
(as with other hormonal contraceptives) DOES
below was obtained in studies with oral contraceptives (the Pill) and
NOT PROTECT against HIV infection (AIDS) and other
it may also apply to NuvaRing®.
Sexually Transmitted Infections (STIs). For protection against
STIs, it is advisable to use latex or polyurethane condoms while
Circulatory disorders (including blood clot in legs, lungs, heart, eyes
using NuvaRing®
.
Blood clots can develop whether or not you are using hormones for
BEFORE you use NuvaRing®
talk to your doctor or pharmacist if:
contraception. They can also happen if you are pregnant. The risk is
you are taking any other prescription or nonprescription drugs
higher in users of combined hormonal contraceptives, including
as these may interfere with the actions of NuvaRing®
NuvaRing® than in non-users, but it is not as high as the risk during
you are or will be having major surgery
pregnancy. You should talk to your doctor about the available options.
you have breast conditions
Blood clots can also occur very rarely in the blood vessels of the heart
you have a family history of breast cancer
(causing a heart attack) or the brain (causing a stroke). Extremely rarely
you have breast disorders including pain, discharge from the
blood clots can occur in the liver, gut, kidney or eye.
nipples, thickenings, or lumps
you have a family history of circulatory disorders including
Following an episode of a blood clot recovery is not always
blood clots, heart attacks or strokes
complete. Very occasionally serious permanent disabilities may
you have diabetes
occur or a blood clot may even be fatal.
you are overweight
you have high blood pressure
If you have to undergo an operation, are bedridden for some time, or
you have abnormal levels of fats in the bloodstream (high
you are not supposed to walk (for example, when you have your leg
cholesterol or triglycerides)
or legs in plaster, or a bandage is put on to treat varicose veins), the
you are a cigarette smoker
risk of having a blood clot may be temporarily higher. In women
you have migraine headaches
who use contraceptive hormones, the risk may be yet higher. In such
you have heart or kidney disease
a case, ask your doctor well in advance about what you should do.
you have a history of seizures or epilepsy
Your doctor may tell you to stop using your hormonal contraception
you have a history of mental depression
several weeks before surgery or at the time of immobilization. Your
you have fibroid tumors of the uterus
doctor will also tell you when you can start using NuvaRing® again
you have gallbladder or pancreatic disease
after you are back on your feet.
you have plans for forthcoming surgery
If you notice possible signs of a blood clot, stop using NuvaRing®
you have a history of jaundice or other liver disease
and consult your doctor immediately (
see the symptoms in section
‘Side Effects and What to do About Them').
Your doctor can advise you if you have any conditions that would
pose a risk to you. The use of combination hormonal contraceptives
Hormonal Contraceptives and Cancer
(including NuvaRing®) should always be supervised by your doctor,
Breast cancer: Breast cancer has been found slightly more often in
with regular follow up to identify side effects associated with its
women that take the Pill than in women of the same age who do not
use. Your visits may include a blood pressure check, a breast exam,
take the Pill. It is not known whether the increased risk of breast
cancer is caused by the use of a hormonal contraceptive. It may be
Irregular Bleeding
that the women taking such a hormonal contraceptive were
During the use of NuvaRing®, in some women, unexpected vaginal
examined more often, so that the breast cancer is noticed earlier.
bleeding (spotting or breakthrough bleeding) between periods may
occur. You may need to use sanitary protection, but continue to use
The most significant risk factors for breast cancer are increasing age
the ring as normal. If the irregular bleeding continues, becomes
and a strong history of breast cancer in the family (mother or sister).
heavy or starts again, tell your doctor.
Other established risk factors include, onset of menstrual periods
before age 12 years, never having children, having your first full-
Ring Disconnection
term pregnancy after the age of 30 years, never having breast fed a
Very rarely NuvaRing® may break. A broken ring is unlikely to
child, and daily alcohol consumption.
cause an overdose because the ring will not release a higher amount
of contraceptive hormones. If NuvaRing® breaks, expulsion is more
You should notify your doctor if you notice any breast lumps. You
likely to occur (see ‘What Should I do if NuvaRing® disconnects?').
should also discuss breast self-examination with your doctor. A
Therefore, if you notice that your NuvaRing® has broken, discard
yearly breast examination by a health care professional is
that ring and replace it with a new ring as soon as possible.
recommended for all women. You should also tell your doctor if a
close relative has or ever had breast cancer (see Warnings &
Risk to the Partner
The effects of hormones released by NuvaRing® on male partners
during sexual intercourse have not been studied.
Cervical cancer: Some studies have found an increase of cancer of
the cervix in women who use hormonal contraceptives, although
During market use, partner penis discomfort (e.g., pain, rash, bruises
this finding may be related to factors other than the use of oral
and abrasions), has been reported.
contraceptives. However, there is insufficient evidence to rule out
the possibility that oral contraceptives may cause such cancers.
INTERACTIONS WITH THIS MEDICATION
Chronic infection with the Human Papilloma Virus (HPV) is
believed to be the most important risk factor for cervical cancer. In
Certain drugs may interact with hormonal contraceptives (including
women who use combined oral contraceptives for a long time the
NuvaRing®) and prevent NuvaRing® from working properly. This
chance of getting cervical cancer may be slightly higher. This
can make hormonal contraceptives less effective in preventing
finding may not be caused by the Pill itself but may be related to
pregnancy or cause unexpected bleeding (spotting or breakthrough
sexual behavior and other factors.
bleeding). Hormonal contraceptives may also interfere with the
working of other drugs.
Liver tumors: In rare cases benign liver tumors and even more
rarely, malignant liver tumors have been reported in users of the
Please inform your doctor or pharmacist if you are taking or have
Pill. These tumors may lead to internal bleeding. Contact your
recently taken any other drugs or herbal products, even those
doctor immediately if you experience severe pain or a lump in the
without a prescription. Also, tell any other doctor or dentist who
prescribes another drug (or the dispensing pharmacist) that you use
NuvaRing®. They can tell you if you need to use an additional
Gallbladder disease
method of contraception and if so, for how long.
Users of hormonal contraceptives have a greater risk of developing
Drugs that may interact with NuvaRing® include:
gallbladder disease requiring surgery within the first year of use.
The risk may double after four or five years of use.
drugs used for the treatment of epilepsy (e.g. lamotrigine,
primidone, phenytoin, barbiturates, carbamazepine, oxcarbazepine,
Use in pregnancy
topiramate, felbamate); tuberculosis (e.g. rifampicin, rifabutin),
Hormonal contraceptives should not be taken by pregnant women.
HIV infections (e.g. ritonavir, nevirapine), and Hepatitis C Virus
There is no evidence, however, that the Pill can damage a
infections (e.g. boceprevir, telaprevir)
developing child. You should check with your doctor about risks to
antibiotics (e.g. penicillins, tetracyclines, metronidazole) for
your unborn child from any medication taken during pregnancy.
infectious diseases
antifungals (e.g. griseofulvin)
Use while breast feeding
anti-coagulants (blood thinners)
The hormones in contraceptives are known to appear in breast milk.
the herbal remedy, St. John's wort
These hormones may decrease the flow of breast milk if hormonal
antihypertensive drugs (for high blood pressure)
contraceptives are not resumed until nursing is established. Some of
antidiabetic drugs and insulin (for diabetes)
the medicine may pass through the milk to the baby and could cause
yellowing of the skin (jaundice) and breast enlargement.
sedatives and hypnotics (e.g. benzodiazepines, barbiturates,
chloral hydrate, glutethimide, meprobamate)
Pregnancy after stopping NuvaRing
®
A woman's menstrual period may be delayed after stopping
other drugs such as phenylbutazone, antihistamines, analgesics,
hormonal contraceptives. There is no evidence that the use of the
antimigraine preparations, or Vitamin E
contraceptive vaginal ring leads to a decrease in fertility. It is wise
cholesterol-lowering drugs (e.g. clofibrate)
to delay starting a pregnancy for at least one menstrual period after
cyclosporine
stopping hormonal contraceptives, so that way the pregnancy can be
antidepressants (e.g. clomipramine)
more accurately dated. Your doctor can recommend a different
(non-hormonal) method of contraception during this time.
Can I use tampons when using NuvaRing®
?
You should not use a NuvaRing® if it was dispensed to you more than
The blood levels of the hormones released by NuvaRing® were not
4 months before or if the expiry date has passed. The dispensing date
changed when women used tampons along with NuvaRing®. It is
and expiry date are both shown on the carton and sachet.
unknown how this affects the safety and the pregnancy protection of
NuvaRing®. Insert NuvaRing® before inserting a tampon. You should
Do not use the ring if you notice a colour change in the ring or any
pay particular attention when removing a tampon to be sure that the
visible signs of deterioration.
ring is not accidentally pulled out. If this should occur, simply rinse the
ring in cool to lukewarm (not hot) water and immediately reinsert it.
While using NuvaRing®, you should not rely upon a diaphragm or
cervical cap when you need a back-up method of birth control
Can I use vaginal medications?
because NuvaRing® may interfere with the correct placement and
The blood levels of the hormones released by NuvaRing® were not
position of these devices.
changed when women used vaginal, water-based spermicides
(nonoxynol or N-9 products) along with NuvaRing®.
When should I start NuvaRing®
?
Follow the instructions in one of the sections below to find out
The blood levels of the hormones released by NuvaRing® were
when to start using NuvaRing®:
increased when women used either an oil-based or water-based
vaginal medication (miconazole nitrate) for a yeast infection while
If you did not use a hormonal contraceptive in the preceding cycle
NuvaRing® was in place. Therefore, this may also happen with
Insert NuvaRing® within the first five days of your cycle. (i.e. Day 1–5
other yeast infection medications. The clinical relevance of this
of the menstrual bleeding). Make sure you also use an extra method of
increase is unknown. It is unknown how long-term use of
birth control (barrier method), such as male condoms or spermicides
spermicide or yeast infection medication with NuvaRing® affects
during the first seven days of NuvaRing® use in the first cycle.
the safety and the pregnancy protection of NuvaRing®.
If you are switching from a combined hormonal contraceptive containing both progestin and estrogen)
PROPER USE OF THIS MEDICATION
Switch from your previous combined hormonal contraceptive on
any day, but at the latest on the day you would have started a new
If you decide to use hormonal contraceptives
cycle, by inserting NuvaRing®. If you have been using your
If you and your doctor decide that, for you, the benefits of hormonal
hormonal contraceptive method consistently and correctly, no extra
contraceptives outweigh the risks, you should be aware of the
birth control method should be needed.
If you are switching from a progestin-only contraceptive (mini-pill,
1. Your doctor will advise you of the appropriate time to start the
implant, injection, or from a progestagen-releasing intra-uterine
use of hormonal contraceptives after childbirth, miscarriage, or
therapeutic abortion.
When switching from a mini-pill, you can stop using the pill on
2. There is no need to stop taking hormonal contraceptives for a
any day of the month and switch to NuvaRing®. Insert
NuvaRing® on the day immediately after your last pill.
When switching from an implant, progestin-containing IUS or
If you want more information about contraceptive vaginal rings, ask
injectable contraceptive, start using NuvaRing® on the same
your doctor or pharmacist.
day you have your implant or IUS removed or on the day your
next injection is due.
NuvaRing® is designed to be a once-a-month contraceptive regimen.
When you are switching from a progestin-only contraceptive, use an
The ring has to be inserted in your vagina. You can verify the
extra method of birth control, such as condoms and/or spermicide,
presence of NuvaRing® yourself, whenever you wish.
for the first seven days after inserting NuvaRing®.
After the ring is inserted, it releases a continuous low dose of
"Use after pregnancy, miscarriage or abortion"
hormones into your body. The ring stays in place for 3 weeks and
Talk to your doctor about using NuvaRing® following an abortion,
then is removed for a one week ring free period. It is not necessary
miscarriage or childbirth or under any other circumstances that are
or recommended to remove NuvaRing® during intercourse.
not listed in this Consumer Information.
READ THESE DIRECTIONS CAREFULLY
For the best protection from pregnancy, use NuvaRing® exactly as directed. Insert one NuvaRing® in the vagina and keep it in place for three weeks in a row. Remove it for a one-week break and then insert a new ring. During the one-week break, you will usually have your menstrual period. Your healthcare provider should examine you at least once a year. Do not use NuvaRing® for a condition for which it was not prescribed. Do not give NuvaRing® to anyone else who may want to use it.
How do I insert NuvaRing®?
Although some women may be aware of NuvaRing® in the vagina,
1. After washing and drying your hands, remove NuvaRing® from
most women do not feel it once it is in place. If you feel discomfort,
its foil pouch. Keep the foil pouch for proper disposal of the
use your finger to gently push NuvaRing® further into your vagina.
ring after use. Choose a position that is most comfortable for
There is no danger of NuvaRing® being pushed too far up in the
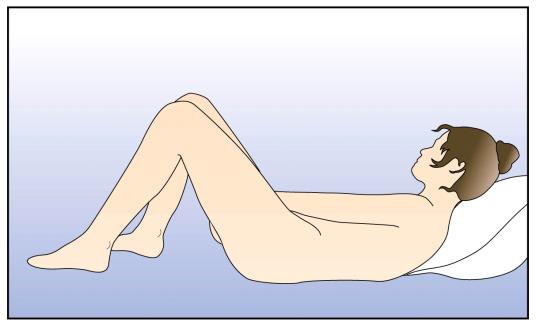
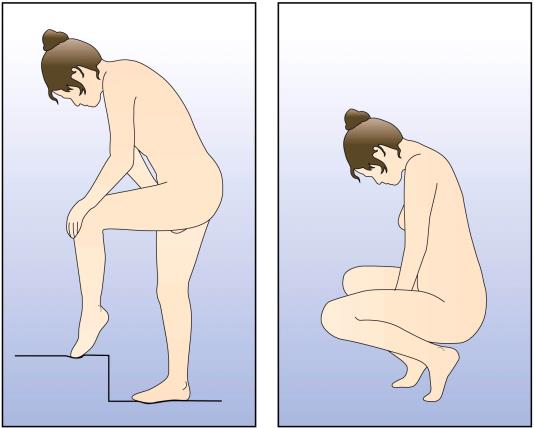
you (e.g., Figure 1).
vagina or getting lost.
3. Once inserted, keep NuvaRing® in place for three weeks in
How do I remove NuvaRing®?
1. Remove the ring three weeks after insertion on the same day of
the week as it was inserted, at about the same time. For example, when NuvaRing® is inserted on a Sunday at about 10:00 PM, the ring should be removed on the Sunday three weeks later at about
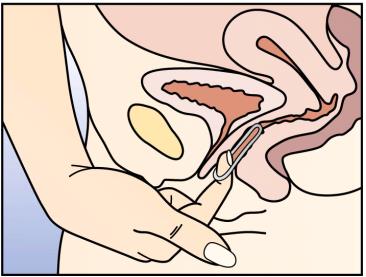
Remove NuvaRing® by hooking the index finger under the forward rim or by holding the rim between the index and middle finger and pulling it out (Figure 4).
Figure 1: Positions for NuvaRing® insertion
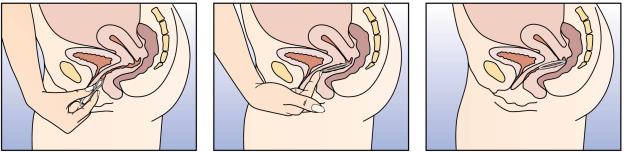
2. Press the sides of NuvaRing® together between your thumb and
2. Place the used ring in the reclosable foil pouch and properly
index finger (Figure 2) and gently push the folded ring into
dispose of it in a waste receptacle, out of the reach of children
your vagina (Figure 3). The exact position of NuvaRing® in the
and pets. Do not throw it in the toilet.
vagina is not important for it to work.
If you are unable to remove NuvaRing®, please contact your
healthcare provider.
Your menstrual period will usually start two to three days after the
ring is removed and may not have finished before the next ring is
inserted. To continue to have pregnancy protection, you must
insert the new ring one week after the last one was removed,
even if your menstrual period has not stopped.
When do I insert a new ring?
After a one-week ring-free break, insert a new ring on the same day, at
the same time of the week as it was removed in the last cycle. For
example, if NuvaRing® was removed on a Sunday at about 10:00 PM,
after the one-week break you should insert a new ring on a Sunday at
about 10:00 PM.
If NuvaRing® is in your vagina too long:
If NuvaRing® has been left in your vagina for an extra week or less
(up to four weeks total), you will remain protected. Remove
Figures 2: Holding NuvaRing® and pressing the sides
NuvaRing® and insert a new ring after a one-week ring-free break.
together.
If NuvaRing® has been left in place for more than four weeks total, there is a possibility that you could become pregnant. You must rule out pregnancy before inserting a new NuvaRing®. You must use an extra method of birth control, such as condoms and/or spermicide, until the new NuvaRing® has been in place for seven days in a row.
Figure 3: Inserting NuvaRing®.
What should I do if NuvaRing® disconnects?
If NuvaRing® has been out of the vagina for more than three
On rare occasions, NuvaRing® may disconnect at the weld joint
hours during the 1st or 2nd week, you may not be adequately
during use. Since the ring's core is solid its contents will remain intact
protected from pregnancy. Re-insert the ring as soon as you
and release of hormones will not be significantly affected. If
remember and use an extra method of birth control, such as
NuvaRing® does disconnect, expulsion (slipping out) is likely to
male condoms or spermicides, until the NuvaRing® has been in
occur (see "If NuvaRing® slips out"). If you discover the ring has
place continuously for seven days in a row.
disconnected you should discard the ring and replace it with a new
If NuvaRing® slips out of the vagina for more than 3 hours during
the 3rd week contraceptive efficacy may be reduced. Throw the ring
How to change the NuvaRing® start day to another day of the
away and choose one of the following two options:
If you wish to change the day on which you start a new NuvaRing®
1. Insert a new ring immediately. Note: Inserting a new ring will
cycle to another day of the week, complete the current cycle,
start the next three-week use period. You may not experience a
removing NuvaRing® on the same day of the week as the one on
period from your previous cycle. However, breakthrough
which you started. During the ring-free period, a new start day may
spotting or bleeding may occur.
be selected by inserting the new NuvaRing® on the first occurrence
2. Have your period and insert a new ring no later than 7 days from
of the desired day. This will be your new Day 1. In no case should
the time the previous ring was removed or expelled. Note: This
there be more than 7 consecutive ring-free days.
option should only be chosen if the ring was used continuously
for the preceding 7 days.
The shorter the ring-free interval, the higher the risk that you do not
have a period from your previous cycle. However, spotting or
In addition, a barrier method such as condoms and/or spermicides
bleeding may occur during the use of the next ring. This practice is
must be used until the new ring has been used continuously for
for a one-time only change and should not to be used as a standard
dosing regimen, as there are no long-term safety data available on
the continuous use of NuvaRing®.
Women with conditions affecting the vagina, such as a prolapsed
uterus, may be more likely to have NuvaRing® slip out of the
If you miss a menstrual period:
You must check to be sure that you are not pregnant if:
If the ring-free period is extended
1. you miss a period and NuvaRing® was out of the vagina for
If the ring-free interval has been extended beyond one week, the
more than three hours during the three weeks of ring use
possibility of pregnancy should be considered and an extra method
2. you miss a period and you had waited longer than one week to
of birth control, such as male condoms or spermicide MUST be
insert a new ring
used until NuvaRing® has been used continuously for seven days.
3. you have followed the instructions and you miss two periods in
Contact your doctor immediately. The longer the ring-free interval,
4. you have left NuvaRing® in place for longer than four weeks
the higher the risk that you have become pregnant
Overdose:
How well tolerated is NuvaRing®?
Overdosage of combination hormonal contraceptives may cause
More than 2,100 women were questioned in a survey of their
nausea, vomiting, vaginal bleeding, or other menstrual irregularities.
experiences using NuvaRing® for several months.
Given the nature and design of NuvaRing® it is unlikely that
overdosage will occur. If NuvaRing® is broken, it does not release a
Nearly all of the women found NuvaRing® easy to insert (96%) and
higher dose of hormones. There are no antidotes and further
remove (98%). Most women did not feel NuvaRing® once it was in
treatment should be symptomatic.
place and 83% of women said they never or rarely felt NuvaRing®
during intercourse. Similarly, 68% of women said their partners
In case of drug overdose, contact a health care practitioner, hospital
never or rarely felt the ring during intercourse, and 91% reported
emergency department or regional Poison Control Centre
that their partner did not mind them using the ring.
immediately, even if there are no symptoms.
Of the 1499 women who completed one year treatment (13 cycles)
Missed Dose:
with NuvaRing®, 96% reported they were satisfied with NuvaRing®,
If NuvaRing® slips out:
and 97% reported they would recommend NuvaRing® to others.
NuvaRing® can slip out of the vagina if it has not been inserted
85% of all women surveyed were satisfied with the use of
properly, or while removing a tampon, during intercourse or
NuvaRing® and 90% would recommend this method to others.
straining during a bowel movement.
Non-contraceptive benefits of hormonal contraceptives
If NuvaRing® slips out of the vagina, and it has been out for
Several health advantages have been linked to the use of hormonal
less than three hours, you should still be protected from
pregnancy. NuvaRing® can be rinsed with cool to lukewarm
Reduction in the incidence of cancer of the uterus and ovaries.
(not hot) water and should be re-inserted as soon as possible,
Reduction in the likelihood of developing benign (non-
and at the latest within three hours of expulsion (slipping out).
cancerous) breast disease and ovarian cysts.
If you have lost NuvaRing®, you must insert a new NuvaRing®
Less menstrual blood loss and more regular cycles. The risk of
and use it on the same schedule as you would have used the lost
developing iron-deficiency anemia is thus reduced.
There may be a decrease in painful menstruation and
weight increase;
premenstrual syndrome (PMS).
Acne, excessive hair growth and male-hormone-related
disorders also may be improved.
mood changes (e.g. depressive moods and emotional
Ectopic (tubal) pregnancy may occur less frequently.
Acute pelvic inflammatory disease may occur less frequently.
painful menstruation;
This may also be the case for NuvaRing® but this has not been
decreased libido;
expulsion of the ring, problems during intercourse and
SIDE EFFECTS AND WHAT TO DO ABOUT THEM
feeling of the ring.
If any of the side effects gets serious, or if you notice any side
Combination hormonal contraceptives (including NuvaRing®) are
effects not listed in this leaflet, please tell your doctor or pharmacist.
not suitable for every woman.
In rare cases the following undesirable effects were reported during
In a small number of women, serious side effects may occur. The
use of NuvaRing®: itching in the genital area, rash, allergic reaction,
most serious side effects of combined hormonal contraceptives
inflammation of the cervix, urinary tract infection, bladder infection,
dizziness, anxiety, diarrhea and vomiting, breast tumor formation,
circulatory disorders (including blood clots in legs, lungs, heart,
breast discharge, back pain, enlarged abdomen, fatigue and penis
discomfort of the partner (such as irritation, rash, itching).
gall bladder disease or liver tumors
SERIOUS SIDE EFFECTS, HOW OFTEN THEY HAPPEN
Contact your doctor as soon as possible if you notice any changes in
AND WHAT TO DO ABOUT THEM
your own health, especially involving any of the items mentioned in this leaflet (see also Warnings and Precautions). Do not forget about
Talk with your doctor
Remove the
the items related to your immediate family.
or pharmacist
ring and call
your doctor or
Be alert for the following symptoms and signs of serious adverse
pharmacist
effects. Call your doctor immediately if they occur:
Uncommon
sharp pain in the chest, coughing blood, or sudden shortness of
sharp pain in chest, coughing
breath. These symptoms could indicate a possible blood clot in
blood, or sudden shortness
of breath/blood clot in the
pain and/or swelling in the calf. These symptoms could indicate
pain in the calf/blood clot in
a possible blood clot in the leg.
crushing chest pain or heaviness. These symptoms could indicate
crushing chest pain or
a possible heart attack.
heaviness/ heart attack
sudden severe or worsening
sudden severe or worsening headache or vomiting, dizziness or
headache or vomiting,
fainting, disturbances of vision or speech, or weakness or
dizziness or fainting,
numbness in an arm or leg. These symptoms could indicate a
disturbance of vision or
possible stroke.
speech, or weakness or
numbness in an arm or
sudden partial or complete loss of vision. This symptom could
indicate a blood clot in the eye.
sudden partial or complete
severe pain or lump in the abdomen. These symptoms could
loss of vision or double vision/blood clot in the eye
indicate a possible tumor of the liver.
severe pain or lump in the
severe depression
abdomen/ liver tumor
yellowing of the skin (jaundice)
severe depression
unusual swelling of the extremities
yellowing of the skin/
breast lumps
unusual swelling of the
With all hormonal contraceptives, for the first few months, you can
breast lumps/ breast cancer
have irregular vaginal bleeding (spotting or breakthrough bleeding)
urinary urgency, frequency,
between your periods. You may need to use sanitary protection, but
burning and/or painful
continue to use NuvaRing® as normal. Irregular vaginal bleeding
urination, and cannot locate
usually stops once your body has adjusted (usually after about
the ring in the vagina/
3 cycles). If it continues, becomes heavy or starts again, tell your
inadvertent insertion of NuvaRing® into the urinary
Users of NuvaRing® have reported the following side effects:
This is not a complete list of side effects. For any unexpected effects
while taking NuvaRing®, contact your doctor or pharmacist.
vaginal discomfort (e.g. vaginal secretion, infection of the

HOW TO STORE IT
MORE INFORMATION
Store NuvaRing® at room temperature (2–30 °C) and avoid direct
This document plus the full product monograph, prepared for
health professionals can be found by contacting the sponsor,
Merck Canada Inc. at: 1-800-567-2594
Do not use a NuvaRing® if it was dispensed to you more than
4 months ago. The dispensing date is shown on the carton and sachet.
This leaflet was prepared by Merck Canada Inc.
Do not use NuvaRing® after the expiry date which is shown on the
Last revised: May 12, 2014
carton and sachet.
® Registered trademark of Merck Sharp & Dohme B.V. Used under
Do not use NuvaRing® if you notice a colour change in the ring or
any visible signs of deterioration.
2011, 2014 Merck Canada Inc., a subsidiary of Merck &Co., Inc.
Keep out of reach of children and pets.
All rights reserved.
If you discover that a child has been exposed to the hormones from
NuvaRing®, ask your doctor for advice.
REPORTING SUSPECTED SIDE EFFECTS
You can report any suspected adverse reactions associated with the use of health products to the Canada Vigilance Program by one of the following 3 ways: • Report online at www.healthcanada.gc.ca/medeffect • Call toll-free at 1-866-234-2345 • Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or - Mail to: Canada Vigilance Program
Postal Locator 0701E
Postage paid labels, Canada Vigilance Reporting Form and the
adverse reaction reporting guidelines are available on the
MedEffect™ Canada Web site at
NOTE: Should you require information related to the
management of side effects, contact your health professional.
The Canada Vigilance Program does not provide medical
advice.
Source: http://www.merck.ca/assets/en/pdf/products/ci/NUVARING-CI_E.pdf
Symposium on Risk versus Hazard Symposium on Risk versus Hazard Risk versus Hazard – How to Regulate in the 21st Century Ragnar E. Lofstedt* In Europe, debate as to whether one should regulate chemicals based on intrinsic hazard or assessment of risk, or possibly a combination of both, has been gaining momentum. This article first provides a brief history of this risk versus hazard debate. Secondly, it ex-amines how European regulators are currently handling the regulation of two chemical compounds, namely Bisphenol A and Deca BDE (a brominated flame retardant), based on forty-five expert interviews with regulators, policy makers and industry representatives in eight Member States, as well as with European Commission officials. The paper shows that there is no clear consensus as to when risk or hazard considerations should be the basis for regulatory decision-making, with wide discrepancies between Member States (e.g. the UK is overall more risk based than Sweden) and between regulatory agencies within Member States. The penultimate section puts forward a series of recommendations to help regula-tors and policy makers develop more consistent and science based regulations for Europe.
MONOGRAPHIE Pr ARIMIDEX® comprimés à 1 mg Inhibiteur de l'aromatase non stéroïdien AstraZeneca Canada Inc. Date de révision : 1004 Middlegate Road 27 avril 2011 Mississauga, Ontario L4Y 1M4 www.astrazeneca.ca Numéro de contrôle : 143918 ARIMIDEX® est une marque de commerce du groupe AstraZeneca.












